Abstract
Obesity and a western diet have been linked to high levels of bile acids and the development of colon cancer. Specifically, increased levels of the bile acid deoxycholic acid (DCA), an established tumor promoter, has been shown to correlate with increased development of colorectal adenomas and progression to carcinoma. Herein we investigate the mechanism by which DCA leads to EGFR-MAPK activation, a candidate mechanism by which DCA may promote colorectal tumorigenesis. DCA treated colon cancer cells exhibited strong and prolonged activation of ERK1/2 when compared to EGF treatment alone. We also showed that DCA treatment prevents EGFR degradation as opposed to the canonical EGFR recycling observed with EGF treatment. Moreover, the combination of DCA and EGF treatment displayed synergistic activity, suggesting DCA activates MAPK signaling in a non-canonical manner. Further evaluation showed that DCA treatment increased intracellular calcium levels and CAMKII phosphorylation, and that blocking calcium with BAPTA-AM abrogated MAPK activation induced by DCA, but not by EGF. Finally we showed that DCA-induced CAMKII leads to MAPK activation through the recruitment of c-Src. Taken together, we demonstrated that DCA regulates MAPK activation through calcium signaling, an alternative mechanism not previously recognized in human colon cancer cells. Importantly, this mechanism allows for EGFR to escape degradation and thus achieve a constitutively active state, which may explain its tumor promoting effects.
Keywords: EGFR activation, DCA, bile acids, colon cancer, calcium signaling
1. Introduction
Obesity and a western diet lead to increased levels of bile acids [1]. High levels of bile acids in the gut have long been linked to the development of many gastroenterological conditions, namely adenocarcinoma of the colon and rectum [2]. Consistent with this, patients diagnosed with colorectal polyps have been shown to also have elevated fecal bile acid concentrations [3]. Specifically, high levels of the secondary bile acid deoxycholic acid (DCA) in fecal matter and patient’s serum correlates with increased adenomatous polyp recurrence and the development of higher grade, and more aggressive disease [4]. Animal studies have confirmed that DCA acts as a tumor promoter, leading to increased tumor development in rodents that have been initiated with the chemical carcinogen, azoxymethane [5–7]. It is unclear how DCA achieves this, but recent studies point to DCA’s role in activating specific cell signaling pathways as a possible explanation. In vitro, DCA is able to stimulate the proliferation of human colonic biopsies as well as induce the activation of mitogenic signaling, which could lead to hyperplasia [8, 9]. Studies have begun to elucidate signaling pathways regulated by bile acids, however, the mechanism of these activities are not well understood.
The main physiological role of bile acids is to facilitate the absorption of dietary lipids and lipid soluble vitamins[10]. However, as mentioned above, recently it has become clear that bile acids can also act as versatile signaling molecules and even regulate gene expression. Bile acids were initially shown to control the expression of the rate limiting enzyme in the bile acid synthetic pathway, cholesterol 7 alpha-hydroxylase, through a bile acid response element that requires protein kinase C activity [11, 12]. Subsequently, several laboratories showed that bile acids could stimulate many molecules including nuclear receptors, G protein coupled receptors and mitogenic signaling pathways [13]. Further analysis demonstrated that bile acids, DCA in particular, could stimulate MAP kinase signaling through activation of the epidermal growth factor receptor (EGFR) in rat hepatocytes [14]. Consistent with this, our lab has demonstrated that the tumor promoting bile acid DCA induces phosphorylation of EGFR in HCT116 colorectal adenocarcinoma cells, resulting in the downstream activation of RAS, RAF, and ERK1/2 [15]; suggesting that DCA could be a key regulator the EGFR-MAPK pathway in colon cancer cells. It is well known that over-activation of EGFR-MAPK signaling in colon cancer cells leads to uncontrolled proliferation and cell survival. Hence, these observations suggested that understanding the mechanism by which DCA regulates this pathway may help elucidate its function as a tumor promoter.
Previous studies are limited and have shown conflicting results when examining the mechanism by which DCA leads to activation of EGFR-MAPK signaling. Some groups have shown that the process is ligand independent, while others have ascertained that DCA can only activate EGFR when natural ligand, such as EGF is present [16, 17]. Moreover, some studies suggest that DCA-induced membrane perturbations may also be involved in the activation of EGFR-MAPK [18–21]. However, the fact that EGFR activation can occur directly or indirectly through a number of converging signaling pathways has led to considerable difficulty in elucidating this mechanism. In this study we were able to show that DCA leads to ligand independent activation of EGFR and subsequent MAPK signaling through a non-traditional mechanism not previously described in colon cancer cells. Furthermore, we present data which shows that this mechanism of activation allows for EGFR to escape degradation, suggesting that increased levels of DCA in the colon may be one mechanism leading to the over-activation of EGFR observed in colon cancers.
2. Materials and methods
2.1 Cell lines and tissue culture
HT-29 human colorectal Adenocarcinoma cells were obtained as a cryopreserved vial from Dr. Donato Romagnolo (University of Arizona, Department of Nutritional Sciences) at passage two after ATCC purchase. The cells are maintained in DMEM with 10% fetal bovin serum (VWR Seradigm Life Sciences, Radnor, PA) and Penicillin-Streptomycin (Corning Inc., Corning, NY ), and incubated at 37°C with 5% CO2. Serum free media was made up of DMEM and Penicillin-Streptomycin.
2.2 Cell treatments and lysate preparation
HT-29 cells were plated in 6 well dishes at a density of 1.0×106 cells/well and cultured in 10% complete media for 24 hours to allow for adherence. After 24 hours, the complete media was replaced with serum-free media for 18 hours prior to any treatment. Cells were then treated with human recombinant EGF (Corning Inc.), DCA (Sigma-Aldrich, St. Louis, MO), or ionomycin (Sigma-Aldrich). In some studies pretreatment of HT-29 cells with inhibitors including PD153035 1μM (Santa Cruz Biotechnology, Dallas, Texas), BAPTA-AM (Santa Cruz Biotechnology), SKI (Santa Cruz Biotechnology) or Kn-93 (Cayman Chemical, Ann Arbor, MI) preceded treatments. Treated cells were washed with PBS, scraped and lysed with RIPA buffer containing HALT protease and phosphatase inhibitor cocktail (ThermoFisher Scientific, Waltham, MA) at various time points. Lysates were sonicated and spun down, removing contaminating DNA and cell debris. Protein was harvested from the supernatant and quantified using a BCA protein assay kit (ThermoFisher Scientific).
