Abstract
BACKGROUND:
Raised intracranial pressure (ICP) may lead to increased stiffness of the optic nerve sheath (ONS).
OBJECTIVE:
To develop a method for analyzing ONS dynamics from transorbital ultrasound and investigate a potential difference between patients with raised ICP vs normal ICP.
METHODS:
We retrospectively analyzed data from 16 patients (≤12 years old) for whom ultrasound image sequences of the ONS had been acquired from both eyes just before invasive measurement of ICP. Eight patients had an ICP ≥20 mm Hg. The transverse motion on each side of the ONS was estimated from ultrasound, and Fourier analysis was used to extract the magnitude of the displacement corresponding to the heart rate. By calculating the normalized absolute difference between the displacements on each side of the ONS, a measure of ONS deformation was obtained. This parameter was referred to as the deformability index. According to our hypothesis, because deformability is inversely related to stiffness, we expected this parameter to be lower for ICP ≥20 mm Hg compared with ICP <20 mm Hg. The one-sided Mann-Whitney U test was used for statistical comparison.
RESULTS:
The deformability index was significantly lower in the group with ICP ≥20 mm Hg (median value 0.11 vs 0.24; P = .002).
CONCLUSION:
We present a method for assessment of ONS pulsatile dynamics using transorbital ultrasound imaging. A significant difference was noted between the patient groups, indicating that deformability of the ONS may be relevant as a noninvasive marker of raised ICP. The clinical implications are promising and should be investigated in future clinical studies.
ABBREVIATIONS:
AUC, area under curve
ICP, intracranial pressure
ONS, optic nerve sheath
ONSD, optic nerve sheath diameter
ROC, receiver operating characteristic
KEY WORDS: Dynamic, Intracranial pressure, Noninvasive, Optic nerve sheath
Assessment of intracranial pressure (ICP) remains a fundamental tool in the care of patients with certain neurological disorders. Invasive monitoring of ICP, using microsensor devices placed within the brain parenchyma or transduced external ventricular drains remain the gold standard. These techniques provide valuable diagnostic information, but have specific limitations, with the most significant of these being the risk of infection and hemorrhage.1-3 The indications for ICP monitoring beyond some of the guidelines for severe traumatic brain injury, however, still remain unclear.4,5 This results in unnecessary invasive procedures being performed and highlights the need for a reliable noninvasive technique to estimate ICP. Numerous noninvasive surrogate markers of ICP have been described,4-7 but none of these have yet been able to replace invasive monitoring as the criterion standard technique.
The optic nerve sheath (ONS) is a continuation of the intracranial meninges, and the perineural subarachnoid space surrounding the optic nerve is a septated, trabeculated region filled with cerebrospinal fluid (CSF).8 The ONS compartment represents a cul-de-sac space, which is in communication with the intracranial subarachnoid space, and an increase in CSF transmission to the ONS occurs when ICP is increased.9-11 Consequently, the accumulation of CSF within the perineural space due to increased ICP can result in distension of the ONS and the optic nerve protrusion or flattening of the posterior globe.12 The relationship between ICP and the optic nerve sheath diameter (ONSD) has been described by several authors with varying accuracy.13 The most distinct limitation of these studies is the difficulty in defining the stiffness of the ONS, a parameter that would be extremely helpful in interpreting the ONSD.
OBJECTIVES
We propose that the increased pressure and subsequent distension may lead to increased stiffness of the nerve sheath. Analyzing ONS dynamics may then provide information that is complementary to the static measurements of ONSD. Therefore, the objectives of this study were to develop a method for analyzing in vivo dynamic properties of the ONS using transorbital ultrasound imaging and to investigate a possible relationship with ICP.
