Abstract
Sequences of the internal transcribed spacers of nuclear ribosomal DNA (ITS-1 and ITS-2) were determined for species of the genus Rugopharynx and Rugonema labiatum, nematodes from the stomachs of macropodid marsupials. Phylogenetic analyses of the aligned sequence data were conducted. The relationships provided molecular support for all species currently recognised, some of which are based on minor morphological differences and on multilocus enzyme electrophoretic data, but also indicated that additional, cryptic species exist within the genus. In addition, the genus Rugonema is placed as a synonym of Rugopharynx, its sole species becoming Rugopharynx labiatum n. comb. The molecular data provided some insights into the evolution of complex buccal capsule morphologies within the genus, but there was no evidence of co-evolution between the macropodid hosts and their parasites.
Keywords: Rugopharynx, Strongylida, Cloacininae, Macropodidae, Phylogeny, Cryptic species
Graphical abstract
Highlights
-
•
There was evidence of cryptic species within the nematode genus Rugopharynx.
-
•
Molecular data provided insights into the evolution of complex buccal capsule morphologies.
-
•
There was no evidence of coevolution between macropodid hosts and their parasites.
1. Introduction
The sacculated forestomachs of kangaroos and wallabies (family Macropodidae) commonly contain large numbers of nematodes belonging to the strongylid sub-family Cloacininae. For example, Vendl and Beveridge (2014) reported means and ranges in numbers of cloacinine nematodes in the stomachs of the red-necked wallaby, Macropus rufogriseus, the eastern grey kangaroo, Macropus giganteus and the swamp wallaby, Wallabia bicolor, as 60,800 (2000–210,000), 20,500 (7000–79,000) and 20,000 (3000–58, 000), respectively. These data support earlier published figures for high intensities of infection in the western grey kangaroo, Macropus fuliginosus, the red kangaroo, Macropus rufus, and M. giganteus (Arundel et al., 1979, Beveridge and Arundel, 1979).
Although there is considerable species diversity in the stomach-inhabiting cloacinine nematodes (Spratt et al., 1991), members of the genus Rugopharynx are prominent representatives of these nematode communities. For instance, Rugopharynx australis dominates the gastric helminth community of M. rufus, with mean and maximum burdens of 47,000 and 266,000 nematodes, respectively (Arundel et al., 1979). In spite of the numerical significance of this genus in the gastric helminth communities of macropodids, continuing taxonomic studies are needed, as it is unlikely that all species have yet been described and the presence of cryptic species could potentially complicate the interpretation of ecological data published to date. The genus Rugopharynx was revised by Beveridge (1982), who recognised nine species. However, Beveridge (1982) noted that R. australis was potentially a complex of a number of species, differentiable only by very minor and often overlapping morphological characteristics. Multilocus enzyme electrophoretic (MEE) studies, combined with morphological evidence, indicated the existence of two new species, R. sigma and Rugopharynx mawsonae, both formerly confused with Rugopharynx zeta (Chilton et al., 1993, Beveridge et al., 1994), while an additional MEE investigation of R. australis by Chilton et al. (1996) provided evidence for at least seven species within this taxon. Subsequently, Beveridge and Chilton (1999) split R. australis into 10 species and resurrected R. alpha as a valid species. The latter revision was based on extrapolating the minor morphological differences identified in samples included in the earlier electrophoretic study across the entire species complex. In spite of this progress, there has been no attempt to independently verify the validity of the species erected to date using molecular methods. A closely related genus, Rugonema was erected by Beveridge (1999) for specimens formerly referred to as R. australis occurring in the stomach of the black-gloved wallaby, Macropus irma, from Western Australia, based on morphological differences in the labial collar. Because of this close association with Rugopharynx, Rugonema labiatum was also included in the present study.
Given the prevalence and abundance of this nematode genus in the stomachs of kangaroos and wallabies, and the diversity of species currently recognised based on minor morphological criteria, this study was undertaken to attempt to establish species boundaries within the genus based on sequences of the first and second internal transcribed spacers (ITS-1 and ITS-2) of nuclear ribosomal DNA. These molecular target regions have proved to be highly informative for the specific identification of a range of strongylid nematodes, including taxa within the Cloacininae (Chilton, 2004), the subfamily to which Rugopharynx belongs.
2. Materials and methods
2.1. Collection, storage and preparation of nematodes
Nematodes were obtained from the stomachs of a range of kangaroos and wallabies (Fig. 1; Table 1), which had been collected as fresh road-kills or from road-kills frozen prior to examination. Host nomenclature follows Van Dyck and Strahan (2008). In instances where a nematode species occurred across a large geographical area, an attempt was made to include samples from different geographical regions of Australia, particularly any occurring on the island of Tasmania (Fig. 1). Australian state names are abbreviated as: NSW, New South Wales; Qld, Queensland; SA, South Australia; Tas, Tasmania; Vic, Victoria; WA, Western Australia. Nematodes were washed in saline, frozen in liquid nitrogen and stored at −80 °C until morphological and molecular studies were undertaken. Nematodes were then thawed, the head and tail of each worm removed, fixed in lactophenol and mounted permanently in polyvinyl lactophenol as voucher specimens. Nematodes were identified according to previous descriptions (Beveridge, 1982, Beveridge et al., 1994, Chilton et al., 1993, Beveridge and Chilton, 1999, Appan et al., 2004). Voucher specimens have been deposited in the South Australian Museum (SAM), Adelaide (Table 1). The mid-body region was used for genetic analyses.
Fig. 1.
Localities within Australia at which specimens of Rugopharynx used in this study were collected. Coordinates for each locality are provided in Table 1. 1, Lake Clifton; 2, Waroona; 3, Collie, Wellington Dam; 4, Perup River; 5, Kalgoorlie; 6, Wallerberdina Station; 7, Port Augusta; 8, Ashbourne; 9, Kangaroo Island; 10, Naracoorte; 11, Hattah Lakes National Park; 12, Yan Yean; 13, The Gurdies; 14, Launceston; 15, Emu Flat, Bondo State Forest; 16, Trangie; 17, Grafton; 18, Lamington National Park; 19, Miles; 20, Dawes; 21, Mt Sebastopol; 22, Rockhampton; 23, Winton; 24, Proserpine; 25, Bowen; 26, Magnetic Island; 27, Lake Barrine.
Table 1.
