Abstract
Objectives
Wideband acoustic transfer functions (WATF) measured in the ear canal have been shown to be effective in the diagnosis of middle ear dysfunction in adults and in newborn infants. Although these measures would be diagnostically useful in older infants, normative data on a large number of older infants are lacking. The goal of this study was to provide such normative data.
Design
The WATF of 458 infants aged 2 to 9 mos and of 210 adults were obtained. Wideband reactance (X), resistance (R), and energy reflectance (ER) were measured in third-octave bands between 250 and 8000 Hz. The effects of age and gender on the WATF were examined, and the WATF in the left and right ears were compared. Test–retest reliability was assessed, and the relationship between the 226-Hz tympanogram and the WATF was examined.
Results
The results agreed well with previous reports testing fewer subjects, which documented age-related change in these measures during infancy and between infancy and adulthood. Test–retest correlations within third octaves were 0.5 to 0.7 at best, but did not vary systematically with age. Infants’ test–retest absolute differences within third octaves for R and ER were similar to those of adults. The shape of the WATF on retest was highly repeatable, and the shapes of the WATF in the ears of the same individual were qualitatively similar. The wideband impedance results were not different in the left and right ears, but ER was slightly, but significantly, lower in the left ear than that in the right ear. Resistance and reactance magnitude were greater for females than males, but there was no effect of gender on ER. Infants whose 226-Hz tympanogram indicated reduced peak admittance (Types As and B) had greater resistance and reactance magnitude than those with normal peak admittance (Types A and C), but no tympanometry group differences were evident in ER.
Conclusions
Age-graded norms are essential to the successful clinical application of WATF. However, the effects of gender and laterality on the WATF are small.
INTRODUCTION
The diagnosis of middle ear disorders in young infants is a long-standing problem. The most common middle ear disorder in infants is otitis media with effusion, defined as fluid in the middle ear without signs or symptoms of acute ear infection (American Academy of Pediatrics 2004). The anatomy of the infant ear makes otoscopic visualization of the tympanic membrane difficult (Jaffe et al. 1970). One approach to assessing middle ear function relies on acoustical measurements in response to the presentation of a sound stimulus in the ear canal. Studies suggest that tympanometry, a commonly used diagnostic tool, is not a sensitive indicator of middle ear disease in infants younger than about 6 mos of age (Paradise et al. 1976; Pestalozza & Cusmano 1980; but see Palmu et al. 1999). Recent articles argue for the use of tympanometry with a 1000-Hz probe tone rather than the typical 226-Hz tone in the assessment of infant middle ear function (Alaerts et al. 2007). However, pressurization of the compliant ear canal of young infants can produce misleading tympanometry results (Holte et al. 1991). Furthermore, most normative studies of 1000-Hz tympanometry have been restricted to newborn infants (Kei et al. 2003; Margolis et al. 2003; Shahnaz et al. 2008), and it is apparent that the admittance of the ear at 1000 Hz changes during the course of infancy (Mazlan et al. 2007). The few studies of infants beyond the neonatal period have reported data on a relatively small number of subjects (Calandruccio et al. 2006).
Wideband acoustic transfer functions (WATF) have been proposed as an alternative diagnostic test to tympanometry (Keefe et al. 2000; Vander Werff et al. 2007; Shahnaz 2008). WATF include wideband energy reflectance (ER) and wideband resistance (R) and reactance (X), which are the real and imaginary components, respectively, of the aural acoustic impedance. The WATF measurements are referenced in each ear to the location of the small probe microphone placed within the ear canal, although the ER is insensitive to its measurement location. These measures have shown promise in the identification of middle ear pathology in infants, children, and adults (Feeney et al. 2003; Keefe & Simmons 2003; Hunter et al. 2008a). WATF have at least three possible advantages over standard types of clinical tympanograms. First, they do not require pressurization of the ear canal. Second, they provide information over a wide frequency range. Third, information over a wide frequency range can be obtained, even from an infant’s ear, in a matter of seconds (Keefe et al. 1993; Vander Werff et al. 2007). However, as with high-frequency tympanometry, WATF norms are available only for newborn infants (Keefe et al. 2000; Shahnaz 2008). It is clear that WATF change significantly over the first year of life (Keefe et al. 1993), indicating the importance of age-graded normative data in the diagnostic application of WATF to infants. Although some normative data are available for infants beyond the newborn period, the age range covered by existing studies is still restricted (e.g., mean age of 12.4 wks in the study by Vander Werff et al. 2007) or a relatively small number of subjects was studied (e.g., 97 infants studied by Hunter et al. 2008b). The available norms in older infants are restricted to ER.
During a period of 4 yrs, we measured WATF in >450 infants ranging from 2 to 9 mos of age and in >200 young adults. These individuals were also participants in psychophysical experiments. Here, we describe these data with a view to providing norms for infants beyond the newborn period. In view of previous reports on neonates (Keefe et al. 2000), the effects of gender and ear on WATF measures were also considered. Test–retest reliability could be assessed for a subset of infants, and the relationship between 226-Hz tympanometry and WATF measures was also examined. The existence of such age-dependent norms in WATF may prove useful in applying WATF to the diagnosis of middle ear disorders such as otitis media with effusion.
MATERIALS AND METHODS
Subjects
Infant subjects were recruited from the Infant Subject Pool, a database of parents who are interested in participating in research and contacted through birth records, at the University of Washington. Infants ranged from 7 to 40 wks of age at the time of test. All were born at term, with birth weight in the normal range and no prenatal or perinatal medical complications. Infants were reported to be developing normally and were healthy on the test date by parent report. Adult subjects were recruited through flyers and newspaper ads. They were between 18 and 30 yrs of age and were healthy by self-report. All subjects passed a screening questionnaire assessing risk of hearing loss, based on the recommendations of the Joint Committee on Infant Hearing (2007). No subject reported more than two episodes of otitis media. Reported episodes of otitis media were diagnosed at least 2 wks before the first test date. All subjects were also assessed using screening tympanometry with a 226-Hz probe tone. As noted in the Introduction section, the validity of 226-Hz tympanometry in middle ear assessment for infants younger than 7 mos of age has been questioned (Paradise et al. 1976). At the time the data described here were collected (1992–1995), unfortunately, devices that allowed 1000-Hz tympanometry to be conducted quickly were not available, and given the lack of norms, it would have been difficult to use 1000-Hz tympanometry as a screening tool in any case. Furthermore, there are reports that 226-Hz tympanometry can be effective in screening for middle ear effusion in infants as young as 2 mos of age (Palmu et al. 1999) and unpublished data from our laboratory indicate that 3-mo-old infants who fail 226-Hz tympanometry (conducted after psychophysical testing) have higher thresholds for detecting a tone in quiet than do infants who pass 226-Hz tympanometry. Thus, despite widespread dissatisfaction with 226-Hz tympanometry in the assessment of young infants, it was the only reasonable alternative at the time. To the extent that some infants slipped through the tympanometric screen, then, the data presented here may not be strictly “normative.” However, we believe for the reasons just stated that the number of such infants was small.
The data were analyzed in three age groups, 2- to 3-mo olds (69 to 105 days), 5- to 9-mo olds (154 to 279 days), and adults (18 to 30 yrs old). The number of subjects in each group was 198, 260, and 210, respectively. The data were subdivided in this way based on the age distribution of the final dataset and to facilitate comparison with previous studies of middle ear development (Keefe et al. 1993). A more detailed distribution of the infant ages included is shown in Figure 1.
Fig. 1.