METHODS
Hypothesis
Our work was based on the idea that cardiovascular pulsation (ie, caused directly by arterial pulsation or transmission of pulsatility through the CSF) leads to motion of the ONS. Hypothesizing that the ONS becomes stiffer with increasing ICP, we suggest that the transverse motion (ie, perpendicular to the ONS) will be more equal on each side of the nerve with high ICP compared with normal ICP. We quantified this by the absolute difference between the magnitude of the transverse pulsatile displacements on the left (dLeft) and right (dRight) side of the ONS, normalized by the sum of the displacements:
 |
(1) |
The value of this dimensionless parameter indicates how much the ONS deforms during cardiovascular pulsation, and was therefore interpreted physically as a measure of deformability and referred to as the deformability index. According to the hypothesis, because the ability to deform is inversely related to stiffness, we expected this parameter to be smaller in the high ICP group than in the normal ICP group.
Participants
We performed an exploratory research study, retrospectively analyzing data from 16 patients (≤12 years old), managed at the Red Cross War Memorial Children's Hospital (Cape Town, South Africa). Inclusion criteria were: (1) invasive ICP measurement via insertion of a parenchymal microsensor or a ventricular catheter was performed during a diagnostic or therapeutic intervention, and (2) concurrent transorbital ultrasound images of the ONS were acquired. Patients with ocular pathology or trauma to the orbit were excluded. The human research ethics committee of the University of Cape Town and the research committee of the Red Cross War Memorial Children's Hospital approved the study. Informed consent was obtained for all patients enrolled in the study. The demographic details are listed in Table 1.
TABLE 1.
Demographic Dataa

Image Acquisition
A single investigator experienced in the use of transorbital ultrasonography acquired ultrasound images from both eyes using a 15 MHz linear array probe (L15-7io; Philips, Bothell, Washington). The images were acquired after the patients were intubated and ventilated, just prior to insertion of the invasive ICP monitor. The initial and most stable ICP reading acquired from the invasive monitoring was used as a spot ICP reading for comparison with the ultrasound images. Patients were always placed in the supine position, head central, slightly flexed, and elevated to about 30°. The image acquisition process was similar to the technique used for ONSD measurement, and, as such, observer experience and variability remain important issues. The heart rate was recorded and ultrasound acquisition was performed when the hemodynamic parameters were stable. The image depth varied from 3 to 5 cm and spatial image resolution varied from 0.06 to 0.11 mm per pixel. The duration of each image sequence was 5 to 10 seconds, and the temporal resolution varied from 40 to 56 frames per second. The ultrasound image depicting the ONS structures and the pixels used for tracking are described in Figure 1.
FIGURE 1.

Axial transorbital ultrasound image demonstrating the optic nerve (ON). Cerebrospinal fluid (CSF) in the perioptic subarachnoid space (SAS), optic nerve sheath (ONS). V represents a manually selected point indicating where the motion is analyzed, and the white rectangle illustrates the pixels used for tracking.
Image Processing
The objective of the image processing was to exploit the high temporal resolution of the ultrasound images for analyzing motion related to cardiovascular pulsation on each side of the optic nerve sheath. The approach is explained in Figure 2 and in the following text.
FIGURE 2.

Illustration of the image processing in patients with ICP <20 mm Hg (Left) and ICP ≥20 mm Hg (Right). Upper, transorbital ultrasound images, zoomed in on the optic nerve sheath complex. Cardiovascular pulsation causes a motion dLeft on the left side of the ONS and a motion dRight on the right side. The rectangles show the regions used for tracking the motion. Middle, transverse pulsatile displacements as a function of time (vertical axis) after extraction of the motion component corresponding to the fundamental heart rate frequency. Note that the curves are strongly zoomed in compared with the images in the upper row (the squares are 25 pixels wide; pulsation is approximately 0.1 pixel). Lower, the same curves superimposed, with transverse pulsatile displacement along the vertical axis and time along the horizontal axis. Note that the displacements are more symmetric on each side of the optic nerve for the patient with ICP ≥20 mm Hg in comparison with the patient with ICP <20 mm Hg. This is consistent with the idea that raised ICP makes the ONS stiffer, which could be observed as more equal transverse motion on each side of the ONS (ie, less deformation). ICP, intracranial pressure; ONS, optic nerve sheath. Color version available online only.
