Abstract
In the field of biomedical optics, optical scattering has traditionally limited the range of imaging within tissue to a depth of one millimetre. A recently developed class of wavefront-shaping techniques now aims to overcome this limit and achieve diffraction-limited control of light beyond one centimetre. By manipulating the spatial profile of an optical field before it enters a scattering medium, it is possible to create a micrometre-scale focal spot deep within tissue. To successfully operate in vivo, these wavefront-shaping techniques typically require feedback from within the biological sample. This Review summarizes recently developed ‘guidestar’ mechanisms that provide feedback for intra-tissue focusing. Potential applications of guidestar-assisted focusing include optogenetic control over neurons, targeted photodynamic therapy and deep tissue imaging.
Most vertebrate organisms, including humans, are optically turbid. Our cellular structures generally exhibit a heterogeneous refractive index. A spatially varying refractive index causes light to scatter, which prevents us from seeing beneath the first few layers of biological material, or from focusing visible radiation deep within biological tissue.
Wavelengths outside the optical spectrum, which scatter less through organic material, are widely available for probing deeper within biological organisms. For example, X-rays offer excellent resolution in computed tomography, and magnetic resonance imaging remains an invaluable tool for medical diagnosis. However, these well-known techniques offer a limited ability to control and manipulate cells or molecules. In contrast, a large suite of recently developed optical methods can now both probe and activate biochemical content using visible light. Example applications include the photochemical activation of drugs1, the photorelease of biomolecules2, stimulation of neural activity through optogenetic tags3 and imaging with fluorescent markers4. Similar insights may also help form images at unprecedented resolution5. However, optical scattering still restricts many of these advances to the outermost layers of tissue.
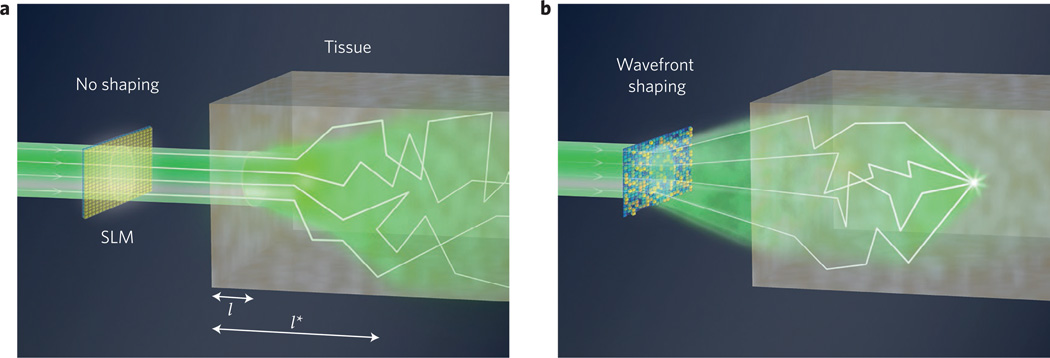
When a beam of light enters skin, its photons bounce around so many times that after a certain distance (the transport mean free path (TMFP), l*), they face an equal probability of moving in any direction. This diffusion of an incident beam of light into a general ‘glow’ happens quite quickly in most biological tissue, typically at a depth6 of l* ≈ 1 mm. Until recently, most optical techniques for imaging deep within tissue would primarily utilize only unscattered (that is, ballistic) photons (for example, confocal imaging, two-photon microscopy7 and optical coherence tomography8). Because the number of ballistic photons decays exponentially with depth, the TMFP has long persisted as a supposedly impassable barrier for diffraction-limited imaging9.
Recently, researchers demonstrated that photons retain a significant amount of information after scattering many times through disordered material10, such as biological tissue11. Instead of throwing these seemingly randomized photons away, a host of new technologies now capture and extract information from multiply scattered light. By measuring the spatial phase profile of an optical field exiting tissue, one can computationally recover an ideal wavefront shape that, when sent back into tissue, will focus to a micrometre-scale spot. This optimization process closely relates to the principle of time reversal, which suggests that a beam of light may be guided backwards through a disordered medium to effectively ‘cancel’ its scattering effects.
Actively correcting random optical wavefront distortions is a common practice in astronomy12. By measuring how the atmosphere perturbs light from a ‘guidestar’ (that is, an approximate point source of light), adaptive optics (AO) systems adjust and sharpen images within most ground-based telescopes. The ‘wavefront-shaping’ technologies covered by this Review share a common foundation with the principle of AO, but extend operation into the biophotonic setting, where heavy optical scattering dominates. Correspondingly, instead of removing low-order aberrations to image ‘across’ distortion clearly, wavefront shaping aims for a generally more useful goal in the field of biology: to send light ‘into’ tissue, thereby forming a tight focus within.
Recent work has adopted one of two guidestar-based strategies. In this Review, we classify these two strategies as using either a ‘feedback’ or ‘conjugation’ guidestar, and detail each in separate sections. Outside of the biological setting, an experimentalist can typically manipulate light on both sides of a scattering material, and thus does not usually need the assistance of a guidestar. Mosk et al. offer a thorough Review of wavefront shaping through non-biological disordered media13. This Review will focus on techniques that hold promise for controlling light inside in vivo tissue, where access is primarily limited to one side of the scattering material. Example guidestars for biological application include fluorescence markers14,15, nonlinear optical particles16, photo-acoustic feedback17–20, ultrasound-enabled focusing21–23 and kinematic targets24,25. Before discussing details about each wavefront-shaping strategy, we first present a simple mathematical model to explain how to account for optical scattering using a properly designed wavefront.
The propagation of light within tissue
First, it is helpful to break down the complex journey of a single photon through tissue into a discrete number of scattering events. Following this short discussion, we will replace our particle viewpoint with a wave model, which allows us to describe wavefront shaping more easily. We may characterize a homogeneous scattering medium by its scattering mean free path l, which specifies the average distance travelled by a photon between two scattering events. A typical value for l in tissue is 100 µm (ref. 6). When imaging tissue samples thicker than l, conventional microscopes will also capture scattered photons, which deteriorate image quality. Techniques like confocal and multiphoton excitation microscopy filter out scattered photons, thereby allowing imaging beyond one scattering mean free path.
Although these techniques have transformed biomedical imaging over several decades, confocal and multiphoton microscopes still face challenges when operating beneath the tissue TMFP depth, l* = l/(1 − g) ≈ 1 mm (refs 9,26). The distance l* depends on both the mean free path l and an anisotropy parameter, g ≈ 0.8–0.98, which takes into account the forward-scattering nature of tissue (Fig. 1a). The TMFP depth matches previously derived penetration upper limits for imaging methods that rely on ballistic photons6,7,9. Although several recent techniques have improved ballistic photon collection using spatial field correlations27 or combined spatial and coherence gating28, many elastically scattered photons remain significantly beyond one millimetre. As the effects of tissue absorption are minimal until centimetre-scale depths29, an ideal technique would account for, as opposed to block, multiply scattered photons.
Figure 1. Principle of wavefront shaping.
a, An unmodified coherent beam of light travels one mean free path (l) with minimal scattering into tissue. A fraction of beam directionality is preserved up to the transport mean free path length, l*. b, By wavefront-shaping the incident field with an SLM, it is possible to focus within tissue beyond l*.
