Abstract
Alcohol abuse and obesity are two known risk factors for hepatocellular carcinoma (HCC) that also synergistically promote HBV/HCV-related carcinogenesis. TLR4, the PAMP for endotoxin participates in inflammatory processes such as M1 activation of hepatic macrophages in alcoholic liver disease. However its role in liver carcinogenesis via ectopic expression and activation, has only recently been revealed in alcohol/HCV-associated HCC models. Alcohol feeding to mice expressing the HCV Ns5a in a hepatocyte specific manner, aggravates liver inflammation via activation of overexpressed TLR4 in the parenchymal cells. Long-term alcohol feeding produces liver tumors in these transgenic mice in a manner dependent on TLR4. From these mice, CD133+/CD49f+ tumor initiating stem cell-like cells (TICs) have been isolated. These TICs exhibit self-renewal and tumorigenic activities driven by TLR4-dependent upregulation of the stem cell factor NANOG. Defective TGF-β tumor suppressor pathway is identified in the TICs and mediated by NANOG target genes Igf2bp3 and Yap1. This TGF-β pathway antagonism is responsible in part for TIC’s tumorigenic activity and chemoresistance. Conversely, mice with attenuated TGF-β pathway due to haploinsufficiency of β2-Spectrin, spontaneously develop liver tumors and alcohol-feeding increases tumor incidence in a TLR4 dependent manner. This reciprocal antagonism between TLR4 and TGF-β pathways may serve as a novel therapeutic target for HCC.
Keywords: TLR4, cancer stem cells, NANOG, TGF-β
Liver cancer epidemiology: importance of synergism among alcohol, obesity and HCV
Hepatocellular carcinoma (HCC) is the most prevalent malignancy of the liver and the 5th most common cancer in men. HCC is diagnosed in over half a million patients world-wide annually and is the second leading cause of the cancer-related death. Cirrhosis is the far most important risk factor for HCC, increasing the risk by 40-fold compared to normal subjects and ~70% of HCC patients have underlying cirrhosis. Despite this tight association, molecular mechanisms by which cirrhosis promotes HCC risk are still elusive. Viral hepatitis (HBV and HCV) is the most common etiological factor for HCC, followed by alcoholic liver disease (ALD). In particular, chronic infection with HCV represents a major risk for HCC (1) as more than 170 million people are infected with HCV globally (1–3). HCV produces proteins which are directly implicated in hepatocyte signaling or metabolic dysregulation, toxicity, and transformation. For instance, the HCV Core protein stimulates production of reactive oxygen species which can cause mitochondrial or nuclear DNA damage (2, 4, 5). The Core protein also inhibits microsomal triglyceride transfer protein and VLDL secretion (6), contributing to the genesis of fatty liver. The Core also induces insulin resistance in vitro and in vivo, and this effect may be mediated by degradation of insulin receptor substrates (IRS) 1 and 2 via upregulation of SOCS3 (7) and IRS serine phosphorylation (8). The latter mechanisms are also relevant to another disease which is increasingly recognized to promote HCC risk: non-alcoholic fatty liver disease (NAFLD) (9) that is a liver phenotype of obesity-associated metabolic syndrome. In fact, HCV/HBV infection, ALD, and NAFLD share common pathophysiological characteristics such as oxidant stress, organelle stress, and metabolic dysregulation which individually or collectively contribute to their oncogenic activities. More importantly, a striking synergism among HCV, alcohol, and obesity exists for the risk of HCC. Existence of co-morbidities such as alcohol abuse or obesity, increases the HCV risk of developing HCC by additional 8 fold, resulting in an overall 45–55 fold increase in the risk as compared to subjects without HCV infection and the co-morbidities (10, 11). As alcohol and obesity continue to dominate as leading life-style factors for disease burden around the world (12), heightened HCC incidence caused by synergistic interactions by these factors and hepatitis virus, represents the most predictable and devastating global health issue.