First Step: Tracking
Tracking was initialized by manually selecting a point at similar depths on both sides of the ONS in the first frame of each image sequence. The motion was then automatically tracked over the entire sequence using normalized 2-dimensional cross-correlation from frame to frame for a region of interest (25 by 61 pixels) around the selected points. The ultrasound data were interpolated, and parabolic approximation was applied to the correlation matrix for subpixel motion estimation. The motion component transverse to the nerve (ie, in the horizontal image direction) was extracted for further analysis.
Second Step: Fourier Analysis
To extract the motion that was related to the cardiovascular pulsation, we applied Fourier analysis to obtain the frequency components of the transverse motion. The magnitude of the (fundamental) frequency component corresponding to the heart rate of each patient was extracted for the left and right side of the ONS in each data set, yielding the transverse pulsatile displacements dLeft and dRight, respectively.
The algorithm was implemented in Matlab (MathWorks, Natick, Massachusetts).
Data Analysis and Statistics
Because the data were retrospectively analyzed, we expected some out-of-plane motion, which is known to deteriorate correlation-based tracking; therefore, a blinded operator graded each data set on a scale from 0 to 2: grade 0: steady acquisition, barely perceivable probe movement; grade 1: perceivable probe motion, no loss of ONS appearance; and grade 2: distinct probe movement with some loss of ONS appearance.
Seven data sets scoring grade 2 were excluded, leaving 25 data sets for further analysis.
The motion analysis was run 5 times for the left and right sides of the optic nerve sheath for each data set to account for variability due to the manual initialization of the tracking region. The mean of the 5 displacement values was used as the motion estimate, and the variation was quantified by using pooled standard deviation.
The 25 data sets were split into a group with ICP ≥20 mm Hg, and a group with ICP <20 mm Hg, comprising 10 and 15 data sets, respectively. The Δ was calculated by using Equation 1, and the one-sided Mann-Whitney U test was used to statistically compare the 2 groups, with significance level P < .05. Diagnostic accuracy was investigated using receiver operating characteristic (ROC).
RESULTS
Descriptive Data
We analyzed a total of 25 data sets. The transverse pulsatile displacement at each side of the ONS was assessed 5 times for each data set. The mean transverse pulsatile displacement of the ONS was 8.3, with a pooled standard deviation of 0.54 measured in percentage of a pixel.
Main Results
The deformability index of the ONS was calculated for each data set. The median was Δ = 0.11 for the high ICP group compared with Δ = 0.24 for the normal ICP group (P = .002). Figure 3 shows a box plot illustrating the median and spread for each group. Results for each patient are included in Table 2.
FIGURE 3.

Box plot illustrating the deformability index (Δ) of the ONS for the high and normal ICP groups. The box plot shows median, 25 and 75 percentiles, and range. ICP, intracranial pressure; ONS, optic nerve sheath.
TABLE 2.
Resultsa,b

ROC analysis gave an area under curve (AUC) of 0.85 (95% confidence interval, 0.61-0.97) (Figure 4). Choosing a cutoff value of Δ = 0.121 yielded a sensitivity of 90% and a specificity of 87%. With the use of this cutoff, 3 of 25 (12%) data sets would be wrongly classified.
FIGURE 4.
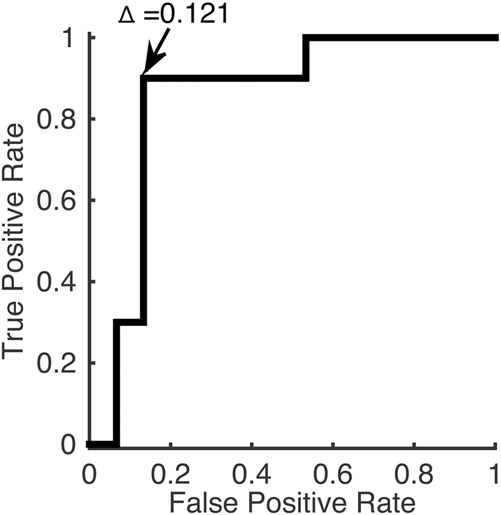
Receiver operator curve. Area under the curve (AUC) was 0.85. A cutoff of Δ = 0.121 gave a sensitivity of 90% and a specificity of 87%.