2.3 Antibodies and western blot
Western blot analysis was performed using 10–20μg of protein. Samples were boiled for 5 minutes with 1x Laemmli sample buffer (Bio-Rad, Hercules, CA) containing 2-Mecaptoethanol (Sigma-Aldrich). Once cooled, samples were loaded with into 10% SDS-PAGE Tris-Glycine gels (Life Technologies, Carlsbad, CA). The gels were run at 125 volts for 1 hour and 45 minutes at room temperature using the Novex Mini cell system (Life Technologies). The gels were then transferred onto a PVDF membrane (Millipore Billerica, Ma) also using the Novex Mini cell system, at 25 volts for 1 hour 30 minutes. The membranes were blocked using 5% blotting grade blocker (Bio-Rad) for 30 minutes at room temperature then incubated in primary antibodies overnight at 4°C. All primary antibodies were used at a 1:800 dilution in 5% BSA (Sigma-Aldrich) in TBS-T with 0.1% Sodium Azide (Sigma-Aldrich). Primary antibodies that were used in this study include: p-ERK1/2 (Cell Signaling Technologies, Danvers, Ma), total ERK1/2 (Cell Signaling Technologies), p-EGFR Tyr1068 (Cell Signaling Technologies), total EGFR (Cell Signaling Technologies), p-EGFR Tyr845 (Cell Signaling Technologies), p-CAMKII (Santa Cruz Biotechnologies), β-actin (Sigma-Aldrich) and p-c-Src (Cell Signaling Technologies). Once blotted with primary antibody, the membranes were washed three times for 5 minutes per wash with TBS-T, and incubated with the appropriate anti-Rabbit or anti-Mouse HRP conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:10,000–1:15,000. Following another wash cycle, chemiluminescence was detected using SuperSignal West Pico chemiluminescent substrate (ThermoFisher Scientific).
2.4 Densitometry
Densitometry was calculated using Image J software and Densitometry 1 Channel plugin. All values shown are the protein of interest divided by the respective β-actin loading control value. Values were normalized by setting the no treatment (NT) value to 1. If the measured values were 0 then no data (ND) was inserted.
2.5 Viability assay
HT-29 cells were plated in 6 well dishes as described above in cell treatments and lysate preparation section. Following treatments, cells were harvested by adding 1ml 0.5% trypsin-EDTA for 5 minutes at 37°C. Trypsin-EDTA was neutralized with 1ml complete media, and cells were pelleted by centrifugation. Cells were then resuspended in 25μl of complete media, followed by treatment with 10 μl of dye mixture (100μg/ml each Acridine Orange and Ethidium Bromide). 10 μl of cells were placed onto a glass slide and covered with a 22mm coverslip. Cells were visualized using a Nikon Eclipse E800 epifluorescent microscope. A minimum of 500 cells were counted per treatment group. All experiments were conducted in triplicate.
2.6 Calcium assay
HT-29 cells were plated in a 96-well clear bottom black plate (Greiner Bio-one, Kremsmünster, Austria), at a density of 40,000 cells/well in complete media and incubated for 24 hrs. The media was removed and cells were incubated with 100μl of 1x dye loading solution from the Fluo-4 NW Calcium assay kit (Life Technologies). The dye solution was then removed and replaced with 100μl of DCA or ionomycin. Plates were read immediately on SpectraMax M2 kinetic plate reader (Molecular Devices, Sunnyvale, California), using SoftMax Pro v6.3 software analysis.
3. Results
3.1 DCA activates EGFR-MAPK signaling in a ligand-independent manner and enhances EGF-induced signaling
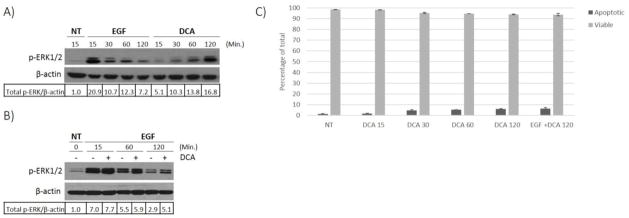
Previous studies have documented that DCA treatment leads to an increase in EGFR-MAPK signaling in colon cancer cells [15, 22–25]. However, it is unclear whether the natural ligand (EGF) is required. There is literature to support both ligand dependent [17] and independent activation [16] of this pathway which was conducted in cholangiocyte cells and hepatocytes respectively. However studies in colon cancer cells has been inconclusive, either due to lack of mechanistic evaluation or failure to perform these studies in the absence of serum containing medium [15, 23–25]. Understanding the precise mechanism in colon cancer will allow for a better understanding of the tumor biology when bile acid concentrations are elevated in the colon. To resolve this we treated HT-29 cells with DCA alone or in combination with EGF under serum starved conditions in order to eliminate the effect of EGF and other growth factors present in serum. Immunoblotting showed that DCA treatment alone was sufficient to cause activation of ERK1/2, although the level of activation was less than that observed with EGF treatment (Figure 1A). Interestingly, co-treating with DCA and EGF resulted in a more pronounced phosphorylation of ERK1/2 that persisted for several hours (Figure 1B), suggesting that DCA may act through a mechanism distinct from, but complementary to MAPK activation induced by EGF.
Fig. 1. DCA induced activation of EGFR-MAPK signaling.
HT-29 cells were grown to 80% confluence and then serum starved for 18 hours prior to treatments. (A) Cell cultures were either not treated (NT) or treated with 100 ng/ml EGF or with 250 μM DCA alone for the times indicated. (B) HT-29 cells were either not treated (NT) or treated with 100 ng/ml EGF only or in combination with 250 μM DCA for the times indicated. Lysates were prepared from the treated cells and phosphorylated ERK1/2 was detected by immunoblotting of the SDS-PAGE fractionated proteins. β-actin was used as the loading control. Densitometry values were calculated as described in methods. The results for a typical experiment are shown. (C) Acridine Orange and Ethidium Bromide assay was performed to detect levels of apoptosis in HT-29 cells treated with 250 μM DCA or DCA plus 100 ng/ml EGF at times indicated (min.).