Inflammation and cancer
Since Rudolf Virchow noted leukocytes in tumor tissues 150 years ago, a link between inflammation and cancer has been an important topic in cancer research (13, 14). Approximately 15–20% of tumor cases are causally associated with infection (15) and chronic inflammation precedes and accompanies tumor development induced by many of tumor causing agents including chemical and physical agents, infectious agents, and autoimmune reactions. Best examples of malignancies caused by infection include gastric cancer caused by Helicobacter pylori, bladder and colon carcinoma caused by schistosomiasis, and HCC caused by HCV and HBV. Further the roles of sterile inflammation in tumorigenesis are also increasingly recognized. Experimental evidence generated during the last two decades implicates the involvement of many inflammatory mediators (e.g., TNF-α, CXCL1, CXCR2, MIF, IKK) in the promotion of various tumors. For instance, macrophage migration inhibitory factor (MIF) released by T lymphocytes and macrophages, is shown to suppress transcriptional activity of p53 to promote tumor formation (16). However, whether and how inflammatory pathways initiate tumorigenesis are still elusive other than a very descriptive concept of DNA damage caused by oxygen and nitrogen radical species produced by infiltrating inflammatory cells. IκB kinase (IKK) which phosphorylates IκB for its degradation and consequent nuclear translocation of active p65/p50, is the most critical mediator of inflammation and cell survival. Deficiency of IKKβ, the catalytic subunit, in hepatocytes predisposes the liver for diethylnitrasamine (DEN)-mediated hepatocarcinogenesis via enhanced hepatocellular death and compensatory proliferation due to sustained JNK activation (17). On the other hand, myeloid specific Ikkb knockout attenuates liver cancer incidence due in part to suppression of MyD88-dependent IL-6 production which also appears to determine the gender disparity in liver cancer development (18). Although this study highlights the role of the macrophage-derived cytokine, cytokines and chemokines released from tumor cells or cells subjected to transformation insults, may also play a role as shown for production of CXCR2-activating chemokines by primary intestinal adenomas and other tumors (19). This study also demonstrates the importance of CXCR2 on Ly6G+ neutrophils for neutrophil recruitment to tumor sites and promotion of tumor growth and expansion. In Ly6G+ depleted mice, these CXCR2 mediated effects are abrogated and tumor cells even undergo apoptosis (19). A similar notion linking neutrophils and HCC needs to be investigated particularly addressing the relationship between alcoholic hepatitis and HCC development.
TLR4 links inflammation to tumorigenesis
The pathogenetic roles of TLR4 and its ligand endotoxin in alcoholic liver disease (ALD) have been established (20–22). Here endotoxin that enters the portal circulation due to alcohol-induced gut permeability, activates TLR4 on hepatic macrophages to stimulate the release of proinflammatory cytokines such as TNF-α and to exert cytotoxic effects on hepatocytes which are sensitized by alcohol (23). Interestingly, TLR4 is ectopically upregulated in hepatocytes in HCV Ns5a transgenic (Tg) mice (24). When these mice are challenged by intraperitoneal injection of a sublethal dose of LPS, fulminant hepatitis with marked increases in plasma AST and TNFα and mortality ensues, but not in wild type (WT) or Ns5a Tg:Tlr4−/− compound mice (24). Similarly, intragastric feeding of ethanol and high fat liquid diet to these Tg mice results in more severe alcoholic steatohepatitis compared to WT mice despite similar endotoxemia, and some mice even develop hepatitis with mid-zonal necrosis and mononuclear and neutrophilic infiltration (24). As expected, this aggravating effect is prevented in Ns5a Tg:Tlr4−/− mice, incriminating the role of ectopically induced hepatocyte TLR4 in accentuating liver inflammation. When Polymixin B and Neomycin are administered to alcohol-fed Ns5a Tg mice, liver injury is attenuated, and enteral administration of LPS to the mice worsens liver injury, supporting the role of gut endotoxin (24). When Ns5a Tg mice are fed alcohol liquid diet ad libitum for 12 months, liver tumor develops in 23% of mice while no tumor incidence is noted in alcohol-fed WT or Ns5aTg:Tlr4−/− mice deficient in TLR4 (24). These results suggest activation of ectopically upregulated TLR4 by alcohol-induced endotoxemia, potentiates alcoholic liver inflammation in the short-term and induces liver tumor in the long-term, supporting an unequivocal role of TLR4 in linking inflammation to tumorgenesis in this model (Figure 1). A subsequent study has extended this observation to HCV Core Tg mice, basically confirming the role of TLR4 in alcohol-induced liver carcinogenesis (25).