DISCUSSION
The aims of this study were to develop a method for analyzing the pulsatile dynamic properties of the ONS using transorbital ultrasound imaging and to investigate a possible relationship with ICP. Specifically, we proposed a hypothesis stating that increased ICP leads to increased stiffness (ie, reduced deformability) of the nerve sheath.
Key Results
The most important finding was the difference between the deformability of the ONS in the group with ICP ≥20 mm Hg compared with the group with ICP <20 mm Hg, thus clearly supporting the hypothesis. ROC analysis showed an AUC = 0.85, and suggested a cutoff value of Δ = 0.121, with lower values indicating raised ICP and higher values indicating normal ICP.
Limitations
The main limitations of this study were the relatively small number of patients, and the fact that the analysis was performed retrospectively and not in a blinded fashion. The pediatric cohort examined in this study warrants further validation of the technique in an adult study population. The study was performed in a controlled environment with patients under general anesthesia for invasive ICP measurement. Although the stability of the hemodynamic parameters were maintained between image acquisition and ICP measurement, the effect of the anesthetic drugs on the measurement is not known. Therefore, the findings in this study also will require validation in awake patients. The image acquisition was performed by a single investigator experienced in the use of transorbital ultrasound, and is thus also limited by the issue of observer variability. Furthermore, although an exploratory research design was suitable for providing insight into the unknown relation between ONS motion and ICP, the nature of the design may limit the ability to draw definite conclusions, particularly regarding issues such as acute change vs long-standing ICP. Compared with invasive ICP monitoring, which can provide continuous, real-time assessment of ICP patterns, the described technique of assessing the pulsatile dynamics of the ONS currently only provides a spot assessment of ICP. Notwithstanding these limitations, the difference between the 2 groups in this study is quite convincing.
Previously, it has been shown that the retrobulbar segment of the ONS is distensible and therefore dilates when ICP is increased.14,15 The technique of ONSD measurement has gained steady support as a noninvasive surrogate marker of raised ICP. However, measurement of the ONSD does not yet provide an accurate assessment of ICP, largely because the optimal cutoff point for the ONSD measurement in patients with normal vs raised ICP has varied considerably.16-25 The noted variation in ONSD between studies is likely attributable to a more complex relationship between the ONS and ICP, involving variability in the stiffness at different ICP thresholds. The magnitude of ONS distension caused by the increase in pressure within the subarachnoid space may depend on a variety of factors, including the degree to which ICP is increased, the rapidity of the increase in ICP, and the elastic characteristics of the ONS, which influence the capability for distension and retraction of the ONS.26 The relationship between ICP and nerve sheath distension is therefore integrally related to the stiffness of the sheath itself.
Although we identified some articles measuring the static diameter at different time points with subsequent comparison of individual measurements,14,27-29 our search did not reveal any published data describing dynamic imaging of the ONS over the cardiac cycle to assess in vivo dynamic characteristics of the ONS.
Interpretation
In this study, we investigated how pulsatile forces deform the ONS dynamically during the cardiac cycle rather than the absolute distention related to the increased pressure within the ONS. Therefore, we believe that our approach may provide information that is complementary to the ONSD and likely contributes to an overall improvement in assessing the ONS in cases of suspected increased ICP, either as an individual marker or by augmenting the interpretation of ONSD measurements. The concept of pulsatile dynamics of the ONS could improve specificity, for example, in comparison with ONSD alone, by differentiating between pathologically distended ONS due to raised ICP and widened ONS not related to raised ICP.
