Abstract
The effect of circadian rhythm (CR) disruption on immune function depends on the method by which CRs are disrupted. Behavioral and thermoregulatory responses induced by lipopolysaccharide (LPS) treatment were assessed in female Siberian hamsters in which circadian locomotor activity (LMA) rhythms were eliminated by exposure to a disruptive phase-shifting protocol (DPS) that sustains arrhythmicity even when hamsters are housed in a light-dark cycle. This noninvasive treatment avoids genome manipulations and neurological damage associated with other models of CR disruption. Circadian rhythmic (RHYTH) and arrhythmic (ARR) hamsters housed in a 16L:8D photocycle were injected with bacterial LPS near the onset of the light (zeitgeber time 1; ZT1) or dark (ZT16) phase. LPS injections at ZT16 and ZT1 elicited febrile responses in both RHYTH and ARR hamsters, but the effect was attenuated in the arrhythmic females. In ZT16, LPS inhibited LMA in the dark phase immediately after injection but not on subsequent nights in both chronotypes; in contrast, LPS at ZT1 elicited more enduring (~4 day) locomotor hypoactivity in ARR than in RHYTH hamsters. Power and period of dark-phase ultradian rhythms (URs) in LMA and Tb were markedly altered by LPS treatment, as was the power in the circadian waveform. Disrupted circadian rhythms in this model system attenuated responses to LPS in a trait- and ZT-specific manner; changes in UR period and power are novel components of the acute-phase response to infection that may affect energy conservation.
Keywords: immune function, circadian rhythms, ultradian rhythms, brain-immune interactions, sickness behavior
Immune function is modulated by the circadian pacemaker in the suprachiasmatic nucleus (SCN). Behavioral, thermoregulatory, and cytokine responses to innate immune challenges (acute-phase responses) reflect an interaction between peripheral cytokine production and brain responsiveness to cytokines (Dantzer et al., 2008). The acute-phase response may be particularly instructive for understanding interactions between the circadian and immune systems. Innate immune responses are robustly modulated by the circadian clock (Franklin et al., 2003; Marpegan et al., 2009): in many animal models, inflammatory responses to bacterial lipopolysaccharide (LPS) are greater during the interval approaching the onset of the active phase and reduced near the beginning of the rest phase (Curtis et al., 2014). Conversely, innate immune activation affects circadian timekeeping. LPS elicits SCN c-fos expression and shifts the circadian pacemaker (Marpegan et al., 2005). Convergent evidence suggests that LPS-induced phase shifts are mediated by proinflammatory cytokines (Marpegan et al., 2005; Paladino et al., 2010; Leone et al., 2012). Thus, time-of-day information derived from the circadian pacemaker modulates responsiveness of the immune system, and conversely, immune mediators modulate clock function.
Whereas circadian rhythms provide circa-24-h temporal order, ultradian rhythms (URs) impose essential temporal structure on subcircadian timescales (periods <8 h; Veldhuis, 2008; Yates and Yates, 2008). URs affect diverse aspects of physiology and behavior, including sleep (Mueller et al., 2012), hormones (Knobil, 1999; Choe et al., 2013; Lloyd et al., 2008), food intake (Warner et al., 2010), and body temperature (Heldmaier et al., 1989), traits that undergo striking changes during the acute response to infection (Hart, 1988). Despite the ubiquity of physiological and behavioral URs, it is unknown whether the temporal structure of URs, like circadian rhythm (CRs), is affected by immune activation.
Induced circadian arrhythmia has been widely used to assess circadian regulation of organismal physiology. Insights regarding circadian influences on immune responses have been gleaned from models of circadian arrhythmia induced by clock gene knockouts (Gibbs et al., 2012), constant illumination (LL; Deprés-Brummer et al., 1997), and ablation of the SCN (SCNx; Filipski et al., 2003; Guerrero-Vargas et al., 2014), but each of these models has limitations. Clock genes are present in all tissues, and resulting immunophenotypes may reflect either arrhythmia or interference with cellular metabolic processes; LL can elevate immunosuppressive glucocorticoids (Welberg et al., 2006) and SCN lesions damage adjacent hypothalamic tissue, increase stress hormones (Buijs et al., 1993; Kalsbeek et al., 2012), and generate glial scars and neuroinflammation that persist for months after the insult (Logan et al., 1992; Silver and Miller, 2004). A general theme emerging from these and other studies is that the circadian system inhibits inflammatory responses and may provide a circadian temporal gating of inflammatory responses (Gibbs et al., 2012); other reports, however, indicate attenuated inflammatory responses in circadian-disrupted animals (Wachulec et al., 1997; Liu et al., 2006; Spengler et al., 2012).
Here we used a noninvasive model of circadian arrhythmia: the disruptive phase-shifted (DPS) hamster, which avoids the aforementioned complications, to examine the consequences of circadian disruption on innate inflammatory responses. DPS hamsters are well suited for translational analyses because, as in humans, dysrhythmia is manifested by genetically and neurologically intact individuals that remain exposed to circadian light-dark cycles. In DPS hamsters, CRs in locomotor activity (LMA), Tb, sleep, hormone secretion, and SCN clock gene expression are eliminated by light treatments administered over the course of 2 days (Ruby et al., 2004; Fernandez et al., 2014).
The present experiment was conducted on female Siberian hamsters; females are understudied in neuroscience research (Beery and Zucker, 2011; Prendergast et al., 2014), an impediment to establishing brain-behavior relations applicable to both sexes.
MATERIALS AND METHODS
Animals
Female Siberian hamsters (Phodopus sungorus) were derived from a breeding colony maintained in a long-day, 15L:9D photoperiod (LD) at the University of Chicago. Hamsters were housed in polypropylene cages, with food (Teklad; Harlan; Madison, WI) and filtered tap water provided ad libitum; cotton nesting material was available in the cages. Ambient temperature and relative humidity were held constant at 19 ± 2°C and 53% ± 10%, respectively. All procedures conformed with principles and guidelines in the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Hamsters were subjected to the circadian DPS procedure at 2 to 8 months of age, and LPS treatments were administered between 8 and 15 months of age, after stable behavioral phenotypes had emerged. Because the DPS procedure induces circadian arrhythmia in a subset of hamsters (see below), middle-aged hamsters 8 to 15 months of age received the DPS treatment at one time point. In some reports, Siberian hamsters are regarded as “aged” at 17 to 24 months (Horton and Yellon, 2001; McKeon et al., 2011) and in others at 24 to 28 months (Reuss and Bürger, 1994; Reuss et al., 2000).