Accurate models of highly scattered light in this deep, diffusive regime exist. Diffuse optical tomography30,31 and fluorescence molecular tomography32 can computationally reconstruct limited-resolution images of macroscopic structure well beyond several TMFP. However, their resolution deteriorates with imaging depth6. Furthermore, such techniques do not take into account the effects of interference, which is the hallmark of coherent light. As we will see, correlations within a complex optical field preserve a relationship between all possible paths that light can follow while scattering. By mapping out the relative optical phase difference between each path, it is possible to control coherent light, deep within tissue, at the diffraction limit.
It is now convenient to treat light as a wave, which, for simplicity, we assume is coherent. A monochromatic optical wave field, u(r), propagates through a linear scattering material with a variable index of refraction, n(r), following the scalar wave equation ∇2u(r) + k2n2(r) u(r) = 0. Here, k = 2π/λ denotes the wavevector and λ is the optical wavelength. Often, one has discrete control over N spatial degrees of freedom of an optical ‘input’ field, ua, before it enters a scattering sample, typically using a spatial light modulator (SLM, Fig. 1). In this scenario, it is useful to define the input field as a row vector ua that contains N complex entries. A limited numerical aperture, or the diffraction limit, allows unambiguous discretization of ua into a vector using the Nyquist–Shannon sampling theorem.
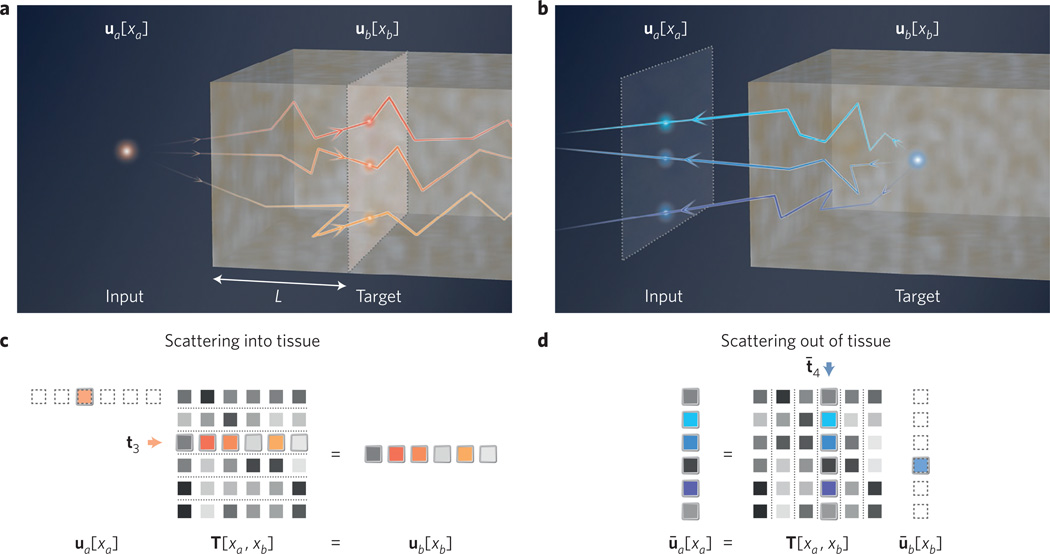
We now consider an arbitrary ‘target’ plane of interest at a depth L within a scattering sample (Fig. 2a), where we hope to focus light. We may spatially discretize this target plane into M entries with a spacing of λ/2n. In many wavefront-shaping experiments, this plane is external to the scatterer and contains an M-pixel detector. Although in practice both input and target fields typically exist along two spatial dimensions, for simplicity we will primarily consider each field along one dimension. Once discretized, the forward-propagating input field vector ua now connects to the optical field at the target plane ub through the matrix equation uaT = ub. Here, we define scattering between the input and target planes, caused by an inhomogeneous refractive index in the wave equation, using a transformation matrix T with N × M complex entries. If light at our embedded target plane is primarily forward-travelling, as in the case of highly anisotropic scattering, then T is the well-known transmission matrix33. Deep within tissue, where scattering becomes isotropic, the total target field includes contributions from all scatterer regions. However, a linear relationship will still connect the total field at both planes. Although not included here for simplicity, any reflected light may also be modelled within the 2N × 2M ‘scattering’ matrix34,35. Finally, for graphical clarity we adopt the convention of multiplying T from the left, with a row vector, to denote light propagating into tissue (Fig. 2a). We will use column vectors to denote light propagation in the opposite direction (Fig. 2b).
Figure 2. Matrix model of scattering in tissue.
a, Forward optical scattering into tissue (distance L, input to target plane). b, Reverse optical scattering out of tissue (target to input plane). c, Transmission matrix model for forward scattering. A discrete input point source at position 3 sets ua[xa] = δ3, the third unit vector. The target field is then t3, the third transmission matrix row. d, Matrix model for scattering from an embedded guidestar point, which sets ūb[xb] = δ̄4 (column vector). Assuming time-reversal symmetry, the input plane field becomes t̄4, the fourth transmission matrix column.
If a point source sequentially shifts across the input plane, then scattering will cause the target optical field to fluctuate. We mathematically express an input field containing a point source in its ith discrete location as ua = δi, where we define δi as the ith unit vector. For any discrete input location xa, this particular input field satisfies ua[xa = i] = 1 and ua[xa ≠ i] = 0. The ith resulting target field is the matrix product δiT = ti, where ti denotes the ith transmission matrix row. Scattering almost always results in a random field with a grainy appearance, called speckle. If the medium is sufficiently thick and turbid, then the embedded speckle field’s spatial variations approach a correlation distance of λ/2n (that is, the speckle becomes ‘fully developed’36). In this limit, each discrete element of ti approaches a complex random variable with a circularly symmetric Gaussian distribution, which is not correlated with its neighbours. If the medium is also thick enough to satisfy L ≫ l, then light is in the multiple-scattering regime37. Here, a small change in the input wavefront (for example, a shift to input unit vector δi+1) will produce a nearly uncorrelated, yet still fully developed, target speckle field. At such depths, it thus becomes fair to approximate each element of the transmission matrix as an uncorrelated random variable.
Remaining sources of correlation within T, caused for example by energy conservation and a finite thickness, are of both practical application and ongoing theoretical interest38. One form of correlation, termed the ‘memory effect’37, helps computational imaging techniques recover objects hidden behind thin, scattering layers39,40 such as tissue41. However, these methods require a finite separation between scatterer and object, and thus cannot directly focus or image to a plane embedded within tissue. The high anisotropy of tissue offers a second useful form of correlation that allows scanning across an embedded plane over a limited range42.
Wavefront shaping using feedback guidestars
The transmission matrix model informs us of the amplitude and phase of light at an embedded target plane, given an arbitrary input field. If the complex matrix T is known, then we can also invert a scattering system to create any desired target field using a specifically designed input field (for example, to focus within tissue). We may express this input field solution mathematically by computing a matrix inverse, ua = ubT−1 (or a pseudo-inverse for rectangular T). If T is unitary, then we can equivalently apply a conjugate transpose, ua = ubT*. Although this is typically not an equivalent operation, conjugation closely approximates inversion when the scattering and detection system has minimal loss43. In practice, combining both matrix inversion and conjugation into one operator helps overcome experimental noise44. Given an SLM to define the input field, we may apply the following three steps to overcome optical scattering. First, scan through N different orthogonal input fields on the SLM and measure each resulting complex target field. For example, if each input field is the ith unit vector δi, then each target field is the ith matrix row ti. Interferometry is typically required to measure the complex target field. Next, construct the transmission matrix T from the N measured target fields (that is, place each ti into the ith row of T). Finally, solve the above inverse matrix equation to create any desired target field ub, by wavefront shaping with the SLM.