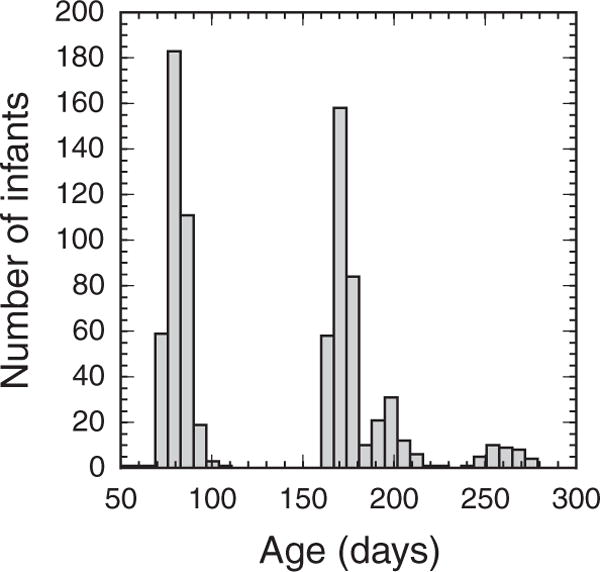
Distribution of age, in days, at which records were acquired from infants.
Acoustic Transfer Function Measurement
Acoustic stimuli were generated in the ear canal using an Etymotic ER-1 insert earphone, and an Etymotic ER-7C microphone was used to record acoustic pressure responses. The microphone probe tube extended 2 to 3 mm beyond the medial edge of the foam ear tip, through which it was threaded, which was affixed to the microphone. Data were obtained using a chirp stimulus (bandwidth 0.25 to 8 kHz) and recorded at a 24-kHz sample rate using a signal-processing board (CAC model DSP32) in a personal computer using custom software. The WATF measurement system was calibrated once daily based on responses measured in a set of six rigid-walled brass cylindrical tubes that were closed at each end. A smaller-diameter (0.485 cm) tube set was used for calibrating the system for ear measurements in infants, and a larger-diameter tube set (0.802 cm) was used for ear measurements in adults. The tube lengths were approximately 26.8, 32.4, 34.5, 41.4, 42.9, and 69.3 cm for both tube sets. The calibration and measurement of WATF were based on methods generally similar to those reported elsewhere (Keefe et al., 1992, 1993, 2000), although these studies used a different ear-canal probe or a different DSP board to deliver stimuli and acquire responses. The reflectance was calculated in terms of the measured acoustic admittance based on the characteristic impedance of the tube set used in calibration (small-diameter area in infants and large-diameter area in adults). Each ear-canal response was recorded as the average of eight chirp presentations, each with a 42-msec duration.
Students and staff members who were also collecting psychophysical data collected the ear-canal data. Some of these individuals were audiologists, but all received training on the collection and interpretation of the ear-canal data. Seven different individuals collected the data described here. The primary goal in every test session was to obtain psychophysical data. In the ear calibration with a 1000-Hz tone was completed at the beginning of each test session to check the position of the foam ear tip. The in the ear calibration was rechecked during the session if the experimenter questioned the ear tip position, or if the ear tip was removed and replaced during a test session. WATF measures were taken at the end of the psychophysical test session. Data were collected with the infant seated on the parent’s or guardian’s lap and with adults seated quietly in a chair. Because stimuli were presented to the right ear for the psychophysical experiments, the WATF measures were taken first in the right ear, with the ear tip still in place. If time and subject’s patience permitted, the experimenter moved the ear tip to the left ear, checked the in the ear calibration, and made WATF measures. Most psychophysical experiments required at least two test sessions. WATF measures were obtained in all sessions in both ears, as much as possible.
Tympanometry was performed after the test session in a quiet room using a Virtual 310 Digital Impedance System, in screening mode, with a 226-Hz probe tone. Ear-canal pressure was swept from positive to negative pressure from +200 to −400 daPa at a sweep rate of 75 daPa/sec. Compensated admittance, the presence of a peak admittance magnitude, and the pressure at the peak admittance were recorded. Each tympanogram was also scored as a “pass” or “fail.” Passing tympanograms had a peak, with peak admittance of at least 0.2 mmhos, at a pressure between −200 and +50 daPa.
Data Processing
Ear-canal measures were measured in 59 twelfth-octave “measurement” bands, with center frequencies ranging from 250 to 8000 Hz. For the analyses reported here, the data were combined into 15 “analysis” bands. The lowest analysis band was the average of the three lowest twelfth-octave measurement bands; the center frequency of this analysis band was 281 Hz. The remaining analysis bands were the average of four consecutive twelfth-octave measurement bands; the center frequency of each these bands was one 24th-octave above the center frequency of the second of the respective measurement bands (i.e., 364, 458, 578, 728, 917, 1155, 1455, 1834, 2311, 2911, 3668, 4622, 5823, and 7336 Hz).
Each record was reviewed by the authors to validate that the data looked reasonable except for relatively narrow frequency ranges described below at which artifact was observed in the form of negative resistances. For measurements in adults and older children, the WATF response is stiffness controlled at low frequencies, which corresponds to a positive equivalent volume. A measurement in an adult ear using a leaky probe fit would lead to a negative equivalent volume below 500 Hz (because of the low-impedance parallel pathway through the leak), so that one criterion of a leak-free probe fit might be that the equivalent volume is positive below 500 Hz. However, some young infants tested with a leak-free probe fit have negative equivalent volumes bounded below by −5 cm3, especially at frequencies <700 Hz (Keefe et al. 1993, 2000), although detailed analyses suggest that their equivalent volume is positive between 700 and 1000 Hz. The criterion of a leak-free probe fit used in this study was that the mean equivalent volume between 700 and 1000 Hz was positive. Of the 1745 records obtained, 65 seemed to have been made without an adequate seal between the ear tip and ear canal. The remaining 1680 records were used for analysis.
In some cases, a negative resistance (R) was measured near the lowest or the highest frequencies. A negative resistance at a particular frequency indicates the presence of some contamination in the measurement. The most likely cause was a smaller error in the calibration at this frequency, which might be caused either by a change in the calibration or by some difference in the status of the yellow foam tip of the probe inserted into the ear canal versus its status when inserted into any of the calibration tubes. The calibration performed daily used a different foam tip from the new tip used in each test ear. Because the extension tube of the ER-1 receiver was threaded individually through each foam tip, any variability in this fitting might lead to variability in calibration.
Records containing negative R were processed as follows. In the case of negative R in the low frequencies, all three ear-canal measures were coded as missing at the highest frequency at which R was negative and for all lower frequencies. In the case of negative R in the high frequencies, all three ear-canal measures were coded as missing at the lowest frequency at which the R was negative and for all higher frequencies. For infant subjects, fewer than 10% of the records contained a negative R in the low frequencies, and only 3 to 5% of the records contained negative R in more than one low-frequency third-octave band. Approximately 25% of infant subjects produced negative R in the highest frequency third octave, but it was rare that an infant had a negative R in more than one high-frequency band. For adults, it was more difficult to obtain data at the lowest frequency. Nearly 83% of adult records had negative R in the band centered at 281 Hz. However, only 24% also had negative R in the next higher third octave (364 Hz), and only a few adult records had negative R in three low-frequency bands (up to 458 Hz). Approximately 38% of adult records had a negative R in the highest frequency band (7336 Hz), and approximately 20% had negative R in the two highest frequency bands (5823 and 7336 Hz). Very few adult records had negative R in more than two high-frequency bands.
For the analyses reported in the following sections, subsets of the large dataset were selected to address the questions at hand. When statistical comparisons were made, each subject in the analysis contributed a single observation in each condition examined. The first record obtained that fit other selection criteria was included. For example, when the effects of age are examined, the first record for each subject was analyzed, even if multiple records were available. Similarly, when the effects of ear (left–right) were examined, only subjects for whom a measurement was made in both ears at the same test session were included, and if more than one pair of records was available, the first one obtained was used. This ensured that the dataset analyzed met the assumption of independence of observations underlying the inferential statistics used to make between-group comparisons. The details of sample selection are reported with each analysis.
RESULTS
Data Obtained
The 1680 acceptable WATF obtained represented data from 668 subjects, including 458 infants and 210 adults. Records were obtained from 494 left ears and 1186 right ears. Of the 198 2- to 3-mo olds included, 193 had at least one right ear record, 115 had at least one left ear record, 40 had multiple left ear records, and 108 had multiple right ear records. Of the 260 5- to 9-mo olds included, 255 had at least one right ear record, 135 had at least one left ear record, 37 had multiple left ear records, and 106 had multiple right ear records. All 210 adults included had at least one right ear record, 69 had at least one left ear record, 30 had multiple left ear records, and 146 had multiple right ear records. These data were obtained in 1205 test sessions. For both infants and adults, failure to collect ear-canal data was nearly always due to time constraints at the end of the session, although there were, of course, times when a fussy or crying infant did not provide WATF data.
