Abstract
AIMS
Deaths associated with cancer metastasis have steadily increased making the need for newer, anti-metastatic therapeutics imparative. Gelsolin and vimentin, actin binding proteins expressed in metastatic tumors, participate in actin remodelling and regulate cell migration. Docosahexaenoic acid (DHA) limits cancer cell proliferation and adhesion but the mechanisms involved in reducing metastatic phenotypes are unknown. We aimed to investigate the effects of DHA on gelsolin and vimentin expression, and ultimately cell migration and proliferation, in this context.
MAIN METHODS
Non-invasive lung epithelial cells (MLE12) and invasive lung cancer cells (A549) were treated with DHA (30 μmol/ml) or/and 8 bromo-cyclic adenosine monophosphate (8 Br-cAMP) (300 μmol/ml) for 6 or 24 h either before (pre-treatment) or after (post-treatment) plating in transwells. Migration was assessed by the number of cells that progressed through the transwell. Gelsolin and vimentin expression were measured by western blot and confocal microscopy in cells, and by immunohistochemistry in human lung cancer biospy samples.
KEY FINDINGS
A significant decrease in cell migration was detected for A549 cells treated with DHA verses control but this same decrease was not seen in MLE12 cells. DHA and 8 Br-cAMP altered gelsolin and vimentin expression but no clear pattern of change was observed. Immunoflorescence staining indicated slightly higher vimentin expression in human lung tissue that was malignant compared to control.
SIGNIFICANCE
Collectively, our data indicate that DHA inhibits cancer cell migration and further suggests that vimentin and gelsolin may play secondary roles in cancer cell migration and proliferation, but are not the primary regulators.
Keywords: lung cancer, migration, docosahexaenoic acid, actin binding proteins, metastasis and actin dynamics
Introduction
Cancer cases resulting in high mortality is reflected by tumor growth, invasion, and metastasis to distant organs [1-3]. Moreover, the lack of curative chemotherapeutic approaches and short median survival (~14 months) indicate the inefficiency of current adjuvant chemotherapy or other interventions such as radiation, against cancer cells possessing the complete malignant phenotype [4-6]. Currently patients with recurring cancer experience high-frequencies of residual micrometastases and relapse [7] with ~50% of surgically treated patients suffering recurrent disease [2, 8-13]. Statistics demonstrate that cancer accounts for 585,720 Americans deaths each year, corresponding to almost 1600 deaths per day. Even after a 20% decline in the death rate from 1991 to 2009 there still exists an intolerable socio-economical toll for this disease [14, 15]. Overall these data indicate the need to explore new anti-cancer treatments such as dietary/nutritional supplements which have few or no effects on normal cell growth, as a novel approach for limiting cancer growth and reoccurrence. Docosahexaenoic acid (DHA), a polyunsaturated fatty acid long approved as dietary supplement, has been shown to offer a broad range of health benefits. DHA has been established as a viable therapeutic for diseases including hypertension, arthritis, atherosclerosis, depression, adult-onset diabetes mellitus, myocardial infarction, thrombosis, and some cancers [16-18]. In relation to our current study, DHA has previously been shown to inhibit cancer cell proliferation and survival [19, 20] but its involvement in cancer metastasis and associated mechanisms are not known. Therefore, we chose to explore the effect of DHA on cancer cell migration, a potent reflector of metastasis while also evaluating non-cancerous cells which should show minimal to no detrimental effects. We chose the lung adenocarcinoma cell line, A549, as they express the invasive metastatic phenotype, and the transformed lung epithelial cell line, MLE12, to represent non-invasive cells.
Mechanistically, actin binding protein (ABP)-mediated cytoskeletal remodelling at the cellular membrane edges facilitates cancer progression and metastasis by inducing the formation of invasive organelles like lamellipodia, filopodia and invadopodia [21-25]. cAMP induces actin polymerization through protein kinase A mediated regulation of ABP. Understanding the role of ABPs in migration processes could provide novel therapeutic targets for anti-metastatic drugs. Our preliminary data indicated that DHA supplementation may modulate the expression and localization of ABPs; therefore, we investigated the effects of DHA supplementation on the ABPs gelsolin and vimentin.
The expression and function of gelsolin and vimentin are well defined in the context of actin dynamics, cancer progression, metastasis, and apoptosis [26-29]. Gelsolin belongs to the actin-severing gelsolin/villin superfamily and plays a key role in actin filament disassembly by severing larger actin filaments into smaller ones [30, 31]. As a viral actin regulator, gelsolin is involved in the formation of cancer cell invasive structures in coordination with other proteins like Arp3, cortactin, and Rho GTPases [30, 32]. Vimentin interacts with tubulin-based microtubules and actin-based microfilaments to comprise the cytoskeleton and thus maintain cellular integrity [33, 34]. Vimentin facilitates the elongation of filamentous structures along with actin in the formation of cell protrusions which are required by cancer cells for migration [35, 36].