Because the data were retrospectively analyzed, image acquisition was not optimized with respect to avoiding out-of-plane motion, which may deteriorate correlation-based tracking. To investigate the effect of the out-of-plane motion, we also processed the excluded data sets. We found that if all data sets were included, the median of the groups with ICP above and below 20 mm Hg would still be significantly different (P = .03). However, the AUC would be reduced to 0.69 (95% confidence interval, 0.46-0.87), and 7 of 32 (22%) data sets would have been wrongly classified. The example in Figure 5 shows the influence of out-of-plane motion for the (excluded) right eye data set of patient A. In this data set, a clear out-of-plane motion occurs at approximately 3 seconds into the sequence. Therefore, an option for this data set may be to constrain the analysis to the period from 4 to 10 seconds. Instead, we chose to let a blinded operator grade the data sets and exclude those interpreted to have extensive out-of-plane motion. Although improving the study, the grading is to some extent subjective, with no definite cutoff. Indeed, some out-of-plane motion could still be seen in some of the remaining images, and therefore may still be a possible explanation to why the approach failed for some of the data sets. The possibility of minimizing out-of-plane motion should be taken into account in protocols for future studies.
FIGURE 5.

Illustrating the effect of out-of-plane motion (patient A, right eye). Upper, the ultrasound image sequence at 2, 3, and 4 seconds into the sequence. The appearance of the ONS at 3 seconds is clearly different compared with the appearance at 2 and 4 seconds, thus scoring an out-of-plane motion grade 2 (and hence excluding it from the analysis). Lower, transverse motion at left (blue line) and right side (black) of the ONS (before extraction of the heart rate frequency component). At approximately 3 seconds, the processing method interpreted the out-of-plane motion as if the left side had a large pulsatile motion, with duration approximately the same as the heart cycle (78 bpm [ie, 0.77 second period]). Therefore, the analysis failed for this data set. ONS, optic nerve sheath. Color version available online only.
Also, the calculated displacements were small, and extracted by extensive spatial (region of interest window) and temporal (Fourier) averaging. Accuracy, reproducibility, and possible technical refinements of this novel ultrasound processing method should be further studied. Finally, it could be of interest to consider additional information, for example, the longitudinal motion, phase content of the Fourier transform (ie, the delay between motion at the left and right side of the nerve), and perhaps motion components other than the fundamental heart rate frequency.
CONCLUSION
This study illustrates the feasibility of noninvasive transorbital ultrasound for assessing optic nerve sheath pulsatile dynamics. The preliminary results demonstrate a significant difference between patient groups with ICP above and below 20 mm Hg, and thus support the suggested hypothesis of a relationship between deformability of the ONS and intracranial pressure. Therefore, the suggested measure may be relevant as a noninvasive marker for distinguishing between raised and normal ICP. To further investigate the hypothesis and the clinical value of the method, a validation in larger prospective clinical studies using blinded analysis is required.
Disclosures
Approval for the study was granted by the Human Research Ethics Committee of the University of Cape Town and the research committee of the Red Cross War Memorial Children's Hospital. Informed consent was granted by the parents/guardians for all patients included in the study. This work was funded by SINTEF (Trondheim, Norway) and University of Cape Town, South Africa. Drs Brekken and Selbekk were funded by SINTEF (Trondheim, Norway). Dr Padayachy was funded by the Harry Crossley Research Fellowship grant and the University of Cape Town. All authors have a patent pending for the method for detecting pulsatile dynamics of the optic nerve sheath, diagnostic methods, medical uses, noninvasive markers, systems and transducer devices. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Footnotes
These authors contributed equally to this work.
COMMENTS
The authors describe the reliability of optic nerve sheath (ONS) stiffness as a noninvasive marker of raised ICP. They use the deformability index as the marker of ONS stiffness. To this purpose, they compare data from the ultrasound image sequences of the ONS with the intracranial pressure values obtained with standard invasive measurement in 16 pediatric patients.
The authors conclude that the deformability index was significantly lower in the group of patients with lower intracranial pressure (ICP ≥20 mm Hg).
The article is well written, concise, and clearly illustrates the methodology and results to the reader.