Comparison with Previous Results
One question of interest was whether the results obtained in this large sample of infants and adults were similar to those reported in previous studies that included a smaller number of subjects. For this analysis, the first record obtained from each subject from the right ear that met tympanometric inclusion criteria was used, providing 174 records from 2- to 3-mo olds, 205 records from 5- to 9-mo olds, and 183 records from adults. Figure 2 plots the average X, R, and ER as a function of frequency from this study and from the study by Keefe et al. (1993) in each of three age groups. The 2- to 3-mo olds are compared with 18 3-mo olds tested by Keefe et al. and the 5-to 9-mo olds are compared with 11 6-mo olds tested by Keefe et al. The error bars represent ±1 SEM in the current results.
Fig. 2.
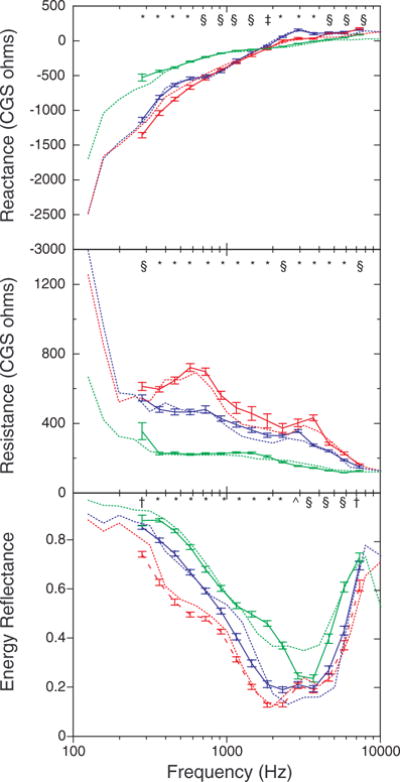
Mean ear-canal reactance (top), resistance (middle), and energy reflectance (bottom) as a function of frequency in this study (solid lines) and as reported by Keefe et al. (1993) (dotted lines) for three age groups. The data for 2- to 3-mo olds are shown in red, for 5- to 9-mo olds in blue, and for adults in green. The data from the study by Keefe et al. were from 3- and 6-mo olds. Error bars represent ±1 SEM. The symbols at the top of each panel represent the significant age differences at each frequency, with an alpha level of 0.05. The symbol * indicates that all groups differ; § indicates that infants differed from adults, but the two infant groups did not differ; † indicates that 2- to 3-mo olds differed from the other age groups, which did not differ; ^ indicates that 2- to 3-mo olds differed from adults, but 5- to 9-mo olds did not differ from either of the groups.
The current results are similar to those of Keefe et al. (1993) in both absolute value and dependence on frequency. In general, when the results of the two studies diverge, the difference is small. Considering that the ages tested in the two studies were slightly different and that the ear-canal measures were not the goal of the test sessions in which they were taken in this study, the correspondence is good. One exception is that reflectance in the 3000 to 4000 Hz range is lower for adults in this study than for those tested by Keefe et al.
Effects of Age on Middle Ear Measures
Given that Keefe et al. (1993) reported age-related changes in WATF during infancy and into adulthood and that the current results are quite similar to those of Keefe et al. (1993), it is no surprise that we also find age-related changes in WATF, as Figure 2 suggests. These data are also presented in Appendix 1 (see Supplemental Digital Content 1, http://links.lww.com/EANDH/A22), separately for the three age groups, with 90th, 75th, 50th, 25th and 10th percentiles at each frequency for X, R, and ER.
An age × frequency analysis of variance (ANOVA) was performed on X, R, and ER. One-way age or frequency ANOVA was used to follow-up significant effects as appropriate, with Bonferroni post hoc pairwise comparisons. An alpha of 0.05 was used.
X changed with age, in a rather complex pattern. Below 700 Hz, X was significantly greatest for 5- to 9-mo olds, intermediate for 2- to 3-mo olds, and lowest for adults. From 700 to 1155 Hz, X was significantly greater for adults than for infants, although at most frequencies the two infant age groups did not differ. No age groups differed in X at 1834 Hz. Above 2000 Hz, X was significantly greater for the infants than the adults, and at some frequencies 2- to 3-mo olds had greater X than 5- to 9-mo olds. R decreased progressively with age across the frequency range. The 2- to 3-mo olds had significantly higher R than the 5- to 9-mo olds who in turn had significantly higher R than the adults, except at 2311 and 7336 Hz, at which the two infant groups did not differ. Below 3000 Hz, ER increased progressively with age with 2- to 3-mo olds significantly lower than 5- to 9-mo olds who in turn were significantly lower than adults. At higher frequencies, infants had significantly lower ER than adults, but the two infant age groups did not differ. The only exceptions were at 281 and 7336 Hz at which the 2- to 3-mo olds had significantly lower ER than the two older groups, who did not differ.
WATF were obtained from 10 infants when they were 2- to 3-mo olds and again when they were 5- to 9-mo olds. Mean R, X, and ER, for this group at each age are plotted as a function of frequency in the three panels of Figure 3. The error bars are SEM. Although not all the age effects are significant with the much smaller n, note that the age effects observed in the cross-sectional data (Fig. 2) are apparent in the longitudinal data. In fact, for X and ER at least, the age-related differences seem to be larger than those seen in the cross-sectional data.
Fig. 3.
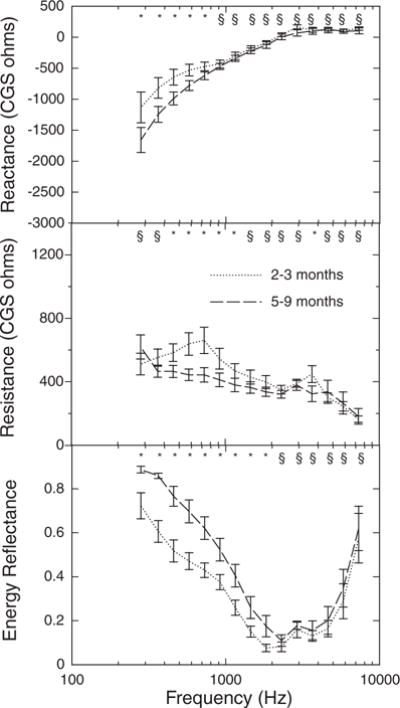
Mean ear-canal wideband acoustic transfer functions as a function of frequency for infants tested longitudinally at two ages (top panel reactance; middle panel resistance; bottom panel energy reflectance). Error bars represent ±1 SEM. Symbols as in Figure 2.
Reliability
To assess the reliability of WATF, four approaches were used. First, test–retest correlations in each third-octave band were calculated for ears that were tested on two separate days, generally within a 2-wk period. Second, the absolute difference between values obtained on different days was examined. Third, the similarity of two wideband measurements from the same ear was assessed using cross-correlation. Fourth, the similarity of two wideband measurements from the ears of the same subject was assessed using cross-correlation (differences between ears are addressed in a subsequent section).
A group of 290 subjects provided WATF from the right ear on 2 days when that ear also passed the tympanometry screening. These were distributed fairly evenly across the three age groups. For 2- to 3-mo olds, the two measures were collected within 11 days for each other; 91% were collected within 7 days of each other. For 5- to 9-mo olds, 80% of the two measures were collected within 7 days of each other, but three were collected 15, 16, and 17 days apart, respectively. For adults, 90% of the two measures were collected within 14 days of each other, but a few were collected as long as a month apart. The number of observations was not the same at each frequency, because some subjects’ data at the lowest and highest frequencies were excluded (see Materials and Methods section). The Pearson product–moment correlation between the two measures of X, R, and ER in each third octave was calculated. The statistical significance of the correlations was assessed by t test with an alpha level of 0.05. The test–retest correlations are listed in Table 1 along with the number of observations contributing each other.