Specimens of Rugopharynx and Rugonema labiatum used in molecular analyses with collection data, deposition of morphological voucher specimens, and GenBank registration numbers for sequences.
| Parasite species | Host species a | Specimen Code | Locality b | Coordinates | SAM voucher no. | GenBank no. | Buccal Capsule Type |
|---|---|---|---|---|---|---|---|
| R. alpha | P. purpureicollis | F886 | Winton, Qld | 22° 23′S 143°3′E | 36137 | LN906946 | I |
| R. australis | M. rufus | F739 | Wallerberdina Stn via Pt Augusta, SA | 31° 43′S 138° 7′E | 36138 | LN906947 | I |
| G97 | Kalgoorlie, WA | 30° 44′S 121° 27′E | 36209 | LN906948 | |||
| M. giganteus | F751 | Trangie, NSW | 32° 2′S 147° 59′E | 36139 | LN906949 | ||
| F754 | Trangie, NSW | 32° 2′S 147° 59′E | 36140 | LN906950 | |||
| M. dorsalis | F758 | Miles, Qld | 26° 40′S 150° 11′E | 36141 | LN906951 | ||
| M. robustus | F704 | Pt Augusta, SA | 32° 30′S 137° 46′E | 36142 | LN906952 | ||
| M. fuliginosus | F784 | Hattah Lakes, National Park, Vic | 34° 42′S 142° 17′E | 36143 | LN906953 | ||
| R. chi | T. billardierii | F716 | Launceston, Tas | 41° 26′S 147° 8′E | 36144 | LN906954 | I |
| R. delta | M. dorsalis | F97 | Rockhampton, Qld | 23° 16′S 150° 49′E | 36145 | LN906955 | III |
| R. epsilon | M. rufogriseus | F72 | Naracoorte, SA | 36° 57′S 140° 44′E | 36146 | LN906956 | IIA |
| F372 | Launceston, Tas | 41° 26′S 147° 8′E | 36147 | LN906957 | |||
| F76 & F77 | Emu Flat, NSW | 34° 35′S 149° 42′E | 36148 | LN906958 | |||
| LN906959 | |||||||
| W. bicolor | F80 | Emu Flat, NSW | 34° 35′S 149° 42′E | 36149 | LN906960 | ||
| R. longibursaris | M. rufogriseus | F373 | Launceston, Tas | 41° 26′S 147° 8′E | 36150 | LN906961 | III |
| F100 | Naracoorte, SA | 36° 57′S 140° 44′E | 36151 | LN906962 | |||
| R. macropodis | M. fuliginosus | F731 | Naracoorte, SA | 36° 57′S 140° 44′E | 36152 | LN906963 | I |
| F736 | Kangaroo Island, SA | 35° 49′S 137° 12′E | 36153 | LN906964 | |||
| M. giganteus | F727 | Emu Flat, NSW | 34° 35′S 149° 42′E | 36212 | LN906965 | ||
| R. mawsonae | M. dorsalis | F108-F110 | Rockhampton, Qld | 23° 23′S 150° 30′E | 36154 | LN906966 | III |
| LN906967 | |||||||
| LN906968 | |||||||
| R. mu | W. bicolor | YE1 | The Gurdies, Vic | 38° 23′S 145° 34′E | 30903 | LN906969 | I |
| F510 | Emu Flat, NSW | 34° 35′S 149° 42′E | 36155 | LN906970 | |||
| R. omega | M. rufogriseus | F107 | Grafton, NSW | 29° 41′S 152° 56′E | 36156 | LN906971 | III |
| R. pi | M. rufogriseus | F710 | Grafton, NSW | 29° 41′S 152° 56′E | 36157 | LN906972 | I |
| F712 | Emu Flat, NSW | 34° 35′S 149° 42′E | 36158 | LN906973 | |||
| M. parryi | F750 | Dawes, Qld | 24° 40′S 151° 15′E | 36159 | LN906974 | ||
| R. rho | M. eugenii | F724 | Kangaroo Island, SA | 35° 49′S 137° 12′E | 36160 | LN906975 | I |
| F905 | Perup River, WA | 34° 24′S 116° 26′E | 36161 | LN906976 | |||
| M. fuliginosus | F780 | Ashbourne, SA | 35° 17′S 138° 46′E | 36162 | LN906977 | ||
| F910 & F911 | Waroona, WA | 32° 51′S 115° 59′S | 36163 | LN906978 | |||
| M. irma | G114 | Lake Clifton, WA | 32° 47′S 115° 40′E | 36164 | LN906979 | ||
| R. rosemariae | M. giganteus | F889 | Yan Yean. Vic | 37° 33′S 145° 10′E | 36165 | LN906980 | I |
| R. rufogrisea | M. rufogriseus | F84 | Emu Flat, NSW | 34° 35′S 149° 42′E | 36166 c | LN906981 | IIB |
| F720 | Launceston, Tas | 41° 26′S 147° 8′E | 36167 | LN906982 | |||
| M. parryi | F381 | Dawes, Qld | 24° 40′S 151° 15′E | 36168 | LN906983 | ||
| R. setonicis | S. brachyurus | F872 | Wellington Dam, WA | 30° 16′S 152° 52′E | 36169 | LN906984 | I |
| R. sigma | T. thetis | F794 | Lamington National Park, Qld | 28° 15′S 153° 8′E | 36170 | LN906985 | III |
| T. stigmatica | G384 | Lamington National Park, Qld | 28° 15′S 153° 8′E | 36210 | LN906986 | ||
| F92 | Lake Barrine, Qld | 17° 15′S 145° 38′E | 36171 | LN906987 | |||
| R. spratti | M. rufogriseus | F375 | Launceston, Tas | 41° 26′S 147° 8′E | 36172 | LN906988 | I |
| F770 | Emu Flat, NSW | 34° 35′S 149° 42′E | 36173 | LN906989 | |||
| R. tau | T. thetis | F793 | Lamington National Park, Qld | 28° 15′S 153° 8′E | 36174 | LN906990 | I |
| R. theta | M. dorsalis | F112 | Bowen, Qld | 20° 1′S 148° 15′E | 36175 | LN906991 | I |
| R. zeta | P. assimilis | F88 | Magnetic Island, Qld | 19° 8′S 146° 50′E | 36176 | LN906992 | III |
| P. inornata | G110 | Proserpine, Qld | 20° 24′S 148° 35′E | 36177 | LN906993 | ||
| P. herberti | G152 | Mt Sebastopol, Qld | 20° 43′S 146° 42′E | 36178 | LN906994 | ||
| Rugonema labiatum | M. irma | F875 | Collie, WA | 33° 22′S 116° 9′E | 36179 | LN906995 | I |
Abbreviations of host generic names: M., Macropus; P., Petrogale; S., Setonix; T., Thylogale; W., Wallabia.