DPS Procedure
The DPS manipulation that destabilizes the hamster circadian pacemaker employs phase-resetting light stimuli that render a substantial proportion of hamsters permanently behaviorally circadian arrhythmic (“ARR”; Ruby et al., 2004) and disrupts SCN clock gene expression (Grone et al., 2011). Hamsters were housed for 4 weeks in a 16L:8D photoperiod, and then on a single night, a 2-h light pulse was administered during the fifth through seventh hours of the dark phase. The next day, the 16L:8D photocycle was phase-delayed by 3 h, by extending the light phase. Ninety-three hamsters were subjected to the DPS protocol, which typically renders >50% of hamsters permanently circadian arrhythmic (Ruby et al., 2004).
Locomotor Activity Monitoring
After DPS treatment, home-cage LMA was assessed using passive infrared motion detectors positioned outside the cage (22 cm above the cage floor). Motion detectors registered activity when 3 of 27 zones were crossed. Activity triggered closure of an electronic relay recorded by a computer running ClockLab software (Actimetrics, Evanston, IL). Cumulative activity counts were collected at 1-min intervals. Activity data were collected in consecutive 10-day blocks, over an interval spanning 2 to 3 months after the DPS treatment (cf. Ruby et al., 1998).
Circadian Phenotyping
Criteria for assessing the presence/absence of CRs were comparable to those in prior reports of DPS-induced CR disruption (Ruby et al., 2004; Ruby et al., 1998). The χ2 periodogram analyses (ClockLab; Actimetrics) were performed on 10-day blocks of activity data (cf. Ruby et al., 1998). Hamsters were designated ARR absent clear and significant (p < 0.001) peaks in the periodogram in the circadian range, with LMA distributed throughout the light-dark cycle, and daily discrete activity onsets and offsets not evident on visual inspection of the periodogram; hamsters with significant circadian activity peaks in the χ2 periodogram and clear daily activity onsets and offsets were considered entrained/ rhythmic (RHYTH) (Ruby et al., 1998). Sixteen RHYTH and 16 ARR hamsters were randomly assigned to receive LPS and saline treatments. LMA records of representative RHYTH and ARR hamsters are illustrated in Figure 1.
Figure 1.
Disruptive phase shifts render hamsters behaviorally arrhythmic. Representative double-plotted activity records of circadian entrained hamsters (RHYTH; A and C) and of hamsters rendered circadian arrhythmic (ARR; B and D) by disruptive phase shift (DPS) light treatments (see “DPS Procedure” for details). Records depict 10 consecutive days of home-cage locomotor activity collected >1 month after the DPS procedure was applied; corresponding χ2 periodogram analyses are depicted below each activity record. Values above the ascending line in the periodogram analysis indicate a significant (p < 0.001) period. Time is indicated on the horizontal axis at the top of each actogram, along with light (white) and dark (black) phases of the 16L:8D photocycle. Shaded area indicates the dark phase.
Surgical Procedures
Upon completion of circadian phenotyping, hamsters were anesthetized with isoflurane vapors (3% in medical O2) and received precalibrated radio-telemetric transmitters intraperitoneally (i.p.) (Mini-Mitter ER-4000, E-mitters; Philips Respironics Murrysville, PA). Postoperatively, subcutaneous (s.c.) buprenorphine analgesic was administered at 12-h intervals for 48 h. E-mitters transmitted mean body temperature (Tb) and cumulative LMA at 6-min intervals to receiver boards placed under the cages. Temperature and LMA data were acquired using Vitalview software (Philips Respironics) and stored for offline analyses.
LPS Treatments
After a postsurgical recovery interval ≥1 month, physiological and behavioral sickness responses to LPS were evaluated as previously described (Prendergast et al., 2008). Estrous cycle (EC) stage is difficult to monitor in this species (Wynne-Edwards and Lisk, 1987); therefore, the day of the EC on which LPS was administered was not known and may have introduced some variability in the data; however, because all hamsters were freely cycling, any such error was randomly distributed across all subjects and all treatment conditions. Body mass and food intake values were recorded daily; body mass data are not reported here. After 3 d of baseline testing, hamsters were injected i.p. with either bacterial LPS (625 µg/kg; isolated from Escherichia coli strain 026:B6, Sigma, St. Louis, MO) or sterile 0.9% saline (0.1 mL). This dose of LPS is commonly used in studies of Siberian hamsters (Wen et al., 2007; Prendergast et al., 2008), which require a somewhat higher dose to elicit robust sickness responses (Bilbo et al., 2002, 2003; Prendergast et al., 2003). Injections in one cohort of hamsters were delivered at ZT16 (during 15 min immediately preceding the onset of darkness) and in a separate cohort at ZT1 (1 h after light onset). Food intake, body mass, Tb, and LMA were recorded for 6 to 7 d after injections. LPS and saline treatments were administered in a block-randomized design. Hamsters were randomly assigned to initial LPS/ saline treatment groups, and treatments were delivered in a counterbalanced design, with successive injections separated by 10 to 14 days. Due to equipment failure, Tb and activity data from a subset of RHYTH (LPS, n = 4; saline, n = 4) and ARR (LPS, n = 4; Saline n = 4) hamsters injected with LPS at ZT1 were collected for 48 h after injection, but no Tb or activity data were recorded for the ensuing 4 days.
Analyses of Ultradian Responses to LPS Treatment
Time series associated with Tb and LMA were analyzed using methods typically applied to oscillatory neural signals (Kay and Beshel, 2010; Kay et al., 2009). Multitaper techniques, adapted to brief events in which only a small number of cycles may be present, were used to quantify URs and CRs. Ultradian and circadian frequency and power analyses were done in MATLAB using multitaper analysis tools (mtspecgramc) from the Chronux toolbox (http://www.chronux.org/; Mitra and Bokil 2008). Ultradian spectral analysis was performed separately on 3 nonoverlapping 8-h windows to enable resolution of periods <4 h and to separate dark and light periods. Five tapers were applied to each 8-h data window, restricting the analysis band to 6.9e-5 to 2.78e-4 Hz (1- to 4-h periods). Power was estimated by summing the power within the analysis band for each 8-h interval. The dark and light phases were analyzed separately; however, complete data for light-phase activity and temperature URs are not reported here due to the absence of systematic differences between chronotypes, except as noted below. This summed power was then transformed to dB, as is standard procedure (Kay and Beshel, 2010), by 10*log10(treatment power/baseline power), so that negative values represent a decrease and positive values an increase from the 8-d baseline means. The peak frequency was estimated using the pmtm multitaper function in MATLAB, and the maximum within the analysis band as described above was taken as the peak frequency.