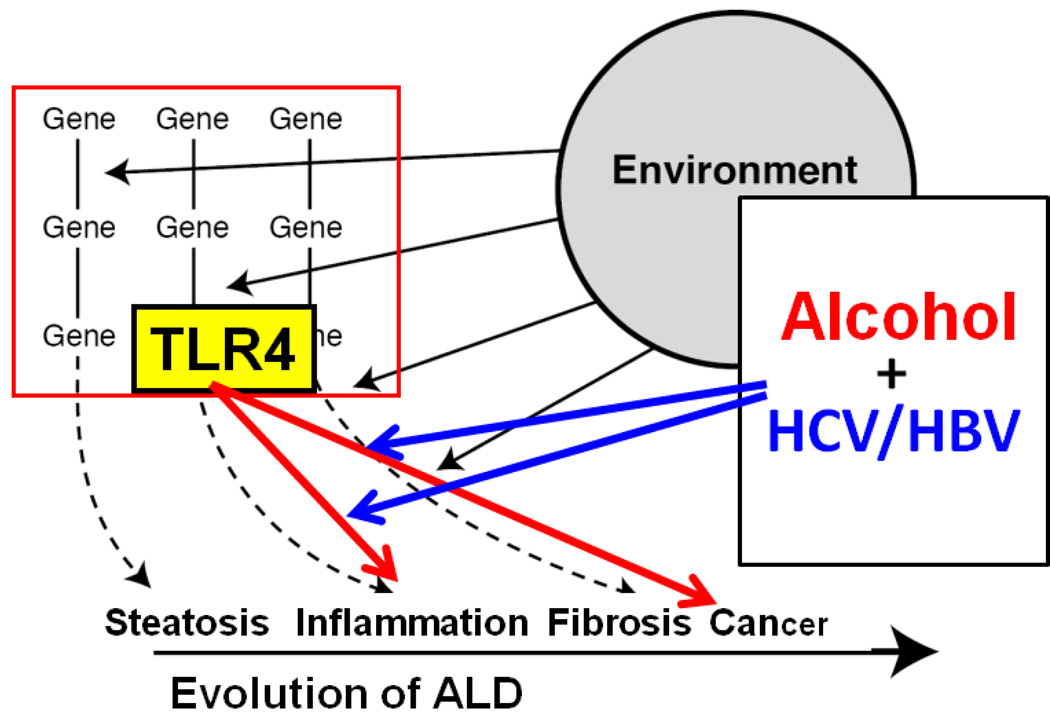
Figure 1.
This schematic diagram depicts that alcohol and HCV interact to render synergistic effects on the evolution of alcoholic liver disease that is governed by gene-environment interactions (dotted arrows and solid black lines). TLR4 mediates promotion of liver inflammation and cancer as depicted by red arrows and alcohol and HCV facilitate these TLR4-dependent mechanisms as shown by blue arrows.
In fact, TLR4 is already implicated in lung (26), colon (27), and skin carcinomas (28). Although we consider macrophages and lymphocytes are the primary cell types which express TLR4, increasing evidence points to the role of TLR4 ectopically expressed in epithelial parenchymal cells in oncogenesis (24, 29). A recent study from Robert Schwabe’s laboratory demonstrates HCC development in mice induced by DEN and carbon tetrachloride requires the intestinal microbiota and TLR activation in non-bone marrow-derived resident liver cells (30), suggesting the importance of TLR4 on hepatocytes, biliary epithelial cells, and/or hepatic stellate cells. Our aforementioned results point to the role of hepatocytes in hepatocarcinogenesis although this has to be confirmed in other non-HCV models. This study from Schwabe’s laboratory also concludes that TLR4 and the intestinal microbiota are required for HCC promotion but not HCC initiation (30). In contrast, our studies on alcohol-fed HCV Tg mice and tumor initiating stem cell-like cells (TICs) isolated from these models demonstrate TLR4 activation induces the key pluripotency factor NANOG and other stem cell factors required for the generation of CD133+/CD49f+ TICs and the development of liver tumors (25).
The involvement of inflammasomes in liver inflammation is increasingly recognized (31) where endogenous damaged-associated molecular patterns (DAMP) triggers sterile inflammation via the assembly of the inflammasome and consequent activation of caspase-1, interleukin-1 and other cytokines. Indeed, recent studies demonstrate the involvement of this inflammatory pathway in alcoholic steatohepatitis in mice (32) and hepatitis associated with HCV infection (33). Whether this pathway also links inflammation to liver oncogenesis, is yet to be tested.
The most recent study has identified and isolated liver cancer progenitors from the pre-malignant lesions in different mouse HCC models, and these cells are shown to give rise to liver cancer when introduced into a liver undergoing chronic damage and compensatory proliferation (34). These cells reside within dysplastic lesions and are suggested to arise from dysplastic hepatocytes and form tumors via autocrine IL-6 signaling. It is yet to be determined whether our TLR4-dependent TICs are traced back to dysplastic cells and whether the cancer progenitors from this study are also driven by the TLR4 pathway.