Abbreviations and contractions of Australian state names: NSW, New South Wales; Qld, Queensland; SA, South Australia; Tas, Tasmania; Vic, Victoria; WA, Western Australia.
Specimen deposited was F87.
2.2. Morphological methods
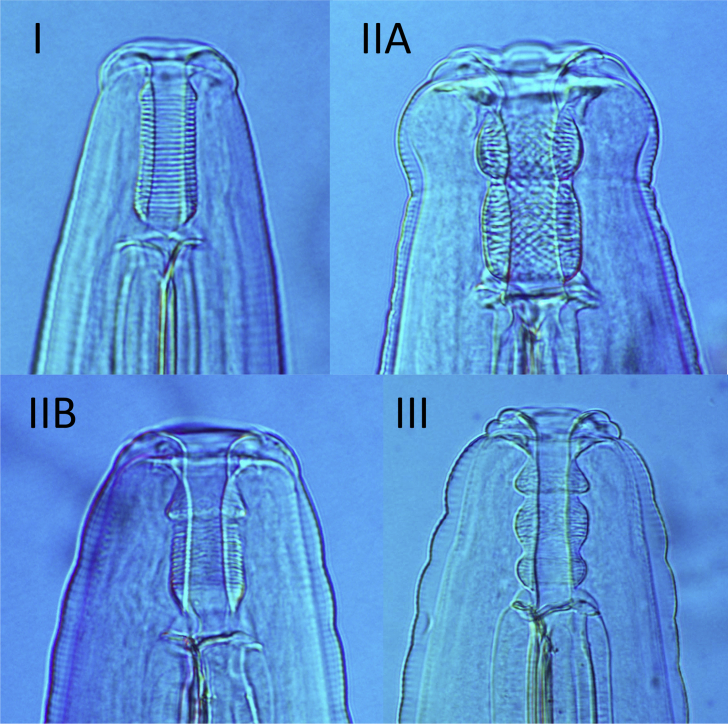
Species within the genus Rugopharynx were divided into three groups based on the morphology of the buccal capsule (Table 1). Nematodes with a simple, cylindrical buccal capsule were designated as type I (Fig. 2), while those with a buccal capsule divided into two sections were designated as type II. These buccal capsules were further subdivided into species with a buccal capsule divided in the mid region (R. epsilon) (type IIA) and those in which the division occurred in the anterior quarter (R. rufogrisea) (type IIB). Species with a buccal capsule divided into three segments were designated as type III. In some species, such as R. spratti and R. tau, the potential division of the buccal capsule is subtle; hence, in these cases, species were classified as type I.
Fig. 2.
Morphological buccal capsule types in the genus Rugopharynx. I, simple cylindrical buccal capsule, R. macropodis; IIA, bilobed buccal capsule with subequal divisions, R. epsilon; IIB, bilobed buccal capsule with anterior lobe shorter, R. rufogrisea; III, trilobed buccal capsule, R. longibursaris.
2.3. Molecular methods and phylogenetic analysis
Genomic DNA was isolated from the mid-body part of each nematode using a small-scale sodium-dodecyl-sulphate/proteinase K extraction method (Gasser et al., 1993), followed by purification using a mini-column (Wizard™ Clean-Up, Promega). The region of rDNA comprising the ITS-1, 5.8S rRNA gene, ITS-2 and flanking sequences (= ITS+) was amplified by the PCR using primers NC16 (forward; 5′-AGTTCAATCGCAATGGCTT-3′) and NC2 (reverse; 5′-TTAGTTTCTTTTCCTCCGCT-3′). PCR was performed in a 50 μl volume for 30 cycles at 94 °C for 30 s (denaturation), 55 °C for 30 s (annealing) and 72 °C for 30 s (extension), followed by one cycle at 72 °C for 5 min (final extension). Negative (no-DNA) controls were included in each set of reactions. Amplicons were purified using mini-columns (using Wizard™ PCR-Preps, Promega), and the ITS+ sequenced in both directions using the same primers (separately) as used for PCR. The sequences generated in the present study have been deposited in the GenBank database (accession numbers LN906946-LN906995; Table 1). Sequences were initially aligned using the program Muscle (Edgar, 2004), and alignments adjusted manually using the program Mesquite v.2.75 (Maddison and Maddison, 2011).
Phylogenetic analyses of the aligned sequence data were conducted by Bayesian inference (BI) using Monte Carlo Markov Chain analysis in the program MrBayes v.3.2.2 (Ronquist and Huelsenbeck, 2003). The likelihood parameters set for the BI analysis of sequence data were based on the Akaike Information Criteria test in jModeltest v.2.1.5 (Posada, 2008). The number of substitutions was set at 6, with a gamma-distribution. For the tree, posterior probability (pp) values were calculated by running 2,000,000 generations with four simultaneous tree-building chains. Trees were saved every 100th generation. At the end of each run, the standard deviation of split frequencies was <0.01, and the potential scale reduction factor approached one. For each analysis, a 50%-majority rule consensus tree was constructed based on the final 75% of trees produced by BI. Analyses were run three times to ensure convergence and insensitivity to priors. Phylogenetic analyses of the ITS+ sequence data were also conducted using the neighbor-joining (NJ) and maximum parsimony (MP) methods in PAUP* 4.0b10 (Swofford, 1999). For the MP analyses, heuristic searches were carried out with random addition of sequences (n = 100), tree-bisection-reconstruction (TBR) branch swapping, the MulTrees option in effect, MaxTrees set at 2000 and saving all equally parsimonious trees. Characters were treated as unordered and were weighted equally. Alignment gaps were treated as a fifth character state in the MP analyses. The tree length (L), consistency index, excluding uninformative characters (CI) and the retention index (RI) were recorded. Bootstrap analyses (1000 replicates) were conducted to determine the relative support for clades in the consensus trees; nodal support was expressed as a percentage. The ITS+ sequences of Labiostrongylus australis (GenBank accession numbers AJ308403-AJ308411) were used as the outgroup in the phylogenetic analyses, because this nematode species belongs to a related tribe within the same subfamily (i.e. Cloacininae) (see Lichtenfels, 1980) to which Rugopharynx also belongs (Beveridge, 1982, Chilton et al., 1997) and because it has been relatively well studied genetically (Chilton et al., 2009b).