Analyses of Circadian Responses to LPS Treatment
To assess CR power, a 2-day window (480 points at 6-min intervals) of Tb or LMA data, stepped by 1 day, was analyzed, resulting in 7 power analyses for an 8-d period. Preceding analysis, data were subjected to a 30-point moving average smoother, and then the mean of the 8 h of signal was subtracted. The multitaper analysis was performed using 5 tapers, a moving window of 480 points (48 h) stepped by 240 points (24 h); analysis was restricted to a band pass of 0.000005 to 0.00005 Hz (5.55- to 55.5-h periods). Preliminary analysis indicated that power from the smoothed signals fell within a restricted range, so power was summed within the period band 51.2 to 12.8 h. Power values were converted into dB using the mean baseline power for each hamster, as described above.
Statistical Analyses
Analyses of variance (ANOVAs) and post hoc pair-wise comparisons were performed with Statview 5.0 (SAS Institute, Cary, NC). Effects of injection treatment and circadian phenotype on Tb LMA and food intake were assessed using ANOVA followed by Fisher’s protected least significant difference (PLSD) tests or 2-tailed t tests. To protect against type I error and α inflation in experiment 1, multiple pairwise comparisons of Tb were only performed after obtaining a significant omnibus F statistic, with α set to 0.01. In all other cases, differences were considered significant if p ≤ 0.05.
RESULTS
Effects of Circadian Arrhythmia on Sickness Responses to LPS
Fever
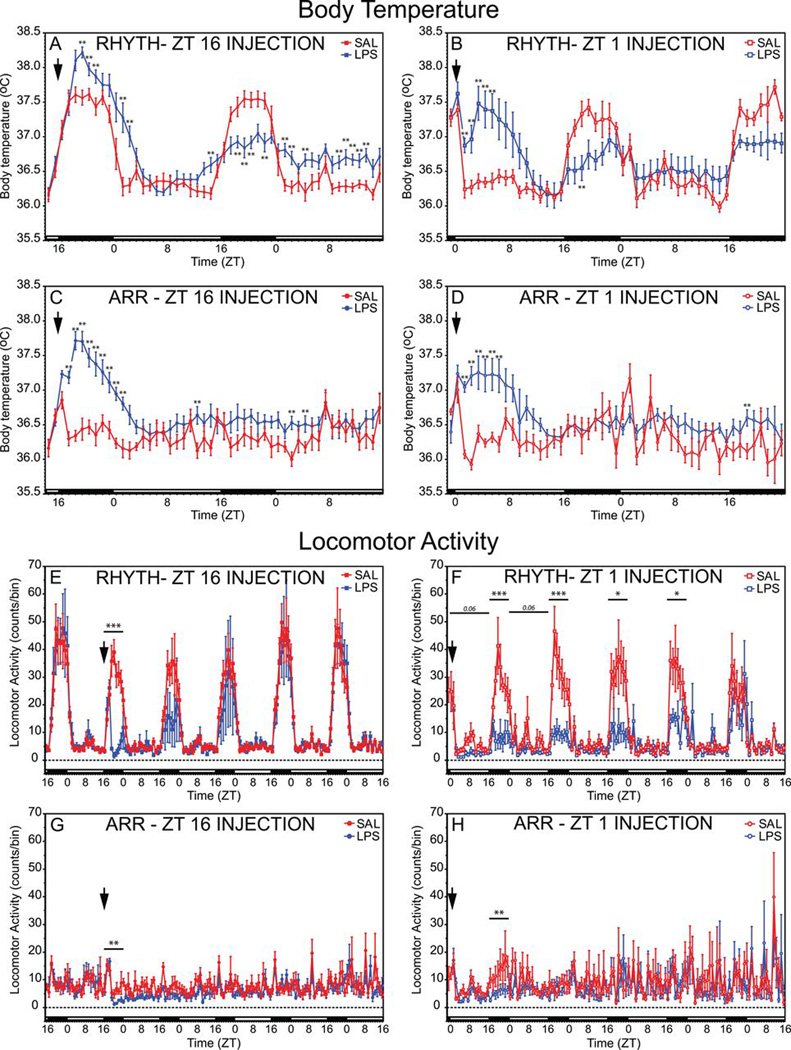
LPS treatments at ZT16 elicited febrile responses, and circadian chronotype significantly modulated effects of LPS on the pattern of change in Tb (chronotype × injection: F49,1372 = 3.87, p ≤ 0.001; Fig. 2). Fevers persisted until the early hours of the next morning (Fig. 2A). During the ensuing dark phase, LPS-treated RHYTH hamsters exhibited hypothermia, whereas ARR hamsters exhibited no residual thermoregulatory response to LPS (Fig. 2C). In the next light phase, Tb was again elevated in RHYTH but not ARR hamsters treated with LPS.
Figure 2.
Lipopolysaccharide (LPS) elicits fever and inhibits locomotor activity. Mean ± SEM body temperature (A–D) and spontaneous home-cage locomotor activity (LMA; E–H) of circadian entrained (RHYTH) and circadian arrhythmic (ARR) female Siberian hamsters injected intraperitoneally (i.p.) with bacterial LPS (625 µg/kg) or sterile saline (SAL) at either ZT16 or ZT1. Chronotype and injection time are indicated within each panel. Each data point indicates the mean of 10 Tb values (6-min sampling interval) obtained during a given hour. The timing of injection treatments is indicated by a downward vertical arrow near the ordinate axis, and the light/dark cycle is indicated with black (dark phase) and white (light phase) horizontal bars along the abscissa. A–D: **p < 0.01 vs. SAL value, within each panel. E–H: *p < 0.05, **p < 0.01, ***p < 0.001 vs. SAL value.