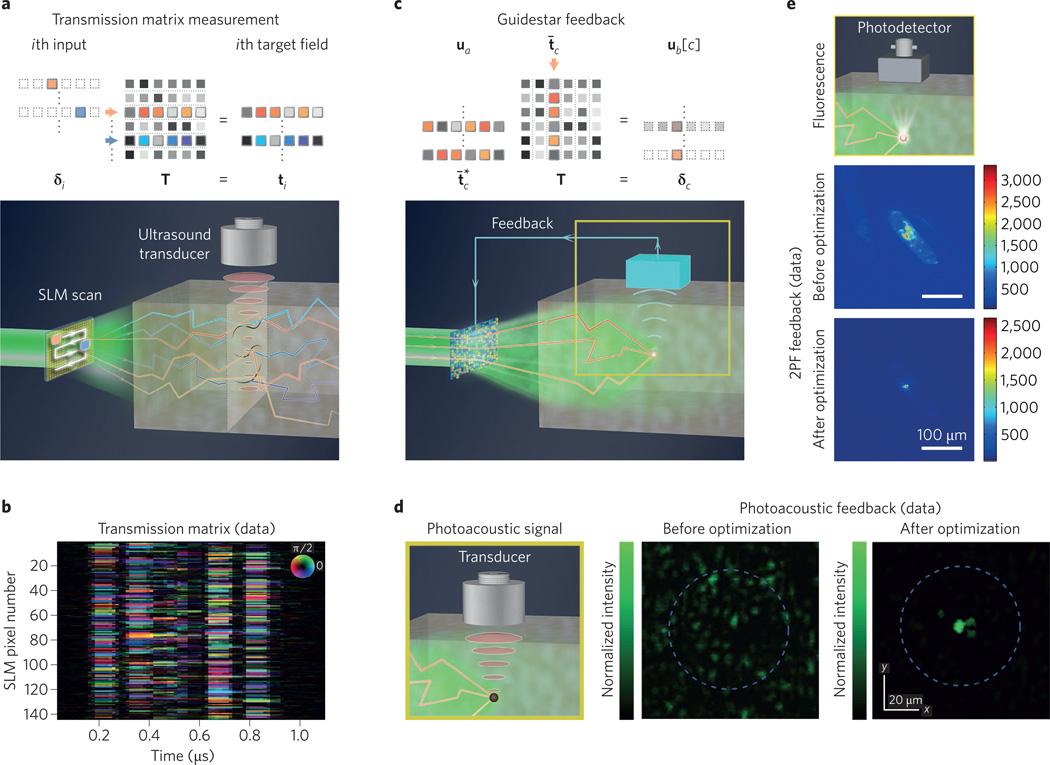
Transmission matrix measurement and inversion can deliver arbitrary images through disordered materials33,44–46. It is also possible to measure the scattering response of reflected light in a similar fashion47. Although this is an elegant way to overcome optical scattering, measuring each target speckle field ti from within tissue is practically challenging. One experimental technique uses the photo-acoustic effect17 (Fig. 3a). Here, light at an embedded plane induces an ultrasound signal through localized thermal expansion48. This time-varying ultrasound response, detected with an external transducer, offers an indirect estimate of local light intensity along one scatterer dimension. Linearly translating the detector system, while scanning through SLM patterns at each unique location, builds up a full transmission matrix (Fig. 3b). If the experimental goal is to form a single focus, however, it is not necessary to measure the entire transmission matrix.
Figure 3. Feedback guidestars.
a, Measuring the optical transmission matrix. Rows of T are sampled by scanning one transparent pixel across an input SLM and detecting each target field. From within tissue, an external transducer obtains indirect measurements via the photo-acoustic effect. b, Compiled together, these photo-acoustic measurements form an optical transmission matrix17. c, Feedback guidestar matrix model, with measurements from one discrete location within tissue, ub[3]. d, Photo-acoustic feedback measured via an ultrasound transducer optimizes light delivery to a tight focus20. e, Fluorescence feedback, such as two-photon fluorescence (2PF). After optimization, 2PF feedback focuses light through L = 1 mm of brain tissue56. Figure reproduced with permission from: e, ref. 56, OSA.
If we are just concerned with the value of the target field ub[c] at one fixed target location xb = c within tissue, we do not need to multiply our input field with the entire transmission matrix. Instead, we must only consider the cth column of the transmission matrix and compute an inner product. We use a bar to distinguish this cth column as the column vector t̄c. We may determine the scalar target field formed at location c by any input field vector ua with the inner product, uat̄c = ub[c] (Fig. 3c). Here, we see that only the N values within t̄c are needed to map fully the scattering relationship between the input plane and the field at the cth embedded target location. Just like measuring and conjugating the transmission matrix to undo scattering across the entire target plane, measuring and computing the conjugate transpose of t̄c enables focusing at the target point xb = c. This is clear if we set in the above inner product, such that , which is the maximum possible normalized amplitude at target location c.
Matching our procedure to measure T, one may determine t̄c by scanning through orthogonal SLM patterns10,49. Now, however, only a single pixel detector records the target field. Or, instead of cycling through orthogonal SLM patterns, feedback between the single detector and the SLM (for example, with a genetic or hill-climbing algorithm) encourages speedy maximization of delivered light intensity35,50. With either approach, the ideal SLM-shaped input field remains the conjugated transmission matrix column vector51 , and the focal spot brightness increases linearly with the number of conjugated optical modes10.
For most biological experiments, it is not possible to embed a physical detector (for example, a photodiode) into tissue to measure t̄c. Similar to indirectly measuring the transmission matrix, one may instead use a signal that is correlated with localized light intensity to provide SLM feedback52. We refer to this mechanism as a ‘feedback guidestar’, of which there are several varieties (Fig. 3c–e). One of the first demonstrations of overcoming scattering with a feedback guidestar used a fluorescent bead embedded within a disordered material. A feedback algorithm that connected the fluorescence intensity (detected outside the material) to an input-shaping SLM experimentally enhanced the fluorescent excitation by a factor of 20 or more14,53.
A fluorescent guidestar often requires invasive positioning and fixes light delivery to a single location. A moveable ultrasound focal spot, which frequency-shifts light54 through refractive index modulation and scatterer displacement, offers a non-invasive feedback alternative55. Because ultrasound scatters weakly in tissue, it provides a useful mechanism for extracting optical information from an embedded target plane. Alternatively, instead of sending in ultrasound and detecting modulated light, an externally measured photo-acoustic signal also indicates the local strength of internal light, as mentioned earlier. Standard photo-acoustic ‘guidestar’ feedback originates from a single spot, and is limited to the acoustic resolution18,19. Nonlinear photo-acoustic feedback20 helps to focus near the optical resolution limit (5–7 µm, Fig. 3d), although not yet within thick biological tissue.
It is also possible to create guidestar-like feedback by monitoring the two-photon fluorescence signal generated directly inside a scattering sample56,57 (Fig. 3e). Or, one may track the coherent interference of backscattered light from different sample layers58,59. These two examples achieve feedback with optical mechanisms that are also shared by other biomedical imaging modalities: two-photon excitation microscopy and optical coherence tomography (OCT), respectively. Next, we briefly detail how AO techniques, which are also used to improve two-photon excitation imaging and OCT performance, apply to wavefront shaping with feedback guidestars.