TABLE 1.
Test–retest reliability of wideband ear-canal measures
| Frequency (Hz) | 2–3 Mo
|
5–9 Mo
|
Adults
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | R | ER | n | X | R | ER | n | X | R | ER | n | |
| 281 | 0.24 | 0.10 | 0.44 | 68 | 0.32 | 0.28 | 0.26 | 64 | 0.71 | 0.65 | 0.84 | 3 |
| 364 | 0.32 | 0.55 | 0.53 | 79 | 0.30 | 0.49 | 0.37 | 66 | 0.11 | 0.16 | 0.95 | 101 |
| 458 | 0.43 | 0.57 | 0.55 | 82 | 0.44 | 0.47 | 0.23 | 72 | 0.70 | 0.20 | 0.43 | 128 |
| 578 | 0.40 | 0.48 | 0.40 | 82 | 0.43 | 0.41 | 0.26 | 72 | 0.68 | 0.16 | 0.62 | 133 |
| 728 | 0.31 | 0.57 | 0.42 | 82 | 0.47 | 0.33 | 0.26 | 73 | 0.68 | 0.28 | 0.69 | 134 |
| 917 | 0.45 | 0.71 | 0.52 | 82 | 0.61 | 0.71 | 0.36 | 73 | 0.75 | 0.45 | 0.67 | 136 |
| 1155 | 0.44 | 0.80 | 0.57 | 82 | 0.68 | 0.67 | 0.44 | 73 | 0.76 | 0.52 | 0.58 | 136 |
| 1455 | 0.52 | 0.77 | 0.56 | 82 | 0.66 | 0.55 | 0.34 | 73 | 0.74 | 0.51 | 0.64 | 135 |
| 1834 | 0.59 | 0.60 | 0.40 | 82 | 0.61 | 0.48 | 0.31 | 73 | 0.68 | 0.56 | 0.62 | 135 |
| 2311 | 0.48 | 0.67 | 0.16 | 82 | 0.58 | 0.30 | 0.36 | 73 | 0.68 | 0.44 | 0.51 | 135 |
| 2911 | 0.29 | 0.53 | 0.33 | 82 | 0.59 | 0.14 | 0.43 | 73 | 0.65 | 0.31 | 0.39 | 135 |
| 3668 | 0.06 | 0.54 | 0.18 | 82 | 0.58 | 0.27 | 0.44 | 73 | 0.69 | 0.61 | 0.30 | 135 |
| 4622 | 0.38 | 0.56 | 0.39 | 81 | 0.54 | 0.09 | 0.30 | 71 | 0.72 | 0.51 | 0.37 | 132 |
| 5823 | 0.39 | 0.43 | 0.39 | 80 | 0.43 | 0.05 | −0.05 | 66 | 0.58 | 0.56 | 0.28 | 94 |
| 7336 | 0.57 | 0.21 | 0.18 | 50 | 0.30 | 0.04 | −0.10 | 48 | 0.69 | 0.61 | 0.37 | 61 |
Significant (p < 0.05) correlations are in bold.
R, resistance; X, reactance; ER, energy reflectance; n, no. subjects.
The test–retest correlations varied with measure, age, and frequency. The test–retest correlations in X were greatest at 1834 Hz for 2- to 3-mo olds, whereas 5- to 9-mo olds and adults produced moderate (0.6 to 0.7) correlations across a broader frequency range, with the largest correlations occurring closer to 1000 Hz. The test–retest correlations in X were greatest for adults (as high as 0.76) and smallest for 2- to 3-mo olds (no higher than 0.59). The test–retest correlations in R were greatest for 2- to 3-mo olds (as high as 0.80) and smallest for adults (no higher than 0.61). Infants’ greatest test–retest correlations in R were at about 1000 Hz, whereas adults’ greatest test–retest correlations were at higher frequencies.
For infants, the lowest test–retest correlations were found in ER. For 2- to 3-mo olds, the highest correlations were again found around 1000 Hz, but the best correlation was only 0.57. For 5- to 9-mo olds, the test–retest correlations were clearly poorer (no higher than 0.44) for ER than for the other WATF, although the correlation was about the same over a fairly broad frequency range. The correlations were higher for adults than infants, approximately 0.7 at 700 to 900 Hz, and comparable with the correlations in X. A very high test–retest correlation (0.95) was found for adults at 364 Hz.
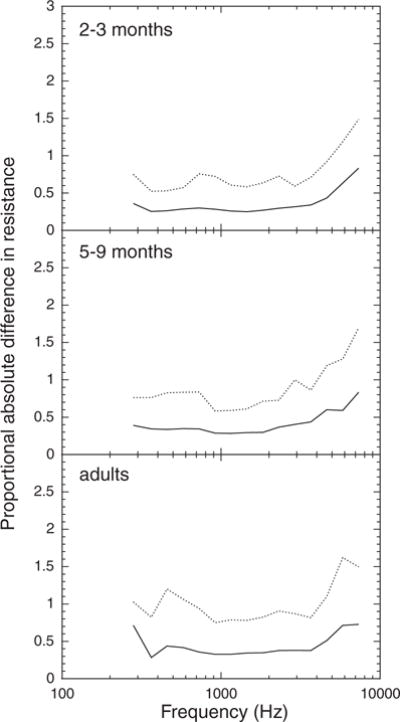
The absolute difference between third-octave values of the WATF was also analyzed. For R and X, the differences were converted to a proportional absolute difference of the average value to allow comparisons across age groups and frequency (i.e., [measure 1 − measure 2]/measure 1). An age × frequency ANOVA of the (proportional) absolute differences was conducted. Significant main effects and interactions were followed up with one-way age or frequency ANOVA as appropriate. Bonferroni post hoc pairwise comparisons were used to establish the nature of significant effects in the one-way ANOVA. An alpha level of 0.05 was used. The absolute differences between the measures obtained on test and retest are plotted as a function of third-octave frequency for infants and adults in Figures 4 to 6. The solid line in each graph is the mean (proportional) absolute difference and the dotted line indicates the 90th percentile.
Fig. 4.
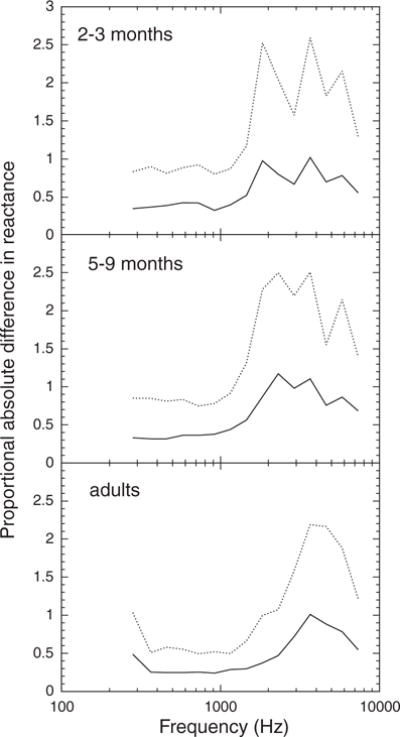
Absolute test–retest difference in reactance, expressed as a proportion of mean reactance, as a function of frequency for three age groups. The solid line represents the mean value and the dashed line represents the 90th percentile value.
Fig. 6.
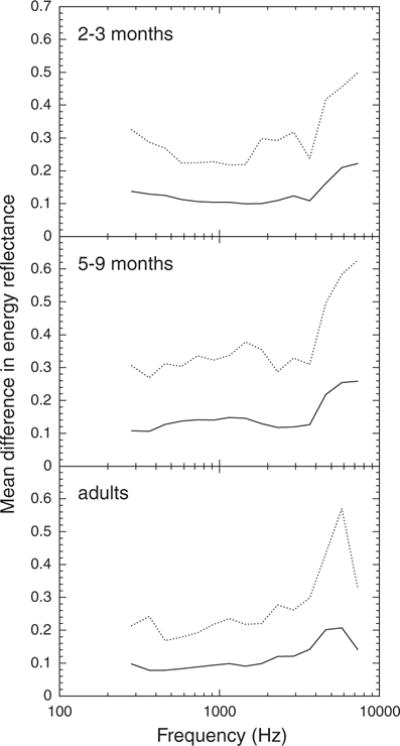
Absolute test–retest difference in energy reflectance as a function of frequency for three age groups. The solid line represents the mean value and the dashed line represents the 90th percentile value.