LPS at ZT1 also elicited fever, and chronotype interacted with injection to affect the change in Tb (F42,1176 = 1.51, p = 0.021; Fig. 2B,D). In RHYTH hamsters, fever persisted for the first half of the light phase (Fig. 2B); during the ensuing dark phase, their nocturnal rise in Tb was reduced (F1,14 = 6.92, p = 0.02; Fig. 2B). Among ARR hamsters, LPS also caused fever for the first half of the initial posttreatment light phase, but thermoregulatory effects were not evident the following night (Fig. 2D).
Locomotor activity
In hamsters injected with LPS at ZT16, chronotype (F119,3332 = 11.7, p ≤ 0.001) and injection (F119,3332 = 1.72, p ≤ 0.001) affected the pattern of LMA over the next 5 days (Fig. 2); the chronotype × injection interaction was marginally significant (F119,3332 = 1.22, p = 0.054). LPS inhibited LMA in RHYTH hamsters during the dark phase immediately after injection (0–8 h postinjection; F1,14 = 46.3, p ≤ 0.0001, Fig. 2E), but on the ensuing 4 nights, LMA did not differ significantly between LPS- and SAL-treated hamsters. In ARR hamsters, LPS at ZT16 inhibited LMA on the night of injection (F1,14 = 16.2, p = 0.0013, Fig. 2G) but not thereafter.
LPS at ZT1 likewise inhibited LMA (chronotype: F119,1428 = 3.52, p ≤ 0.001; injection: F119,1428 = 1.46, p = 0.0013; chronotype × injection: F119,1428 = 1.73, p ≤ 0.001; Fig. 2). Among RHYTH hamsters, LPS tended to decrease LMA during the first and second light phases postinjection (F1,14 = 4.31, p = 0.057, Fig. 2F) but not thereafter. LPS also inhibited activity during the first dark phase after treatment (F1,14 = 18.3, p = 0.0008), and nocturnal hypoactivity persisted for the next 3 nights (F1,14 > 6.4, p ≤ 0.04, all comparisons; Fig. 2F).
In ARR hamsters, inhibitory effects of ZT1 LPS were not apparent during the light phase on any day after injection (Fig. 2H). In common with ZT16 injections, ZT1 LPS treatment decreased activity during the first dark phase postinjection (F1,14 = 10.7, p = 0.0056) but not during subsequent dark phases (Fig. 2H).
Anorexia
LPS reduced food intake (F6,336 = 36.2, p ≤ 0.0001; Fig. 3), which depended on treatment ZT (injection × ZT: F6,336 = 2.81, p = 0.011). Anorexic responses were reduced in hamsters injected with LPS at ZT1 relative to ZT16 (F6,336 = 14.3, p ≤ 0.0001). LPS-induced anorexia at ZT16 was comparable in RHYTH and ARR hamsters (Fig. 3A, F6,168 = 0.89, p = 0.51). Food intake was lower in LPS-treated hamsters on posttreatment days 1 to 3 (p ≤ 0.013, Fig. 3A), but not thereafter.
Figure 3.
Lipopolysaccharide (LPS)–induced anorexia. Mean ± SEM change in food intake (A and B) of circadian entrained (RHYTH; squares) and circadian arrhythmic (ARR; circles) female Siberian hamsters injected intraperitoneally (i.p.) with bacterial LPS (625 µg/ kg) or sterile saline (SAL) at either ZT16 (A) or ZT1 (B). *p < 0.05, **p < 0.01, ***p < 0.001 vs. SAL value, within chronotype. #p < 0.05 vs. RHYTH-LPS value.
In contrast, LPS at ZT1 elicited different anorexic responses in RHYTH and ARR hamsters (F6,168 = 3.44, p = 0.0031). On day 1, LPS elicited anorexia in RHYTH hamsters (Fig. 3B, p = 0.001) but not ARR hamsters. Food intake in RHYTH ZT1-treated hamsters returned to normal values by the second posttreatment day (Fig. 3B).
Effects of LPS on Ultradian and Circadian Rhythms
UR power—ZT16 injections
Locomotor activity
Chronotype and injection interacted to affect power of dark-phase URs (F6,168 = 3.13, p = 0.0062; Fig. 4A). In RHYTH hamsters, LPS decreased UR power on the first 3 nights after treatment (p ≤ 0.009, all comparisons; Fig. 4A) but did not significantly inhibit UR power in ARR hamsters (Fig. 4A).
Figure 4.
Lipopolysaccharide (LPS) decreases power of dark-phase ultradian rhythms. Mean ± SEM power of the ultradian rhythm (UR) in locomotor activity (A and C) and body temperature (B and D) of circadian entrained (RHYTH; squares) and circadian arrhythmic (ARR; circles) female Siberian hamsters injected intraperitoneally (i.p.) with bacterial LPS (625 µg/kg) or sterile saline (SAL) at ZT16 (A and B) or ZT1 (C and D). *p < 0.05, **p < 0.01, ***p<0.001 vs. SAL value, within chronotype.
Body temperature
LPS treatments affected the pattern of change in dark-phase Tb UR power (F6,168 = 12.0, p ≤ 0.0001; Fig. 4B), but chronotype did not. On the second and third nights posttreatment, Tb UR power was reduced in LPS-treated RHYTH and ARR hamsters (p < 0.05, all comparisons; Fig. 4B).
UR power—ZT1 injections
Locomotor activity
LPS altered the pattern of change in dark-phase UR power over time (F6,72 = 3.25, p = 0.007; Fig. 4C), decreasing power on nights 1 to 4 after injection in RHYTH hamsters (p < 0.05, all comparisons) but only on night 1 (p = 0.006) in ARR hamsters (Fig. 4C).
Body temperature
LPS altered the pattern of change in dark-phase Tb UR power over time (F6,72 = 7.46, p ≤ 0.0001; Fig. 4D), attenuating power on night 1 in both chronotypes (p < 0.003, both comparisons; Fig. 4D). UR power was reduced on night 2 in RHYTH (p = 0.008) but not ARR hamsters (Fig. 4D).
UR period—ZT16 injections
Locomotor activity
There was a significant main effect of circadian chronotype on dark-phase UR period (τ’) (F1,28 = 61.8, p ≤ 0.0001), with longer τ’s in saline-treated ARR than saline-treated RHYTH hamsters on most testing days (Fig. 5A). The effect of LPS on τ′ was restricted to the dark phase immediately after treatment and was evident in RHYTH (p = 0.0005) but not ARR (Fig. 5A) hamsters.