A consensus tree depicting the phylogenetic relationships of the nematodes was compared with a composite tree of the hosts. The host phylogenetic tree was based primarily on the molecular analyses of Meredith et al. (2008); however, as some species (e.g., Thylogale billardierii, Macropus dorsalis, Petrogale herberti, Petrogale assimilis and Petrogale inornata) were not included in that study, the tree was modified to incorporate these hosts based on the studies of Campeau-Péloquin et al., 2001, Cardillo et al., 2004 and Eldridge et al. (2011). The host phylogenetic tree also includes three species, Macropus (Notamacropus) agilis. M. (Osphranter) bernardus and M. (O.) antilopinus, from which no species of Rugopharynx have been reported. Not all species of Petrogale and Thylogale are included in the phylogenetic tree, only those included in the present study.
3. Results
The length of the ITS+ region, excluding flanking regions was 744–777 bp for all taxa within the genus Rugopharynx and Rugonema labiatum. The length of the ITS-1 sequences ranged from 373 bp (Rugopharynx mawsonae, R. sigma from Thylogale thetis and Rugonema labiatum) to 387 bp (R. mu, R. rufogrisea and some individuals of R. australis), whereas the ITS-2 sequences were much shorter, ranging from 216 bp (R. longibursaris, R. omega and R. zeta) to 313 bp (R. alpha and R. mawsonae) (Supplementary Table 1). The length (153 bp) and nucleotide sequence of the 5.8S rRNA gene was the same for all morphospecies within the genus Rugopharynx and Rugonema labiatum.
Each morphospecies had a unique set of ITS+ sequences. Rugonema labiatum was most genetically similar to R. pi. There were five fixed differences (i.e. where there are no shared nucleotides at an alignment position) between these two taxa; two in the ITS-1 and three in the ITS-2. The magnitude of fixed differences in ITS+ sequence among morphospecies within the genus Rugopharynx ranged from eight (i.e. between R. omega and R. longibursaris) to 84 (i.e. between R. alpha and R. rufogrisea). Only one fixed difference in ITS-1 sequence was detected between R. setonicis and Rugopharynx rho compared with 10 fixed differences between these two morphospecies for the ITS-2, while the lowest number of fixed differences in ITS-2 sequence among morphospecies (i.e. five) was between R. omega and R. longibursaris.
For some morphospecies, there was variation among individuals in the DNA sequences of the ITS-1 and/or ITS-2 (see Supplementary Tables 2–8). Three variable positions (two in the ITS-1 and one in the ITS-2) were detected in the aligned sequences of R. mu individuals from hosts (W. bicolor) collected in New South Wales and Victoria; however, none of these mutations represented a fixed difference (Supplementary Table 2). Similarly, there were no fixed differences in ITS+ sequence among three specimens of R. mawsonae from the same host (M. dorsalis), or among two specimens of R. pi from two host species (M. rufogriseus and M. parryi), even though variations were detected at nine (four in the ITS-1 and five in the ITS-2) and three (two in ITS-1 and one in the ITS-2) alignment positions, respectively. A similar pattern of intraspecific variation was found for three specimens of R. macropodis from two host species (M. giganteus and M. fuliginosus), except for a single nucleotide insertion in the ITS-1 sequence for one of two individuals (i.e. F736) collected from M. giganteus. Specimens of R. longibursaris from M. rufogriseus collected from Launceston (Tasmania) and Emu Flat (New South Wales) differed in ITS+ sequence at eight alignment positions (five in the ITS-1 and three in the ITS-2), whereas no genetic variation occurred in the DNA sequence of R. spratti, specimens of which were collected from the same host species and localities as R. longiburaris. Similarly, there were no fixed differences in ITS+ sequence of five R. epsilon individuals collected on the mainland (i.e. from M. rufogriseus and W. bicolor) and in Tasmania (from M. rufogriseus), except for a single insertion in the ITS-1 sequence of specimen F77 collected in New South Wales (Supplementary Table 3).
In contrast, fixed differences in both the ITS-1 and ITS-2 sequences were detected among individuals of four morphospecies, R. rufogrisea, R. australis, R. zeta and R. rho, collected from different host species and/or geographical regions (see Supplementary Tables 4–8, respectively). In the case of R. rufogrisea, although there were no fixed nucleotide differences in ITS+ sequences between specimens from M. rufogriseus collected in New South Wales (F84) and Tasmania (F720), there were seven (of 12) variable nucleotide positions (two in the ITS-1 and five in the ITS-2) when compared to a specimen from M. parryi collected in Queensland. Similarly, there were 52 variable nucleotide positions in ITS+ between R. sigma specimens from Thylogale stigmatica and those from T. thetis, most (25 in ITS-1 and 24 in ITS-2) representing putative fixed differences between nematodes from the two host species; 20 of these differences (five in ITS-1 and 15 in ITS-2) represented 1-10 bp nucleotides. In addition, nucleotide variation was detected at 20 positions in ITS+ sequences among individuals of R. zeta collected from different species of Petrogale in Queensland. There was only one fixed nucleotide difference in ITS-1 sequence between specimens of R. zeta and those from P. herberti and P. inornata, whereas these nematodes differed unequivocally at 13 (11 in ITS-1 and two in ITS-2) of 20 variable positions when compared with the ITS+ sequence from a specimen of R. zeta from P. assimilis. Genetic variation was also detected at 52 nucleotide positions in the ITS+ sequences among seven individuals of R. australis. This morphospecies could be separated into two groups (clades) based on their ITS+ sequences. Nematodes in clade 1 had fixed differences at 38 (14 in ITS-1 and 24 in ITS-2) alignment positions when compared to those in clade 2. One eastern grey kangaroo (M. giganteus) collected from New South Wales contained taxa from both clades 1 and 2 (i.e. specimens F751 and F754). Likewise, in the case of R. rho, individuals from Macropus eugenii from South Australia (F724) and Western Australia (F905) had almost identical ITS+ sequences, which differed at 16 positions (eight in ITS-1 and eight in ITS-2) from specimens from M. fuliginosus from South Australia (F780) and Western Australia (F910), and from M. irma (G114) from Western Australia. Eighteen (five in ITS-1 and 13 in ITS-2) of these differences represented 1–4 nucleotides.