These studies tested the hypothesis that the anti-metastatic properties of DHA are through modulation of ABPs, specifically gelsolin and vimentin. Our findings indicate that DHA inhibits cAMP-induced cell migration in A549 cancer cells and has little to no effect on MLE12 control cells. Expression of both gelsolin and vimentin were altered with cAMP treatment and DHA supplementation, but no clear patterns were observed. Collectively, these data indicate that DHA suppresses cell migration induced by cAMP in cancerous cells but the ABPs gelsolin and vimentin may not be involved directly in DHA-mediated inhibition.
Materials and Methods
Cell culture and treatment
Non-invasive lung epithelial cells (MLE12) and invasive lung cancer cells (A549) (American Type Culture Collection, Manassas, VA) were cultured at 37 °C in a humidified atmosphere containing 5% CO2. MLE12 cells were maintained in HITES media previously published by our laboratory [37]. A549 cells were grown in DMEM 1X with 10% FBS supplemented with 4.5 g/L-glucose. Cells were treated when ~80% confluent (for 6 h or 24 h) with either PBS (the vehicle control), cell permeant analogs of cAMP (8Br-cAMP, 300 μM) (Sigma Chemical Co., St. Louis, MO), DHA (30 μM), or both cAMP and DHA prior to plating (pre-treatment) or post plating (post-treatment).
Transwell analysis
As a measurement of metastatic phenotype, the cell migration rate was studied using transwell apparatus. Six well plates were set-up using Falcon's Transparent PET Membrane, 8.0 μm pore size, 1 × 105 pores/cm2 inserts, according to the manufacturer's instructions. Cells were treated with 8Br-cAMP and/or DHA either just prior to plating (pre-treatment) or immediately after (post-treatment) to mimic the pre- and post-metastasis treatment of cancers. The cAMP analogue, 8Br-cAMP, was used to induce a proliferative state in our cells. A549 and MLE12 cells were plated at 1×105 cells per well and were incubated in a humidified tissue culture incubator at 37°C with 5% CO2 atmosphere. After 24 h of growth, cells that migrated to the lower compartment were counted using a Neubauer cell counting chamber and the percentage of cells that migrated were calculated.
Western blot
Lysis buffer (Nupage®LDS sample buffer) containing PMSF (0.3 nmol/ml), okadoic acid (0.01 nmol/ml), leupeptin (0.1 nmol/ml) and aprotonin (0.005 - 0.01 TIU/ml) was used to prepare total cell lysates. Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-human rabbit monoclonal antibodies targeting gelsolin (dilution, 1:1000) (Cell Signaling Technology, Inc., Danvers, MA), anti-human mouse monoclonal antibodies targeting Vimentin (dilution, 1:1000) (Sigma Chemical Co., St. Louis, MO), and mouse monoclonal antibodies targeting β-actin (1:10,000) (Abcam, Cambridge, MA). Finally, membranes were probed with horseradish peroxidase-conjugated secondary antibody (dilution, 1:1000) (BD Pharminogen®, Franklin lakes, NJ) for 1 h at room temperature and specific bands were visualized employing Amersham™ECL™ Prime Western Blotting Detection Reagent (GE Healthcare, Buckinghamshire, UK). The band intensity was measured by densitometry.
Immunofluorescence (IF) Microscopy
Cells were plated in 6-well plates containing cover slips (Corning Incorporated Corning, New York, NY) at 1 × 105 cells/well and treated with 8Br-cAMP and/or DHA as previously described. A wound was induced across the cell monolayer, and incubated for an additional 24h. The cover slips were then removed and the cells were stained with anti-gelsolin (1:500) or anti-vimentin (1:500) followed by the secondary antibody, Alexa 488 labeled IgG (1:1000). Nuclei were stained with DAPI (Invitrogen, Carlsbad, CA). Images were acquired by confocal microscopy (LSM510, Carl Zeiss, Jena, Germany) in a blinded fashion using identical settings for all images (200X). Percent change in protein intensity was quantified using NIH Image J analysis.
Human control (n = 4) and malignant (n = 4) lung tissue slides were obtained from Lung Cancer Biospecimen Research Network (LCBRN)(University of Virginia, Charlottesville, VA). Tissues were processed for immunofluorescence labelling using standard protocols [38]. Tissue sections were stained with primary anti-human rabbit monoclonal antibodies for gelsolin (1:100) or anti-human mouse monoclonal antibodies for vimentin (1:100) followed by secondary antibody Alexa 488 labeled IgG (1:1000) and nuclei were stained with DAPI (Invitrogen, Carlsbad, CA). Four images from each slide were obtained using Carl Zeiss's Axio Scope A1 Polarized Light Fluorescent Microscope (Carl Zeiss, Jena, Germany) with 200X magnification and identical settings. The intensity of image color was quantified using NIH image J software.