DCA has both mitogenic and apoptotic effects on colorectal cells. In fact, these biological features lend to the molecules tumor promoting abilities. Simultaneous stimulation of apoptosis as well as cell growth allows for DCA to exert a selective pressure on colonic epithelium [16, 25]. Consequently, cells that are sensitive to DCA’s apoptotic effects die off and can be replaced with cells that have developed resistance to this pressure while at the same time receiving mitogenic stimulation [26]. In order to insure that the cells we are studying are still viable at the concentration and time of DCA treatment used in figure 1A and 1B, we performed an acridine orange and propidium iodine viability assay, on samples prepared under the same conditions. These results are presented in figure 1C, showing that the cells are clearly viable. HT-29 cells are more resistant to the effects of DCA-induced apoptosis than other human colorectal adenocarcinoma cells, such as HCT116. Furthermore, they can withstand the requirement for serum starvation, mandated when studying a mitogenic signaling pathway, such as EGFR. These results show that the conditions used in this manuscript for DCA treatment are optimal for studying the effects of DCA on mitogenic signaling in colorectal cancer.
3.2 DCA activation of ERK1/2 requires EGFR but does not result in tyrosine 1068 phosphorylation of the receptor
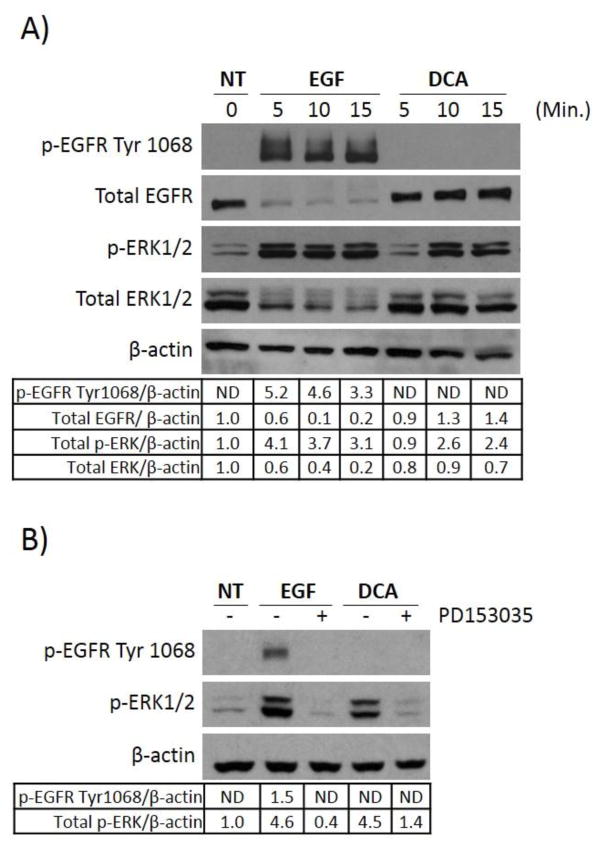
The epidermal growth factor receptor is directly upstream of the MAP kinase signaling cascade. Binding with the EGF ligand leads to dimerization and autophosphorylation at tyrosine 1068 (Tyr1068), resulting in activation of MAPK signaling [27]. Given that DCA treatment induces downstream MAPK activation with increased phosphorylation of ERK1/2, we sought to determine whether DCA’s effects are occurring upstream of the MAPK signaling cascade at the EGFR. Current studies of bile acid signaling in human colorectal adenoma cells have been insufficient in their examination of mechanisms leading to DCA-induced MAPK activation [22]. In particular, the role of EGFR in this process has been inadequately investigated. To examine this directly, HT-29 cells were serum starved and exposed to DCA. The phosphorylation status of EGFR was evaluated at the traditional site Tyr1068 as well as downstream at ERK1/2 phosphorylation. Because previous studies in hepatocytes have shown that ligand-independent activation of EGFR can occur rapidly in response to DCA [16], we examined phosphorylation at early time points of 5, 10, and 15 minutes post DCA treatment. Figure 2A shows that DCA alone could induce ERK1/2 phosphorylation as early as 10 minutes after treatment, however, EGFR did not become phosphorylated at Try1068 as was observed with EGF treatment. This observation could be interpreted in two ways: 1) EGFR is not involved in DCA-induced ERK1/2 activation or 2) DCA results in activation of EGFR in the absence of inducing phosphorylated Try1068.
Fig. 2. DCA activation of ERK1/2 requires EGFR but does not result in phosphorylation of the receptor at Tyr1068.
(A) HT-29 cells were grown as described in Figure 1 and either not treated (NT) or treated with 100ng/ml EGF or 250μM DCA for the times indicated. Extracts were prepared and probed for the presence of phosphorylated EGFR at Tyr1068, total EGFR, phosphorylated ERK1/2, total ERK1/2, or β-actin protein using immunoblotting. (B) HT-29 cells were either not treated (NT), treated with 100 ng/ml EGF for 15 minutes, or with 250 μM DCA for 60 minutes. Parallel cultures were pretreated with 1μM PD153035 for two hours prior to being exposed to either EGF or DCA. Protein extracts were fractionated by SDS-PAGE and probed either for phosphorylated EGFR, phosphorylated ERK1/2, or β-actin. These experiments were done three times. The results for a typical experiment are shown.
To test these possibilities we pretreated HT-29 cells with PD153035, a selective ATP competitive inhibitor of EGFR activation, before exposing to EGF or DCA. Pretreating with the EGFR inhibitor led to a complete blockade of DCA-induced ERK1/2 phosphorylation (Figure 2B), indicating that EGFR is required for downstream activation of ERK1/2 by DCA. In conclusion, DCA-mediated activation of MAPK signaling requires EGFR. However, DCA does not result in phosphorylation of EGFR on Tyr1068. These data support the notion that DCA results in MAPK activation through an alternative mechanism.
Interestingly, when examining total protein levels of EGFR and ERK1/2 in figure 2A we observed that EGF, but not DCA treatment, leads to degradation of these proteins. This is import because it demonstrates that DCA can lead to activation of MAPK signaling in a manner which is not regulated by traditional negative regulator processes present with ligand dependent activation. This further exemplifies the differences between DCA’s activation mechanism and EGF, and suggests that constitutive activation of this pathway may be one manner by which DCA exerts its tumor promoting effects. We therefore further investigated how DCA is capable of leading to unregulated activation of the EGFR-MAPK pathway.