Phylogenetic analyses were conducted to determine whether individual morphospecies from different host species and/or geographical regions represented monophyletic assemblages. The ITS+ sequences were aligned over 824 positions (404 for ITS-1, 153 for 5.8S rDNA and 267 for ITS-2), 175 of which were informative for the MP analysis. The topology of the strict consensus tree of the MP analysis (not shown), based on 1217 equally most parsimonious trees (L = 579, CI = 0.58, and RI = 0.83), was very similar to that produced from the BI (Fig. 3) and the NJ analyses (Fig. 4). In all phylogenetic analyses, there was no support for all specimens of R. australis representing a monophyletic assemblage. However, there was absolute support (pp = 1.0 in the BI analysis and bs = 100% in both the NJ and MP trees) for the separation of R. australis into two clades. Similarly, there was no support for R. sigma from the two host species (T. stigmatica and T. thetis) forming a monophyletic assemblage. There was strong support (pp = 0.999 and 1, and bs = 95–100%) for the separation of R. rho into two clades, one containing individuals from M. eugenii and the other containing individuals from M. fuliginosus and M. irma. However, there was support in the BI analysis (pp = 0.994) for a sister taxa relationship for the two clades of R. rho, and absolute support (pp = 1) for a sister relationship of the two clades with R. setonicis. In contrast, in the MP and NJ analyses, there was no support for a sister taxon relationship between the two clades of R. rho. However, there was strong support (bs = 90%) in the NJ analysis for an assemblage comprising R. setonicis and the two clades of R. rho. There was also strong support (pp = 1.0 in the BI analysis and bs = 96–97% in the MP and NJ analyses) for R. zeta from the three host species, with support (bs = 70–95%, pp = 0.831) for R. zeta from P. inornata and P. herberti forming a clade to the exclusion of R. zeta from P. assimilis. Similarly, there was absolute support (pp = 1.0; bs = 100%) for R. rufogrisea representing a monophyletic clade, and support (bs = 85–100%; pp = 0.882) for R. rufogrisea collected from M. rufogriseus in Tasmania and New South Wales forming a clade to the exclusion of R. rufogrisea collected from M. parryi in Queensland.
Fig. 3.
Phylogenetic relationships of species of Rugopharynx and Rugonema labiatum based on a Bayesian analysis of the sequence data of the ITS+ nuclear ribosomal DNA. Values above branches indicate posterior probabilities that were greater than 0.8. Abbreviations of Australian state names are provided in Table 1.
Fig. 4.
Phylogenetic relationships of species of Rugopharynx and Rugonema labiatum based on a neighbor-joining analysis of the sequence data of the ITS+ nuclear ribosomal DNA. Values above and below branches represent the NJ and MP bootstrap values (respectively) that were greater than 70%.
In the phylogenetic analysis of ITS+ sequence data for the morphospecies, R. alpha was the sister species to the remaining congeners. There was support in the BI and MP analyses (i.e. pp = 0.973; bs = 86%) for a clade consisting of R. macropodis, R. rosemariae, R. rufogrisea and R. theta forming a clade with respect to the other species. Rugonema labiatum nested in a clade with R. pi and R. mu. There was also some support (i.e. pp = 0.995; bs = 71–75%) for a clade containing R. longibursaris, R. omega, R. tau and R. spratii (Fig. 3, Fig. 4).
Comparison of the phylogeny of the nematodes, derived from the analysis of ITS+ sequence data, with that currently available for the host species (Fig. 5) provides little evidence for co-speciation between nematodes and hosts. Also included on the nematode tree were the different buccal capsule types. This comparison revealed that a simple buccal capsule (I) appears to be the plesiomorphic state (as for R. alpha). Of the three principal clades depicted in Fig. 5, type I buccal capsules occurred in all clades, being the exclusive buccal capsule type in one clade, but was mixed with type II capsules in a second clade and mixed with type III capsules in the third clade.
Fig. 5.
Molecular phylogeny of species of Rugopharynx and Rugonema labiatum based on a consensus of the BI, NJ and MP trees (Fig. 3, Fig. 4), and the relationships of their hosts. This figure includes species of Macropus (M. agilis, M. antilopinus, M. bernardus) which are not hosts to species of Rugopharynx, as well as M. parma, from which no material could be obtained for genetic studies. Only those species of Petrogale included in this study are shown on the host tree. The morphology of the buccal capsule for each nematode taxon is also shown.
4. Discussion
To date, species of the genus Rugopharynx found in the stomachs of macropodid marsupials have been identified solely on the basis of morphological criteria (Beveridge, 1982) or the combination of morphological and MEE data (Chilton et al., 1993, Beveridge et al., 1994). In describing new species within the R. australis complex, Beveridge and Chilton (1999) relied on limited MEE data (Chilton et al., 1996) and extrapolated from this base in describing nine new species based on small but potentially significant morphological characters.
The present study represents the first detailed examination of the genus using DNA sequence data and therefore represents the first test of the validity of the range of species currently recognised either exclusively on morphological grounds or on the basis of morphological and MEE data. The current molecular analyses included most of the currently known species, apart from R. longispicularis and R. petrogale. Both of these species occur in hosts, such as the parma wallaby, Macropus parma, and the brush-tailed rock wallaby, Petrogale penicillata, which are currently considered to be rare or vulnerable species (Maynes, 2008, Eldridge and Close, 2008); therefore, it is challenging to obtain parasite material from these host species for molecular studies. No species of Rugopharynx are known to occur in M. (N.) agilis, M.(O.) antilopinus and M. (O.) bernardus (Spratt et al., 1991).
In the current study, all of the species of Rugopharynx presently recognised based on morphological differences (i.e. morphospecies), in some cases with supporting MEE data, had a unique set of ITS+ sequences, thereby providing additional evidence in support of their validity. Fixed differences in the ITS-1 and ITS-2 sequences between or among morphospecies were limited (e.g., 0.3% between R. setonicis and R. rho, and 2.3% between R. omega and R. longibursaris, for the ITS-1 and ITS-2, respectively), and data from a single pair of ribosomal DNA spacers may not always provide unequivocal support for specific status (Nadler and Pérez-Ponce de Léon, 2011). However, given the reliability of ITS-1 and ITS-2 sequences for identifying and distinguishing nematode species from macropodids to date (e.g., Chilton et al., 2009a, Chilton et al., 2012), the evidence presented here is relatively strong. In some instances, the morphological and genetic differentiation is supported by the occurrence of formerly cryptic species in the same host individual. In the case of R. australis and R. macropodis (i.e. previously included within R. australis), the former occurring in kangaroos in arid environments and the latter in areas of higher rainfall (Beveridge and Chilton, 1999), both species were found at one intermediate location in Victoria (Pine Plains Station) (Beveridge and Chilton, 1999), indicating genetic isolation and providing further support for the validity of the two species.