Results
DHA inhibits cAMP-induced cell migration in A549 cells
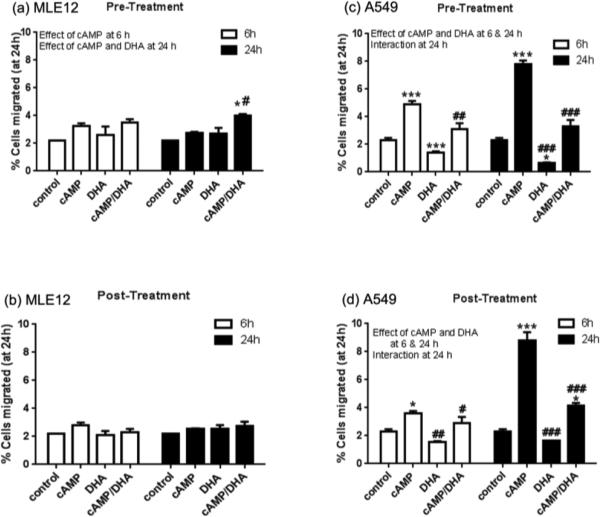
MLE 12 and A549 cells were cultured and treated with 8Br-cAMP and/or DHA for 6 or 24 h either prior to or after plating into the transwell apparatus. In MLE12 cells, no changes in cell migration were observed due to treatments with the exception of a minor increase in the cAMP/DHA pre-treated cells at 24 h (Fig. 1, a and b). In A549 cells, a significant increase in cell migration was observed with cAMP and cAMP/DHA treatment at 6 and 24 h in both pre- and post-treatment groups. DHA supplementation alone decreased cell migration and attenuated the increase in the cAMP/DHA treated cells at both time points and with pre- and post-treatment (Fig. 1, c and d). These data indicate that DHA supplementation, both before (pre-) or immediately after (post-) induction is able to suppress the pro-migratory effects of cAMP.
Fig. 1. Cell migration analysis by transwell assay.
MLE12 and A549 cells were treated with 8Br-cAMP and/or DHA for 6 or 24 h pre- and post-plating (1×105) in the transwell apparatus. After an additional 24 h, the cells that migrated to lower compartment were counted using neubauer cell counting chamber and the percent of cell migration was calculated. Data represent the average of 3 experiments and is presented as mean ± SE. Data were analyzed by 2-way ANOVA with Tukey's post hoc. The results of the 2-way ANOVA (effects or interactions) are indicated on each graph. Post hoc analyses indicated individual differences as *p<0.05; **p<0.005 and ***p<0.001, compared to respective controls and #p<0.05; ##p<0.005 and ###p<0.001 compared to cAMP group.
DHA modulates the expression of gelsolin and vimentin
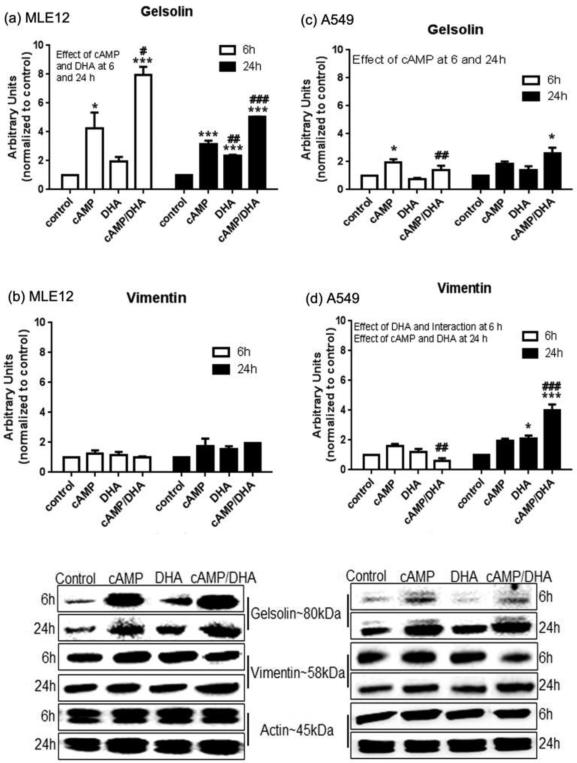
To determine whether DHA supplementation suppressed the expression of gelsolin or vimentin, western blot analyses were performed on whole cell lysates obtained at 6 or 24 h after treatments. cAMP increased the expression of gelsolin in both MLE12 and A549 cell at 6 h and in MLE12 cells at 24 h; the increase in MLE12 cells was substantially greater than A549 cells at 24 h (Fig. 2, a and c). DHA was able to attenuate the cAMP-induced increases in gelsolin in A549 cells at 6h. Vimentin expression was unaffected by any treatment in MLE12 cells (Fig. 2, b). In A549 cells, a modest decrease in vimentin expression was observed in the cAMP/DHA double treated cells at 6 h and increases in vimentin with DHA alone and cAMP/DHA at 24 h. Overall, the data indicate that DHA supplementation has a suppressive effect on gelsolin in both cell lines at both time points. DHA supplementation did not affect expression of vimentin in MLE12 cells and caused a modest increase in vimentin levels in A549 cells.
Fig. 2. Western blot of actin binding proteins (ABP).
Total cell lysate of MLE12 and A549 cells were analyzed by western blot using anti-gelsolin (a and c), and anti-vimentin (b and d) antibodies. Data represent the average of 3 experiments and are presented as mean ± SE. Data were analyzed with statistical significance noted as in Figure 1.