Infants tended to have larger proportional absolute differences in X than adults and to be much more variable than adults, as indicated by the 90th percentiles (Fig. 4). The two infant groups were similar in proportional absolute difference and variability in X. The average proportional absolute difference in X at 1115 Hz was approximately 0.4 for the infants and 0.3 for the adults. The age effect on proportional absolute difference in X (infants versus adults) was statistically significant from 458 to 2911 Hz.
The mean and variance of the proportional absolute difference in R tended to increase with age (Fig. 5). The average proportional absolute difference in R at 1115 Hz was 0.26 for the 2- to 3-mo olds, 0.29 for 5- to 9-mo olds, and 0.32 for adults. However, although the 2- to 3-mo olds had statistically smaller proportional absolute differences in R than older subjects, the 5- to 9-mo olds and adults did not differ significantly.
Fig. 5.
Absolute test–retest difference in resistance, expressed as a proportion of mean resistance, as a function of frequency for three age groups. The solid line represents the mean value, and the dashed line represents the 90th percentile value.
The mean and variance of the absolute difference in ER were greatest in 5- to 9-mo olds, intermediate in 2- to 3-mo olds, and smallest in adults (Fig. 6). The average absolute difference in ER at 1115 Hz was 0.10 for 2- to 3-mo olds, 0.15 for 5- to 9-mo olds, and 0.09 for adults. This age effect was statistically significant between 384 and 1455 Hz, although the 2- to 3-mo olds and adults did not differ at some frequencies.
The (proportional) absolute difference in all three measures increased with increasing frequency. The increase in proportional absolute difference in X at high frequencies was more pronounced for infants than for adults. For infants, the proportional absolute difference in X was significantly greater for frequencies >1834 Hz than for lower frequencies, whereas for adults, the statistical “breakpoint” was at 2311 Hz. The increase in proportional absolute difference in R due to frequency was similar for the three age groups; the proportional absolute difference in R increased significantly at 4622 Hz. The absolute difference in ER increased significantly >3688 Hz for adults, >4622 Hz for 5- to 9-mo olds, and >5823 Hz for 2- to 3-mo olds.
Thus, both the test–retest correlations and absolute differences indicate greater stability of the WATF in the middle frequencies. Although the test–retest correlations in X tend to be poorer for 2- to 3-mo olds than for older subjects, the absolute differences in X are similar for the two infant age groups. Both the test–retest correlations and absolute differences indicate that R is more reliable for 2- to 3-mo olds than for older subjects. This may be related to the fact that R is larger for 2- to 3-mo olds than for older subjects (see Fig. 2). Both analyses also indicate that ER is less reliable for 5- to 9-mo olds than for 2- to 3-mo olds or adults.
Another way to think about reliability of WATF is in terms of the within-subject similarity of the shape of the function. Four examples of individual WATF on test (solid line) and retest (dotted line) are shown in Figure 7. Curves obtained from different individuals were somewhat different, and note that the peculiar features of an individual’s WATF also tended to appear on retest. To assess the similarity of the shape of the curves on test and retest, the records included in the reliability analysis above were cross-correlated with each other. For each pair of test–retest records recorded in the same ear, the cross-correlation was calculated as one function was shifted along the frequency axis; thus, frequency was the “lag” variable. The maximum cross-correlation obtained across lags was taken as the correlation for that subject. Of course, any two randomly chosen WATF would be expected to be correlated, because the records share a general dependence on frequency. Thus, to provide a comparison, ear-canal records were randomly chosen, two at a time and with replacement, from the sample of records in the cross-correlation analysis. The cross-correlation of 1000 randomly chosen pairs of WATF was calculated. The significance of the average test–retest cross-correlation was assessed against the cross-correlation of the randomly paired records, using the z-score calculated from the Fisher Z-transformed correlations (Papoulis 1990). This process was completed for all three WATF. Because the results were similar in the three age groups, only the results for the combined age groups are reported.
Fig. 7.
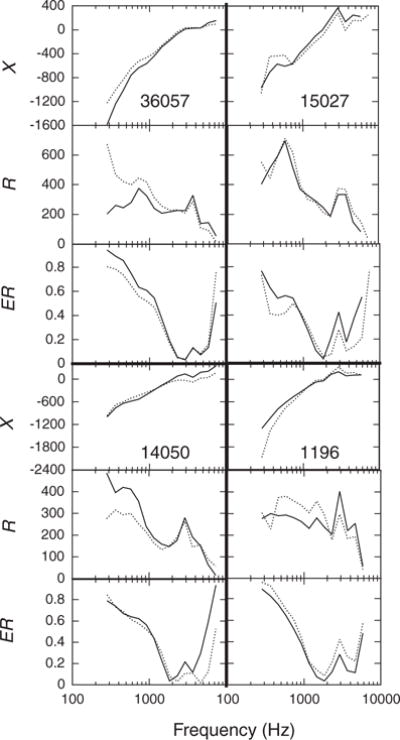
Examples of individual ear-canal WATF at test and retest for four subjects. The thick lines separate the data of different subjects. Within a single subject’s data, the top panel shows reactance (X), the middle panel shows resistance (R), and the bottom panel shows energy reflectance (ER). The subject number is given in the top panel of each section. Subject 36057 was 5.5-mo old; subject 15027 was 2.5-mo old; subject 14050 was 2.5-mo old; and subject 1196 was 6.5-mo old. The solid line shows the initial test and the dashed line shows the retest values.
For X, the average test–retest cross-correlation within subject was 0.96 (SEM = 0.004), compared with 0.91 (SEM = 0.003) for the random pairings. For R, the average test–retest correlation was 0.83 (SEM = 0.009), whereas the average cross-correlation for random pairings was 0.68 (SEM = 0.06). Finally, the average test–retest cross-correlation for ER was 0.85 (SEM = 0.007), compared with 0.73 for the random pairings (SEM = 0.005). All three differences were very highly significant (all p < 10−8). Thus, the results of this analysis demonstrate that there are stable individual differences in the acoustic response of the ear that are evident in WATF.
The similarity of response between the ears of the same individual was also examined, reasoning that if there are stable individual differences in the acoustic response of the ear, then the two ears of the same person might share the properties that are responsible for the individual differences. First, the partial correlation between the left and right ear responses in each third-octave band, controlling for age, was calculated. These correlations were statistically significant (p<0.005) for all three ear-canal measures in all frequency bands, with only two exceptions (X at 728 Hz and R at 7336 Hz). The correlations between left and right ear X were less (~0.25) in the lower frequencies, but increased to 0.4 to 0.5 at frequencies >1000 Hz. For R, the left–right correlations were 0.4 to 0.5 at all frequencies, except for the two lowest and two highest third octaves. For ER, the correlations were <0.4 at frequencies <917 Hz, in the range 0.5 to 0.7 between 1000 and 4000 Hz, and then decreased to approximately 0.3 at higher frequencies. Thus, in many cases, particularly in the middle frequency range, there was a low to moderate correlation between the acoustic responses of the two ears.
A cross-correlation analysis similar to the one used to assess the test–retest similarity of the WATF was also used to assess the similarity of the responses of the two ears of the same individual. The cross-correlation between the left and right ears of the same subject was compared with the cross-correlation between 1000 pairs of left and right ears randomly selected with replacement from the same dataset. Because the results were similar in the three age groups, only the results for the combined age groups are reported. For X, the average between-ear cross-correlation was 0.95 (SEM = 0.006) for same subjects, compared with 0.91 (SEM = 0.001) for the random pairings. For R, the average between-ear correlation was 0.85 (SEM = 0.009) for same subjects, whereas the average cross-correlation for random pairings was 0.68 (SEM = 0.02). Finally, the average between-ear cross-correlation for ER was 0.84 (SEM = 0.009) for same subjects, compared with 0.73 for the random pairings (SEM = 0.002). The differences were all significant (all p<0.002). Note that there is little difference between the cross-correlations of the response of the same ear on different days and those of the left and right ear on the same day. Thus, the frequency dependences of the acoustic responses of the left and right two ears are apparently as similar as those of the same ear measured on two occasions.