TLR4-deopendent TICs and TLR4-TGF-β antagonism
Immunostaining of liver tumor sections from alcohol-fed Ns5a Tg mice reveals small cells with an increased nuclear to cytoplasmic ratio positively stained for both NANOG and CD133, the marker for progenitor cells (24). This prompted us to isolate CD133-positive cells via FACS. Using this approach, a very small yet significantly increased percentage of CD133+/CD49f+ cells is isolated from liver tumors of alcohol-fed Ns5a Tg mice compared to normal livers of WT mice (1.11% vs. 0.05%) (25). Analysis of the CD133+/CD49f+ cells show enhanced expression of stemness genes such as Nanog, Oct4, Sox2 as compared to CD133−/Cd49f+ cells, and these inductions are abrogated by knockdown of Tlr4. Further, the cells grow anchorage independently in soft agar and form spheroids in methylcellulose culture in a manner dependent on TLR4 and NANOG (25). Finally, the tumor-initiating property of CD133+/CD49f+ cells is confirmed by tumor development by serial transplantation in NOG mice. As predicted, this activity is also TLR4 and NANOG dependent. Of interest, these TLR4/NANOG-dependent TICs are defective in the TGF-β tumor suppressor pathway because NANOG induces IGF2BP3 and YAP1 which block the TGF-β pathway at the level of SMAD3 phosphor-activation and p-SMAD3 nuclear translocation, respectively (25). Further, IGF2BP3-mediated AKT activation phosphorylates YAP1 to enhance YAP1’s ability to retain SMAD in the cytosol. Knockdown of these two proteins restores TGF-β pathway, suppresses self-renewal and tumorigenic activity, and enhances chemosensitivity in TICs. Conversely, reduced TGF-β tumor suppressor pathway caused by haploinsufficiency of β2-Spectrin, the chaperon protein required for p-SMAD nuclear translocation, results in spontaneous liver tumor development in a manner dependent on ectopically induced and activated TLR4, and this effect is aggravated by alcohol intake. Knockdown of β2-Spectrin in Huh7 cells significantly increases TLR4 expression, and the cells’ self-renewal and tumorigenic activities. The latter effects are abrogated by concomitant knockdown of TLR4, extending the relevance of the TLR4-TGF-β antagonism to humans. In essence, tipping the balance of the reciprocal antagonism between TLR4 oncogenic and TGF-β tumor suppressor pathways, appears to determine whether tumor initiating occurs, and this notion signifies the importance of this antagonism as a novel potential therapeutic target for HCC (see a schematic diagram shown in Figure 2).
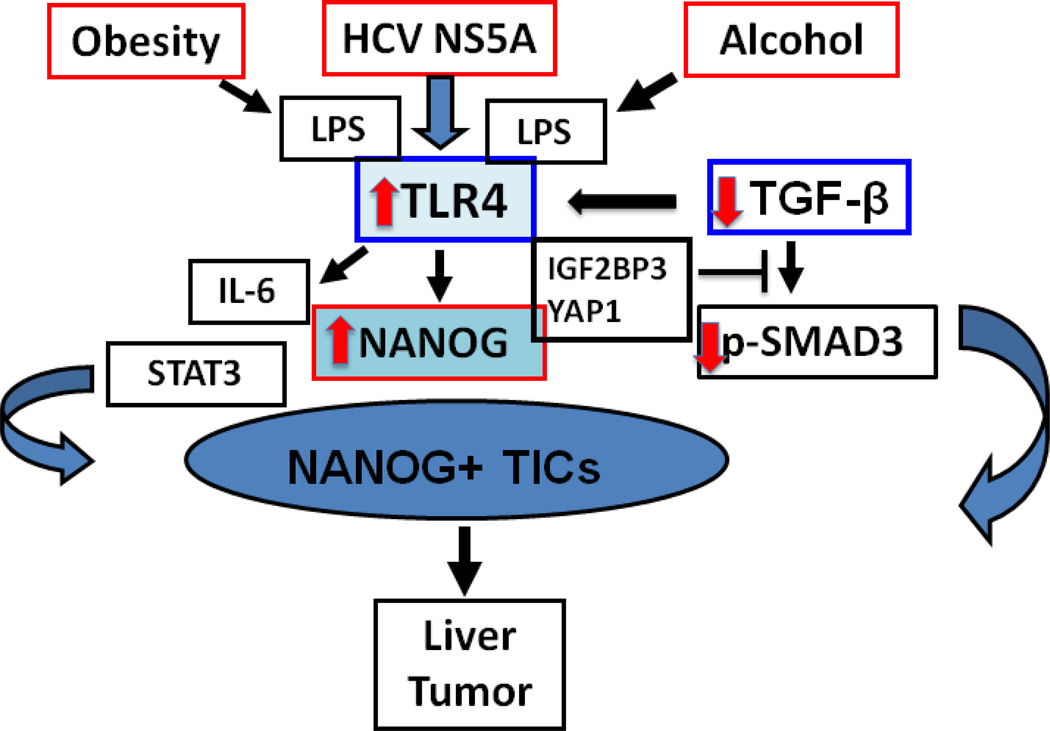
Figure 2.