Confocal Images at the wound edge
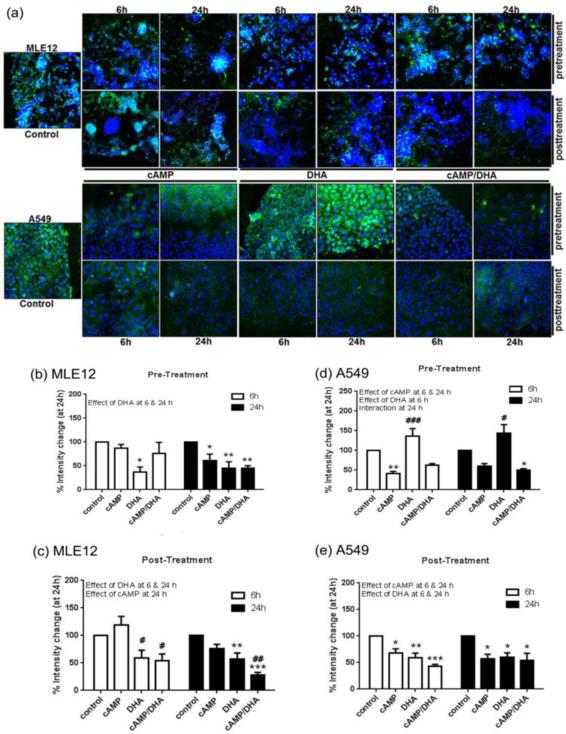
Confocal analysis of gelsolin (Fig. 3, a) demonstrated a decrease in gelsolin expression at the leading edges of wound closure in MLE12 cells with both cAMP and DHA for both pre- and post-treatment at 6 h and 24 h (Fig. 3, b and c). A similar decrease was observed in A549 cells after cAMP pre-treatment (Fig. 3, d) however, DHA pre-treatment alone caused an increase in gelsolin expression. The post-treatment of A549 cells demonstrated a decrease in gelsolin expression much like that observed in the MLE12 cells (Fig. 3, e).
Fig. 3. Confocal analysis of gelsolin.
Gelsolin levels in MLE12 and A549 cells were assessed by confocal microscopy followed by assessment with NIH Image J software. Images are at 200X magnification. Green represents gelsolin and blue represents nuclei (DAPI). Data are expressed as mean ± SE. Data were analyzed with statistical significance noted as in Figure 1.
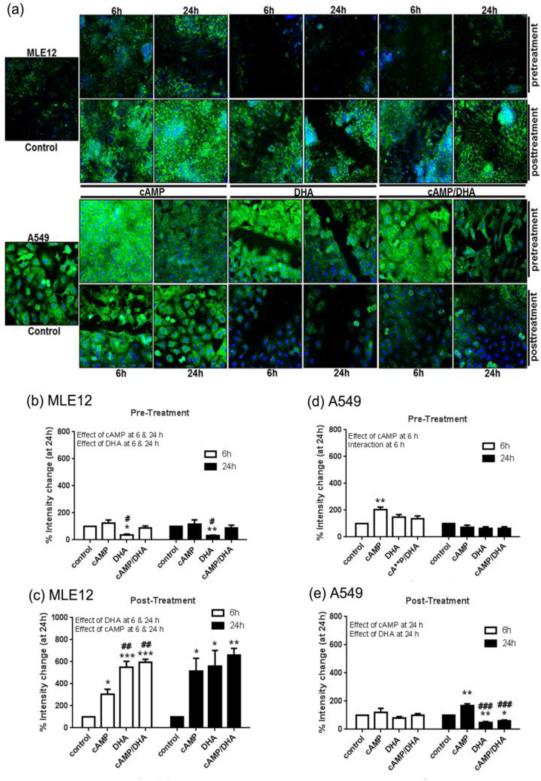
Vimentin expression analysis in MLE12 cells by confocal microscopy (Fig. 4, a) revealed a decrease in expression levels with DHA pre-treatment at 6 h and 24 h (Fig. 4, b). Conversely, an increase in vimentin expression was observed in MLE12 cells that were post-treated with cAMP, DHA, or cAMP/DHA double treatment at both 6 h and 24 h (Fig. 4, c). In A549 cells pre-treated with DHA, cAMP induced vimentin expression at 6 h with no effect at 24 h (Fig. 4, d). In post-treated A549 cells, cAMP increased and DHA decreased expression at 24 h (Fig. 4, e).
Fig. 4. Confocal analysis of vimentin.
Vimentin levels in MLE12 and A549 cells were assessed by confocal microscopy followed by assessment with NIH Image J software. Images are at 200X magnification. Green represents vimentin and blue represents nuclei (DAPI). Data are expressed as mean ± SE. Data were analyzed with statistical significance noted as in Figure 1.