Effects of Gender and Ear
The effect of gender on wideband middle ear measures was assessed using the first record obtained from each subject’s right ear, as in the previous analyses. The numbers of males and females were about the same in the two infant age groups; about two thirds of the adult subjects were female. In the analyses conducted to assess the effects of gender, age and frequency were included as independent variables. Age × frequency × gender ANOVA of each dependent variable was conducted. Follow-up two-way and one-way ANOVA were conducted as appropriate, given the significant main effects and interactions in the omnibus analysis. Bonferroni post hoc pairwise comparisons were ultimately used to determine which means were significantly different from each other. An alpha level of 0.05 was used. The effects of age and frequency were no different from those reported above. The description below focuses on the gender effects.
Mean (±1 SEM) X is plotted as a function of frequency for males and females in Figure 8; each panel shows the data of one age group. X was slightly greater for females at the lower frequencies. The effect of gender was only significant in the four third-octave bands <600 Hz. Mean (±1 SEM) R as a function of frequency for males and females is plotted in Figure 9; again, each panel shows the data of one age group. R was significantly higher for females in the 2- to 3-mo group and in the adult group, but not in the 5- to 9-mo group. No significant effects of gender on ER were found.
Fig. 8.
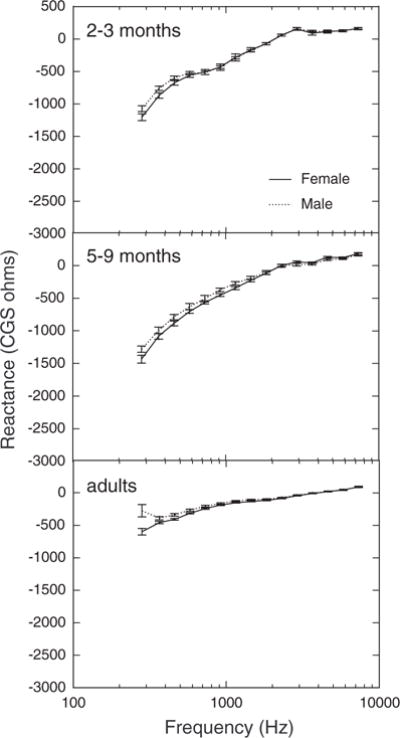
Mean reactance as a function of frequency for females and males in three age groups. Error bars represent ±1 SEM.
Fig. 9.
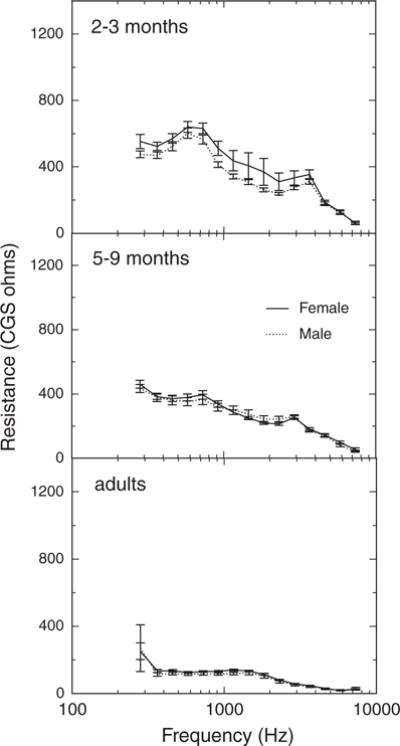
Mean resistance as a function of frequency for females and males in three age groups. Error bars represent ±1 SEM.
WATF were recorded in both ears in a single test session for 92 infants 2- to 3-mo olds, 110 infants 5- to 9-mo olds, and 65 adults, when both ears of a subject passed the tympanometric screening. These data were used to assess the effect of ear on WATF and to examine any interactions between frequency, age, and ear. Age × frequency × ear ANOVA of each dependent variable were conducted, with follow-up two-way and one-way ANOVA as appropriate. Bonferroni post hoc pairwise comparisons were used to identify differences between specific means. An alpha level of 0.05 was used. Frequency and age were independent variables in these analyses, but the focus here is on ear and interaction with ear effects. The effects of age and frequency were similar to those reported earlier.
The difference between ears was not significant in X or R, and the effect of ear did not interact with age or frequency for X or R. However, ER in the right ear was significantly higher than that in the left ear (p<0.00005). Mean (±1 SEM) ER is plotted in Figure 10 as a function of frequency for the left and right ears. The effect is relatively small: the mean difference across all frequencies is approximately 0.02. Although it seems that there is no difference between the ears at some frequencies, the effect of ear on ER did not interact significantly with frequency or age. Thus, although the frequency dependence of ER is similar in the left and right ears, as a rule, ER is higher in the right ear than that in the left ear.
Fig. 10.
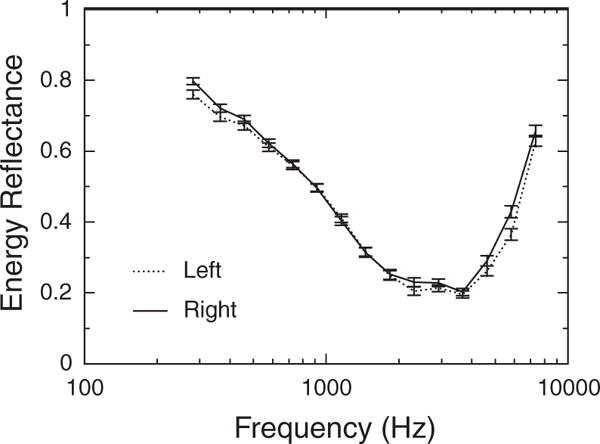
Mean energy reflectance as a function of frequency in left and right ears, collapsed across age group. Error bars represent ±1 SEM.
Relationship Between WATF and 226 Hz Tympanometry
As noted earlier, 226-Hz tympanograms were collected for each subject, as long as the subject remained cooperative, at the end of each session. A few infants provided both WATF and tympanometry data on 2 days when the tympanometry outcome, which was used as an inclusion criterion in the analyses, was different. An example of WATF data of a 2.5-mo-old infant who passed tympanometry at the first visit with a single-peaked tympanogram (type A) in the normal pressure range but had a flat tympanogram (type B) on the second visit is shown in Figure 11. The differences between the visits in all three wideband WATF measures are obvious: X, R, and ER are all higher over much of the frequency range at the visit with the flat tympanogram. However, R was lower at frequencies >1500 Hz in the ear with a flat tympanogram. Of course, there were subjects who had different tympanometry outcomes on 2 days, but indistinguishable WATF, as well as those who had different WATF with the same tympanometry outcome. Whether the tympanogram or the WATF was more accurate in these cases is impossible to determine in the absence of a gold standard measure of conductive status.
Fig. 11.
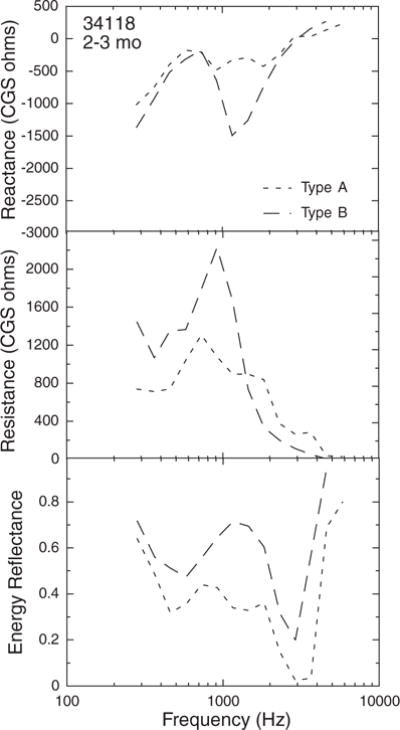
Example of wideband acoustic transfer functions from one ear of a 2.5-mo-old infant, with normal tympanogram (“Type A”) on one visit and a flat tympanogram (“Type B”) on the second.