As explained previously, AO is the imaging complement of wavefront shaping: the former passively removes aberrations after light has propagated from an object of interest, whereas the latter actively shapes light so that it can pass through an aberrative medium (for example, to form a focus). In early applications, AO applied similar feedback algorithms to measure and correct image distortions caused by the atmosphere12 or the eye’s lens60. Later, AO microscopes applied fluorescence feedback to remove low-order aberrations from ballistic light61. As with wavefront shaping, AO does not necessarily require a specific physical feedback guidestar. Systems may also apply feedback from two-photon62 or fluorescent63 image intensities. In addition to removing image aberrations, AO microscopes also commonly correct for distortions within their illumination path64. This closely matches the goal of wavefront shaping. Furthermore, recent AO microscopes now also correct for complex image distortions caused by non-ballistic light57,65–67.
Although conceptually similar, three key differences help distinguish guidestar-based wavefront shaping from AO techniques. First, many AO set-ups focus light into a guidestar from the input side (for example, laser guidestars in astronomy and ophthalmology). Significant scattering precludes the ability to focus light directly into a guidestar within tissue. This gives rise to our list of inventive feedback mechanisms (Table 1). Second, a wavefront correction map in AO can remove aberrations over a finite area known as the isoplanatic patch68. The isoplanatic patch beneath 1 TMFP in tissue is limited to several square micrometres or less. This limits the relevance of one optimal input wavefront to a specific target location. Finally, the number of parameters (and hence measurements) required to fit an AO aberration map is typically small. An AO transmission matrix contains of the order of N ≈ 102 modes. In contrast, deep-tissue guidestars require many more measurements to map accurately the N ≈ 105 or more independent optical modes in the diffusive regime13.
Table 1.
List of biological tissue guidestars for feedback and conjugation.
| Guidestar mechanism | Ref. | Minimum spot size |
Non-invasive? | Translate position? |
PBR | Required time (s) |
Coupling efficiency |
|
|---|---|---|---|---|---|---|---|---|
| Conjugation guidestar |
Fluorescence | 14, 53, 114 | 1 µm | No | No | 20–40 | 103 | Low |
| Ultrasound | 55 | 1 mm | Yes | Yes | 10 | 103–104 | Low | |
| Photo-acoustic | 18, 19 | 35 µm | Yes | Yes | 5–10 | 103 | Moderate | |
| Nonlinear photo-acoustic | 20 | 5 µm | Yes | Yes | 6,000 | 103 | Moderate | |
| Two-photon | 56, 57 | 1–5 µm | Yes* | Yes | 20 | 103–104 | Moderate | |
| Coherence gating | 58, 59 | 1 µm | Yes | Yes | 50 | 1–10 | Moderate | |
| Feedback guidestar |
Second harmonic | 16 | 2 µm | No | No | 400 | 1 | Moderate |
| Fluorescence | 15 | 1 µm | No | No | 10–100 | 102 | Moderate | |
| Ultrasound | 21–23 | 30 µm | Yes | Yes | 5 | <1 | Low | |
| Iterative ultrasound | 88–90 | 10 µm | Yes | Yes | 30 | 1–10 | Low | |
| Variance-encoded ultrasound (TROVE) | 91 | 5 µm | Yes | Yes | 100 | 103–104 | Low | |
| Particle displacement (TRAP, TRACK) | 24, 25 | 5–10 µm | Yes* | Yes | 300 | <1 | High | |
Minimum spot size selected as minimum across all similar demonstrations. Peak-to-background ratio (PBR) selected as maximum across all similar demonstrations. Required time is an order of magnitude approximation. Coupling efficiency p, defined as the percentage of incident optical power transferred into a detectable guidestar signal, is high if p > 0.5, moderate if 0.5 > p > 0.01, and low if p < 0.01.
Possible invasive insertion used in experiment, but not required in principle.
Time reversal and phase conjugation
Feedback guidestar techniques measure the scattering response of tissue sequentially over time. Unfortunately, accurate focusing can require millions of unique measurements, and the scattering response of in vivo tissue changes on a sub-second timescale69,70. To overcome this time constraint, a helpful strategy would involve measuring the scattering response of tissue in a parallel manner. By adopting the principle of time reversal, or ‘optical phase conjugation’ (OPC), it is possible to refocus light to a guidestar spot from a single snapshot.
To outline the principle of OPC, let us assume that an ideal guidestar emits a monochromatic optical wave with frequency ω. At any position r and time t within the scatterer, we may express this emitted scalar field as u(r, t) = Re{A(r) exp[i(φ(r) − ωt)]}. Here, A specifies the field amplitude and φ defines its spatially varying phase. Given propagation within a lossless medium, the wave equation remains time-invariant. For any forward-propagating field u(r, t) that is scattering out of the medium, there also exists a wavefunction u′ = u(r, −t) that will precisely retrace the path of u back through every scattering interaction to its original guidestar location. If the constant phase lines for u must satisfy φ(r) = ωt, then the constant phase lines of u′ must satisfy φ(r) = −ωt. It is clear the phase lines of u′ also satisfy the relation −φ(r) = ωt.The left-hand side of this equation represents the spatial phase conjugate of u. We can therefore ‘time reverse’ an arbitrary field u back into its original guidestar spot by conjugating its spatial phase, and then allowing it to continue travelling ahead in time.