Francesco Tomasello
Messina, Italy
The authors report to us on the “Pulsatile Dynamics of the Optic Nerve Sheath and Intracranial Pressure: An Exploratory In Vivo Investigation” and describe their experience with a rather novel technique for assessing the stiffness of the optic nerve sheath (ONS) as a noninvasive marker of raised intracranial pressure (ICP) using analysis of ONS dynamics from transorbital ultrasound. They claim that this technique adds significantly to current understanding of the response of the ONS to changes in ICP. While preliminary, this is rather interesting and important research. Measurements of ICP to date require the invasive insertion of a probe of some kind in the cranial vault, preferably in the brain parenchyma or the ventricular cavities of the lateral ventricles. Equally known is the anatomic relationships that make the optic nerve a part of the central nervous system, which is surrounded by the dura mater, the subarachnoid space, and cerebrospinal fluid. The ONS provides a window into the CNS and any change in ICP affects the optic nerve sheath, in effect, changing its diameter.
The authors retrospectively analyze 16 patients where intraparenchymal ICP measurements along with concurrent transorbital ultrasound images of the ONS were acquired. Patients with ocular pathology or trauma to the orbit were excluded. While the placement of an invasive ICP monitor may suggest traumatic pathology, it is important to note that the majority of the patients were reported to have an underlying diagnosis affecting CSF dynamics and associated with possible long-standing intracranial pressures (11 of 16 patients, 69%), 3 patients had large tumors (2 in the posterior fossa) and 2 patients had sustained trauma without further specification. The authors hypothesized that the ONS becomes stiffer with increasing ICP, we suggest that the transverse motion (ie, perpendicular to the ONS will be more equal on each side of the nerve with high ICP compared with normal ICP). Given the collection of pathologies, it seems that this technique is able to anecdotally distinguish between low and high ICP due to long-standing or chronic ICP elevation with a fairly high degree of certainty. It remains rather unclear whether this technique can differentiate between patients with acute elevations of ICP, possibly even compartmentalized ICP problems, and patients without such problems. The issues surrounding the retrospective study type and low number of patients (especially the possibly most interesting patients with a trauma) are predictable shortcomings of this report. Blaivas et al1 performed a prospective, blinded observational study on emergency department patients with a suspicion of elevated ICP due to possible focal intracranial pathology in 35 patients and reported that the sensitivity and specificity for ONS diameter based ICP range prediction, when compared with CT results, were 100% and 95%, respectively.
The technique may well have usability in acute changes in ICP, but this will need to be evaluated in a prospective study where, ideally, the measurement is performed before and after treatment of raised ICP, allowing assessment after an acute change in ICP.2 Other, perhaps minor issues that would have to be worked out in larger patient populations are that accurate fundoscopy can be vital to the appropriate diagnosis and treatment of patients with suspected elevated intracranial pressure, but it is often technically difficult or poorly tolerated by the photophobic patients. It also remains uncertain whether there is a way to calibrate the findings against an absolute pressure scale in a given patient without requiring concurrent invasive pressure measurements and how fine of a scale would be possible based on the average ultrasound resolution and dynamic measurement errors.
Clemens M. Schirmer
Wilkes-Barre, Pennsylvania
- 1.Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003;10(4):376-381. [DOI] [PubMed] [Google Scholar]
- 2.Amin D, McCormick T, Mailhot T. Elevated intracranial pressure diagnosis with emergency department bedside ocular ultrasound. Case Rep Emerg Med. 2015;2015:385970. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Czosnyka M, Pickard J. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75(6):813-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiegand C, Richards P. Measurement of intracranial pressure in children: a critical review of current methods. Dev Med Child Neurol. 2007;49(12):935-941. [DOI] [PubMed] [Google Scholar]
