Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect of cancer treatment. CIPN is the most frequent cause of dose reduction or treatment discontinuation in patients treated for cancer with commonly used drugs including taxanes and platinum-based compounds. No FDA-approved treatments for CIPN are available. In rodents, CIPN is represented by peripheral mechanical allodynia in association with retraction of intraepidermal nerve fibers (IENFs). The mechanism of chemotherapy-induced neurotoxicity is unclear but it has been established that mitochondrial dysfunction is an important component of the dysregulation in peripheral sensory neurons.
We have shown earlier that inhibition of mitochondrial p53 accumulation with the small compound pifithrin-μ (PFT-μ) prevents cerebral neuronal death in a rodent model of hypoxic-ischemic brain damage. We now explore whether PFT-μ is capable of preventing neuronal mitochondrial damage and CIPN in mice. We demonstrate for the first time that PFT-μ prevents both paclitaxel- and cisplatin-induced mechanical allodynia. Electron microscopic analysis of peripheral sensory nerves revealed that PFT-μ secured mitochondrial integrity in paclitaxel-treated mice. In addition, PFT-μ administration protects against chemotherapy-induced loss of intra-epidermal nerve fibers (IENF) in the paw. To determine whether neuroprotective treatment with PFT-μ would interfere with the anti-tumor effects of chemotherapy, ovarian tumor cells were cultured in vitro with PFT-μ and paclitaxel. PFT-μ does not inhibit tumor cell death but even enhances paclitaxel-induced tumor cell death. These data are the first to identify PFT-μ as a potential therapeutic strategy for prevention of CIPN to combat one of the most devastating side effects of chemotherapy.
Keywords: chemotherapy, neuropathy, pifithrin-μ, mitochondria, peripheral nerves
Introduction
The American Cancer Society estimates 1.6 million people will be diagnosed with cancer in 2014 and nearly 14.5 million cancer survivors live in the U.S. today. Of these newly diagnosed cases and cancer survivors nearly 60% will experience pain, tingling, and numbness in hands and feet due to the side effects of chemotherapy [9; 32; 33; 47–49]. This chemotherapy-induced peripheral neuropathy (CIPN) is the major dose-limiting toxicity associated with cancer treatment. The highest incidence of CIPN is associated with taxanes (paclitaxel) and platinum-based (e.g. cisplatin) treatment [32; 33; 47]. With no FDA-approved drugs to prevent or treat CIPN, a significant number of cancer patients receive sub-optimal anti-cancer treatment due to dose reduction needed because of neuropathic pain. In addition, CIPN frequently persists long after completion of chemotherapy thereby reducing quality of life of cancer survivors [45]. Furthermore, new anti-cancer treatments frequently used in combination with chemotherapy can exacerbate symptoms of CIPN. Vascular endothelial growth factor (VEGF) inhibitors (bevacizumab, sorafenib and sunitinib) as well as immune modulating compounds (thalidomide) have been reported to increase incidence of CIPN which can often leads to early treatment cessation[23; 41; 44].
In rodent models of CIPN increased sensitivity to a mechanical stimulus (mechanical allodynia) is commonly used as a read out for pain [7; 13; 16; 27; 34; 46]. Paclitaxel stabilizes microtubules thereby inhibiting with cell division [35]. Cisplatin exerts its anti-tumor effect by binding to and crosslinking DNA which triggers cellular apoptosis. Additionally, both drugs have been shown to interfere with mitochondrial function in tumor cells as well as in non-dividing cells [2; 10; 17; 43]. In peripheral sensory neurons increased oxidative stress, excessive calcium levels, and ATP depletion [1; 11; 37; 53–55] can lead to spontaneous discharge of peripheral sensory neurons. Spontaneous discharge of both A- and C- fiber sensory neurons has been linked to neuronal sensitization and hyperalgesic responses [50–52]. One of the mechanisms that can lead to alterations in oxidative stress, calcium levels, and ATP, is improper mitochondrial function [1]. Furthermore, disruption of mitochondrial function has also been associated with a reduction in IENF density [14]. In this work we propose that prevention of chemotherapy-induced mitochondrial dysfunction may be a promising avenue for inhibition of CIPN.
The small molecule inhibitor, Pifithrin-μ (PFT-μ) has been identified for its capacity to inhibit mitochondrial p53 accumulation without impacting p53 transcriptional activity [39]. Our group demonstrated previously that disruption of the p53 mitochondrial pathway as well as intraperitoneal administration of PFT-μ protects against cerebral neuronal loss in a rodent model of neonatal ischemic brain damage [28–31]. In this model, PFT-μ prevented mitochondrial accumulation of p53 in the brain, thereby reducing oxidative stress and maintaining ATP production.
We hypothesized that protection of neuronal mitochondria by PFT-μ might also be a means to prevent CIPN. When investigating potential therapeutics for CIPN it is most critical that these drugs do not interfere with cancer therapy. Therefore, we also explored possible interference of PFT- μ with in vitro tumor cell killing induced by paclitaxel.
Methods
Animals
Adult female C57BL/6 mice were housed at the Texas A&M Health Science Center Program for Animal Resources. Mice were housed on a regular 12 h light/dark cycle with free access to food and water. All procedures were consistent with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and the Ethical Issues of the International Association for the Study of Pain[56] and were approved by the local IACUC.
Drug Administration
Paclitaxel (6 mg/ml) in 50% El Kolipher (Sigma Aldrich, St. Louis, MO) and 50% ethanol (Sigma Aldrich) was diluted in sterile saline to final concentration of 1 mg/ml and administered i.p. at a dose of 10 mg/kg every other day for two weeks. Cisplatin (Sigma Aldrich) was diluted in sterile saline and administered i.p. at a dose of 2.3 mg/kg daily for 5 days, followed by 5 days of rest, and as second round of 5 doses of cisplatin. PFT-μ (25 mg/ml in DMSO (Sigma Aldrich)) was diluted in sterile saline. PFT-μ was administered 1 hour prior to chemotherapy treatment intraperitoneally (i.p.) at a dose of 8 mg/kg [30; 40]. PFT-μ was administered one hour prior to each dose of chemotherapy and not on any of the other days. We have shown that these chemotherapy dosing schedules induce CIPN in mice [34]. Additionally, this dosage of PFT-μ was effective in protecting rodent neuronal mitochondria in ischemia [30].