3.3 DCA induces calcium from extracellular stores
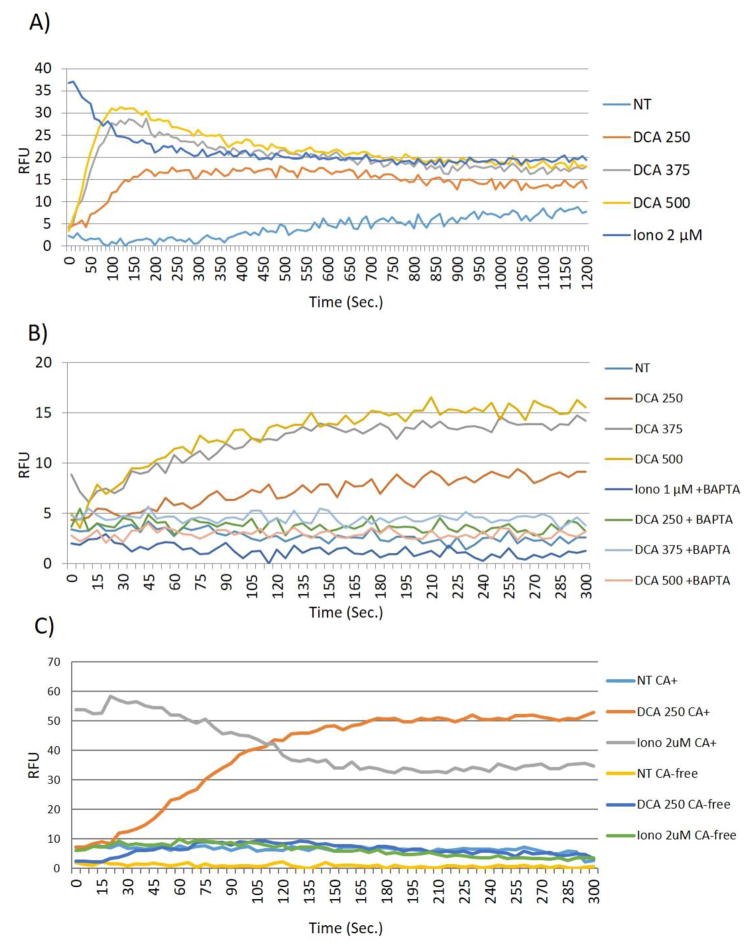
The data above indicated that DCA leads to the activation of EGFR-MAPK signaling, but that the mechanism of EGFR activation by DCA is very different from that observed with EGF treatment. Most notably, EGFR is not phosphorylated at Tyr1068. The EGFR-MAPK pathway interacts with a number of other signaling cascades. Non-canonical activation of this pathway may be achieved by DCA through any number of direct or indirect interactions. One option is the regulation of EGFR through the induction of second messengers. It has been shown that DCA induces calcium in colorectal cancer cells and that the ability of DCA to induce cellular apoptosis is directly mediated through the effects of calcium [28, 29]. However, the effects of calcium-related signaling on other cellular processes in colon cancer cells, namely EGFR-MAPK activation, have not been examined Previous research in vascular smooth muscle cells has demonstrated that the second messenger, calcium, is able to induce EGFR-MAPK activation in a non-traditional fashion [30–32]. Therefore we postulated that DCA-induced calcium may be playing a role in the non-canonical activation of EGFR-MAPK pathway. First we examined the ability of DCA to induce calcium in our system. Not surprisingly we demonstrated that DCA caused increased levels of calcium. Specifically, measurements of calcium levels in the cells showed that DCA induced a rapid and sustained change in calcium levels in a dose dependent manner (Figure 3A), which could be blocked using the cell permeable calcium chelator BAPTA-AM (Figure 3B). To determine the source of DCA-induced calcium we examined the ability of DCA to induce calcium fluctuations in the absence of calcium containing buffer. Figure 3C shows that when calcium is absent DCA is unable to induce a rise in calcium levels in HT-29 cells. These data demonstrate that DCA is utilizing extracellular stores to modify calcium levels in HT-29 cells. Similar data is observed with ionomycin treatment, suggesting that DCA, like ionomycin, has the ability to mobilize calcium across biological membranes (Figure 3C).
Fig. 3. DCA induces Ca2+ influx.
(A) HT-29 cells were grown in 96 well plates, serum starved as described in Figure 1, and then treated with DCA at the concentrations indicated. Thirty reads per well were taken every 10 seconds for 20 minutes. (B) HT-29 cells were preincubated with 20μM BAPTA-AM for 30 minutes prior to treating with DCA. Sixteen readings per well were taken every 5 seconds for 5 minutes total. C) Calcium assay was performed as previously described (methods), Calcium-free conditions were created by utilizing calcium-free assay buffer in place of traditional assay buffer. Treatment with DCA or ionomycin at the concentrations indicated, were carried out in calcium free conditions (CA-free) where indicated or conventional calcium-containing assay buffer (CA+). Kinetic reads were taken every 10 sec for 20 min. Data points show the average of two wells and all points are normalized to the lowest read per plate. HT-29 cells incubated with DCA and ionomysin at the concentrations indicated were used for comparison. These experiments were performed nine times. The results from a typical experiments are depicted.
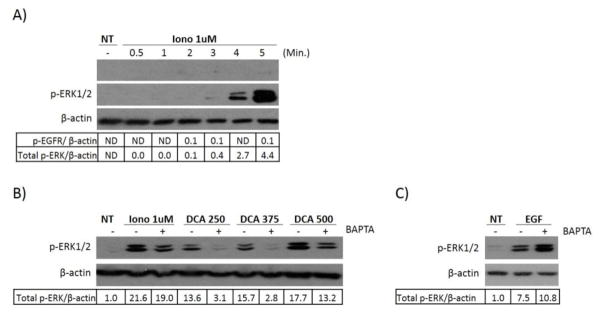
We predicted that DCA-induced calcium was responsible for the induction of EGFR-MAPK activation, so we hypothesized that ionomycin, an ionophore which raises the intracellular levels of calcium, would also be capable of inducing MAPK activation. We therefore treated cells with ionomycin and investigated both ERK1/2 phosphorylation as well as phosphorylation of EGFR at Tyr1068. As expected we saw that ionomycin could indeed lead to phosphorylation of ERK1/2 and that this, similar to DCA treatment, occurred in the absence of EGFR phosphorylation at Tyr1068 (Figure 4A). These data suggested that calcium itself could lead to non-canonical activation of EGFR-MAPK signaling, and therefore may be a key player in understanding DCA’s ability to regulate this pathway. In order to directly evaluate this we tested whether blocking calcium fluctuations by using BAPTA-AM could abrogate the activation of ERK1/2 induced by DCA and ionomycin. We found that pretreatment with BAPTA-AM, which blocked the increase in calcium (Figure 3B), also suppressed ERK1/2 phosphorylation induced by DCA and ionomycin (Figure 4B). Moreover, BAPTA-AM did not suppress EGF-induced ERK1/2 activation (Figure 4C). These data confirm that calcium plays a role in the non-canonical activation of mitogenic signaling induced by DCA and supports the hypothesis that a change in the level of calcium in HT-29 cells is necessary for the induction of EGFR-MAPK activation.