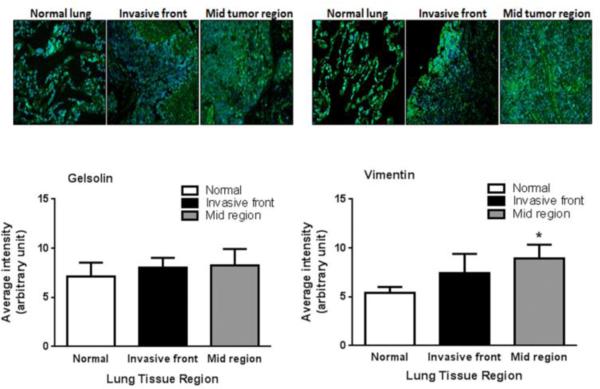
Gelsolin and Vimentin upregulated in lung cancer tissues
The translational potential of our findings was assessed by measuring gelsolin and vimentin levels in human lung cancer tissues. Tissue sections were treated to quantify the levels of gelsolin and vimentin in control tissue, the tumor invading front, and the tumor mid region, to differentiate the distribution of gelsolin and vimentin in relation to tumor growth and progression (Fig. 5). A non-significant increase in gelsolin levels at both tumor sites was observed. Vimentin expression was significantly greater in the tumor mid region of and a modest, non-significant increase in vimentin was observed at the tumor invasive front.
Fig. 5. Immunofluorescence of ABP in human lung tissue.
Control and lung tumor (invasive front and mid region) tissues were analyzed by fluorescence microscopy for expression levels of gelsolin or vimentin (green, blue represents nuclei (DAPI)). Representative IF images (magnification, 200x) were analyzed using NIH Image J software. Each bar is an average of 4 lung tissues (each tissue section was imaged at 4 separate regions). Value expressed as mean ± SE. Statistical analysis was performed using 1-way ANOVA followed by student-t-test and significance is indicated as *p<0.05 compared to respective controls.
Statistical Analysis
Data are presented as mean ± SE and were analyzed by one way or two-way ANOVA followed by Tukey's post-hoc analyses or by Student's t test using GraphPad PRISM 5 (La Jolla, CA). Each data set represents three independent experiments.
Discussion
Much of the current cancer research is focused on the identification of novel therapeutic modalities targeting processes that facilitate the metastatic phenotype [39-42]. Unfortunately, many anti-metastasis regimens have adverse effects on non-cancerous cell growth [43-45], therefore current efforts are being employed to search for unique agents that can selectively inhibit metastatic molecules/pathways in cancer cells. DHA supplementation has been shown to be efficacious in some cancers [20, 46, 47], indicating a potential for DHA to be developed as an anti-cancer therapy in the future. The mechanism by which DHA effects cancer cell growth and progression, especially metastatic phenotypes including migration, has not yet been explored. Therefore, this study set out to identify the mechanism associated with the observed effect of DHA on the metastatic potential of cancer cells using MLE 12 and A549 cells. DHA supplementation alone decreased cell migration and attenuated the increase by cAMP in A549 but not MLE12 cells, at both 6 h and 24 h both with pre- and post-treatment (Fig. 1). While we did not assess apoptosis as a potential variable in the migration studies, we observed no acute accumulation of dead or floating cells indicating massive cells death was not likely responsible for our observations. In support of our findings, DHA has been shown to suppresses the onset, growth, and progression of several diseases including cancers [16, 17, 20, 48]. Studies on human breast cancer cells in athymic nude mice fed diets rich in linoleic acid reported stimulated growth and metastasis, whereas mice fed diets enriched in fish oil reported growth suppression [49]. DHA has also been shown to restrict the proliferation of breast and prostate cancer cell lines and could serve as a future chemopreventive agent [reviewed in 47, 50]. In a separate study, increased DHA intakes lowered the prostate cancer risk in a cohort study of 47,866 US men aged 40-75 years, followed for 14 years [48]. Collectively, these studies support a role for DHA in metastatic phenotype inhibition as reported here.
On a large scale omega-3 fatty acids may act as an anti-cancer agent by manipulating multiple targets responsible for cancer development, including cell proliferation, cell survival, angiogenesis, inflammation, metastasis, and epigenetic abnormalities [46]. Zhang, et al. in 2013 reported that DHA inhibited angiogenesis, tumor growth, and metastasis in vitro via a VEGF receptor 2-dependent mechanism [20]. In other examples, DHA was found to suppress EGF-induced metastasis through an inhibition of EGFR and ErbB2 protein expression and the downstream target uPA and MMP-9 activation in SK-BR3 human breast cancer cells [51].
Our studies were designed to explore more basic pathways relevant to the development and progression of the “metastatic phenotype”, specifically focusing on actin binding protein-mediated actin dynamics. In this regard, actin binding proteins such as gelsolin and vimentin have been shown to participate in actin remodeling, a process known to enhance the formation of cell protrusive structures and thus metastatic phenotypes [52-56]. Therefore, in the current study, we tested the hypothesis that DHA treatment would alter the expression of the actin binding proteins gelsolin and vimentin and thus suppress the metastatic phenotype.