Figure 5.
Lipopolysaccharide (LPS) treatments decrease period of ultradian rhythms. Mean ± SEM period of the ultradian rhythm (UR) in locomotor activity of circadian entrained (RHYTH; squares) and circadian arrhythmic (ARR; circles) female Siberian hamsters injected intraperitoneally (i.p.) with bacterial LPS (625 µg/kg) or sterile saline (SAL) at ZT16 (A) or ZT1 (B). The timing of injection treatments is indicated by a downward vertical arrow near the ordinate axis. Gray symbols preceding injection treatments depict mean baseline values, averaged across the week prior to the initiation of injection treatments. *p < 0.05, ***p < 0.001 vs. SAL value, within chronotype; #p < 0.05, ##p < 0.01 RHYTH-SAL vs. ARR-SAL values.
Body temperature
Chronotype did not affect dark-phase Tb τ′ (not illustrated). Tb τ′ was not significantly affected by LPS treatment.
UR period—ZT1 injections
Locomotor activity
Chronotype significantly affected τ′ of the dark-phase UR: periods were longer in ARR than RHYTH hamsters (F1,12 = 26.9, p = 0.0002; Fig. 5B). LPS, however, did not affect dark-phase τ′ as a function of chronotype.
Body temperature
Dark-phase Tb τ′ was not affected by chronotype or injection (not illustrated).
Circadian Rhythms
ZT16—CR power
LPS reduced circadian power in the LMA waveform (F6,84 = 7.47, p ≤ 0.0001; Fig. 6A), with effects evident during the first and second 24-h intervals after injection (p < 0.05, both comparisons) but not thereafter. LPS also reduced Tb circadian power (F6,84 = 10.4, p ≤ 0.0001; Fig. 6B) on the second through the fourth posttreatment days (p ≤ 0.002, all comparisons).
Figure 6.
Lipopolysaccharide (LPS) inhibits power of behavioral and thermoregulatory circadian rhythms. Mean ± SEM power of the circadian rhythm in locomotor activity (A and C) and body temperature (B and D) of circadian entrained (RHYTH) female Siberian hamsters injected intraperitoneally (i.p.) with bacterial LPS (625 µg/kg) or sterile saline (SAL) at either ZT16 (A and B) or ZT1 (C and D). *p < 0.05, **p < 0.01, ***p < 0.001 vs. SAL value. +p < 0.05, ++p < 0.01, +++p < 0.001 vs. LPS-ZT16 value for corresponding measure and time point.
ZT1—CR power
Locomotor activity
LPS decreased circadian power (F6,36 = 3.68, p = 0.0059; Fig. 6C) during the first 4 posttreatment days (p < 0.05, all comparisons) with a greater reduction in ZT1 than ZT16 hamsters on days 3 to 7 posttreatment (p < 0.05, all comparisons; Fig. 6A,C). In contrast, LPS at ZT1 did not affect power in the Tb circadian rhythm (Fig. 6D).
DISCUSSION
LPS-induced activation of the hamster innate immune system induced fever and sickness behaviors. Novel findings in the present report include evidence that power in locomotor activity (LMA) and Tb URs was enduringly altered, UR period was transiently lengthened, and CR power was chronically attenuated by LPS. Many of these responses were influenced by the timing of the immune challenge and/or the integrity of the circadian pacemaker. These data add to an emerging literature on effects of circadian organization and timing of immune challenges on multiple aspects of the acute-phase response to infection.
Despite unavoidable limitations in clock gene knockout, LL, and SCNx models of circadian arrhythmia, results from the DPS hamster model used here (which is genetically and neurologically intact) underscore a theme that has emerged from these other models, namely, that the circadian clock has important influences on the immune system. That these different approaches converge in establishing a strong circadian-immune connection suggests that effects observed after gene knockouts or SCN ablation may indeed reflect loss of timing information per se rather than pleiotropic effects or nonspecific lesion confounds.
Time of Injection Effects Were Dissimilar on LMA vs. Tb
The severity of the response to LPS varied over circadian time in a trait-specific manner. In RHYTH hamsters, LPS at ZT16 elicited more enduring febrile responses than at ZT1, whereas LMA responses were more persistent at ZT1 than at ZT16. These data add to work in rodents (Liu et al., 2006; Marpegan et al., 2009; Keller et al., 2009; Weil et al., 2009; Spengler et al., 2012) and humans (Petrovsky et al., 1998) documenting circadian variation in responses to LPS and other inflammatory challenges. The time of peak responsiveness to inflammogens depends on species, type of inflammatory challenge, and outcome measure.
A minority of responses to LPS in ARR hamsters varied as a function of injection ZT (e.g., anorexia). This may be attributable to masking effects of ZT treatment. There is precedent for circadian arrhythmic animals to exhibit trait-specific masking responses in a full L:D cycle: 42% of Syrian hamsters with large SCN lesions, behaviorally arrhythmic in running wheels in LL, synchronized locomotor activity, and ingestive behavior in the presence of a light-dark cycle (Rusak 1977).Synchronization of wheel-running behavior by the light-dark cycle also is evident in multiple gene knockout mice (e.g., Bae et al., 2001; Izumo et al., 2014) that are circadian arrhythmic in constant darkness. Although LMA and Tb are arrhythmic in DPS hamsters exposed to a full light-dark cycle, a complete inventory of traits that exhibit masking responses to light (or darkness) in this model does not yet exist.
Circadian Arrhythmia Attenuated Behavioral and Thermoregulatory Responses to LPS
Compared to their respective saline-injected controls, Tb responses to LPS at ZT16 were attenuated in ARR compared to RHYTH hamsters, as was true of diminished LMA in response to LPS at ZT1. LPS has been reported to attenuate (Wachulec et al., 1997) or exacerbate (Guerrero-Vargas et al., 2014) Tb responses to LPS in SCNx rats. The dose of LPS and route of administration may account for these divergent results. Species and sex differences (Wachulec et al., 1997; Guerrero-Vargas et al., 2014) and the procedures employed to induce circadian arrhythmia also may affect responses to LPS. For example, the effects of circadian arrhythmia on memory processing differ categorically depending on whether arrhythmia is induced via SCNx or DPS (Fernandez et al., 2014). The direction and magnitude of effects of circadian arrhythmia on the inflammatory response to LPS may similarly depend on the methods employed to render animals arrhythmic.