Fig. 4. Ionomycin or DCA-induced ERK1/2 phosphorylation can be blocked by BAPTA-AM.
HT-29 cells were grown and serum starved as described in Figure 1. (A) The cells were then treated with 1 μM ionomycin for the times indicated. (B) HT-29 cells were either pretreated with 20μM BAPTA-AM for 30 minutes or left untreated and then incubated with increasing doses of DCA for 60 minutes, ionomycin for 5 minutes or (C) 100ng/ml EGF for 15 minutes. Results for a typical immunoblot experiment are shown.
3.4 DCA induces activation of downstream signaling targets
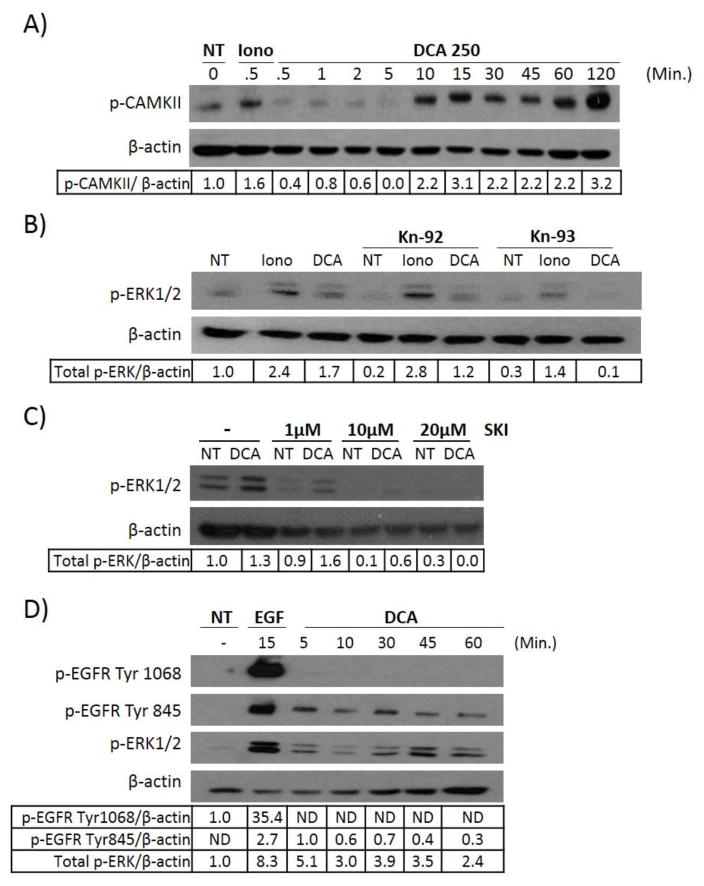
Calcium signaling has been extensively studied and it has been shown that calcium fluctuations can lead to the activation of a molecule known as calmodulin. Calmodulin is a calcium binding protein that propagates calcium signaling by binding calcium ions and facilitating the interactions between calcium and other target proteins [33]. Calmodulin activation can be detected simply by looking at phosphorylation of one of its target proteins Calmodulin-related kinase II (CAMKII), which is directly phosphorylated in response to calmodulin activation. Furthermore, data gathered in vascular endothelial cells has shown that calcium-regulated EGFR-MAPK activation is mediated through CAMKII, followed by the recruitment of the oncoprotein c-Src. We therefore examined the effects of DCA and ionomycin on CAMKII phosphorylation. Figure 5A shows that DCA does indeed lead to phosphorylation of the molecule CAMKII, further demonstrating that the bile acid DCA can increase intracellular calcium levels that activate calcium-related signaling. To test if CAMKII participates in DCA-induced MAPK activation we treated HT-29 cells with the CAMKII inhibitor Kn-93 or the control compound Kn-92. Neither ionomycin nor DCA-induced ERK1/2 phosphorylation was affected by the control compound, but Kn-93 was able to suppress DCA-induced ERK1/2 phosphorylation completely (Figure 5B) demonstrating the necessity of CAMKII for DCA’s regulation of MAPK signaling.
Fig. 5. DCA stimulates ERK1/2 phosphorylation through c-Src.
(A) HT-29 cells were either left untreated or treated with 1 μM ionomycin or 250 μM DCA for the times indicated. For all experiments protein extracts were prepared from the treated cells and probed by immunoblotting for phosphorylated ERK1/2, phosphorylated EGFR, or phosphorylated CAMKII. (B) Serum starved HT-29 cells were treated with 25μM of either Kn-29 (control compound) or Kn-93 (CAMKII inhibitor) for 60 minutes. Treatment with ionomycin (1μM) for 5 minutes or DCA (250μM) for 60 minutes followed. Cell lysates were harvested and levels of p-ERK1/2 were evaluated by western blotting. (C) HT-29 cells were either left untreated (−) or pre-incubated with increasing concentrations of Src Kinase Inhibitor (SKI), followed by no treatment (NT) or 250 μM DCA treatment for 60 minutes. (D) HT-29 cells, grown and prepared as before, were treated with EGF (100ng/ml) or DCA (250 μM) at the times indicated. Extracts prepared from the cells were then probed for phosphorylated EGFR (Tyr1068 or Tyr845), or phosphorylated ERK1/2 using the appropriate antibodies. β-actin was used as a loading control for all experiments A–D. Examples of typical results are shown.