Western blot and confocal analysis indicated that DHA modulates expression of both gelsolin and vimentin differently in A549 cancer cells than in MLE12 control cells (Fig. 2). Western analyses showed that in MLE12 cells, cAMP exposure at 6 and 24 h induced gelsolin protein levels. This increase in gelsolin may be due to the dual role of gelsolin in both polymerizing as well as stabilizing actin filaments [57]. There was no effect of cAMP or DHA on vimentin in MLE12 cells (Fig. 2, b). As a major contributor to the metastatic phenotype [27, 28], changes in vimentin would not be expected in noninvasive MLE12 cells. In A549 cells, cAMP induced gelsolin while DHA was able to modestly attenuate this increase at 6 h but had no effect at 24 h. DHA alone and in combination with cAMP induced vimentin at 24 h which is in line with the invasive nature of A549 cells. Both proteins are necessary to maintain the high actin turn over and invasive phenotype and both gelsolin and vimentin are known to be highly expressed in primary cancer cells [26, 36]. Suppression of the effect of cAMP by DHA at 6 h on both proteins in A549 cells indicate the potential that DHA may be effective at attenuating the increased expression of these proteins at shorter time periods or lower cell population numbers.
Confocal analysis of gelsolin (Fig. 3) and vimentin (Fig. 4) at the edges of wounds indicates a negative correlation between the expression of these proteins and metastatic phenotype. This data is in contrast with the whole cell homogenate protein levels in Figure 2 and represents the difference between measuring gelsolin and vimentin expression in an entire plate of cells verses measuring the expression at the leading edge of a wound, where the cells are highly proliferative and potentially invasive. The pre- and post-treatments were performed to mimic the pre- and post-metastasis treatment of cancers. In MLE12 cells, DHA pre and post treatment decreased the levels of gelsolin at the leading edges of the wound and in this context gelsolin could be promoting actin stabilization (Fig. 3 a, b and c). In the case of A549 cells, a decrease in gelsolin levels with cAMP exposure was surprising, and indicates that the role of gelsolin in this process is not straightforward (Fig. 3 a, d and e). An increase in gelsolin with DHA at 6 h or 24 h pre-treatment could be due to DHA accumulation within the cell membrane and DHA is functioning to increase gelsolin levels to stabilize actin and to restrict cell migration in A549 cells.
Vimentin expression was increased by cAMP in DHA pre-treated A549 cells at 6h and was induced by cAMP and DHA in DHA post-treated MLE12 cells at 24 h (Fig. 4 a, b, and c). DHA pre-treatment at 6 or 24 h caused a decrease in vimentin expression in MLE12. In contrast, DHA, pre- and post-treatment, 6 or 24 h, maintained or decreased normal expression of vimentin. While the results of the confocal analysis of the wound assay do not reflect a direct correlation between cAMP treatment, expression of gelsolin and vimentin, and alterations with DHA supplementation, they do support the anti-migratory effects of DHA treatment.
To investigate the translational relevance of our findings, we measured gelsolin and vimentin expression in human control and malignant lung tissues (Fig. 5). Immunofluorescence analysis of gelsolin and vimentin expression in human lung tissues indicates a modest but significant increase in vimentin in malignant lung tissue while gelsolin levels were not different. These findings are in line with our current cell data as well as previous studies [26, 27, 29, 36, 58].
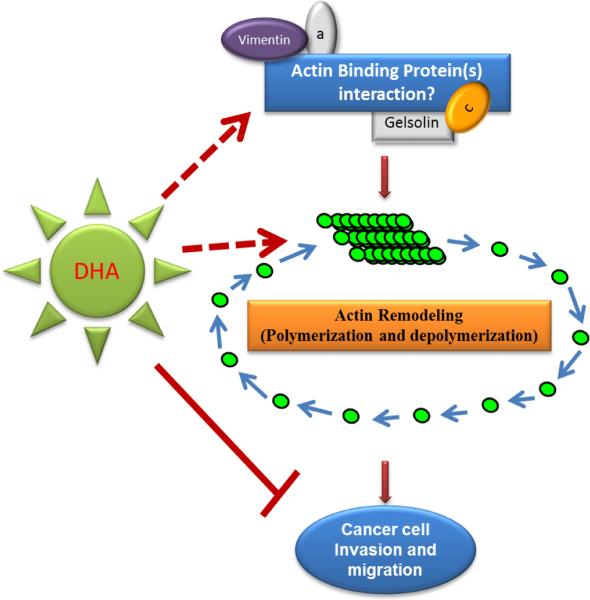
Overall, our findings clearly indicate the DHA inhibits the lung cancer cell metastatic phenotype. While our data do not indicate a straightforward role for gelsolin or vimentin in cAMP induced cell migration, they do not rule out a possible role for other ABPs in this process (Fig. 6). Further investigations are needed using in-vivo and in-vitro experimental models to explore the mechanisms associated with the role of ABPs and the effects of DHA supplementation on these activities. Importantly, the ability of DHA to precisely regulate lung cancer cell metastatic phenotype represents innovative therapeutic for patients with metastatic cancers.
Fig. 6. Schematic representation of the experimental hypothesis.
DHA treatment inhibits cells migration and ABPs are likely to be involved. While our data indicate that gelsolin and vimentin are not key regulators, we suggest that their interaction with other ABPs play an important regulatory role in cell migration and proliferation.