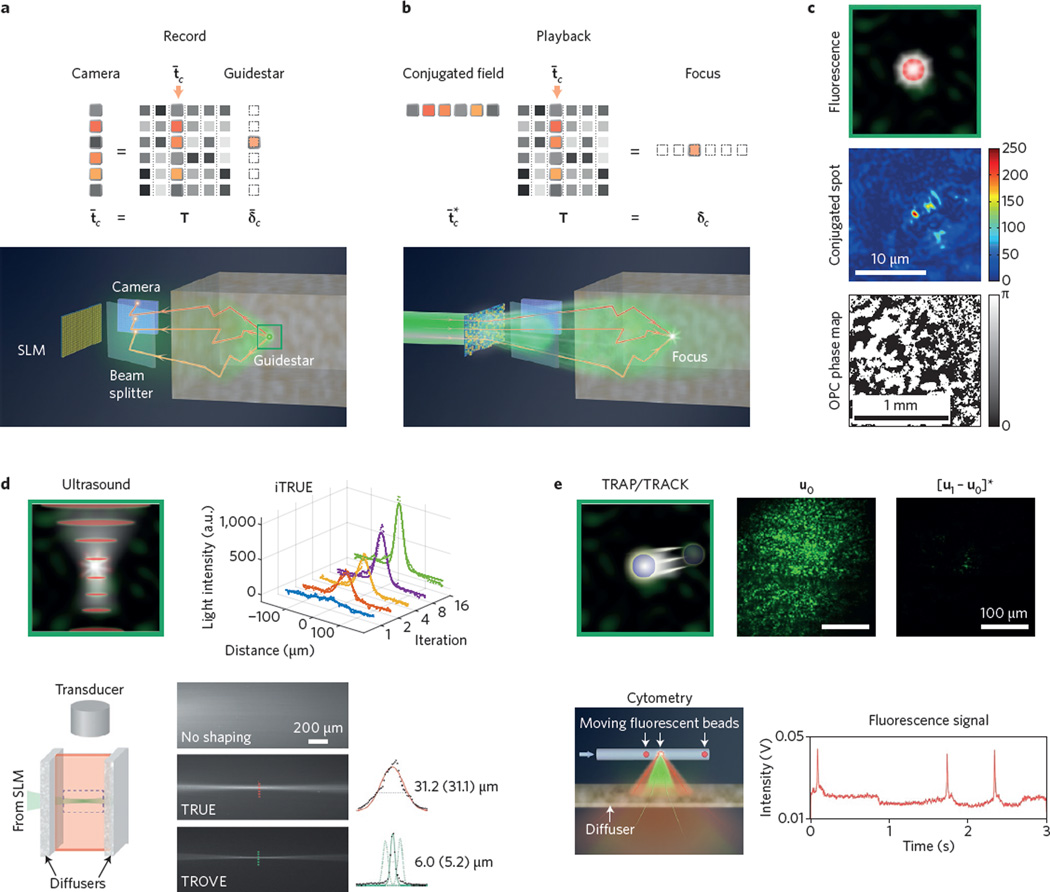
To describe OPC within our linear algebra framework, we represent the light emerging from the target plane’s guidestar as ūb, where the bar denotes a column vector. We assume time-reversal symmetry to express its transformation to the input plane as ūa = Tūb. Light propagating from the target to input plane, as a column vector, now multiplies into our transmission matrix from the right (Fig. 2b,d). In practice, sample absorption and a limited detection aperture prevent collection of the complete guidestar field35. Despite this challenge, the following phase-conjugation strategy can still be used to focus light into tissue. First, create a discrete point source of light at a specific location xb = c within tissue: ūb = δ̄c, the cth unit column vector. Next, capture the resulting scattered field at the input plane: ūa = Tδ̄c = t̄c, the cth column of T (Fig. 4a). Finally, phase-conjugate the detected field into and send the conjugate-transposed field back towards the target plane: . This conjugated field re-focuses to a discrete spot at the guidestar origin (Fig. 4b). Incomplete measurement and imperfect conjugation of t̄c also typically introduces a finite background field ε. Note this expression closely matches the final result of our previous ‘feedback guidestar’ strategy. Here, OPC directly measures and conjugates the vector t̄c, whereas feedback from the target plane determines the elements of in sequence.
Figure 4. Conjugation guidestars.
a, Matrix model and set-up for detecting an embedded guidestar field. Light from target field spot δ̄3 forms the speckle field t̄3 at the input plane camera. b, Matrix model and set-up for conjugation guidestar focusing. SLM-shaping an incident wavefront into conjugate field refocuses to δ3. c, Fluorescent conjugation guidestar experiment (0.2 µm bead), with resulting focus and conjugate phase map15. d, Ultrasound conjugation guidestar experiments. iTRUE sharpens the conjugated spot size90 by a factor of three. TROVE reduces the focal spot width91 from 31 µm to 5 µm. e, Kinematic target conjugation guidestar experiments (TRAP/TRACK). The resulting focus enables particle counting25. Figure reproduced with permission from: c, ref. 15, AIP; e, ref. 25, OSA.
Several experimental techniques are available to implement OPC. Early work used a deformable mirror to display the conjugate map of distorted light from a guidestar, which corrected for atmospheric turbulence12. Typical deformable mirrors can only reshape a small number of low-order optical modes. Similarly early experiments also tested holograms for conjugating light through fixed distortions71,72. Unlike a deformable mirror, holograms can record and modify millions of wavefront modes. Nonlinear optical phenomena may also act like a ‘conjugate mirror’73 to conjugate light through dynamic distortions74. Examples include stimulated Brillouin scattering, multiple-wave mixing and the photorefractive effect75,76. Although these approaches offer quick response times, the analog nature of nonlinear techniques precludes the ability to adjust their conjugation maps computationally.
The first OPC experiment to focus light through thick biological tissue involved using a hologram to record and conjugate the scattered field11. Shortly thereafter, focusing through tissue was achieved with digital optical phase conjugation (DOPC), which interferometrically measures an optical field on a digital detector, and then creates its phase conjugate using a pixel-to-pixel matched SLM77. A similar strategy can compensate for and combine multiple beams within an optical fibre78. Here, unlike early phase-conjugation work with deformable mirrors, DOPC uses a modern SLM that contains several million individually addressable elements. Thus, DOPC may compensate for complex optical transformations that involve millions of propagation modes, such as diffusive scattering in tissue13. Furthermore, unlike analog holograms, DOPC can digitally correct for minor setup misalignments79 and can in principle shape either continuous or pulsed beams of arbitrary power.
Wavefront shaping with conjugation guidestars
With direct access to the target plane, OPC can measure and conjugate arbitrary fields through ex vivo tissue11. Without access, for instance during an in vivo experiment, OPC requires an effective ‘conjugation guidestar’that can emit light for subsequent refocusing. Fluorescent excitation within the retina is one of the first conjugation guidestars applied in a biological context80. Here, a focused beam stimulates a small incoherent light source at the retina, which is externally measured to compensate for aberrations. Embedded fluorescent microspheres81 and proteins82,83 serve as similar conjugation beacons in AO microscopy. It is also possible to implement conjugation-based correction using coherence gating84 or OCT85. Here, we review recent extensions of this early conjugation work into the regime of wavefront shaping, where wavefront distortions arise from deep within tissue. We summarize our list of conjugation guidestars in Table 1.
The first guidestar for conjugation-based focusing through turbid media used the nonlinear second-harmonic generation signal from a nanoparticle16. Shortly thereafter, Vellekoop et al. conjugated light from a fluorescent marker embedded beneath 0.5 mm of tissue15 (Fig. 4c). Although the conjugated spot sizes of these techniques may approach the diffraction limit, both nonlinear and fluorescent guidestar signals originate from a fixed, spatially confined spot. Furthermore, their emission signals tend to be weak, and thus require long exposure times (≥1 s).
Focused ultrasound offers a conjugation guidestar that may be easily moved to different positions (Fig. 4d). As discussed in the context of feedback guidestars, a confined ultrasound focus can frequency-shift light at a defined spot within in vivo tissue86. Several experiments detected and phase-conjugated the ultrasound-modulated wavefront scattered from this finite spot (also termed a ‘virtual source’)21–23. The resolution of early time-reversed ultrasonically encoded (TRUE) demonstrations was fixed by the size of the ultrasound focus (25–50 µm). Although increasing the ultrasound frequency shrinks this spot size, it also decreases the number of photons modulated from deep within the tissue, thus presenting a trade-off between resolution and depth87. A potential solution to this resolution trade-off is iterative TRUE88–90, whereby a feedback loop between the conjugated and detected fields can experimentally shrink the focus spot size by a factor of three (Fig. 4d).
Instead of improving TRUE focusing resolution with iterative feedback, one may also adopt a statistical approach known as the time reversal of variance-encoded light (TROVE)91. Conceptually, the relatively large TRUE focus modulates many optical speckles. The modulated field emerging from the tissue is thus a superposition of weighted optical fields, each originating from a unique location within the ultrasound spot. Standard TRUE simply detects and conjugates this entire superposition. Alternatively, through a series of measurements and application of Gaussian statistics, TROVE computationally decomposes this superposition back into its individually weighted components. By conjugating the appropriate component, it is possible to refocus to a single optical mode (that is, a focal spot one speckle wide, Fig. 4d, bottom). In combination with recent methods to perform TRUE over timescales of <10 ms (ref. 92), TROVE concepts may help establish a translatable, optical diffraction-limited guidestar for in vivo use.