The number of subjects for whom a within-subject comparison of WATF associated with different tympanometry outcomes was small, so a between-subject analysis was used to assess the relationship between tympanometry and WATF. The tympanometry screening results were negative for only a few adults, so adults were excluded from the analysis. The subjects included in the analysis were identified as follows. First, all subjects who had a type B (“flat”) tympanogram were identified. Second, all subjects who were not included in the type B group, but who had a type A tympanogram with compensated peak admittance magnitude <0.2 mmho were identified; such tympanograms were classified as type As. Third, all subjects who were not included in the type B or As groups, who had a tympanogram with peak admittance at a pressure <−200 daPa, type C, were identified. Finally, all subjects with type A (“normal”) tympanograms who were not in any of the other groups were identified. One wideband record obtained in the same session and ear as the qualifying tympanogram for each of these subjects was included in the between-subject analysis. If more than one record was available, the first record obtained was used. If records in both ears were available, with the same tympanogram type in the same session, then one of these records was randomly chosen to be included. The final dataset included records from 325 infants with type A, 39 infants with type B, 27 infants with type As, and 26 infants with type C tympanograms. Frequency × tympanogram type ANOVA was performed for each dependent measure. One-way ANOVA and Bonferroni post hoc pairwise comparisons were used to establish differences between specific means as appropriate. An alpha level of 0.05 was used. The results showed that WATF are sensitive to differences in middle ear function that are reflected in the 226-Hz tympanogram of infants. Mean X and R differed significantly across tympanogram groups, but ER did not differ significantly across groups.
Mean X (top) and R (bottom) are plotted as a function of frequency for the four types of tympanograms in Figure 12. The average standard error for each group is shown in the left portion of each panel for clarity. X tended to be greater for the type As group than for the other tympanogram groups. The difference between type As and A groups was significant at all frequencies except 1455 and 1834 Hz. The type As group also tended to have greater X than the type B and C groups, but the differences were only significant at frequencies >2311 Hz.
Fig. 12.
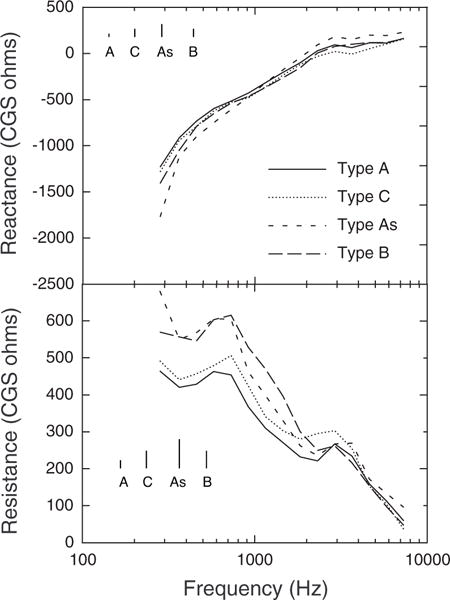
Mean reactance and resistance as a function of frequency in ears grouped by 226-Hz tympanogram type. Type A: normal tympanogram; Type C: tympanogram with admittance peak at a pressure <−200 daPa; Type As: peaked tympanogram peak admittance <0.2 mmho; Type B: flat tympanogram (no defined peak admittance). For clarity, the inset lines show the average standard error of the mean for the tympanogram type indicated below the respective line.
The type B tympanogram group had significantly higher R than the type A group at all frequencies <2311 Hz. The type C tympanogram group had significantly higher R than the type A group between 1834 and 2911 Hz. The type As tympanogram group had significantly higher R than the type A group across the frequency range.
CONCLUSIONS
The major finding of this study is the remarkable similarity of average WATF measured in different laboratories for infants of the same age. Although we did not include their data in the comparison plots in Figure 2, Sanford and Feeney (2008) have recently reported WATF from 12- and 24-wk-old infants, which are also similar to those reported here and those in the study by Keefe et al. (1993), using a different measurement system. That samples of 20 or so infants produce similar mean results as samples of 200 or so infants speaks to the stability of WATF across subjects. Furthermore, seven different experimenters, who varied widely in knowledge of and investment in middle ear assessment, collected the WATF in this study. The similarity of results suggests that published norms will provide sufficient information to guide diagnostic decisions based on WATF and that extensive training is not required to obtain reasonable WATF information. However, it should be noted that Vander Werff et al. (2007) reported ER values from infants that are somewhat higher than those described in this study or in the study by Sanford and Feeney or Keefe et al. Data in the study by Vander Werff et al. came from 98 infants ranging from 2 to 36 wks of age, with a median age of about 10 wks. The frequency dependence of the data resembles that seen in younger infants (e.g., 4-wk olds in Sanford & Feeney 2008), but the ER values are nonetheless about 0.1 higher across the frequency range. This difference may be related to the measurement system used by Vander Werff et al. which was different from that used in the other studies. Thus, any statement about the general repeatability of ER measures in infants must be tempered by this apparent discrepancy.
The only notable difference between the current results and those of Keefe et al. (1993) was that for adult ER around 4000 Hz: ER was lower in this study than that reported by Keefe et al. around that frequency. This difference may result from differences in the age range of adults included in the two studies. Keefe et al. included adults older than 30 yrs in their adult sample, and it seems that WATF change with age in adulthood. Specifically, ER has been shown to increase around 4000 Hz in older adults (Feeney et al. 2003; Sanford & Feeney 2008), consistent with the difference in ER shown in Figure 2. It should be noted that although ER was apparently sensitive to this age-related change during adulthood, X and R were not. Nevertheless, results in adults show variability in the mean or median ER near 4000 Hz that may involve other sources of variability besides age. ER results in older studies are summarized by Farmer-Fedor and Rabbitt (2002), and in newer studies by Keefe and Abdala (2007) and Liu et al. (2008).
It is clear from these results, as well as those studies based on smaller subject numbers, that WATF changes with age during infancy, particularly in the midfrequency range (Keefe et al. 1993; Sanford & Feeney 2008). The mechanisms that could contribute to these age-related changes in the WATF include changes in the compliance of the ear-canal wall and tympanic membrane as well as increases in middle ear volume. These mechanisms have been discussed in some detail in previous articles (Keefe et al. 1993; Sanford & Feeney 2008). The major implication of these findings is the need for age-graded WATF norms.
Gender and ear, as well as age, affect WATF values. Females had slightly higher ear-canal impedance than males, even among infants. Keefe et al. (2000) reported similar gender differences in newborn infants, as well as small gender differences in ER. Gender differences in ear-canal area may be responsible for this effect (Keefe et al. 1993). Newborn infant boys tend to be longer (National Center for Health Statistics 2000) and heavier (Tamimi et al. 2003) than newborn baby girls, and this may be associated with sex-specific differences in external- and middle-ear anatomy. In any case, the gender difference in impedance is small and no gender difference is observed in ER, so this effect would have no consequence for establishing norms for these measures.
Similarly, the observed difference between left and right ears in ER is small and likely to be of little consequence to establishing norms for ER. Keefe et al. (2000) also reported small ear differences in both impedance and ER in newborn infants, but the direction of the effect was different in the low frequencies at which larger ER was observed in the left than the right ear. The difference between the current results and those of Keefe et al. may be related to the differences in infant age. In addition, because WATF were always measured in the left ear after they were measured in the right ear in the current study, the order of measurement could contribute to apparent ear differences in the WATF. However, there is no known reason to expect such a contribution.