Inflammatory responses in rodents with CR disruption induced by clock gene mutations likewise suggest that the method by which CRs are disrupted can affect the immunophenotype. Some circadian clock genes appear to enhance inflammatory responses to LPS: Clock-deficient mice exhibit reduced tumor necrosis factor α (TNFα)–mediated NF-κB activation (Spengler et al., 2012), and per2 mutant mice are resistant to LPS-induced sepsis (Liu et al., 2006). On the other hand, macrophages rendered arrhythmic via bmal1−/− failed to exhibit CRs in LPS-induced interleukin (IL)–6 production, an effect also evident in macrophages from mice deficient in reverbα; macrophages of rev-erbα−/− mice exhibit enhanced IL-6 responses to LPS (Gibbs et al., 2012). The macrophage clock likely inhibits cytokine production at certain circadian phases. Assessment of the proinflammatory cytokine responses to LPS in DPS-ARR hamsters would be instructive in ascertaining whether effects of chronotype reported here reflect ARR-induced decrements in peripheral production of inflammatory mediators.
Ultimately, disentangling the effects of loss of temporal order per se from the direct (noncircadian) effects of interventions that induce temporal disorder (Gibbs et al., 2012; Spengler et al., 2012; Sato et al., 2014) remains a challenge to understanding how the circadian timing system affects inflammatory responses and, more broadly, organismal-level immune function. Nevertheless, the present data, derived from neurologically and genetically “intact” hamsters, underscore the importance of the circadian system in the maintenance of normal immune responses.
LPS-Induced Inflammatory Responses Dampen Circadian Power
Multiple aspects of the circadian waveform changed after LPS treatment, which immediately decreased power of LMA activity CRs, regardless of injection ZT. Effects of LPS on power of CRs in Tb were only evident after ZT16 injections, however, and were delayed in appearance. LPS causes modest but stable shifts in the phase of the circadian pacemaker in the SCN (Marpegan et al., 2005), effects mimicked by intracerebroventricular (i.c.v.)treatments with IL-1β or TNFα, and blocked by inhibitors of TNFα signaling (Leone et al., 2012), suggesting that TNFα plays a central role in LPS-induced phase shifts. Decreases in overall LMA levels by LPS or live bacterial infections have been reported in several rodent models (reviewed in Harrington, 2012), as has inhibition by the viral mimetic Poly I:C (Katafuchi et al., 2005) and treatment with proinflammatory cytokines (e.g., IL-1β; Anisman et al., 2008). Initial global disruption of LMA, followed by a resumption of circadian activity patterns, is commonly reported (Harrington, 2012). Decreases in global LMA levels and decrements in the circadian waveform may reflect dampening of the underlying circadian pacemaker (Okada et al., 2008) and may constitute an underrecognized symptom of the acute-phase response to infection. Changes in CRs during acute illness may constitute an adaptive component of the acute-phase response to infection, conserving energy by decreasing the amount or amplitude of LMA and peak Tb.
Ultradian Rhythms Are Enduringly Altered by Inflammatory Responses
Effects of LPS on URs were evident in both RHYTH and ARR chronotypes, but there was a general pattern of diminished effects of LPS in ARR hamsters. In RHYTH females, the temporal structure (period) of URs was disrupted shortly after LPS treatment at ZT16. A ~1-h increase in the period of the LMA UR was evident during the first dark phase, immediately after injection. Normal UR periods were restored by the following night. Period-lengthening effects of LPS on LMA URs were not evident after ZT1 injections, and the periods of URs in Tb were unaffected by LPS. Thus, LPS has a potent, transitory period-lengthening effect on select URs and may be limited to inflammatory responses initiated around the time of activity onset.
In contrast, effects of LPS on UR power were evident at both ZTs; in many instances, decreases in UR power persisted for several days depending on trait and chronotype. At ZT16, LPS inhibited dark-phase LMA and Tb UR power of RHYTH hamsters for 2 to 3 nights. ZT1 LPS likewise inhibited UR power in both chronotypes, with more enduring effects in RHYTH hamsters.
To our knowledge, these are the first quantitative descriptions of changes in the UR waveform during the acute-phase response to simulated infection. Numerous reports describe effects of LPS on sleep (Krueger et al., 1986; Exton et al., 1995; Kapás et al., 1998; Schiffelholz and Lancel, 2001; Jakubcakova et al., 2011), a behavior with clear ultradian characteristics, but information on LPS-induced changes in the sleep ultradian waveform (period, power) is lacking.
URs are ubiquitous in vertebrate physiology, evident during both the active (food intake, LMA, Tb) and inactive (sleep, rest) phases. An important functional role for URs may lie in higher-frequency temporal partitioning of activity during each phase of the circadian cycle, conserving energy by consolidating behavior into bouts of relative activity and inactivity (Aschoff and Gerkema, 1985). Disorganization of ultradian temporal order normally present in well individuals may reflect novel, energetically costly aspects of the acute-phase response to infection.
Quantification of CNS cytokine or monoaminergic responses to LPS, although beyond the scope of the present study, could provide mechanistic insights into the chronobiological consequences of innate immune activation (cf. Blum et al., 2014).
Summary
In a novel model of circadian arrhythmia, with relevance to human circadian disruption, we investigated the role of circadian phase and integrity on multiple components of the behavioral response to infection. We confirmed and extended existing reports of circadian phase-specific effects of inflammation on sickness responses. Circadian arrhythmia attenuated several sickness responses, suggesting that temporal disorganization of the internal milieu in the DPS model may interfere with the generation of robust behavioral and immune responses to infection. The DPS treatment does not involve surgical or genomic manipulations; nevertheless, DPS-ARR hamsters exhibited altered inflammatory responses to LPS. These data offer novel, convergent evidence toward the importance of the circadian system in maintaining normal immune responses and suggest that immunological changes observed in knockout and lesion models may indeed be consequences of the elimination of circadian time information. Novel inhibitory effects of LPS on the generation of CRs and URs in multiple traits also were documented. Inflammation-induced changes in URs may reflect potentially costly components of the acute-phase response to infection.