Von Frey test for mechanical allodynia
Mechanical allodynia as a readout for CIPN was measured as the hind paw withdrawal response to von Frey hair stimulation using the up-and-down method as we described previously[34; 46]. Mice were placed in a plastic cage (10×10×13 cm3) with a mesh floor for 30 min prior to testing. Subsequently, a series of von Frey hairs (0.02, 0.07, 0.16, 0.4, 0.6, 1.0 and 1.4 g) (Stoelting, Wood Dale, Illinois, USA) were applied perpendicular to the mid-plantar surface of hind paw. A trial began with the application of the 0.16 g hair. A positive response was defined as a clear paw withdrawal or shaking. Whenever a positive response occurred, the next lower hair was applied, and whenever a negative response occurred, the next higher hair was applied. The testing consisted of five stimuli after the first change in response occurred, and the pattern of response was converted to a 50% von Frey threshold using the method described previously [6] by an investigator blinded to treatment until the end of the experiment.
Transmission Electron Microscopy for Mitochondrial Morphology
To investigate mitochondrial integrity, dorsal root ganglia (DRG) and peripheral nerve axons (sciatic nerve removed by mid-thigh removal method) were fixed in a solution containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.3. Samples were washed in 0.1 M cacodylate buffer and treated with 0.1% Millipore-filtered buffered tannic acid, post fixed with 1% buffered osmium tetroxide for 30 min, and stained and blocked with 1% Millipore-filtered uranyl acetate. The samples were washed in water, dehydrated with increasing concentrations of ethanol, infiltrated, embedded in LX-112 medium and polymerized in a 60 C oven for 2–3 days. Ultrathin sections were cut in a Leica Ultra cut microtome (Leica, Deerfield, IL), stained with uranyl acetate and lead citrate in a Leica EM Stainer, and examined in a JEM 1010 transmission electron microscope (JEOL, USA, Inc., Peabody, MA) at an accelerating voltage of 80 kV. Digital images were obtained using AMT Imaging System (Advanced Microscopy Techniques Corp, Danvers, MA).
A blinded scorer rated mitochondria as typical or atypical. This chemotherapy-induced mitochondria rating tool was established by Flatters et al. [11]. Percentage of atypical mitochondria was calculated for each mouse (n=3). Only mitochondria fully in the field of view were scored and mitochondria under 165 nm in diameter were excluded from scoring. Atypical mitochondria were identified by 2-fold increases in diameter or excessive vacuolization (more 50% translucent). For DRG tissues 10–25 mitochondria scored/mouse, for peripheral nerve axons 7–18 mitochondria scored/mouse. Preliminary studies have shown that there are no differences in mitochondrial morphology between vehicle-alone or PFT- μalone treated mice. Therefore PFT- μ-alone treated mice were used for baseline comparisons. Furthermore, percentages of atypical mitochondria in PFT- μ alone treated mice were comparable to what has been reported by others under baseline conditions in previously published studies [54].
Immunostaining for IENFs
For quantification of IENFs, 3 mm2 biopsies from the plantar surface of the hind paws were collected 3 weeks after completion of paclitaxel treatment) and processed as described [4; 5; 34; 38]. Biopsies were immediately placed in Zamboni’s fixative for 24 hrs., transferred to 20% sucrose, frozen in Optimal Cutting Temperature compound (OCT), and sliced into 25 μm sections. The sections were blocked for 2 hrs. At room temperature in 0.1 M PBS containing 5% normal donkey serum/0.3% Triton X-100. Sections were incubated with an antibody against the pan neuronal marker PGP9.5 (AbD Serotec; Rabbit, 1:1000) along with anti-Collagen IV antibody (Southern Biotech; Goat, 1:100) followed by Alexa-594-donkey anti-rabbit (Life Technologies, 1:500) and Alexa-488-donkey anti-goat (Invitrogen, 1:500). Secondary antibodies alone did not give a signal, indicating specificity of the staining. Three randomly chosen sections from each paw were quantified under a Leica fluorescence microscope. Nerve fibers that crossed the collagen stained dermal/epidermal junction into the epidermis were counted in three fields of each slice using the 40× objective. The length of the epidermis within each field was measured using ImageJ. IENFs density was determined as the total number of fibers/length of epidermis (IENFs/mm). A scorer blinded to the experimental set up rated the IENF density.
Tumor Cell Assay
Human ovarian tumor cells (HeyA8) were a gift from Dr. Anil Sood, MD Anderson Cancer Center [12; 18]. Tumor cells were maintained in 5% CO2 at 37 °C in RPMI-1640 media plus 15% fetal bovine serum and 0.1 % antibiotics. Tumor cell viability was measured by an enzymatic MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) assay. HeyA8 cells (3,000 cells /well) were seeded in flat bottom 96 well plates. After 24 hours fresh media was added to the wells +/− PFT-μ (5–20 μM) and +/− paclitaxel (15 nM) for 72 hours. After 72 hrs. MTT (0.15%) was added to each well and incubated for 2 hrs. followed by DMSO. Colorimetric changes were measured at 570 nm on a Biotek Epoch plate reader.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was carried out using repeated measure analysis of variance by two-way ANOVA or one-way ANOVA followed by Bonferroni analysis. P < 0.05 was considered statistically significant.