- 3.Padayachy L, Figaji A, Bullock RS. Intracranial pressure monitoring for traumatic brain injury in the modern era. Childs Nerv Syst. 2010;26(4):441-452. [DOI] [PubMed] [Google Scholar]
- 4.Bratton SL, Chestnut RM, Ghajar J, et al. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical care, AANS/CNS. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(suppl 1):S37-S44. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg JB, Shiloh AL, Savel RH, Eisen LA. Non-invasive methods of estimating intracranial pressure. Neurocrit Care. 2011;15(3):599-608. [DOI] [PubMed] [Google Scholar]
- 6.Kristiansson H, Nissborg E, Bartek J, Jr, Andresen M, Reinstrup P, Romner B. Measuring elevated intracranial pressure through noninvasive methods: a review of the literature. J Neurosurg Anesthesiol. 2013;25(4):372-385. [DOI] [PubMed] [Google Scholar]
- 7.Beau B. Non-invasive assessment of cerebrospinal fluid pressure. J Neuroophthalmol. 2014;34(3):288-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 2003;87(6):777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18(4):323-328. [DOI] [PubMed] [Google Scholar]
- 10.Killer HE, Jaggi GP, Flammer J, Miller NR, Hubner AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain. 2007;130(pt 2):514-520. [DOI] [PubMed] [Google Scholar]
- 11.McAuley D, Paterson A, Sweeney L. Optic nerve sheath ultrasound in the assessment of paediatric hydrocephalus. Childs Nerv Syst. 2009;25(1):87-90. [DOI] [PubMed] [Google Scholar]
- 12.Kramer LA, Sargsyan AE, Hasan KM, Polk JD, Hamilton DR. Orbital and intracranial effects of microgravity: findings at 3-T MR Imaging. Radiology. 2012;263(3):819-827. [DOI] [PubMed] [Google Scholar]
- 13.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068. [DOI] [PubMed] [Google Scholar]
- 14.Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87(1):34-40. [DOI] [PubMed] [Google Scholar]
- 15.Geeraerts T, Merceron S, Benhamou D, Vigue B, Duranteau J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008;34(11):2062-2067. [DOI] [PubMed] [Google Scholar]
- 16.Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. Pediatr Radiol. 1996;26(10):701-705. [DOI] [PubMed] [Google Scholar]
- 17.Newman W, Hollman A, Dutton G, Carachi R. Measurement of the optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 2002;86(10):1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimberly H, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008;15(2):201-204. [DOI] [PubMed] [Google Scholar]
- 19.Soldatos T, Chatzimichali M, Papathanasiou M, Gouliamos A. Optic nerve sonography: a new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J. 2009;26(9):630-634. [DOI] [PubMed] [Google Scholar]
- 20.Moretti R, Pizzi B, Cassini F, Vivaldi N. Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care. 2009;11(3):406-410. [DOI] [PubMed] [Google Scholar]
- 21.Rajajee V, Vanaman M, Fletcher J, Jacobs T. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15(3):506-515. [DOI] [PubMed] [Google Scholar]
- 22.Strumwasser A, Kwan R, Yeung L, et al. Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res. 2011;170(2):265-271. [DOI] [PubMed] [Google Scholar]
- 23.Malayeri A, Bavarian S, Mehdizadeh M. Sonographic evaluation of optic nerve diameter in children with raised intracranial pressure. J Ultrasound Med. 2005;24(2):143-147. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe A, Kinouchi H, Hirokoshi T, Uchida M, Ishigame K. Effect of intracranial pressure on the diameter of the optic nerve sheath. J Neurosurg. 2008;109(2):255-258. [DOI] [PubMed] [Google Scholar]
- 25.Shofty B, Ben-Sira L, Constanini S, Freedman S, Kesler A. Optic nerve sheath diameter on MR Imaging: establishment of norms and comparison of pediatric patients with idiopathic intracranial hypertension with healthy controls. Am J Neuroradiol. 2012;33(2):366-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen HC, Lagreze W, Krueger O, Helmke K. Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure: an experimental study. Acta Ophthalm. 2011;89(6):528-532. [DOI] [PubMed] [Google Scholar]
- 27.Driessen C, van Veelen ML, Lequin M, Joosten KF, Mathijssen IM. Nocturnal ultrasound measurements of optic nerve sheath diameter correlate with intracranial pressure in children with craniosynostosis. Plast Reconstr Surg. 2012;130(3):448e-451e. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Min HG, Ha SI, Jeong HW, Seo H, Kim JU. Dynamic optic nerve sheath diameter responses to short-term hyperventilation measured with sonography in patients under general anesthesia. Korean J Anesthesiol. 2014;67(4):240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33(6):860.e5-860.e7. [DOI] [PubMed] [Google Scholar]