Acknowledgments
We thank Priyesh Patel for technical assistance, Dr. Betty Theriault for expert veterinary care, and 3 anonymous reviewers for constructive criticism. This work was supported by grant R01 AI-67406.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Anisman H, Gibb J, Hayley S. Influence of continuous infusion of interleukin-1beta on depression-related processes in mice: Corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacology (Berl) 2008;199:231–244. doi: 10.1007/s00213-008-1166-z. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Gerkema M. On diversity and uniformity of ultradian rhythms. In: Schulz H, Lavie P, editors. Ultradian Rhythms in Physiology and Behavior. Berlin: Springer; 1985. pp. 321–334. [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Drazen DL, Quan N, He L, Nelson RJ. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc Biol Sci. 2002;269:447–454. doi: 10.1098/rspb.2001.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Quan N, Prendergast BJ, Bowers SL, Nelson RJ. Photoperiod alters the time course of brain cyclooxygenase-2 expression in Siberian hamsters. J Neuroendocrinol. 2003;15:958–964. doi: 10.1046/j.1365-2826.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- Blum ID, Zhu L, Moquin L, Kokoeva MV, Gratton A, Giros B, Storch KF. A highly tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. Elife. 2014 Dec 29;:3. doi: 10.7554/eLife.05105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264:R1186–R1192. doi: 10.1152/ajpregu.1993.264.6.R1186. [DOI] [PubMed] [Google Scholar]
- Choe HK, Kim HD, Park SH, Lee HW, Park JY, Seong JY, Lightman SL, Son GH, Kim K. Synchronous activation of gonadotropin-releasing hormone gene transcription and secretion by pulsatile kisspeptin stimulation. Proc Natl Acad Sci USA. 2013;110:5677–5682. doi: 10.1073/pnas.1213594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprés-Brummer P, Bourin P, Pages N, Metzger G, Lévi F. Persistent T lymphocyte rhythms despite suppressed circadian clock outputs in rats. Am J Physiol. 1997;273:R1891–R1899. doi: 10.1152/ajpregu.1997.273.6.R1891. [DOI] [PubMed] [Google Scholar]
- Exton MS, Bull DF, King MG, Husband AJ. Modification of body temperature and sleep state using behavioral conditioning. Physiol Behav. 1995;57:723–729. doi: 10.1016/0031-9384(94)00314-9. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Lu D, Ha P, Costacurta P, Chavez R, Heller HC, Ruby NF. Circadian rhythm: Dysrhythmia in the suprachiasmatic nucleus inhibits memory processing. Science. 2014;346:854–857. doi: 10.1126/science.1259652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipski E, King VM, Li X, Granda TG, Mormont MC, Claustrat B, Hastings MH, Lévi F. Disruption of circadian coordination accelerates malignant growth in mice. Pathol Biol (Paris) 2003;51:216–219. doi: 10.1016/s0369-8114(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Franklin AE, Engeland CG, Kavaliers M, Ossenkopp KP. Lipopolysaccharide-induced hypoactivity and behavioral tolerance development are modulated by the light-dark cycle in male and female rats. Psychopharmacology (Berl) 2003;170:399–408. doi: 10.1007/s00213-003-1554-3. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone BP, Chang D, Bourgin P, Cao V, Fernald RD, Heller HC, Ruby NF. Acute light exposure suppresses circadian rhythms in clock gene expression. J Biol Rhythms. 2011;26:78–81. doi: 10.1177/0748730410388404. [DOI] [PubMed] [Google Scholar]
- Guerrero-Vargas NN, Salgado-Delgado R, Basualdo Mdel C, García J, Guzmán-Ruiz M, Carrero JC, Escobar C, Buijs RM. Reciprocal interaction between the suprachiasmatic nucleus and the immune system tunes down the inflammatory response to lipopolysaccharide. J Neuroimmunol. 2014;273:22–30. doi: 10.1016/j.jneuroim.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Harrington ME. Neurobiological studies of fatigue. Prog Neurobiol. 2012;99:93–105. doi: 10.1016/j.pneurobio.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Steinlechner S, Ruf T, Wiesinger H, Klingenspor M. Photoperiod and thermoregulation in vertebrates: Body temperature rhythms and thermogenic acclimation. J Biol Rhythms. 1989;4:251–265. [PubMed] [Google Scholar]
- Horton TH, Yellon SM. Aging, reproduction, and the melatonin rhythm in the Siberian hamster. J Biol Rhythms. 2001;16:243–253. doi: 10.1177/074873040101600307. [DOI] [PubMed] [Google Scholar]
- Izumo M, Pejchal M, Schook AC, Lange RP, Walisser JA, Sato TR, Wang X, Bradfield CA, Takahashi JS. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife. 2014 Dec 19;:3. doi: 10.7554/eLife.04617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubcakova V, Flachskamm C, Deussing JM, Kimura M. Deficiency of corticotropin-releasing hormone type-2 receptor alters sleep responses to bacterial lipopolysaccharide in mice. Brain Behav Immun. 2011;25:1626–1636. doi: 10.1016/j.bbi.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Liu J, Lei J, Timmermans L, Foppen E, Cailotto C, Fliers E. Differential involvement of the suprachiasmatic nucleus in lipopolysaccharide-induced plasma glucose and corticosterone responses. Chronobiol Int. 2012;29:835–849. doi: 10.3109/07420528.2012.699123. [DOI] [PubMed] [Google Scholar]
- Kapás L, Hansen MK, Chang HY, Krueger JM. Vagotomy attenuates but does not prevent the somnogenic and febrile effects of lipopolysaccharide in rats. Am J Physiol. 1998;274:R406–R411. doi: 10.1152/ajpregu.1998.274.2.R406. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Kondo T, Take S, Yoshimura M. Enhanced expression of brain interferon-alpha and serotonin transporter in immunologically induced fatigue in rats. Eur J Neurosci. 2005;22:2817–2826. doi: 10.1111/j.1460-9568.2005.04478.x. [DOI] [PubMed] [Google Scholar]
- Kay LM, Beshel J. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task. J Neurophys. 2010;104:829–839. doi: 10.1152/jn.00166.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Líbano D, Kopell N. Olfactory oscillations: The what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobil E. The wisdom of the body revisited. News Physiol Sci. 1999;14:1–11. doi: 10.1152/physiologyonline.1999.14.1.1. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Kubillus S, Shoham S, Davenne D. Enhancement of slow-wave sleep by endotoxin and lipid A. Am J Physiol. 1986;251:R591–R597. doi: 10.1152/ajpregu.1986.251.3.R591. [DOI] [PubMed] [Google Scholar]