Even without ultrasonic frequency encoding, multiple measurements of a time-varying optical field can still lead to a sharp, phase-conjugated focus within tissue. Two recent works (TRAP24 and TRACK25) demonstrate this concept using a kinematic target embedded within a scattering material. First, a scattered field is measured when the target is absent from a volume of interest (u0 in Fig. 4e). Second, a different scattered field is measured after the target has moved into the volume (u1). Subtracting the first field from the second and conjugating the result will focus to the target location ([u1 − u0]* in Fig. 4e). An immediate application of the TRAP/TRACK principle is for in vivo flow cytometry. From two measurements of one ‘guidestar’ cell in motion, one may conjugate light to a tight focal spot. It is then possible to monitor the passage of any subsequent cells, for example, within the vein of interest, via external detection (Fig. 4e, bottom). Acquiring a sequence of speckle measurements may also help compute an image of such an embedded moving object, as recently considered in ref. 93.
Experimental development
Before achieving widespread biological application, guidestar techniques must first address tissue motion (Fig. 5). Any unknown change in scattering response between guidestar signal measurement and wavefront playback is a source of error. Macroscopic effects, like heartbeat and blood flow, are the primary cause of tissue movement. Even with an ideal playback wavefront, a sharp focus will quickly spread into random speckle during in vivo conjugation69. Studies indicate a system response time of less than 50 ms is needed to overcome movements in unconstrained tissue, or several seconds when the tissue is immobilized (that is, pinched)69,70.
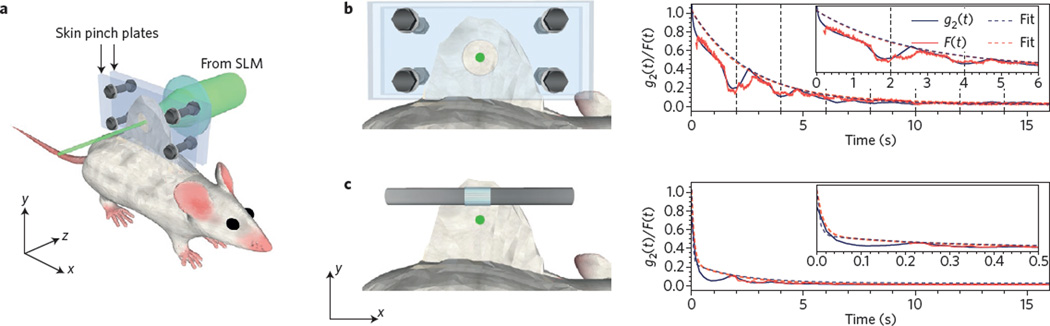
Figure 5. Tissue motion dims an OPC focus.
a, Diagram of OPC decorrelation experiment, where wavefront shaping forms a tight focus through pinched, in vivo mouse tissue. b, Focusing light through partially immobilized dorsal skin. Both the speckle autocorrelation (g2(t), black) and OPC focal spot intensity (F(t), red) decay in magnitude over the course of several seconds, with fitted curves. c, In unconstrained skin, decorrelation occurs on a much faster (sub-second) timescale. Figure reproduced with permission from ref. 70, OSA.
A select number of current setups, using either digital feedback49 or nonlinear optical conjugation92, operate within a 50 ms time window. An integrated detector and SLM94, along with feedback algorithms that account for the temporal dynamics of the scattering medium95,96, may lead to system response times below 1 ms. If the tissue sample can fit within an optical cavity, all-optical feedback can execute wavefront shaping at extremely fast, sub-microsecond timescales97.
In parallel with increased speed, future wavefront-shaping technologies hope to push their focusing depth below one centimetre (that is, into the macroscopic regime6). At such depths, optical absorption becomes a limiting factor. Fluorescent and nonlinear guidestars with improved efficiencies will help overcome signal-to-noise ratio challenges. Models predict that ultrasound guidestars, which become attenuated at large depths, may currently extend to centimetre depth scales87,98.
The computational nature of most recent wavefront-shaping work is one of its strongest assets. Mirroring the field of computational imaging, co-designed measurement and post-processing strategies can overcome experimental challenges. Examples include quick transmission matrix measurement without a reference beam99, overcoming large amounts of experimental noise50,100, shrinking the OPC focus width91, and the related notion of a ‘computational’ guidestar, created through a combination of multiple measured fields exiting the tissue (for example, TRAP/TRACK).
Finally, most of the guidestar techniques reviewed here achieve refocusing with monochromatic light. The spectral dependence of optical scattering introduces additional degrees of freedom for wavefront control101,102. The related ability to temporally recompress an optical pulse using time reversal, as already well-studied with ultrasound103, may also greatly enhance focused energy confinement. Although several recent electromagnetic implementations show promising benefits for spectral/temporal control104–108, few achieve this control from just one side of the scattering material, which most biological settings require.
Biological applications
A variety of applications await both feedback and conjugation guidestar focusing in tissue. Many of these applications fall into one of two categories: energy delivery and information extraction. A well-established example from the former category is photodynamic therapy, whose goal is to illuminate a diseased body region that contains a pre-administered photosensitizing drug. Applications include treatment of dermatological and ophthalmic disease, as well as solid tumors1. Highly concentrated optical power can also directly ablate tissue109 or power implanted devices110. Photodynamic therapy, tissue ablation and power delivery currently suffer from limited optical control at directly accessible tissue regions (that is, less than one TMFP from the surface). A guidestar-based focus offers micrometre-scale optical control that can extend down multiple TMFP into the body, thereby possibly enabling new treatments.
Delivering energy to specific neurons within the brain can also help uncover how they communicate. When illuminated with sufficient light, optogenetic markers may activate or deactivate various physiological processes3. However, precise control over which neurons are optically activated is highly desired — inefficiencies and residual heating are a current limitation111. Initial demonstrations suggest that guidestar-based focusing may improve both the resolution and penetration depth of current optogenetic excitation techniques67,112,113. Furthermore, it may also assist with the joint goal of localizing fluorescence emitted from active neurons, potentially with subwavelength precision114. Although DOPC currently images arbitrary fluorescent structure at limited resolution23, the above list of alternative goals will likely precede any significant improvement to its imaging performance. Finally, apart from exciting and detecting functionalized cells, shaped wavefronts can also manipulate particles within a scattering material66,115. Such optical manipulation within thick tissue may offer new tools for the active control of cellular and subcellular functions. In summary, guidestar-based methods can now successfully overcome turbidity to focus light deep within tissue. We will see many exciting biological applications over the next several years, which will likely grow in both number and impact as their technological foundation continues to develop.
Acknowledgments
We thank M. Jang, E. Zhou, B. Judkewitz, I. M. Vellekoop, J. Brake, H. Deng and M. Harfouche for helpful feedback during manuscript preparation. This work is supported by the National Institutes of Health (1DP2OD007307-01), the National Institutes of Health BRAIN Initiative (1U01NS090577-01) and a GIST-Caltech Collaborative Research Proposal (CG2012).