The data presented here suggest that additional cryptic (i.e. genetically distinct but morphologically similar) species of Rugopharynx remain to be described. Specimens of R. australis occurred in two quite distinct clades, although some specimens from the two clades were collected from the same individual host (M. giganteus). The magnitude of fixed differences in ITS-1 and ITS-2 sequences between members of the two R. australis clades (3.6% and 10.4%, respectively) was greater than that between related morphospecies (e.g., 0.8% and 2.3%, respectively, between R. omega and R. longibursaris). Furthermore, there was no support for the two R. australis clades forming a monophyletic assemblage. Examination of the voucher specimens involved in this study suggested that the two specimens were differentiable based on spicule lengths, with those from M. giganteus from Trangie, NSW (F751) being 2.0 mm, and those of the additional specimen from the same individual host (F754) being only 1.28 mm long; the spicules of a similar specimen from M. fuliginosus from Hattah Lakes, Victoria (F784), were 1.36 mm long. Beveridge and Chilton (1999) gave the spicule lengths of R. australis as 1.44–1.95 mm, which virtually encompasses the range of the specimens used in this study. The spicule lengths of the most (morphologically) similar species to R. australis, (i.e. R. macropodis) are 1.14–1.23 mm (Beveridge and Chilton, 1999). In addition, Beveridge and Chilton (1999) noted significant differences in bursal morphology within this species. Therefore, it appears that R. australis, as currently defined, is a composite of at least two species.
In the case of R. sigma, there was limited variation (i.e. two fixed differences in ITS-2 but none in ITS-1) in the ITS+ sequences of two specimens from T. stigmatica, collected 1200 km from one another in Queensland, whereas they had 49 fixed differences (25 in ITS-1 and 24 in ITS-2) when compared with R. sigma from T. thetis. This magnitude of sequence difference (6.5% and 10.3% for ITS-1 and ITS-2, respectively) exceeded that among many morphospecies within the genus. Furthermore, specimens F794 from T. thetis and G384 from T. stigmatica were collected at the same locality (Lamington National Park, Qld), thus being in sympatry. The phylogenetic analyses also showed that R. sigma from the two host species did not form a monophyletic clade, providing additional support that they represent cryptic species. Examination of the female voucher specimen of R. sigma from T. thetis (F794) indicates a tail length of 0.21 mm compared with 0.39–0.45 mm for specimens from T. stigmatica and the distance of the vulva from the posterior end as 0.30 mm compared with 0.60–0.70 mm in specimens from T. stigmatica (see Chilton et al., 1993). Hence, there appear to be morphological features supporting the molecular differences for these specimens. Griffith et al. (2000) concluded that the helminth communities of these two host species in southern Queensland, where they are sympatric, were essentially similar. The current genetic studies suggest that these conclusions may need to be revised.
Specimens of R. zeta from P. inornata and P. herberti formed a strongly supported clade to the exclusion of specimens from P. assimilis. These three closely related species of rock wallabies have parapatric distributions along the eastern coast of Queensland (Potter et al., 2012). Chilton et al. (2009a) examined three species of cloacinine nematodes, Cloacina caenis, C. pearsoni and C. robertsi, which occur in these related rock wallaby species and demonstrated genetic differences between them, suggesting the existence of cryptic species. Current data also suggest the possible existence of cryptic species within R. zeta. There was one fixed difference in ITS+ between specimens of R. zeta from P. inornata and P. herberti (but none in the ITS-2), whereas they had 13 fixed differences (11 in ITS-1 and 2 in ITS-1) when compared to the R. zeta from P. assimilis. The magnitude of sequence difference in ITS-1 (2.8%) exceeds that detected between R. omega and R. longibursaris, whereas the 0.9% sequence differences in the ITS-2 is less than between these two morphospecies. Therefore, additional molecular investigations are required to test the hypothesis that R. zeta represents a species complex. The current data suggest the existence of one species in P. assimilis and a second species in both P. inornata and P. herberti. Rugopharynx zeta also occurs in a number of related species of rock wallabies (Petrogale mareeba, Petrogale sharmani and P. penicillata) (Spratt et al., 1991) and specimens from these additional hosts would need to be included in future studies.
Rugopharynx rho from M. fuliginosus from both South Australia and Western Australia, together with M. irma from Western Australia, formed a clade distinct from the same morphospecies obtained from M. eugenii in South Australia and Western Australia. There was also no support in the MP and NJ analyses for these two clades forming a monophyletic assemblage. As M. fuliginosus and M. eugenii are sympatric at both localities (Van Dyck and Strahan, 2008), the data suggest that cryptic species may also exist within this taxon. In Western Australia, M. irma and M. fuliginosus are sympatric (Van Dyck and Strahan, 2008) and the occurrence of this species in M. irma may have resulted from host switching.
Consequently the data presented here suggest that additional cryptic species may exist within R. australis, R. rho, R. sigma and R. zeta. There is also a possibility of cryptic species within R. rufogrisea. There were no fixed differences in ITS+ sequence between specimens of R. rufogrisea from M. rufogriseus collected on the mainland of Australia and the island state of Tasmania, which is consistent with the findings for other morphospecies (e.g., R. epsilon and R. spratti) that parasitise M. rufogriseus. However, the magnitude of the fixed sequence differences between R. rufogrisea from M. rufogriseus and M. parryi (0.5% and 2.1% in ITS-1 and ITS-2, respectively) is very similar to that between R. omega and R. longibursaris, two other species that parasitise M. rufogriseus (Beveridge, 1982). This aspect needs to be explored further using multiple specimens from both host species.
The current analysis also provides some insight into host specificity within the genus, Although many of the species examined in this study appear to be moderately host specific, occurring in one or two host species (i.e. R. alpha, R. chi, R. delta, R. longibursaris, R. mawsonae, R. mu, R. omega, R. pi, R. spratti, R. tau and R. theta), other species included in this study appear to have a wide host range. Thus, R. australis was identified in M. rufus (the type host for the species), as well as in M. fuliginosus, M. giganteus, Macropus robustus and M. dorsalis. Specimens from the first four host species were collected in arid or semiarid regions of Australia (Table 1) where these host species are sympatric and R. australis is a common parasite in each of them (Arundel et al., 1979, Beveridge et al., 1998). However, R. australis is an uncommon parasite of M. dorsalis (see Beveridge et al., 1998) and the specimen collected here was in an area in which M. dorsalis is sympatric with kangaroo species commonly parasitized by this nematode.
Analyses of ITS+ sequence data of R. epsilon specimens from different host species (i.e. M. rufogriseus and W. bicolor) suggest that it represents a single species with a broad host range, given that R. epsilon parasitizes a variety of macropodid hosts (Spratt et al., 1991). Therefore, examination of additional specimens of R. epsilon from other host species needs to be studied to test this proposal.