Results
The effect of PFT-μ administration on paclitaxel-induced peripheral neuropathy
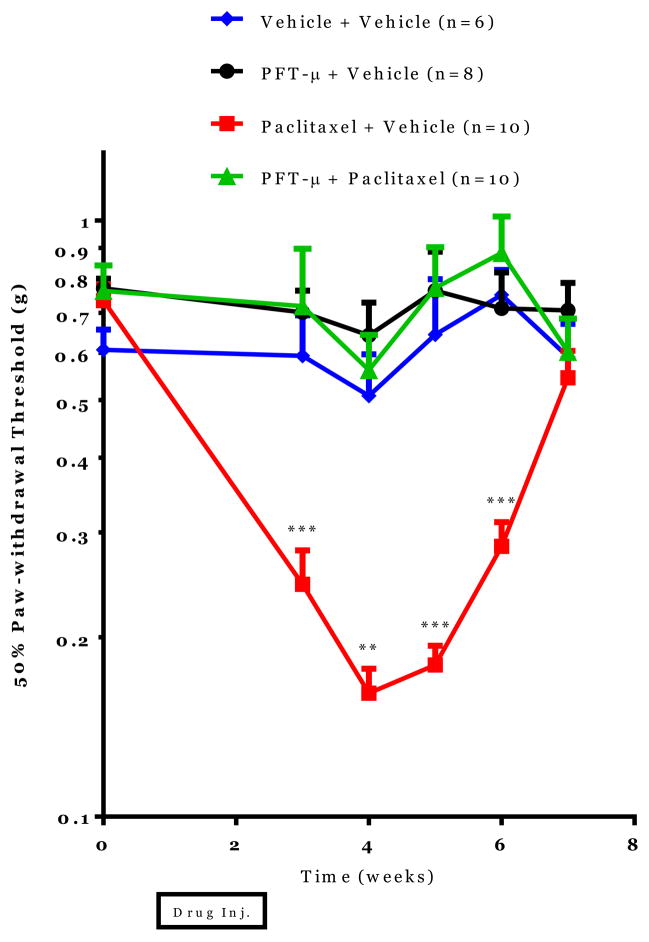
To investigate if PFT-μ administration prevents paclitaxel-induced mechanical allodynia, adult C57/Bl6 mice were treated with 10 mg/kg paclitaxel +/− 8 mg/kg PFT-μ every other day for two weeks. In mice treated with paclitaxel alone, decreased paw-withdrawal thresholds were observed as measured with the von Frey test for five weeks after paclitaxel administration. This decrease in paw-withdrawal threshold is indicative of chemotherapy-induced mechanical allodynia. PFT-μ completely prevented development of paclitaxel-induced decreases in paw-withdrawal thresholds. No differences in paw-withdrawal threshold were detected between mice treated with vehicle alone, PFT-μ alone or PFT-μ + paclitaxel. These data demonstrate that systemic administration of PFT-μ completely prevents paclitaxel-induced mechanical allodynia (see Figure 1).
Figure 1. Effect of PFT-μ administration on paclitaxel-induced peripheral neuropathy.
Mechanical allodynia was measured using von Frey hairs and the 50% paw withdrawal threshold was calculated by the up-down method. Statistical analysis using two-way repeated measures ANOVA revealed a significant group effect (p=0.0001), time effect (p=0.0006) and a time by group interaction (p=0.0006). Bonferroni post-hoc analysis was used to determine differences between groups at specified time points. *p<0.05, **p<0.01, ***p<0.001 between Paclitaxel+Vehicle and Paclitaxel + PFT-μ. n=6–10/group.
The effect of PFT-μ administration on mitochondrial morphology in peripheral sensory neuron cell bodies and axons
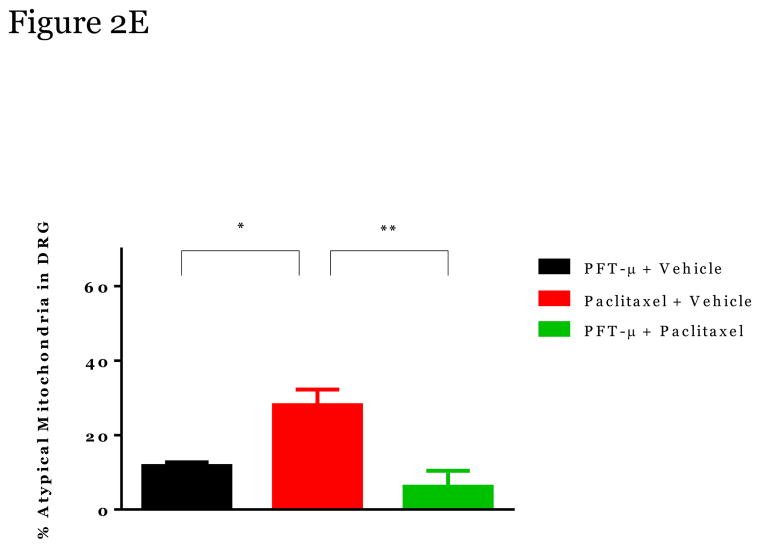
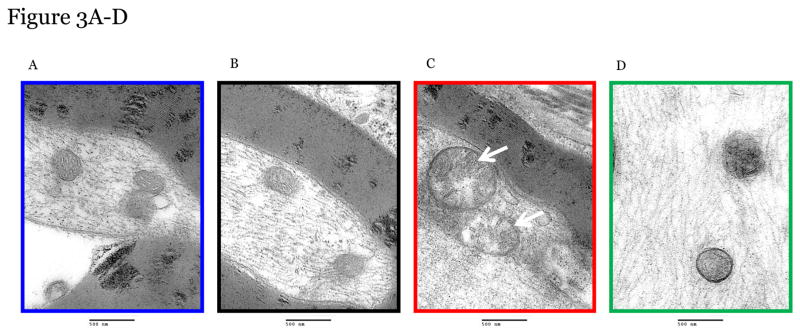
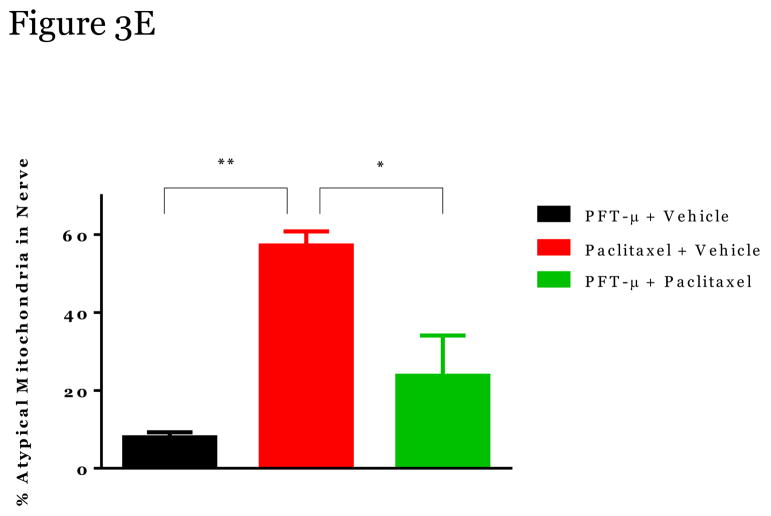
Paclitaxel induces swollen and vacuolated mitochondria in peripheral sensory neurons which may contribute to CIPN [11; 21; 26; 54]. Using transmission electron microscopy we confirmed that mitochondria in peripheral sensory nerve cell bodies in lumbar dorsal root ganglia (DRGs) and axons in the sciatic nerve of paclitaxel-treated mice were enlarged in shape, had disorganized cristae, displayed swelling/vacuolization, and loss of double membranes (denoted by white arrows) (Figure 2 and 3). Furthermore, the number of atypical mitochondria in DRGs and nerves was increased following paclitaxel treatment (Figure 2E, 3E).