Acknowledgement
The authors acknowledge the financial support of NIH funding, R01AT006880 to Lynette Rogers, and Molly Augustine for manuscript editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunter K. Journal of molecular medicine. 2015;93:719. doi: 10.1007/s00109-015-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold RS, Fedewa SA, Goodman M, Osunkoya AO, Kissick HT, Morrissey C, True LD, Petros JA. Bone. 2015;78:81. doi: 10.1016/j.bone.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung LW, Baseman A, Assikis V, Zhau HE. The Journal of urology. 2005;173:10. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 4.Petruzzelli GJ, Howell JB, Pederson A, Origitano TC, Byrne RW, Munoz L, Emami B, Clark JI. American journal of otolaryngology. 2015;36:547. doi: 10.1016/j.amjoto.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Noh JM, Choi DH, Park H, Huh SJ, Park W, Seol SW, Jeong BK, Nam SJ, Lee JE, Kil WH. Journal of radiation research. 2014;55:553. doi: 10.1093/jrr/rrt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrecca K, Guiot MC, Panet-Raymond V, Souhami L. Journal of neuro oncology. 2013;111:19. doi: 10.1007/s11060-012-0983-4. [DOI] [PubMed] [Google Scholar]
- 7.Pantel K, Cote RJ, Fodstad O. Journal of the National Cancer Institute. 1999;91:1113. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 8.Nini A, Gandaglia G, Fossati N, Suardi N, Cucchiara V, Dell'Oglio P, Cazzaniga W, Luzzago S, Montorsi F, Briganti A. European urology. 2015 doi: 10.1016/j.eururo.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky JT, Cohen AM. Diseases of the colon and rectum. 1991;34:723. doi: 10.1007/BF02050360. [DOI] [PubMed] [Google Scholar]
- 10.Willett CG, Tepper JE, Cohen AM, Orlow E, Welch CE. Annals of surgery. 1984;200:685. doi: 10.1097/00000658-198412000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estes NC, Giri S, Fabian C. The American surgeon. 1996;62:546. [PubMed] [Google Scholar]
- 12.Kim TG, Park W, Choi DH, Park HC, Kim SH, Cho YB, Yun SH, Kim HC, Lee WY, Lee J, Park JO, Park YS, Lim HY, Kang WK, Chun HK. International journal of colorectal disease. 2014;29:193. doi: 10.1007/s00384-013-1797-3. [DOI] [PubMed] [Google Scholar]
- 13.Barreto SG, Pawar S, Shah S, Talole S, Goel M, Shrikhande SV. World journal of surgery. 2014;38:484. doi: 10.1007/s00268-013-2266-4. [DOI] [PubMed] [Google Scholar]
- 14.Siegel R, Ward E, Brawley O, Jemal A. CA: a cancer journal for clinicians. 2011;61:212. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 15.Siegel R, Ma J, Zou Z, Jemal A. CA: a cancer journal for clinicians. 2014;64:9. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 16.Horrocks LA, Yeo YK. Pharmacological research : the official journal of the Italian Pharmacological Society. 1999;40:211. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- 17.Swanson D, Block R, Mousa SA. Advances in nutrition. 2012;3:1. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kromhout D, Giltay EJ, Geleijnse JM, Alpha Omega Trial G. The New England journal of medicine. 2010;363:2015. [Google Scholar]
- 19.Blanckaert V, Ulmann L, Mimouni V, Antol J, Brancquart L, Chenais B. International journal of oncology. 2010;36:737. doi: 10.3892/ijo_00000549. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6530. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinlan MP. Journal of cellular physiology. 2004;200:277. doi: 10.1002/jcp.20009. [DOI] [PubMed] [Google Scholar]
- 22.Pfaendtner J, De La Cruz EM, Voth GA. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7299. doi: 10.1073/pnas.0911675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Cell. 2004;118:363. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Annual review of cell and developmental biology. 2003;19:541. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi H, Condeelis J. Biochimica et biophysica acta. 2007;1773:642. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L. The Journal of biological chemistry. 2004;279:36680. doi: 10.1074/jbc.M405197200. [DOI] [PubMed] [Google Scholar]
- 27.Toiyama Y, Yasuda H, Saigusa S, Tanaka K, Inoue Y, Goel A, Kusunoki M. Carcinogenesis. 2013;34:2548. doi: 10.1093/carcin/bgt282. [DOI] [PubMed] [Google Scholar]
- 28.Wang CI, Wang CL, Wu YC, Feng HP, Liu PJ, Chang YS, Yu JS, Yu CJ. Journal of proteome research. 2015;14:1739. doi: 10.1021/pr501097a. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto K, Kyoda Y, Tanaka T, Maeda T, Kobayashi K, Uchida K, Kitamura H, Hirata K, Tsukamoto T, Masumori N. Laboratory investigation; a journal of technical methods and pathology. 2015;95:283. doi: 10.1038/labinvest.2014.165. [DOI] [PubMed] [Google Scholar]