Footnotes
Author contributions
All authors contributed equally to this work.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Huang Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. T. 2005;4:283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis-Davies GCR. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nature Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang F, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtman JW, Conchello JA. Fluorescence microscopy. Nature Methods. 2005;2:910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MA, Lew MD, Moerner WE. Extending microscopic resolution with single-molecule imaging and active control. Annu. Rev. Biophys. 2012;41:321–342. doi: 10.1146/annurev-biophys-050511-102250. [DOI] [PubMed] [Google Scholar]
- 6.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nature Methods. 2010;7:603–611. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 7.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nature Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 8.Huang D, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theer P, Denk W. On the fundamental imaging-depth limit in two-photon microscopy. J. Opt. Soc. Am. A. 2006;23:3139–3149. doi: 10.1364/josaa.23.003139. [DOI] [PubMed] [Google Scholar]
- 10.Vellekoop IM, Mosk AP. Focusing coherent light through opaque strongly scattering media. Opt. Lett. 2007;32:2309–2311. doi: 10.1364/ol.32.002309. [DOI] [PubMed] [Google Scholar]
- 11.Yaqoob Z, Psaltis D, Feld MS, Yang C. Optical phase conjugation for turbidity suppression in biological samples. Nature Photon. 2008;2:110–115. doi: 10.1038/nphoton.2007.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy JW. Adaptive Optics for Astronomical Telescopes. Oxford Univ. Press; 1998. [Google Scholar]
- 13.Mosk AP, Lagendijk A, Lerosey G, Fink M. Controlling waves in space and time for imaging and focusing in complex media. Nature Photon. 2012;6:283–292. [Google Scholar]
- 14.Vellekoop IM, van Putten EG, Lagendijk A, Mosk AP. Demixing light paths inside disordered metamaterials. Opt. Express. 2008;16:67–80. doi: 10.1364/oe.16.000067. [DOI] [PubMed] [Google Scholar]
- 15.Vellekoop IM, Cui M, Yang C. Digital optical phase conjugation of fluorescence in turbid tissue. Appl. Phys. Lett. 2012;101:081108. doi: 10.1063/1.4745775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh CL, Pu Y, Grange R, Psaltis D. Digital phase conjugation of second harmonic radiation emitted by nanoparticles in turbid media. Opt. Express. 2010;18:12283–12290. doi: 10.1364/OE.18.012283. [DOI] [PubMed] [Google Scholar]
- 17.Chaigne T, et al. Controlling light in scattering media non-invasively using the photoacoustic transmission matrix. Nature Photon. 2014;8:58–64. [Google Scholar]
- 18.Kong F, et al. Photoacoustic-guided convergence of light through optically diffusive media. Opt. Lett. 2011;36:2053–2055. doi: 10.1364/OL.36.002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caravaca-Aguirre AM, et al. High contrast three-dimensional photoacoustic imaging through scattering media by localized optical fluence enhancement. Opt. Express. 2013;21:26671–26676. doi: 10.1364/OE.21.026671. [DOI] [PubMed] [Google Scholar]
- 20.Lai P, Wang L, Tay JW, Wang LV. Photoacoustically guided wavefront shaping for enhanced optical focusing in scattering media. Nature Photon. 2015;9:126–132. doi: 10.1038/nphoton.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X, Liu H, Wang LV. Time-reversed ultrasonically encoded optical focusing into scattering media. Nature Photon. 2011;5:154–157. doi: 10.1038/nphoton.2010.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Si K, Fiolka R, Cui M. Fluorescence imaging beyond the ballistic regime by ultrasound-pulse-guided digital phase conjugation. Nature Photon. 2012;6:657–661. doi: 10.1038/nphoton.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YM, Judkewitz B, Dimarzio CA, Yang C. Deep-tissue focal fluorescence imaging with digitally time-reversed ultrasound-encoded light. Nature Commun. 2012;3:928. doi: 10.1038/ncomms1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Xu X, Liu Y, Wang LV. Time-reversed adapted-perturbation (TRAP) optical focusing onto dynamic objects inside scattering media. Nature Photon. 2014;8:931–936. doi: 10.1038/nphoton.2014.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou EH, Ruan H, Yang C, Judkewitz B. Focusing on moving targets through scattering samples. Optica. 2014;1:227–232. doi: 10.1364/OPTICA.1.000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton NG, et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nature Photon. 2013;7:205–209. doi: 10.1038/nphoton.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang S, et al. Imaging deep within a scattering medium using collective accumulation of single-scattered waves. Nature Photon. 2015;9:253–258. [Google Scholar]
- 28.Matthews TE, et al. Deep tissue imaging using spectroscopic analysis of multiply scattered light. Optica. 2014;1:105–111. [Google Scholar]
- 29.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nature Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 30.den Outer PN, Lagendijk A, Nieuwenhuizen TM. Location of objects in multiple-scattering media. J. Opt. Soc. Am. A. 1993;10:1209–1218. [Google Scholar]
- 31.Arridge SR. Optical tomography in medical imaging. Inverse Probl. 1999;15:R41–R93. [Google Scholar]
- 32.Ntziachristos V, Tung CH, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nature Med. 2002;8:757–760. doi: 10.1038/nm729. [DOI] [PubMed] [Google Scholar]
- 33.Popoff SM, et al. Measuring the transmission matrix in optics: an approach to the study and control of light propagation in disordered media. Phys. Rev. Lett. 2010;104:100601. doi: 10.1103/PhysRevLett.104.100601. [DOI] [PubMed] [Google Scholar]
- 34.Beenakker CWJ. Random-matrix theory of quantum transport. Rev. Mod. Phys. 1997;69:731–808. [Google Scholar]
- 35.Vellekoop IM. PhD thesis. Univ. Twente; 2008. Controlling the propagation of light in disordered scattering media. [Google Scholar]
- 36.Goodman JW. Speckle Phenomena in Optics: Theory and Applications. Roberts and Co; 2007. [Google Scholar]
- 37.Feng S, Kane C, Lee PA, Stone AD. Correlations and fluctuations of coherent wave transmission through disordered media. Phys. Rev. Lett. 1988;61:834–837. doi: 10.1103/PhysRevLett.61.834. [DOI] [PubMed] [Google Scholar]
- 38.Wiersma DS. Disordered photonics. Nature Photon. 2013;7:188–196. [Google Scholar]
- 39.Bertolotti J, et al. Non-invasive imaging through opaque scattering layers. Nature. 2012;491:232–234. doi: 10.1038/nature11578. [DOI] [PubMed] [Google Scholar]
- 40.Katz O, Heidmann P, Fink M, Gigan S. Non-invasive single-shot imaging through scattering layers and around corners via speckle correlations. Nature Photon. 2014;8:784–790. [Google Scholar]
- 41.Yang X, Pu Y, Psaltis D. Imaging blood cells through scattering biological tissue using speckle scanning microscopy. Opt. Express. 2014;22:3405–3413. doi: 10.1364/OE.22.003405. [DOI] [PubMed] [Google Scholar]
- 42.Judkewitz B, Horstmeyer R, Vellekoop IM, Papadopoulos IN, Yang C. Translation correlations in anisotropically scattering media. Nature Phys. 2015;11:684–689. [Google Scholar]