Figure 2. The effect of PFT-μ administration on altered mitochondria morphology in peripheral sensory neuron cell bodies.
Representative Electron Microscopy Images from Mitochondria in DRG from Vehicle alone (A) PFT-μ+Vehicle-Treated mice (B), from Paclitaxel-Treated mice (C); and from Paclitaxel+PFT-μ–Treated mice (D). DRG tissues were harvested at week 5 at the moment that paclitaxel-treated mice showed mechanical allodynia. Percentage of atypical mitochondria/mouse (E). n=3 mice/treatment 10–25 mitochondria scored/mouse. Magnification 50,000 x. Statistical analysis using one-way ANOVA revealed a significant difference between groups (p<0.01). Bonferroni post-hoc analysis was used to determine differences between groups. *p<0.05, **p<0.01.
Figure 3. The effect of PFT-μ administration on altered mitochondria morphology in peripheral sensory neuron axons.
Representative Electron Microscopy Images from Mitochondria in peripheral sensory neuron axons (sciatic nerve) from Vehicle alone (A) PFT-μ+Vehicle-Treated mice (B), from Paclitaxel-Treated mice (C); and from Paclitaxel+PFT-μ–Treated mice (D). DRG tissues were harvested at week 5 at the moment that paclitaxel-treated mice showed mechanical allodynia. Percentage of atypical mitochondria/mouse (E). n=3 mice/treatment 7–18 mitochondria scored/mouse. Magnification 50,000x. Statistical analysis using one-way ANOVA revealed a significant difference between groups (p<0.01). Bonferroni post-hoc analysis was used to determine differences between groups. *p<0.05, **p<0.01.
Administration of PFT-μ completely prevented these paclitaxel-induced alterations in mitochondrial morphology in the DRGs (2C, D) and sciatic nerve (3C, D) with similar percentages of atypical mitochondria as the mice treated with PFT-μ alone. No differences in mitochondrial size were measured when comparing the DRG and sciatic nerves of mice that received PFT-μ alone or PFT-μ + paclitaxel, 2E and 3E. Preliminary analysis of mitochondrial abnormalities did not show differences between vehicle alone (2a, 3A) or PFT- μ alone (2B, 3B) treated mice, therefore PFT- μ alone treated mice were used as baseline. The percentage of atypical mitochondria in samples from mice treated with PFT- μ alone was consistent with the percentage reported under baseline conditions in previously published studies [54].” An image of mitochondrial morphology of a vehicle alone animal can be observed in Figure 2 and 3. These data demonstrate that PFT-μ efficiently preserves mitochondrial morphological integrity of peripheral sensory neurons in paclitaxel-treated mice.
The impact of PFT-μ on paclitaxel-induced IENF loss
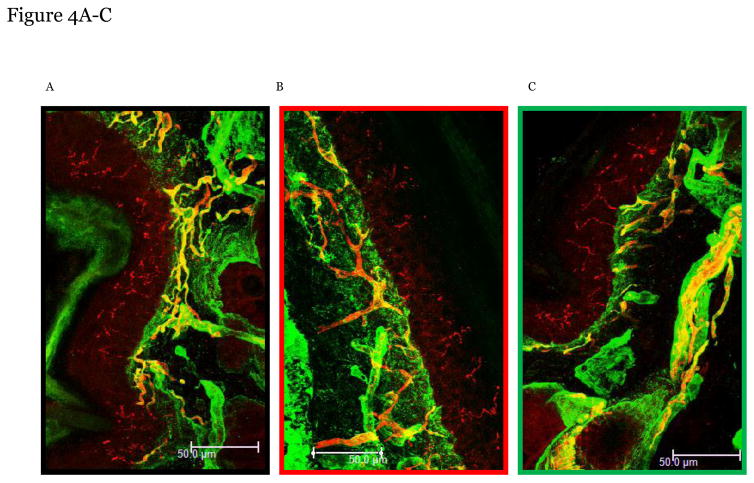
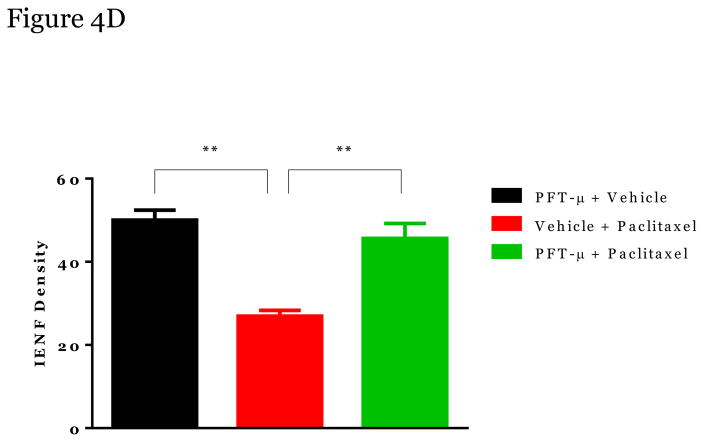
We analyzed the potential protective effect of PFT-μ on loss of IENFs in the hind paws of paclitaxel-treated mice. The density of nerve fibers entering the epidermis was significantly reduced in the paclitaxel-treated mice (Figure 4B) when compared to PFT-μ + vehicle- treated mice (Figure 4A). Notably, PFT-μ administration significantly reduced paclitaxel-induced IENF retraction, Figure 4c and 4d. No differences in IENF levels were observed when comparing the PFT-μ and the PFT-μ + paclitaxel treated mice, Figure 4D. These data demonstrate that PFT-μ administration protects completely against paclitaxel-induced IENFs loss.
Figure 4. The impact of PFT-μ on paclitaxel-induced IENF retraction.