The genus Rugonema was erected (Beveridge, 1999) for a single species of nematode, Ru. labiatum, from the stomach of M. irma which resembled Rugopharynx but in which the labial collar, instead of forming an annulus, was prominently four-lobed, similar to the genus Wallabinema. However, Wallabinema lacks a striated buccal capsule and differs in the morphology of the oesophagus. The genetic data presented here show that Ru. labiatum is clearly nested within the genus Rugopharynx. The sole distinguishing morphological feature of Rugonema, that is the four lip-like lobes of the labial collar, is an autapomorphy within the tribe Pharyngostrongylinea. Based on the molecular data and a reconsideration of its morphological differentiation, Rugonema is here made a synonym of Rugopharynx with its sole species becoming Rugopharynx labiatum (Beveridge, 1999) n. comb. This species was most genetically similar to R. pi and belonged to a clade that also included R. mu.
Beveridge (1982) divided Rugopharynx into three groups based on the morphology of the buccal capsule, with either a simple cylindrical buccal capsule, a bilobed or a trilobed buccal capsule. The group with bilobed buccal capsules consisted of R. epsilon and R. rufogrisea, with the indentation occurring in the mid region in R. epsilon and in the anterior quarter in R. rufogrisea. However, among the new species described by Beveridge and Chilton (1999), some were difficult to allocate to a particular group (i.e., R. tau and R. petrogale) because the division of the buccal capsule was subtle (R. tau) or variable (R. petrogale). The mapping of the morphological data on to the consensus nematode phylogenetic tree (Fig. 5) suggest that the simple buccal capsule is the plesiomorphic state within the genus and that bilobed buccal capsules have evolved independently. The findings of the present study also suggest that the trilobed buccal capsules are a derived character. The lack of resolution in the cladogram prevents more detailed conclusions from being drawn on the evolution of buccal capsule shapes within the genus.
There appears to be no co-evolutionary relationship between nematodes and hosts. Based on molecular evidence, Setonix diverged within the macropodine lineage about 10 million years ago, while Thylogale and Petrogale are sister taxa to the clade that contains Macropus and Wallabia, their estimated time of divergence being about eight million years (Meredith et al., 2008). The largest clade of the molecular phylogenetic tree of the nematodes contains species from each of these macropodine genera apart from Setonix. Beveridge and Chilton (2001) undertook a morphological phylogenetic analysis of the R. australis complex, which produced a completely unresolved tree, and consequently these authors concluded that there was no obvious co-evolutionary association with hosts. Comparable studies of other cloacinine genera also supported the hypothesis that evolution within this group of nematodes was primarily by host switching (Beveridge and Chilton, 2001), a hypothesis concordant with the data presented above. Chilton et al. (2016) have provided molecular evidence for host switching in the related cloacinine genus Cyclostrongylus.
In summary, the molecular data presented here support the earlier morphological studies of the genus Rugopharynx and provide additional evidence that the species of Rugopharynx currently established, many of them based on minor morphological differences and MEE data, are indeed valid. The study has also revealed the existence of additional cryptic species within the genus that need to be characterised morphologically. Comparisons of buccal capsule morphology with the phylogenetic tree provided some insights into the evolution of more complex buccal capsules, but there were no obvious co-evolutionary associations with hosts, the data instead suggesting a pattern of host switching in the evolution of the genus.
Conflicts of interest
The authors declare that there is no conflict of interest.
Acknowledgements
This study was funded largely by grants from the Australian Research Council and the Australian Biological Resources Study. Specimens were collected under the following permits: Victorian Department of Sustainability and Environment 90-053, 91-095, 93-016, 10000434, 100003649), Queensland National Parks and Wildlife Service T00436, T1131), New South Wales National Parks and Wildlife Service A68.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijppaw.2016.04.003.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Appan A., Bergfeld J., Beveridge I. New species of parasitic nematodes from macropodid marsupials in Western Australia. Trans. R. Soc. S. Aust. 2004;128:77–84. [Google Scholar]
- Arundel J.H., Beveridge I., Presidente P.J.A. Parasites and pathological findings in enclosed and free-ranging populations of Macropus rufus (Desmarest) (Marsupialia) at Menindee, New South Wales. Aust. Wildl. Res. 1979;6:361–379. [Google Scholar]
- Beveridge I. A taxonomic revision of the Pharyngostrongylinea Popova (Nematoda: Strongyloidea) from macropodid marsupials. Aust. J. Zool. Suppl. Ser. No. 1982;83 1–150. [Google Scholar]
- Beveridge I. Rugonema labiatum n.g., n.sp. (Nematoda: Strongyloidea) from the stomach of Macropus irma (Marsupialia: Macropodidae) from Western Australia. Syst. Parasitol. 1999;44:229–234. doi: 10.1023/a:1006202029345. [DOI] [PubMed] [Google Scholar]
- Beveridge I., Arundel J.H. Helminth parasites of grey kangaroos, Macropus giganteus Shaw and M. fuliginosus (Desmarest), in eastern Australia. Aust. Wildl. Res. 1979;6:69–77. [Google Scholar]
- Beveridge I., Chilton N.B. Revision of the Rugopharynx australis (Moennig, 1926) complex (Nematoda: Strongyloidea) from macropodid marsupials. Invert. Taxon. 1999;13:805–843. [Google Scholar]
- Beveridge I., Chilton N.B. Co-evolutionary relationships between the nematode subfamily Cloacininae and its macropodid marsupial hosts. Int. J. Parasitol. 2001;31:976–996. doi: 10.1016/s0020-7519(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Beveridge I., Chilton N.B., Andrews R.H. A morphological and electrophoretic study of Rugopharynx zeta (Johnston & Mawson, 1939) (Nematoda: Strongyloidea) with the description of a new species, R. mawsonae, from the black-striped wallaby, Macropus dorsalis (Marsupialia: Macropodidae) Syst. Parasitol. 1994;27:159–172. [Google Scholar]