Reciprocal antagonism by TLR4 oncogenic and TGF-β tumor suppressor pathways. TLR4 is ectopically upregulated in hepatocytes by HCV NS5A and activated by endotoxemia caused by alcohol intake or obesity. Activated TLR4 induces the pluripotency factor NANOG to generate tumor-initiating stem cell-like cells (TICs) partly via compromised TGF-β pathway by IGF2BP3 and YAP1 induced by NANOG. Conversely, defective TGF-β pathway upregulates TLR4 and causes liver tumorigenesis.
Acknowledgments
The authors’ research described in this review was supported by NIH grants 1R01AA018857, 5RC2AA019392, and P50AA011999, and Department of Veterans Affairs.
Footnotes
Compliance with Ethical Requirements
The studies were also conducted following full approval of IRB and IACUC protocols by respective institutions for appropriate involvement of human and animal subjects.
Hidekazu Tsukamoto, Lopa Mishra, and Keigo Machida declare no conflict of interest.
References
- 1.Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32(1 Suppl):225–237. doi: 10.1016/s0168-8278(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 2.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002 Feb;122(2):366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 3.Yao F, Terrault N. Hepatitis C and hepatocellular carcinoma. Curr Treat Options Oncol. 2001 Dec;2(6):473–483. doi: 10.1007/s11864-001-0069-6. [DOI] [PubMed] [Google Scholar]
- 4.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005 Nov 11;280(45):37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 5.Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, et al. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001 Jun 1;61(11):4365–4370. [PubMed] [Google Scholar]
- 6.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chretien Y, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002 Feb;16(2):185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004 Nov;165(5):1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee S, Saito K, it-Goughoulte M, Meyer K, Ray RB, Ray R. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J Virol. 2008 Mar;82(6):2606–2612. doi: 10.1128/JVI.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular Cancer - the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2013 Aug 23; doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002 Nov;36(5):1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 11.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004 Sep 1;101(5):1009–1017. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto H. Conceptual importance of identifying alcoholic liver disease as a lifestyle disease. J Gastroenterol. 2007 Aug;42(8):603–609. doi: 10.1007/s00535-007-2075-3. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001 Feb 17;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkin DM, Pisani P, Munoz N, Ferley F. Microbes and Malignancy: Infection as a cause of human cancers. New York: Cold Spring Harbor Laboratory Press; 1999. Infections and human cancer. [Google Scholar]
- 16.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999 Nov 15;190(10):1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007 Jul 6;317(5834):121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012 Sep 4;122(9):3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001 Jul;34(1):101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 21.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000 Nov;32(5):1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 22.Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, et al. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001 Jun;25(6 Suppl):51S–54S. doi: 10.1097/00000374-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 23.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001 Jun;15(8):1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 24.Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CL, Tsukamoto H, Liu JC, Kashiwabara C, Feldman D, Sher L, et al. Reciprocal regulation by TLR4 and TGF-beta in tumor-initiating stem-like cells. J Clin Invest. 2013 Jul 1;123(7):2832–2849. doi: 10.1172/JCI65859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Bauer AK, Dixon D, DeGraff LM, Cho HY, Walker CR, Malkinson AM, et al. Toll-like receptor 4 in butylated hydroxytoluene-induced mouse pulmonary inflammation and tumorigenesis. J Natl Cancer Inst. 2005 Dec 7;97(23):1778–1781. doi: 10.1093/jnci/dji403. [DOI] [PubMed] [Google Scholar]
- 27.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007 Dec;133(6):1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010 Jul 7;29(13):2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukata M, Shang L, Santaolalla R, Sotolongo J, Pastorini C, Espana C, et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis. 2011 Jul;17(7):1464–1473. doi: 10.1002/ibd.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012 Apr 17;21(4):504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012 Nov;143(5):1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012 Oct 1;122(10):3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1beta production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013 Apr;9(4):e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He G, Dhar D, Nakagawa H, Font-Burgada J, Ogata H, Jiang Y, et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell. 2013 Oct 10;155(2):384–396. doi: 10.1016/j.cell.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]