Paw biopsies were obtained from the hind paws of mice in Week 5 (when mechanical allodynia and mitochondria alterations were measured). Paws were fixed and stained with antibodies for intra-epidermal nerve fibers (PGP9.5; red) and collagen (green). To control for a-specific staining, paws (from each treatment group) were stained with secondary antibodies alone, which never resulted in positive staining. Representative images PFT-μ+Vehicle-Treated mice (A), from Paclitaxel-Treated mice (B); and from Paclitaxel+PFT-μ–Treated mice (C). Arrows denote intraepidermal nerve fibers (show in red) crossing the basement membrane (shown in green). Quantification of all paws obtained (n=3–4 mice/group) shown in (D). IENF density = number of nerve fibers crossing the basement membrane/length of the basement member (mm). **p<0.01. Magnification 40x.
Potential effects of PFT on Tumor cell viability
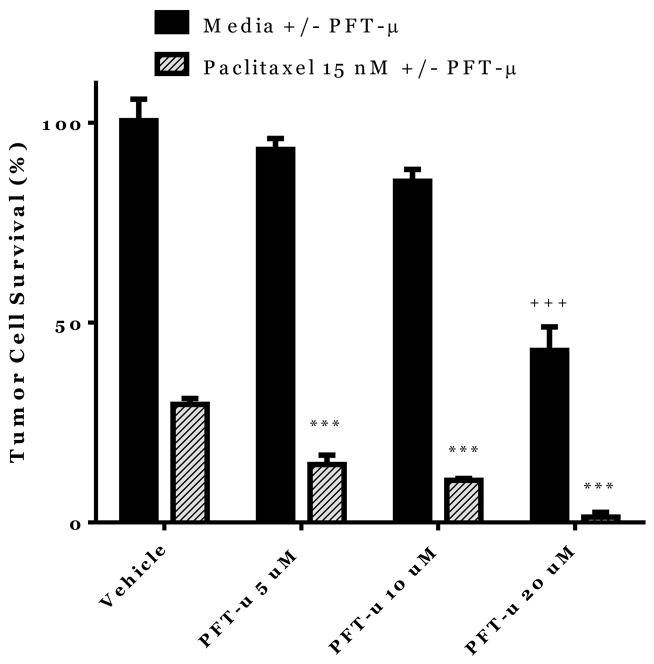
One of the potential risks of treating CIPN with PFT-μ may be that the drug may also protect against paclitaxel-induced tumor cell death by preventing accumulation of p53 at the mitochondria. Therefore, we assessed the effect of PFT-μ on ovarian tumor cells treated in vitro with paclitaxel. Human ovarian (p53+) tumor cells (HeyA8) were incubated with paclitaxel alone or in combination with PFT-μ. Tumor cell survival was measured by an enzymatic assay. Culturing HeyA8 cells with paclitaxel alone led to a reduction in tumor cell survival of more than 50% (Figure 5). Addition of PFT-μ to the cultures of tumor cells and paclitaxel further reduced ovarian tumor cell survival in comparison to paclitaxel alone (stripped bars). Additionally, PFT-μ (20 μM) alone also decreased tumor cell survival (solid bars). These data provide evidence that PFT-μ does not inhibit but even enhances the anti-tumor effect of paclitaxel.
Figure 5. Potential effects of PFT on Tumor cell viability.
Tumor cell (HeyA8 cells) survival as measured by MTT assay in the presence and absence of PFT-μ and paclitaxel (72 hrs.). Data were analyzed by two-way ANOVA. ***p<0.001 between Paclitaxel alone (Vehicle) and Paclitaxel+PFT-μ. +++ p<0.001 between PFT-μ alone (Vehicle) and PFT-μ 20 μM. n=4–12/group.
The effect of PFT-μ administration on cisplatin-induced neuropathy
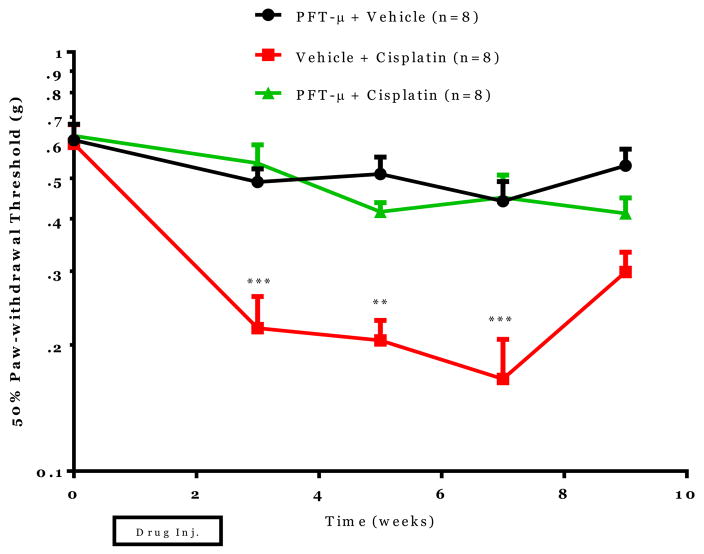
Next, we investigated if PFT-μ also prevented cisplatin-induced neuropathy. Mice were treated with cisplatin (2.3 mg/kg) alone or in combination with PFT-μ and mechanical allodynia was measured. In mice treated with cisplatin alone decreased paw-withdrawal thresholds were measured following cisplatin administration at weeks 3, 5, and 7 (Figure 6). Systemic PFT-μ administration completely prevented cisplatin-induced changes in paw-withdrawal threshold and thereby cisplatin-induced mechanical allodynia.
Figure 6. The effect of PFT-μ administration on cisplatin-induced neuropathy.
Mechanical allodynia was measured using von Frey hairs and the 50% paw withdrawal threshold was calculated by the up-down method. Statistical analysis using two-way repeated measures ANOVA revealed a significant group effect (p=0.0001), time effect (p=0.0001) and a time by group interaction (p=0.0053). Bonferroni post-hoc analysis was used to determine differences between groups at specified time points. *p<0.05, **p<0.01, ***p<0.001 between Cisplatin+Vehicle and Cisplatin + PFT-μ. n=8/group.
Discussion
We have identified the small molecule inhibitor PFT-μ as an efficacious agent to prevent chemotherapy-induced peripheral neuropathy (CIPN) in mice treated with paclitaxel or cisplatin. Moreover, PFT-μ completely prevented paclitaxel-induced changes in mitochondrial morphology in peripheral sensory neurons. Furthermore, PFT-μ administration protected against the loss of IENF in mice treated with paclitaxel. From these data we can conclude that PFT-μ prevents CIPN in mice treated with paclitaxel or cisplatin. Notably, in vitro, PFT-μ does not inhibit but rather enhances the anti-tumor effects of paclitaxel.