As mentioned above, one of the downstream targets of CAMKII is c-Src, a non-receptor bound tyrosine kinase protein. This oncoprotein has been linked to the progression and development of colon, breast and prostate cancer. In colon cancer c-Src levels are found to be increased 5–8 fold in polyps and increased expression correlates with advanced stage and malignant cancer potential. Most importantly, c-Src activation by CAMKII has been linked to transactivation of EGFR, and subsequent MAPK signaling in vascular smooth muscle cells [30–32]. In this situation c-Src leads to EGFR activation through the phosphorylation of unspecified tyrosine residues [30]. Bile acids have also been shown to induce c-Src activation, through unknown mechanisms, in hepatocytes [34], human cholangiocarcinoma cells [35], and colorectal cells [36, 37]. c-Src has been suggested as one potential mechanism of DCA-induced EGFR-MAPK activation in hepatocytes and gastrointestinal cancers although no data testing this hypothesis directly has been conducted in colorectal cells [35, 38]. Based on the current information we directly evaluated the role of c-Src in DCA-induced EGFR-MAPK activation using the c-Src specific inhibitor (Src kinase inhibitor 1) SKI. SKI is a potent competitive inhibitor of c-Src, competing at both the ATP binding site and the peptide binding site of the kinase. Figure 5C demonstrates that when cells are treated with SKI, DCA-induced phosphorylation of ERK1/2 is lost. These data show that c-Src plays a key role in the ability of DCA to induce EGFR- MAPK activation. Previous research demonstrated that unspecified phosphorylation of EGFR by calcium-induced c-Src mediated EGFR-MAPK activity. It is now known that c-Src is capable of phosphorylating EGFR on four major tyrosine residues: 845, 891, 920, and 1101 [32]. Of these, phosphorylation site tyrosine 845 (Tyr845), and to a lesser extent 1101, have been demonstrated to be important in MAPK activation, while 891 and 920 are associated with PI3K activation [32, 39]. We therefore predicted that DCA treatment would lead to phosphorylation of the c-Src-specific phosphorylation site Tyr845 on EGFR, if c-Src was in fact binding to and causing phosphorylation of the receptor. Figure 5D clearly demonstrates that DCA induces phosphorylation of Tyr845, but not Tyr1068 which further supports the notion that c-Src is a mediator of DCA-induced EGFR modification. .
Taken together our data shows that the bile acid DCA is capable of inducing ligand independent activation of EGFR and subsequent MAPK signaling through a novel mechanism in colon cells that is dependent on calcium, CAMKII, and c-Src. Moreover, we have elucidated a novel non-canonical activation pathway of EGFR-MAPK signaling regulated by calcium fluctuations, which has previously not been described in colorectal adenocarcinoma cells.
4. Discussion
In these studies we have demonstrated that treatment of human colon cancer cells with 250μM DCA, similar to levels induced by a western diet, results in the calcium dependent activation of p-CAMKII, c-Src and phosphorylation of EGFR at the c-Src dependent phosphorylation site Tyr845. This activation mediated the downstream activity of MAPK and subsequent ERK1/2 activation. Furthermore, we showed that EGF and DCA together lead to a strong and prolonged MAPK activation compared to either treatment alone, demonstrating that both the traditional and non-canonical signaling can occur simultaneously. These data elucidate the previously unidentified mechanism by which DCA leads to activation of the pro-tumorigenic EGFR-MAPK pathway in colon cancer, and clearly demonstrates DCA’s diversity as an activator of this pathway.
The levels of fecal bile acids in healthy individuals range from 50–150μM [40, 41] with increased levels of DCA observed in patients diagnosed with colorectal cancers or adenomas [42]. Moreover, individuals who consume a high fat diet can often present with levels of DCA ranging from 200–300μM in their fecal water [43–46]. Levels within this range are associated with an increased risk of colorectal adenoma development and colorectal cancer [2–4]. Colon cancer cells treated with DCA at levels found within this range are sufficient to induce strong activation of EGFR-MAPK signaling, [15, 24, 25, 47]. Individuals on a vegetarian diet have lower levels of DCA in their fecal water and are also at a lower risk for developing colon cancer [48]. Consistent with this, DCA at less than 100μM does not induce apoptosis [49] or activation of mitogenic signaling [24]. Hence, the levels of bile acids used in our studies, which show stimulation of mitogenic signaling, are associated with increased tumorigenesis and may promote tumor development in humans.
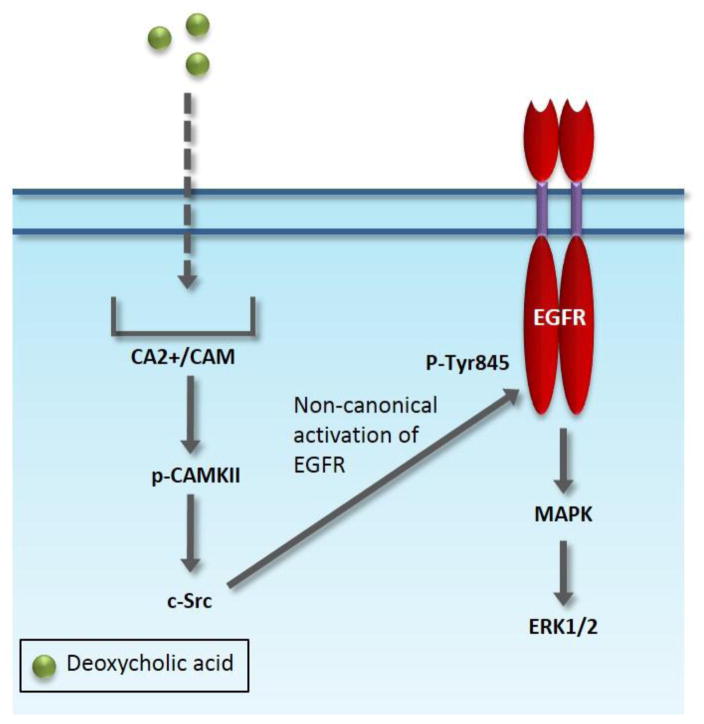
We also determined during our characterization of DCA mediated signaling that its activation of EGFR did not trigger degradation of the receptor as is observed when EGFR is activated by EGF. All of our studies were performed in serum-free conditions which allowed us to tease out what effects were due solely to DCA treatment as opposed to the contamination of other growth factors. We are the first to clearly show that DCA can activate EGFR-MAPK in a ligand independent manner as well as synergize with the natural ligand EGFR. These data clearly address the ambiguity in the field and show that DCA can modulate ERK1/2 activity in both ligand dependent and ligand independent manner. Our demonstration that DCA can stimulate activity of EGFR that is not accompanied by degradation supports the notion that DCA can promote hyper-stimulation of EGFR-mediated signaling and that this may be important for this bile acid’s tumor promoting properties. Finally, because we have determined that DCA may be activating cellular signaling through calcium mobilization it is plausible that calcium inhibitors may help ameliorate DCA’s tumor promoting effects in patients with increased DCA levels. Figure 6 summarizes our model of DCA-induced MAPK activation mediated through calcium. Under these circumstances we have demonstrated that calcium fluctuations lead to the activation of calcium-related signaling molecule CAMKII and subsequent c-Src activation, resulting in alternative activation of EGFR at Tyr845. This signaling cascaded results in activation of MAPK signaling. These findings suggest one possible explanation for the clinically observed link between high levels of DCA and increased adenoma development and imply that targeted therapies against molecules within the non-canonical activation pathway of EGFR regulation may reduce the risk of colon cancer in patients with increased levels of DCA. Moreover, therapies which solely target the ligand binding domain of EGFR, such as Cetuximab, used to treat stage IV colorectal cancer, would not be sufficient if this alternative pathway is intact. Our research demonstrated that calcium has the previously unappreciated function of regulating EGFR activity in colon cancer. These unexpected results warrant further evaluation of the alternative activation pathway of EGFR in cancer patients, particularly because it seems to bypass traditional negative regulatory processes.