- Beveridge I., Chilton N.B., Johnson P.M., Smales L.R., Speare R., Spratt D.M. Helminth parasite communities of kangaroos and wallabies (Macropus spp. and Wallabia bicolor) from north and central Queensland. Aust. J. Zool. 1998;46:473–495. [Google Scholar]
- Campeau-Péloquin A., Kirsch J.A.W., Eldridge M.D.B., La Pointe F.J. Phylogeny of the rock-wallabies, Petrogale (Marsupialia: Macropodidae) based on DNA/DNA hybridisation. Aust. J. Zool. 2001;49:463–486. [Google Scholar]
- Cardillo M., Bininda-Emonds O.R.P., Boakes E., Purvis A. A species-level phylogenetic supertree of marsupials. J. Zool. (Lond.) 2004;264:11–31. [Google Scholar]
- Chilton N.B. The use of nuclear ribosomal DNA markers for the identification of bursate nematodes (order Strongylida) and for the diagnosis of infections. Anim. Health Res. Rev. 2004;5:173–187. doi: 10.1079/ahr200497. [DOI] [PubMed] [Google Scholar]
- Chilton N.B., Andrews R.H., Beveridge I. Genetic evidence for a species complex within Rugopharynx australis (Mönnig, 1926) (Nematoda: Strongyloidea) from macropodid marsupials. Syst. Parasitol. 1996;34:125–133. [Google Scholar]
- Chilton N.B., Beveridge I., Andrews R.H. Electrophoretic comparison of Rugopharynx longibursaris Kung and Rugopharynx omega Beveridge (Nematoda: Strongyloidea), with the description of R. sigma n. sp. from pademelons, Thylogale spp. (Marsupialia: Macropodidae) Syst. Parasitol. 1993;26:159–170. [Google Scholar]
- Chilton N.B., Gasser R.B., Beveridge I. Phylogenetic relationships of Australian strongyloid nematodes inferred from ribosomal DNA sequence data. Int. J. Parasitol. 1997;27:1481–1494. doi: 10.1016/s0020-7519(97)00134-3. [DOI] [PubMed] [Google Scholar]
- Chilton N.B., Huby-Chilton F., Gasser R.B., Koehler A.V., Beveridge I. Phylogenetic relationships of species of the oesophageal parasitic nematode genera Cyclostrongylus and Spirostrongylus (Strongyloidea: Chabertiidae: Cloacininae) with their wallaby hosts (Marsupialia: Macropodidae) Mol. Cell. Probes. 2016;30:93–99. doi: 10.1016/j.mcp.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Chilton N.B., Huby-Chilton F., Johnson P.M., Beveridge I., Gasser R.B. Genetic variation within species of the nematode genus Cloacina (Strongyloidea: Cloacininae) parasitic in the stomachs of rock wallabies, Petrogale spp. (Marsupialia: Macropodidae) in Queensland. Aust. J. Zool. 2009;57:1–10. [Google Scholar]
- Chilton N.B., Huby-Chilton F., Smales L.R., Gasser R.B., Beveridge I. Genetic divergence between island and continental populations of the parasitic nematode Labiostrongylus australis in Australia. Parasitol. Res. 2009;104:229–236. doi: 10.1007/s00436-008-1178-y. [DOI] [PubMed] [Google Scholar]
- Chilton N.B., Jabbar A., Huby-Chilton F., Jex A., Gasser R.B., Beveridge I. Genetic variation within the Hypodontus macropi (Nematoda: Strongyloidea) complex from macropodid marsupial hosts in Australia. Electrophoresis. 2012;33:3544–3554. doi: 10.1002/elps.201200364. [DOI] [PubMed] [Google Scholar]
- Van Dyck S., Strahan R. third ed. Reed New Holland; Sydney: 2008. The Mammals of Australia; p. 887. [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge M.D.B., Close R.L. Brush-tailed rock-wallaby: Petrogale penicillata (Gray, 1852) In: Van Dyck S., Strahan R., editors. The Mammals of Australia Third edition. Reed New Holland; Sydney: 2008. pp. 382–384. [Google Scholar]
- Eldridge M.D.B., Heckenberg K., Neaves L.E., Metcalfe C.J., Hamilton S., Johnson P.M., Close R.L. Genetic differentiation and introgression amongst Thylogale (pademelons) taxa in eastern Australia. Aust. J. Zool. 2011;59:103–117. [Google Scholar]
- Gasser R.B., Chilton N.B., Hoste H., Beveridge I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucl. Acids Res. 1993;21:2525–2526. doi: 10.1093/nar/21.10.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J.E., Beveridge I., Chilton N.B., Johnson P.M. Helminth communities of pademelons, Thylogale stigmatica and T. thetis (Marsupialia: Macropodidae) from eastern Australia and Papua New Guinea. J. Helminthol. 2000;74:307–314. doi: 10.1017/s0022149x00000457. [DOI] [PubMed] [Google Scholar]
- Lichtenfels J.R. Keys to genera of the superfamily Strongyloidea No. 7. In: Anderson R.C., Chabaud A.G., Willmott S., editors. CIH Keys to the Nematode Parasites of Vertebrates. Commonwealth Agricultural Bureaux; Farnham Royal: 1980. p. 41. [Google Scholar]
- Maddison W., Maddison D. 2011. Mesquite: a Modular System for Evolutionary Analysis. v2.75 ed. [Google Scholar]
- Maynes G. Parma wallaby: Macropus parma Waterhouse, 1845. In: Van Dyck S., Strahan R., editors. The Mammals of Australia. third ed. Reed New Holland; Sydney: 2008. pp. 341–343. [Google Scholar]
- Meredith R.W., Westerman M., Springer M.S. A phylogeny and timescale for the living genera of kangaroos and kin (Macropodiformes: Marsupialia) based on nuclear DNA sequences. Aust. J. Zool. 2008;56:395–410. [Google Scholar]
- Nadler S.A., Pérez-Ponce de Léon G. Integrating molecular and morphological approaches for characterizing parasitic cryptic species: implications for parasitology. Parasitology. 2011;138:1688–1709. doi: 10.1017/S003118201000168X. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Potter S., Cooper S.J.B., Metcalfe C.J., Taggart D.A., Eldridge M.D.B. Phylogenetic relationships of rock-wallabies, Petrogale (Marsupialia: Macropodidae) and their biogeographic history within Australia. Mol. Phylogen. Evol. 2012;62:640–652. doi: 10.1016/j.ympev.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Spratt D.M., Beveridge I., Walter E.L. A catalogue of Australasian monotremes and marsupials and their recorded helminth parasites. Rec. S. Aust. Mus. Monogr. Ser. 1991;1 1–105. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland: 1999. PAUP*4.0b10. [Google Scholar]
- Vendl C., Beveridge I. Estimation of species richness in the complex communities of nematode parasites found in the stomachs of kangaroos and wallabies (Family Macropodidae) Trans. Roy. Soc. South Aust. 2014;138:105–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.