Paclitaxel and oxaliplatin treatment have previously been shown to induce mitochondrial swelling and vacuolization in peripheral nerves[10; 52; 53]. Ex vivo analysis of peripheral sensory neurons demonstrated that chemotherapy-induced mitochondrial swelling resulted in decreased cellular respiration and ATP production [54]. Furthermore altered mitochondrial function was linked to increased spontaneous discharge of sensory neurons. This spontaneous neuronal discharge has been suggested to underlie neuropathic pain [50–52]. Loss of IENFs is associated with chemotherapy treatment as measured by a decrease in the density of nerve fibers entering into the epidermis [4; 5; 34; 38]. Mechanisms of how chemotherapy treatment leads to IENF retraction are less well understood. Some authors have proposed that IENF retraction is due to decreased mitochondrial transport in peripheral sensory axons [3; 54]. Others have suggested that the energy rich and high mitochondrial density specifically in nerve terminals renders these sites most sensitive to chemotherapy leading to retraction of the nerve ending [22]. The retraction of nerve fibers may leave the remaining nerve fibers highly sensitized and primed for spontaneous discharge. Here we report that PFT-μ administration preserves both mitochondrial morphology as well as IENF density following paclitaxel treatment. We hypothesize that PFT-μ is preventing p53-mediated mitochondrial disruption in peripheral sensory neurons. This prevention of mitochondrial disruption prevents further downstream consequences such as neuronal sensitization and IENF retraction.
When investigating potential therapeutics for CIPN, it is important to determine if the therapeutic candidates interfere with tumor cell-killing of chemotherapy. When we tested PFT-μ in vitro for its capacity to regulate tumor cell growth, we observed that PFT-μ does not impair, but actually enhances paclitaxel-induced tumor cell death. This observation is in line with other recent publications investigating PFT-μ as a potential anti-cancer agent. These studies showed that PFT-μ administration to human tumor cells in combination with other anti-tumor therapies (hyperthermia and chemotherapy) enhanced tumor cell death [15; 20; 24; 36]. Our work is the first to demonstrate that PFT-μ enhances paclitaxel-tumor cell death of HeyA8 human tumor cells. Additionally, we are the first to have identified PFT-μ as a potential efficacious therapeutic for CIPN. These results further highlight the importance of PFT-μ and its potential therapeutic benefit for CIPN and tumor growth.
The tumor suppressor gene p53 is upregulated following cellular stress signaling including DNA damage, hypoxia, and chemotherapy treatment [30; 42]. Mitochondrial accumulation of p53 regulates numerous cellular processes including apoptosis and autophagy. PFT-μ was discovered for its ability to prevent p53 accumulation at the mitochondria without impacting transcriptional activity of p53 [39]. In these initial drug screens Strom et al. investigated the protective capabilities of PFT-μ for radiation toxicity in mice [39] and showed that PFT-μ prevented weight loss and death of the animal associated with sub-lethal and lethal doses of whole body gamma radiation. PFT-μ has also been shown to prevent accumulation of p53 at mitochondria and to reduce mitochondrial oxidative stress in response to toxic chemical excitation of striatal neurons [8]. We previously investigated the protective effects of PFT-μ in a model of neonatal hypoxic-ischemic (HI) brain damage [30]. We showed that HI increases p53 mitochondrial accumulation, reactive oxygen species (ROS) and cytosolic accumulation of pro-apoptotic proteins. These p53-mediated mitochondrial alterations led to neuronal apoptosis as measured by increased cytosolic cytochrome-c and active caspase-3 levels in association with white and grey matter damage in the brain. Systemic administration of PFT-μ prevented p53 mitochondrial accumulation, ROS production, release of pro-apoptotic proteins as well as HI-induced neuronal loss. In addition, PFT-μ administration prevented behavioral deficits such as motoric and cognitive function associated with HI-induced brain damage.
The data presented herein shows that paclitaxel administration leads to altered mitochondrial morphology and that this is prevented by co-administration of PFT-μ. Furthermore, this protection of mitochondria corresponds with prevention of chemotherapy-induced mechanical allodynia as well as IENF retraction. As a result, we have identified a means to prevent adverse behavioral and neuronal side-effects of chemotherapy. However, at present, we do not know whether PFT-μ exerts this protective effect by preventing p53 accumulation at the mitochondria as has been shown in our experiments focusing on neuronal protection by PFT-μ after hypoxic-ischemic brain damage.
It is surprising that PFT-μ protects neurons against chemotherapy-induced mitochondrial damage while at the same time enhancing tumor cell death. One reason for this dichotomy might be explained by studies which identified that PFT-μ can influence tumor cell death via interfering with the association between lysosome-associated membrane protein 2 (LAMP2) and HSP70 leading to accumulation of the waste recognizing molecule p62 which is involved in autophagia and tumor cell death [19]. The enhanced tumor cell death in response to PFT-μ was independent of p53 as PFT-μ- also induced tumor cell death in p53-null tumor cell lines. These data demonstrate that PFT-μ can exhibit tumor cell killing effects independent of p53. However, it should be noted that the effect of PFT-μ on p62 was observed in highly dividing (p53-) tumor cell lines. When Leu et al. investigated effects of PFT-μ on non-transformed fibroblasts they measured only limited effects on cell viability with no mention of increases in autophagy proteins. These data in combination with our study suggest that PFT-μ may have differential effects depending on either cell type or proliferative state of the cell [19]. We hypothesize that in a non-dividing cell such as a peripheral neuron, PFT-μ is predominantly interfering with chemotherapy-induced p53 mitochondrial accumulation thereby preventing sensitization and symptoms of CIPN. In tumor cells which are known to have increased levels of HSP70 [25], PFT-μ may primarily prevent HSP70 interaction with LAMP2 leading to accumulation of p62 proteins, dysregulation of autophagy and enhanced tumor cell death. Further studies will be required to determine what the mechanism via which PFTm protects against paclitaxel-induced mechanical allodynia.