- 30.Ghoshdastider U, Popp D, Burtnick LD, Robinson RC. Cytoskeleton. 2013;70:775. doi: 10.1002/cm.21149. [DOI] [PubMed] [Google Scholar]
- 31.Kiselar JG, Janmey PA, Almo SC, Chance MR. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3942. doi: 10.1073/pnas.0736004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun HQ, Yamamoto M, Mejillano M, Yin HL. The Journal of biological chemistry. 1999;274:33179. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. The Journal of clinical investigation. 2009;119:1763. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs E, Weber K. Annual review of biochemistry. 1994;63:345. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 35.Esue O, Carson AA, Tseng Y, Wirtz D. The Journal of biological chemistry. 2006;281:30393. doi: 10.1074/jbc.M605452200. [DOI] [PubMed] [Google Scholar]
- 36.Park SB, Ryu YJ, Chung YS, Kim CH, Chung CK. Journal of Korean Neurosurgical Society. 2015;57:329. doi: 10.3340/jkns.2015.57.5.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrell MR, Rogers LK, Liu Y, Welty SE, Tipple TE. Free radical biology & medicine. 2010;49:1361. doi: 10.1016/j.freeradbiomed.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snegovskikh V, Mutlu L, Massasa E, Taylor HS. Reproductive sciences. 2014;21:1460. doi: 10.1177/1933719114553448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan SH, Wang YY, Wu ZY, Zhang ZF, Lu J, Li MQ, Shan Q, Wu DM, Sun CH, Hu B, Zheng YL. Oncotarget. 2015 doi: 10.18632/oncotarget.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng J, Qi J, Li XT, Zhou K, Xu JH, Zhou Y, Zhang GQ, Xu JP, Zhou RJ. International journal of clinical and experimental medicine. 2015;8:4220. [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Z, Duan X, Cai H, Wang L, Li M, Qu J, Li W, Wang Y, Wang J. Oncology reports. 2015 doi: 10.3892/or.2015.4044. [DOI] [PubMed] [Google Scholar]
- 42.Deep G, Kumar R, Jain AK, Agarwal C, Agarwal R. Mutation research. 2014;768:35. doi: 10.1016/j.mrfmmm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun GC, Yang X, Yu Y, Zhao DW. Anti-cancer agents in medicinal chemistry. 2015 doi: 10.2174/1871520615666150318101845. [DOI] [PubMed] [Google Scholar]
- 44.Bansal S, Sardana K, Singh K, Garg VK. Indian journal of dermatology. 2014;59:588. doi: 10.4103/0019-5154.143525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheppard RJ, Berger J, Sebag IA. Frontiers in pharmacology. 2013;4:19. doi: 10.3389/fphar.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jing K, Wu T, Lim K. Anti-cancer agents in medicinal chemistry. 2013;13:1162. doi: 10.2174/18715206113139990319. [DOI] [PubMed] [Google Scholar]
- 47.Karmali RA. Journal of internal medicine. Supplement. 1989;731:197. doi: 10.1111/j.1365-2796.1989.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 48.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. The American journal of clinical nutrition. 2004;80:204. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 49.Rose DP, Connolly JM, Rayburn J, Coleman M. Journal of the National Cancer Institute. 1995;87:587. doi: 10.1093/jnci/87.8.587. [DOI] [PubMed] [Google Scholar]
- 50.Terry PD, Rohan TE, Wolk A. The American journal of clinical nutrition. 2003;77:532. doi: 10.1093/ajcn/77.3.532. [DOI] [PubMed] [Google Scholar]
- 51.Li CC, Yao HT, Cheng FJ, Hsieh YH, Lu CY, Wu CC, Liu KL, Chang JW. Nutrition and cancer. 2015:1. doi: 10.1080/01635581.2015.1037961. [DOI] [PubMed] [Google Scholar]
- 52.Bayo P, Jou A, Stenzinger A, Shao C, Gross M, Jensen A, Grabe N, Mende CH, Rados PV, Debus J, Weichert W, Plinkert PK, Lichter P, Freier K, Hess J. Molecular oncology. 2015 doi: 10.1016/j.molonc.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capote C, Maccioni RB. Experimental cell research. 1998;239:202. doi: 10.1006/excr.1997.3902. [DOI] [PubMed] [Google Scholar]
- 54.Khaitlina S, Fitz H, Hinssen H. The FEBS journal. 2013;280:4600. doi: 10.1111/febs.12431. [DOI] [PubMed] [Google Scholar]
- 55.Zhuo J, Tan EH, Yan B, Tochhawng L, Jayapal M, Koh S, Tay HK, Maciver SK, Hooi SC, Salto-Tellez M, Kumar AP, Goh YC, Lim YC, Yap CT. PloS one. 2012;7:e43594. doi: 10.1371/journal.pone.0043594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ida CM, Yan X, Jentoft ME, Kip NS, Scheithauer BW, Morris JM, Dogan A, Parisi JE, Kovacs K. Endocrine pathology. 2013;24:149. doi: 10.1007/s12022-013-9254-y. [DOI] [PubMed] [Google Scholar]
- 57.Tomas A, Yermen B, Min L, Pessin JE, Halban PA. Journal of cell science. 2006;119:2156. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 58.Nomura H, Uzawa K, Ishigami T, Kouzu Y, Koike H, Ogawara K, Siiba M, Bukawa H, Yokoe H, Kubosawa H, Tanzawa H. BMC cancer. 2008;8:39. doi: 10.1186/1471-2407-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]