The test–retest correlations between WATF values within third-octave bands were moderate at best, and generally poorer for infants than for adults. The absolute test–retest differences in X values were larger in infants than adults, but relatively small age differences in absolute test–retest differences were found in R and ER. Vander Werff et al. (2007) reported absolute test–retest differences in ER for infants and adults. The present results agree with those of Vander Werff et al. insofar as the absolute differences in ER were smaller in the midfrequency range than at higher frequencies and the absolute differences in infants were larger than in adults. However, the absolute differences here were considerably larger than those described by Vander Werff et al. They report 90th percentile differences of just >0.1 for infants in the midfrequency range; the 90th percentile differences were about 0.4 for ER in both groups of infants tested here. There are several likely contributors to this discrepancy. First, Vander Werff et al. tested, on average, younger infants than those tested here. In our results, the absolute test–retest difference in ER was smaller for younger infants. Second, none of the infants tested in this study was asleep or even very quiet, whereas many subjects in the study by Vander Werff et al. were asleep or at least very quiet. In fact, many of the infants tested in this study were probably losing patience with the research experience. In that regard, however, the infants in this study may be more similar to older infants who are tested in common middle-ear screening situations. However, if the individual changes in the WATF associated with middle-ear dysfunction are similar to those seen in the subject whose data are plotted in Figure 11, then even a relatively large test–retest difference could be tolerated.
To summarize, this study obtained normative WATF data on a sample population of infants of age 2 to 9 mo and in a large number of adults. The results were compared with previously reported measurements on smaller groups of subjects, and a high degree of similarity across studies was observed. Effects on WATF due to age, gender, and ear were identified. Variability within the same subject at different test dates and across subjects was also assessed. The relationships between WATF and 226-Hz tympanometric responses were evaluated in subjects grouped according to the status of the tympanometric test. A limitation of this research is the lack of comparison between WATF measurements and middle-ear status assessed by pneumatic otoscopy. Despite this limitation, these normative data may be useful in continuing research on the use of WATF to screen and diagnose middle-ear disorders. It may be important to determine the appropriate age groupings for norms and to ensure that all infants in the sample have normal middle ear status at the time of test.
Supplementary Material
Acknowledgments
The authors thank Janelle Constantino, Nicole Formisano, Beth Kopyar, Karin Perlazzo, Sara Teller, and Ellen Wilcox for their assistance in data collection, as well as the participants and their parents.
This work was supported by NIH grants DC00520 (to L. W. and D. K.), DC00396 (to L. W.), and DC003784 (to D. K.).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ear-hearing.com).
References
- Alaerts J, Luts H, Woulters J. Evaluation of middle ear function in young children: Clinical guidelines for the use of 226- and 1,000-Hz tympanometry. Otol Neurotol. 2007;28:727–732. doi: 10.1097/mao.0b013e3180dca1e5. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Diagnosis and management of acute otitis media. Pediatrics. 2004;113:1451–1465. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- Calandruccio L, Fitzgerald TS, Prieve BA. Normative multifrequency tympanometry in infants and toddlers. J Am Acad Audiol. 2006;17:470–480. doi: 10.3766/jaaa.17.7.2. [DOI] [PubMed] [Google Scholar]
- Farmer-Fedor BL, Rabbitt RD. Acoustic intensity, impedance and reflection coefficient in the human ear canal. J Acoust Soc Am. 2002;112:600–620. doi: 10.1121/1.1494445. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Grant IL, Marryott LP. Wideband energy reflectance measurements in adults with middle-ear disorders. J Speech Lang Hear Res. 2003;46:901–911. doi: 10.1044/1092-4388(2003/070). [DOI] [PubMed] [Google Scholar]
- Holte L, Margolis RH, Cavanaugh RM. Developmental changes in multifrequency tympanograms. Audiology. 1991;30:1–24. doi: 10.3109/00206099109072866. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Bagger-Sjoback D, Lundberg M. Wideband reflectance associated with otitis media in infants and children with cleft palate. Int J Audiol. 2008a;47:S57–S61. doi: 10.1080/14992020802294057. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Tubaugh L, Jackson A, et al. Wideband middle ear power measurement in infants and children. J Am Acad Audiol. 2008b;19:309–324. doi: 10.3766/jaaa.19.4.4. [DOI] [PubMed] [Google Scholar]
- Jaffe BF, Hurtado F, Hurtado E. Tympanic membrane mobility in the newborn (with seven months’ follow-up) Laryngoscope. 1970;80:36–48. doi: 10.1288/00005537-197001000-00004. [DOI] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing. Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. Rockville, MD: American Speech-Language-Hearing Association; 2007. [PubMed] [Google Scholar]
- Keefe DH, Abdala C. Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears. J Acoust Soc Am. 2007;121:978–993. doi: 10.1121/1.2427128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Arehart KH, et al. Ear-canal impedance and reflection coefficient in human infants and adults. J Acoust Soc Am. 1993;94:2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Folsom RC, Gorga MP, et al. Identification of neonatal hearing impairment: Ear-canal measurements of acoustic admittance and reflectance in neonates. Ear Hear. 2000;21:443–461. doi: 10.1097/00003446-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Ling R, Bulen JC. Method to measure acoustic impedance and reflection coefficient. J Acoust Soc Am. 1992;91:470–485. doi: 10.1121/1.402733. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Simmons JL. Energy transmittance predicts conductive hearing loss in older children and adults. J Acoust Soc Am. 2003;114:3217–3238. doi: 10.1121/1.1625931. [DOI] [PubMed] [Google Scholar]
- Kei J, Mazlan R, Hickson L, et al. Measuring middle ear admittance in newborns using 1000 Hz tympanometry: A comparison of methodologies. J Am Acad Audiol. 2003;14:20–28. doi: 10.3766/jaaa.18.9.3. [DOI] [PubMed] [Google Scholar]
- Liu YW, Sanford CA, Ellison JC, et al. Wideband absorbance tympanometry using pressure sweeps: System development and results on adults with normal hearing. J Acoust Soc Am. 2008;124:3708–3719. doi: 10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH, Bass-Ringdahl S, Hanks WD, et al. Tympanometry in newborn infants—1 kHz norms. J Am Acad Audiol. 2003;14:383–392. [PubMed] [Google Scholar]
- Mazlan R, Kei J, Hickson L, et al. High frequency immittance findings: Newborn versus six-week-old infants. Int J Audiol. 2007;46:711–717. doi: 10.1080/14992020701525858. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. 2000 CDC Growth Charts for the United States: Methods and Development. Washington, D.C.: U.S. Government Printing Office; 2000. [PubMed] [Google Scholar]
- Palmu A, Puhakka H, Rahko T, et al. Diagnostic value of tympanometry in infants in clinical practice. Int J Pediatr Otorhinolaryngol. 1999;49:207–213. doi: 10.1016/s0165-5876(99)00207-4. [DOI] [PubMed] [Google Scholar]
- Papoulis A. Probability and Statistics. New York: Prentice-Hall; 1990. [Google Scholar]
- Paradise JL, Smith CG, Bluestone CD. Tympanometric detection of middle ear effusions in infants and young children. Pediatrics. 1976;58:198–209. [PubMed] [Google Scholar]
- Pestalozza G, Cusmano G. Evaluation of tympanometry in diagnosis and treatment of otitis media of the newborn and of the infant. Int J Pediatr Otorhinolaryngol. 1980;2:73–82. doi: 10.1016/0165-5876(80)90031-2. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Feeney MP. Effects of maturation on tympanometric wideband acoustic transfer functions in human infants. J Acoust Soc Am. 2008;124:2106–2122. doi: 10.1121/1.2967864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnaz N. Wideband reflectance in neonatal intensive care units. J Am Acad Audiol. 2008;19:419–429. doi: 10.3766/jaaa.19.5.4. [DOI] [PubMed] [Google Scholar]
- Shahnaz N, Miranda T, Polka L. Multifrequency tympanometry in neonatal intensive care unit and well babies. J Am Acad Audiol. 2008;19:392–418. doi: 10.3766/jaaa.19.5.3. [DOI] [PubMed] [Google Scholar]
- Tamimi RM, Lagiou P, Mucci LA, et al. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326:1245–1246. doi: 10.1136/bmj.326.7401.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Werff KR, Prieve BA, Georgantas LM. Test-retest reliability of wideband reflectance measures in infants under screening and diagnostic test conditions. Ear Hear. 2007;28:669–681. doi: 10.1097/AUD.0b013e31812f71b1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