The symptoms of CIPN can severely interfere with effective cancer treatment but can also persist long into survivorship thereby reducing quality of life [45]. The staggering numbers of individuals affected by CIPN make the discovery of efficacious therapeutics for treatment of CIPN extremely important. The findings in this work demonstrate that PFT-μ is an attractive candidate drug for prevention of CIPN since it has the potential to prevent CIPN and to enhance tumor-cell killing.
Acknowledgments
This study was supported by TU System: Star Award and NIH R21 CA183736. The research was also supported in part by the MD Anderson Cancer Center Support Grant (NCI P30 CA016672). Electron microscopy images were obtained at the MD Anderson Cancer Center High Resolution Electron Microscopy Facility with the assistance of Kenneth Dunner Jr.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- 1.Ahmed LA, Shehata NI, Abdelkader NF, Khattab MM. Tempol, a Superoxide Dismutase Mimetic Agent, Ameliorates Cisplatin-Induced Nephrotoxicity through Alleviation of Mitochondrial Dysfunction in Mice. PloS one. 2014;9(10):e108889. doi: 10.1371/journal.pone.0108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andre N, Braguer D, Brasseur G, Goncalves A, Lemesle-Meunier D, Guise S, Jordan MA, Briand C. Paclitaxel induces release of cytochrome c from mitochondria isolated from human neuroblastoma cells. Cancer research. 2000;60(19):5349–5353. [PubMed] [Google Scholar]
- 3.Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nature reviews Neurology. 2014;10(6):326–336. doi: 10.1038/nrneurol.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyette-Davis J, Dougherty PM. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Experimental neurology. 2011;229(2):353–357. doi: 10.1016/j.expneurol.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152(2):308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Deuis JR, Lim YL, Rodrigues de Sousa S, Lewis RJ, Alewood PF, Cabot PJ, Vetter I. Analgesic effects of clinically used compounds in novel mouse models of polyneuropathy induced by oxaliplatin and cisplatin. Neuro-oncology. 2014;16(10):1324–1332. doi: 10.1093/neuonc/nou048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong XX, Wang YR, Qin S, Liang ZQ, Liu BH, Qin ZH, Wang Y. p53 mediates autophagy activation and mitochondria dysfunction in kainic acid-induced excitotoxicity in primary striatal neurons. Neuroscience. 2012;207:52–64. doi: 10.1016/j.neuroscience.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109(1–2):132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Evtodienko YV, Teplova VV, Sidash SS, Ichas F, Mazat JP. Microtubule-active drugs suppress the closure of the permeability transition pore in tumour mitochondria. FEBS letters. 1996;393(1):86–88. doi: 10.1016/0014-5793(96)00875-7. [DOI] [PubMed] [Google Scholar]
- 11.Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gharpure KM, Chu KS, Bowerman CJ, Miyake T, Pradeep S, Mangala SL, Han HD, Rupaimoole R, Armaiz-Pena GN, Rahhal TB, Wu SY, Luft JC, Napier ME, Lopez-Berestein G, DeSimone JM, Sood AK. Metronomic docetaxel in PRINT nanoparticles and EZH2 silencing have synergistic antitumor effect in ovarian cancer. Molecular cancer therapeutics. 2014;13(7):1750–1757. doi: 10.1158/1535-7163.MCT-13-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guindon J, Deng L, Fan B, Wager-Miller J, Hohmann AG. Optimization of a cisplatin model of chemotherapy-induced peripheral neuropathy in mice: use of vitamin C and sodium bicarbonate pretreatments to reduce nephrotoxicity and improve animal health status. Molecular pain. 2014;10:56. doi: 10.1186/1744-8069-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin HW, Flatters SJ, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Experimental neurology. 2008;210(1):229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser M, Kuhnl A, Reins J, Fischer S, Ortiz-Tanchez J, Schlee C, Mochmann LH, Heesch S, Benlasfer O, Hofmann WK, Thiel E, Baldus CD. Antileukemic activity of the HSP70 inhibitor pifithrin-mu in acute leukemia. Blood cancer journal. 2011;1(7):e28. doi: 10.1038/bcj.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsuyama S, Sato K, Yagi T, Kishikawa Y, Nakamura H. Effects of repeated milnacipran and fluvoxamine treatment on mechanical allodynia in a mouse paclitaxel-induced neuropathic pain model. Biomedical research. 2013;34(2):105–111. doi: 10.2220/biomedres.34.105. [DOI] [PubMed] [Google Scholar]
- 17.Kidd JF, Pilkington MF, Schell MJ, Fogarty KE, Skepper JN, Taylor CW, Thorn P. Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. The Journal of biological chemistry. 2002;277(8):6504–6510. doi: 10.1074/jbc.M106802200. [DOI] [PubMed] [Google Scholar]
- 18.Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, Mangala LS, Merritt WM, Lin YG, Gao C, Schmandt R, Kamat AA, Li Y, Thaker P, Gershenson DM, Parikh NU, Gallick GE, Kinch MS, Sood AK. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. Journal of the National Cancer Institute. 2006;98(21):1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 19.Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Molecular cell. 2009;36(1):15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Sato F, Sato R, Matsubara T, Hirai K, Yamasaki M, Shin T, Shimada T, Nomura T, Mori K, Sumino Y, Mimata H. Dual targeting of heat shock proteins 90 and 70 promotes cell death and enhances the anticancer effect of chemotherapeutic agents in bladder cancer. Oncology reports. 2014;31(6):2482–2492. doi: 10.3892/or.2014.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, Taroni F, Lauria G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Experimental neurology. 2008;214(2):276–284. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer treatment reviews. 2014;40(7):872–882. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Mina LA, Yu M, Johnson C, Burkhardt C, Miller KD, Zon R. A phase II study of combined VEGF inhibitor (bevacizumab+sorafenib) in patients with metastatic breast cancer: Hoosier Oncology Group Study BRE06–109. Investigational new drugs. 2013;31(5):1307–1310. doi: 10.1007/s10637-013-9976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monma H, Harashima N, Inao T, Okano S, Tajima Y, Harada M. The HSP70 and autophagy inhibitor pifithrin-mu enhances the antitumor effects of TRAIL on human pancreatic cancer. Molecular cancer therapeutics. 2013;12(4):341–351. doi: 10.1158/1535-7163.MCT-12-0954. [DOI] [PubMed] [Google Scholar]