- Leone MJ, Marpegan L, Duhart JM, Golombek DA. Role of proinflammatory cytokines on lipopolysaccharide-induced phase shifts in locomotor activity circadian rhythm. Chronobiol Int. 2012;29:715–723. doi: 10.3109/07420528.2012.682681. [DOI] [PubMed] [Google Scholar]
- Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D, Rossi E, Roussel MR. The temporal organization of living systems from molecule to mind. In: Lloyd D, Rossi E, editors. Ultradian Rhythms from Molecules to Mind. Berlin: Springer Science & Business; 2008. pp. 1–8. [Google Scholar]
- Logan A, Frautschy SA, Gonzalez AM, Baird AA. A time course for the focal elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following a localized cortical brain injury. J Neurosci. 1992;12:3828–3837. doi: 10.1523/JNEUROSCI.12-10-03828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Bekinschtein TA, Costas MA, Golombek DA. Circadian responses to endotoxin treatment in mice. J Neuroimmunol. 2005;160:102–109. doi: 10.1016/j.jneuroim.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- McKeon GP, Nagamine CM, Ruby NF, Luong RH. Hematologic, serologic, and histologic profile of aged Siberian hamsters (Phodopus sungorus) J Am Assoc Lab Anim Sci. 2011;50:308–316. [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. New York: Oxford University Press; 2008. [Google Scholar]
- Mueller JC, Steinmeyer C, Kempenaers B. Individual variation in sleep-wake rhythms in free-living birds. Chronobiol Int. 2012;29:1216–1226. doi: 10.3109/07420528.2012.705404. [DOI] [PubMed] [Google Scholar]
- Okada K, Yano M, Doki Y, Azama T, Iwanaga H, Miki H, Nakayama M, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Ishida N, Monden M. Injection of LPS causes transient suppression of biological clock genes in rats. J Surg Res. 2008;145:5–12. doi: 10.1016/j.jss.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Paladino N, Leone MJ, Plano SA, Golombek DA. Paying the circadian toll: The circadian response to LPS injection is dependent on the Toll-like receptor 4. J Neuroimmunol. 2010;225:62–67. doi: 10.1016/j.jneuroim.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: Regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Baillie SR, Dhabhar FS. Gonadal hormone-dependent and -independent regulation of immune function by photoperiod in Siberian hamsters. Am J Physiol. 2008;294:R384–R392. doi: 10.1152/ajpregu.00551.2007. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Bilbo SD, Kinsey SG, Nelson RJ. Photoperiodic adjustments in immune function protect Siberian hamsters from lethal endotoxemia. J Biol Rhythms. 2003;18:51–62. doi: 10.1177/0748730402239676. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Reuss S, Bürger K. Substance P-like immunoreactivity in the hypothalamic suprachiasmatic nucleus of Phodopus sungorus—relation to daytime, photoperiod, sex and age. Brain Res. 1994;638:189–195. doi: 10.1016/0006-8993(94)90649-1. [DOI] [PubMed] [Google Scholar]
- Reuss S, Schaeffer DF, Laages MH, Riemann R. Evidence for increased nitric oxide production in the auditory brain stem of the aged dwarf hamster (Phodopus sungorus): An NADPH-diaphorase histochemical study. Mech Ageing Dev. 2000;112:125–134. doi: 10.1016/s0047-6374(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Barakat MT, Heller HC. Phenotypic differences in reentrainment behavior and sensitivity to nighttime light pulses in Siberian hamsters. J Biol Rhythms. 2004;19:530–541. doi: 10.1177/0748730404268055. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Joshi N, Heller HC. Phase shift magnitude and direction determine whether Siberian hamsters reentrain to the photocycle. J Biol Rhythms. 1998;13:506–517. doi: 10.1177/074873049801300606. [DOI] [PubMed] [Google Scholar]
- Rusak B. Role of suprachiasmatic nuclei in generation of circadian rhythms in golden hamster, Mesocricetus auratus . J Comp Physiol. 1977;118:145–164. [Google Scholar]
- Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- Schiffelholz T, Lancel M. Sleep changes induced by lipopolysaccharide in the rat are influenced by age. Am J Physiol. 2001;280:R398–R403. doi: 10.1152/ajpregu.2001.280.2.R398. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, Antoch MP. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc Natl Acad Sci USA. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD. Pulsatile hormone secretion: mechanisms, significance, and evaluation. In: Lloyd D, Rossi E, editors. Ultradian Rhythms from Molecules to Mind. Berlin: Springer Science & Business; 2008. pp. 229–248. [Google Scholar]
- Wachulec M, Li H, Tanaka H, Peloso E, Satinoff E. Suprachiasmatic nuclei lesions do not eliminate homeostatic thermoregulatory responses in rats. J Biol Rhythms. 1997;12:226–234. doi: 10.1177/074873049701200304. [DOI] [PubMed] [Google Scholar]
- Warner A, Jethwa PH, Wyse CA, I’anson H, Brameld JM, Ebling FJ. Effects of photoperiod on daily locomotor activity, energy expenditure, and feeding behavior in a seasonal mammal. Am J Physiol. 2010;298:R1409–R1416. doi: 10.1152/ajpregu.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Karelina K, Su AJ, Barker JM, Norman GJ, Zhang N, Devries AC, Nelson RJ. Time-of-day determines neuronal damage and mortality after cardiac arrest. Neurobiol Dis. 2009;36:352–360. doi: 10.1016/j.nbd.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L, Thrivikraman KV, Plotsky PM. Combined pre- and postnatal environmental enrichment programs the HPA axis differentially in male and female rats. Psychoneuroendocrinology. 2006;31:553–564. doi: 10.1016/j.psyneuen.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Wen JC, Dhabhar FS, Prendergast BJ. Pineal-dependent and -independent effects of photoperiod on immune function in Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;51:31–39. doi: 10.1016/j.yhbeh.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Lisk RD. Behavioral interactions differentiate Djungarian (Phodopus campbelli) and Siberian (Phodopus sungorus) hamsters. Can J Zool. 1987;65:2229–2235. [Google Scholar]
- Yates FE, Yates LB. Ultradian rhythms as the dynamic signature of life. In: Lloyd D, Rossi E, editors. Ultradian Rhythms from Molecules to Mind. Berlin: Springer Science & Business; 2008. pp. 249–260. [Google Scholar]