Fig. 6.
Non-canonical signaling activation of EGFR-MAPK
Highlights.
Non-canonical activation of EGFR-MAPK signaling by deoxycholic acid is elucidated.
The mechanism is dependent on calcium influx.
Activation of EGFR in this manner allows for it to escape degradation.
Acknowledgments
Funding: National Cancer Institute (R01 CA129688 to J.M., R01 CA129688-03S1 to SMC)
Special thanks to Derrick Broka and the Camenisch and Schroeder labs for their time and assistance with the data collection, use of reagents, and equipment.
Footnotes
Conflict of Interest: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–31. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World journal of gastroenterology. 2009;15:3329–40. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imray CH, Radley S, Davis A, Barker G, Hendrickse CW, Donovan IA, et al. Faecal unconjugated bile acids in patients with colorectal cancer or polyps. Gut. 1992;33:1239–45. doi: 10.1136/gut.33.9.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayerdorffer E, Mannes GA, Richter WO, Ochsenkuhn T, Wiebecke B, Kopcke W, et al. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology. 1993;104:145–51. doi: 10.1016/0016-5085(93)90846-5. [DOI] [PubMed] [Google Scholar]
- 5.Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. Journal of the National Cancer Institute. 1974;53:1093–7. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- 6.Reddy BS, Narasawa T, Weisburger JH, Wynder EL. Promoting Effect of Sodium Deoxycholate on Colon Adenocarcinomas in Germfree Rats. 1976:441–2. doi: 10.1093/jnci/56.2.441. [DOI] [PubMed] [Google Scholar]
- 7.Reddy BS, Watanabe K, Weisburger JH, Wynder EL. Promoting Effect of Bile Acids in Colon Carcinogenesis in Germ-free and Conventional F344 Rats. 1977:3238–42. [PubMed] [Google Scholar]
- 8.Deschner EE, Cohen BI, Raicht RF. Acute and chronic effect of dietary cholic acid on colonic epithelial cell proliferation. Digestion. 1981;21:290–6. doi: 10.1159/000198579. [DOI] [PubMed] [Google Scholar]
- 9.Bartram HP, Scheppach W, Schmid H, Hofmann A, Dusel G, Richter F, et al. Proliferation of human colonic mucosa as an intermediate biomarker of carcinogenesis: effects of butyrate, deoxycholate, calcium, ammonia, and pH. Cancer research. 1993;53:3283–8. [PubMed] [Google Scholar]
- 10.Vlahcevic ZR, Heuman DM, Hylemon PB. Regulation of bile acid synthesis. Hepatology (Baltimore, Md. 1991;13:590–600. [PubMed] [Google Scholar]
- 11.Stravitz RT, Vlahcevic ZR, Gurley EC, Hylemon PB. Repression of cholesterol 7 alpha-hydroxylase transcription by bile acids is mediated through protein kinase C in primary cultures of rat hepatocytes. Journal of lipid research. 1995;36:1359–69. [PubMed] [Google Scholar]
- 12.Ramirez MI, Karaoglu D, Haro D, Barillas C, Bashirzadeh R, Gil G. Cholesterol and bile acids regulate cholesterol 7 alpha-hydroxylase expression at the transcriptional level in culture and in transgenic mice. Molecular and cellular biology. 1994;14:2809–21. doi: 10.1128/mcb.14.4.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. Journal of lipid research. 2009;50:1509–20. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao YP, Studer EJ, Stravitz RT, Gupta S, Qiao L, Dent P, et al. Activation of the Raf-1/MEK/ERK cascade by bile acids occurs via the epidermal growth factor receptor in primary rat hepatocytes. Hepatology (Baltimore, Md. 2002;35:307–14. doi: 10.1053/jhep.2002.31104. [DOI] [PubMed] [Google Scholar]
- 15.Im E, Martinez JD. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J Nutr. 2004;134:483–6. doi: 10.1093/jn/134.2.483. [DOI] [PubMed] [Google Scholar]
- 16.Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, et al. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Molecular biology of the cell. 2001;12:2629–45. doi: 10.1091/mbc.12.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werneburg NW, Yoon JH, Higuchi H, Gores GJ. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. American journal of physiology. 2003;285:G31–6. doi: 10.1152/ajpgi.00536.2002. [DOI] [PubMed] [Google Scholar]
- 18.Akare S, Martinez JD. Bile acid induces hydrophobicity-dependent membrane alterations. Biochim Biophys Acta. 2005;1735:59–67. doi: 10.1016/j.bbalip.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Rao YP, Stravitz RT, Vlahcevic ZR, Gurley EC, Sando JJ, Hylemon PB. Activation of protein kinase C alpha and delta by bile acids: correlation with bile acid structure and diacylglycerol formation. Journal of lipid research. 1997;38:2446–54. [PubMed] [Google Scholar]
- 20.Looby E, Long A, Kelleher D, Volkov Y. Bile acid deoxycholate induces differential subcellular localisation of the PKC isoenzymes beta 1, epsilon and delta in colonic epithelial cells in a sodium butyrate insensitive manner. Int J Cancer. 2005;114:887–95. doi: 10.1002/ijc.20803. [DOI] [PubMed] [Google Scholar]
- 21.Jean-Louis S, Akare S, Ali MA, Mash EA, Jr, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. The Journal of biological chemistry. 2006;281:14948–60. doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- 22.Centuori SM, Martinez JD. Differential Regulation of EGFR-MAPK Signaling by Deoxycholic Acid (DCA) and Ursodeoxycholic Acid (UDCA) in Colon Cancer. Digestive diseases and sciences. doi: 10.1007/s10620-014-3190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Crawley S, Hokari R, Kwon S, Kim YS. Bile acid regulates MUC2 transcription in colon cancer cells via positive EGFR/PKC/Ras/ERK/CREB, PI3K/Akt/IkappaB/NF-kappaB and p38/MSK1/CREB pathways and negative JNK/c-Jun/AP-1 pathway. Int J Oncol. 36:941–53. doi: 10.3892/ijo_00000573. [DOI] [PubMed] [Google Scholar]