- 25.Nanbu K, Konishi I, Mandai M, Kuroda H, Hamid AA, Komatsu T, Mori T. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer detection and prevention. 1998;22(6):549–555. doi: 10.1046/j.1525-1500.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 26.Nieto FR, Cendan CM, Canizares FJ, Cubero MA, Vela JM, Fernandez-Segura E, Baeyens JM. Genetic inactivation and pharmacological blockade of sigma-1 receptors prevent paclitaxel-induced sensory-nerve mitochondrial abnormalities and neuropathic pain in mice. Molecular pain. 2014;10(1):11. doi: 10.1186/1744-8069-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieto FR, Cendan CM, Sanchez-Fernandez C, Cobos EJ, Entrena JM, Tejada MA, Zamanillo D, Vela JM, Baeyens JM. Role of sigma-1 receptors in paclitaxel-induced neuropathic pain in mice. The journal of pain : official journal of the American Pain Society. 2012;13(11):1107–1121. doi: 10.1016/j.jpain.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Nijboer CH, Bonestroo HJ, Zijlstra J, Kavelaars A, Heijnen CJ. Mitochondrial JNK phosphorylation as a novel therapeutic target to inhibit neuroinflammation and apoptosis after neonatal ischemic brain damage. Neurobiology of disease. 2013;54:432–444. doi: 10.1016/j.nbd.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia-ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke; a journal of cerebral circulation. 2008;39(7):2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- 30.Nijboer CH, Heijnen CJ, van der Kooij MA, Zijlstra J, van Velthoven CT, Culmsee C, van Bel F, Hagberg H, Kavelaars A. Targeting the p53 pathway to protect the neonatal ischemic brain. Annals of neurology. 2011;70(2):255–264. doi: 10.1002/ana.22413. [DOI] [PubMed] [Google Scholar]
- 31.Nijboer CH, van der Kooij MA, van Bel F, Ohl F, Heijnen CJ, Kavelaars A. Inhibition of the JNK/AP-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain, behavior, and immunity. 2010;24(5):812–821. doi: 10.1016/j.bbi.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(30):3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 33.Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain medicine. 2001;2(1):8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- 34.Qi-Liang Mao-Ying AK, Krukowski Karen, Huo Xiao-Jiao, Price Theodore J, Cleeland Charles, Heijnen Cobi J. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. CLINICAL CANCER RESEARCH. 2014 doi: 10.1371/journal.pone.0100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. Journal of the National Cancer Institute. 1990;82(15):1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 36.Sekihara K, Harashima N, Tongu M, Tamaki Y, Uchida N, Inomata T, Harada M. Pifithrin-mu, an inhibitor of heat-shock protein 70, can increase the antitumor effects of hyperthermia against human prostate cancer cells. PloS one. 2013;8(11):e78772. doi: 10.1371/journal.pone.0078772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesthesia and analgesia. 2006;102(5):1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Experimental neurology. 2006;201(2):507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, Gudkov AV. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nature chemical biology. 2006;2(9):474–479. doi: 10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 40.Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Molecular pain. 2010;6:15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tacchetti P, Terragna C, Galli M, Zamagni E, Petrucci MT, Pezzi A, Montefusco V, Martello M, Tosi P, Baldini L, Peccatori J, Ruggieri M, Pantani L, Lazzaro A, Elice F, Rocchi S, Gozzetti A, Cavaletti G, Palumbo A, Cavo M. Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: clinical and molecular analyses of a phase 3 study. American journal of hematology. 2014 doi: 10.1002/ajh.23835. [DOI] [PubMed] [Google Scholar]
- 42.Tavernarakis N, Pasparaki A, Tasdemir E, Maiuri MC, Kroemer G. The effects of p53 on whole organism longevity are mediated by autophagy. Autophagy. 2008;4(7):870–873. doi: 10.4161/auto.6730. [DOI] [PubMed] [Google Scholar]
- 43.Varbiro G, Veres B, Gallyas F, Jr, Sumegi B. Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free radical biology & medicine. 2001;31(4):548–558. doi: 10.1016/s0891-5849(01)00616-5. [DOI] [PubMed] [Google Scholar]
- 44.Verheyen A, Peeraer E, Nuydens R, Dhondt J, Poesen K, Pintelon I, Daniels A, Timmermans JP, Meert T, Carmeliet P, Lambrechts D. Systemic anti-vascular endothelial growth factor therapies induce a painful sensory neuropathy. Brain : a journal of neurology. 2012;135(Pt 9):2629–2641. doi: 10.1093/brain/aws145. [DOI] [PubMed] [Google Scholar]
- 45.Vichaya EG, Wang XS, Boyette-Davis JA, Mendoza TR, He Z, Thomas SK, Shah N, Williams LA, Cleeland CS, Dougherty PM. Subclinical pretreatment sensory deficits appear to predict the development of pain and numbness in patients with multiple myeloma undergoing chemotherapy. Cancer chemotherapy and pharmacology. 2013;71(6):1531–1540. doi: 10.1007/s00280-013-2152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Heijnen CJ, Eijkelkamp N, Garza Carbajal A, Schedlowski M, Kelley KW, Dantzer R, Kavelaars A. GRK2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain. 2011;152(7):1649–1658. doi: 10.1016/j.pain.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. Journal of the peripheral nervous system : JPNS. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 48.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. European journal of cancer. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Wolf SL, Barton DL, Qin R, Wos EJ, Sloan JA, Liu H, Aaronson NK, Satele DV, Mattar BI, Green NB, Loprinzi CL. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20(3):625–632. doi: 10.1007/s00520-011-1141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetylL-carnitine. Pain. 2008;135(3):262–270. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain. 2012;153(3):704–709. doi: 10.1016/j.pain.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM. Olesoxime (cholest-4-en-3-one, oxime): analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain. 2009;147(1–3):202–209. doi: 10.1016/j.pain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao WH, Zheng H, Zheng FY, Nuydens R, Meert TF, Bennett GJ. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience. 2011;199:461–469. doi: 10.1016/j.neuroscience.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Yuan F, Cao X, Zhai Z, GangHuang Du X, Wang Y, Zhang J, Huang Y, Zhao J, Hou W. P2X7 receptor blockade protects against cisplatin-induced nephrotoxicity in mice by decreasing the activities of inflammasome components, oxidative stress and caspase-3. Toxicology and applied pharmacology. 2014 doi: 10.1016/j.taap.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]