- 24.Qiao D, Chen W, Stratagoules ED, Martinez JD. Bile acid-induced activation of activator protein-1 requires both extracellular signal-regulated kinase and protein kinase C signaling. The Journal of biological chemistry. 2000;275:15090–8. doi: 10.1074/jbc.M908890199. [DOI] [PubMed] [Google Scholar]
- 25.Qiao D, Stratagouleas ED, Martinez JD. Activation and role of mitogen-activated protein kinases in deoxycholic acid-induced apoptosis. Carcinogenesis. 2001;22:35–41. doi: 10.1093/carcin/22.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Powell AA, Akare S, Qi W, Herzer P, Jean-Louis S, Feldman RA, et al. Resistance to ursodeoxycholic acid-induced growth arrest can also result in resistance to deoxycholic acid-induced apoptosis and increased tumorgenicity. BMC Cancer. 2006;6:219. doi: 10.1186/1471-2407-6-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984;311:483–5. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- 28.Gerbino A, Ranieri M, Lupo S, Caroppo R, Debellis L, Maiellaro I, et al. Ca2+-dependent K+ efflux regulates deoxycholate-induced apoptosis of BHK-21 and Caco-2 cells. Gastroenterology. 2009;137:955–64. 64 e1–2. doi: 10.1053/j.gastro.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti MC, Migliorati G, Moraca R, Riccardi C, Nicoletti I, Fabiani R, et al. Possible mechanisms involved in apoptosis of colon tumor cell lines induced by deoxycholic acid, short-chain fatty acids, and their mixtures. Nutr Cancer. 1997;28:74–80. doi: 10.1080/01635589709514555. [DOI] [PubMed] [Google Scholar]
- 30.Ginnan R, Singer HA. CaM kinase II-dependent activation of tyrosine kinases and ERK1/2 in vascular smooth muscle. American journal of physiology Cell physiology. 2002;282:C754–61. doi: 10.1152/ajpcell.00335.2001. [DOI] [PubMed] [Google Scholar]
- 31.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. The Journal of biological chemistry. 1998;273:8890–6. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 32.Stover DR, Becker M, Liebetanz J, Lydon NB. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. The Journal of biological chemistry. 1995;270:15591–7. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 33.Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends in neurosciences. 1994;17:406–12. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 34.Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Molecular endocrinology. 2010;24:1151–64. doi: 10.1210/me.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon JH, Werneburg NW, Higuchi H, Canbay AE, Kaufmann SH, Akgul C, et al. Bile acids inhibit Mcl-1 protein turnover via an epidermal growth factor receptor/Raf-1-dependent mechanism. Cancer research. 2002;62:6500–5. [PubMed] [Google Scholar]
- 36.Debruyne PR, Bruyneel EA, Karaguni IM, Li X, Flatau G, Muller O, et al. Bile acids stimulate invasion and haptotaxis in human colorectal cancer cells through activation of multiple oncogenic signaling pathways. Oncogene. 2002;21:6740–50. doi: 10.1038/sj.onc.1205729. [DOI] [PubMed] [Google Scholar]
- 37.Khare S, Holgren C, Samarel AM. Deoxycholic acid differentially regulates focal adhesion kinase phosphorylation: role of tyrosine phosphatase ShP2. American journal of physiology. 2006;291:G1100–12. doi: 10.1152/ajpgi.00008.2006. [DOI] [PubMed] [Google Scholar]
- 38.Debruyne PR, Bruyneel EA, Li X, Zimber A, Gespach C, Mareel MM. The role of bile acids in carcinogenesis. Mutation research. 2001;480–481:359–69. doi: 10.1016/s0027-5107(01)00195-6. [DOI] [PubMed] [Google Scholar]
- 39.Sato K. Cellular functions regulated by phosphorylation of EGFR on Tyr845. International journal of molecular sciences. 2013;14:10761–90. doi: 10.3390/ijms140610761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govers MJ, Termont DS, Lapre JA, Kleibeuker JH, Vonk RJ, Van der Meer R. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer research. 1996;56:3270–5. [PubMed] [Google Scholar]
- 41.Ditscheid B, Keller S, Jahreis G. Faecal steroid excretion in humans is affected by calcium supplementation and shows gender-specific differences. European journal of nutrition. 2009;48:22–30. doi: 10.1007/s00394-008-0755-2. [DOI] [PubMed] [Google Scholar]
- 42.Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977;39:2533–9. doi: 10.1002/1097-0142(197706)39:6<2533::aid-cncr2820390634>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 43.Reddy BS. Diet and excretion of bile acids. Cancer research. 1981;41:3766–8. [PubMed] [Google Scholar]
- 44.Ou J, DeLany JP, Zhang M, Sharma S, O'Keefe SJ. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr Cancer. 64:34–40. doi: 10.1080/01635581.2012.630164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon SJ, Miller LJ, Haeffner LJ, Kinsey MD, Kowlessar OD. Abnormal intestinal bile acid distribution in azotaemic man: a possible role in the pathogenesis of uraemic diarrhoea. Gut. 1976;17:58–67. doi: 10.1136/gut.17.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. Journal of lipid research. 2006;47:241–59. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Qiao D, Gaitonde SV, Qi W, Martinez JD. Deoxycholic acid suppresses p53 by stimulating proteasome-mediated p53 protein degradation. Carcinogenesis. 2001;22:957–64. doi: 10.1093/carcin/22.6.957. [DOI] [PubMed] [Google Scholar]
- 48.van Faassen A, Hazen MJ, van den Brandt PA, van den Bogaard AE, Hermus RJ, Janknegt RA. Bile acids and pH values in total feces and in fecal water from habitually omnivorous and vegetarian subjects. The American journal of clinical nutrition. 1993;58:917–22. doi: 10.1093/ajcn/58.6.917. [DOI] [PubMed] [Google Scholar]
- 49.Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, et al. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998;31:111–8. doi: 10.1080/01635589809514689. [DOI] [PubMed] [Google Scholar]