- 43.Tanter M, Thomas JL, Fink M. Time reversal and the inverse filter. J. Acoust. Soc. Am. 2000;108:223–234. doi: 10.1121/1.429459. [DOI] [PubMed] [Google Scholar]
- 44.Popoff S, Lerosey G, Fink M, Boccara AC, Gigan S. Image transmission through an opaque material. Nature Commun. 2010;1:81. doi: 10.1038/ncomms1078. [DOI] [PubMed] [Google Scholar]
- 45.Kim M, et al. Maximal energy transport through disordered media with the implementation of transmission eigenchannels. Nature Photon. 2012;6:581–585. [Google Scholar]
- 46.Cizmar T, Dholakia K. Exploiting multimode waveguides for pure fibre-based imaging. Nature Commun. 2012;3:1027. doi: 10.1038/ncomms2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi Y, et al. Measurement of the time-resolved reflection matrix for enhancing light energy delivery into a scattering medium. Phys. Rev. Lett. 2013;111:243901. doi: 10.1103/PhysRevLett.111.243901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conkey DB, Caravaca-Aguirre AM, Piestun R. High-speed scattering medium characterization with application to focusing light through turbid media. Opt. Express. 2012;20:1733–1740. doi: 10.1364/OE.20.001733. [DOI] [PubMed] [Google Scholar]
- 50.Conkey DB, Brown AN, Caravaca-Aguirre AM, Piestun R. Genetic algorithm optimization for focusing through turbid media in noisy environments. Opt. Express. 2012;20:4840–4849. doi: 10.1364/OE.20.004840. [DOI] [PubMed] [Google Scholar]
- 51.Vellekoop IM, Mosk AP. Universal optimal transmission of light through disordered materials. Phys. Rev. Lett. 2008;101:120601. doi: 10.1103/PhysRevLett.101.120601. [DOI] [PubMed] [Google Scholar]
- 52.Vellekoop IM. Feedback-based wavefront shaping. Opt. Express. 2015;23:12189–12206. doi: 10.1364/OE.23.012189. [DOI] [PubMed] [Google Scholar]
- 53.Vellekoop IM, Aegerter CM. Scattered light fluorescence microscopy: imaging through turbid layers. Opt. Lett. 2010;35:1245–1247. doi: 10.1364/OL.35.001245. [DOI] [PubMed] [Google Scholar]
- 54.Leutz W, Maret G. Ultrasonic modulation of multiply scattered light. Physica B. 1995;204:14–19. [Google Scholar]
- 55.Tay JW, Lai P, Suzuki Y, Wang LV. Ultrasonically encoded wavefront shaping for focusing into random media. Sci. Rep. 2014;4:3918. doi: 10.1038/srep03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katz O, Small E, Guan Y, Silberberg Y. Noninvasive nonlinear focusing and imaging through strongly scattering turbid layers. Optica. 2014;1:170–174. [Google Scholar]
- 57.Tang J, Germain RN, Cui M. Superpenetration optical microscopy by iterative multiphoton adaptive compensation technique. Proc. Natl Acad. Sci. USA. 2012;109:8434–8439. doi: 10.1073/pnas.1119590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiolka R, Si K, Cui M. Complex wavefront corrections for deep tissue focusing using low coherence backscattered light. Opt. Express. 2012;20:16532–16543. [Google Scholar]
- 59.Jang J, et al. Complex wavefront shaping for optimal depth-selective focusing in optical coherence tomography. Opt. Express. 2013;21:2890–2902. doi: 10.1364/OE.21.002890. [DOI] [PubMed] [Google Scholar]
- 60.Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A. 1997;14:2884–2892. doi: 10.1364/josaa.14.002884. [DOI] [PubMed] [Google Scholar]
- 61.Neil MA, et al. Adaptive aberration correction in a two-photon microscope. J. Microsc. 2000;200:105–108. doi: 10.1046/j.1365-2818.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- 62.Albert O, Sherman L, Mourou G, Norris TB, Vdovin G. Smart microscope: an adaptive optics learning system for aberration correction in multiphoton confocal microscopy. Opt. Lett. 2000;25:52–54. doi: 10.1364/ol.25.000052. [DOI] [PubMed] [Google Scholar]
- 63.Booth MJ, Neil MAA, Juškaitis R, Wilson T. Adaptive aberration correction in a confocal microscope. Proc. Natl Acad. Sci. USA. 2002;99:5788–5792. doi: 10.1073/pnas.082544799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Booth MJ. Adaptive optical microscopy: the ongoing quest for a perfect image. Light Sci. Appl. 2014;3:e165. [Google Scholar]
- 65.Ji N, Milkie DE, Betzig E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nature Methods. 2010;7:141–147. doi: 10.1038/nmeth.1411. [DOI] [PubMed] [Google Scholar]
- 66.Cizmar T, Mazilu M, Dholakia K. In situ wavefront correction and its application to micromanipulation. Nature Photon. 2010;4:388–394. [Google Scholar]
- 67.Kong L, Cui M. In vivo fluorescence microscopy via iterative multi-photon adaptive compensation technique. Opt. Express. 2014;22:23786–23794. doi: 10.1364/OE.22.023786. [DOI] [PubMed] [Google Scholar]
- 68.Fried DL. Anisoplanatism in adaptive optics. J. Opt. Soc. Am. 1982;72:52–61. [Google Scholar]
- 69.Meng C, McDowell EJ, Yang C. An in vivo study of turbidity suppression by optical phase conjugation (TSOPC) on rabbit ear. Opt. Express. 2010;18:25–30. doi: 10.1364/OE.18.000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jang M, et al. Relation between speckle decorrelation and optical phase conjugation (OPC)-based turbidity suppression through dynamic scattering media: a study on in vivo mouse skin. Biomed. Opt. Express. 2015;6:72–85. doi: 10.1364/BOE.6.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leith EN, Upatnieks J. Holographic imagery through diffusing media. J. Opt. Soc. Am. 1966;56:523–523. [Google Scholar]
- 72.Goodman JW, Huntley WH, Jackson DW, Lehmann M. Wavefront-reconstruction imaging through random media. Appl. Phys. Lett. 1966;8:311–313. [Google Scholar]
- 73.Yariv A. Phase conjugate optics and real-time holography. IEEE J. Quantum Electron. 1978;14:650–660. [Google Scholar]
- 74.Giuliano CR. Applications of optical phase conjugation. Phys. Today. 1981;34(4):27–35. [Google Scholar]
- 75.Pepper DM. Nonlinear optical phase conjugation. Opt. Eng. 1982;21:212156. doi: 10.1364/ol.4.000052. [DOI] [PubMed] [Google Scholar]
- 76.Fisher RA. Optical Phase Conjugation. Academic; 1983. [Google Scholar]
- 77.Cui M, Yang C. Implementation of a digital optical phase conjugation system and its application to study the robustness of turbidity suppression by phase conjugation. Opt. Express. 2010;18:3444–3455. doi: 10.1364/OE.18.003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bellanger C, Brignon A, Colineau J, Huignard JP. Coherent fiber combining by digital holography. Opt. Lett. 2008;33:2937–2939. doi: 10.1364/ol.33.002937. [DOI] [PubMed] [Google Scholar]
- 79.Jang M, Ruan H, Zhou H, Judkewitz B, Yang C. Method for auto-alignment of digital optical phase conjugation systems based on digital propagation. Opt. Express. 2014;22:14054–14071. doi: 10.1364/OE.22.014054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diaz Santana Haro L, Dainty JC. Single-pass measurements of the wave-front aberrations of the human eye by use of retinal lipofuscin autofluorescence. Opt. Lett. 1999;24:61–63. doi: 10.1364/ol.24.000061. [DOI] [PubMed] [Google Scholar]
- 81.Azucena O, et al. Wavefront aberration measurements and corrections through thick tissue using fluorescent microsphere reference beacons. Opt. Express. 2010;18:17521–17532. doi: 10.1364/OE.18.017521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tao X, et al. Adaptive optics microscopy with direct wavefront sensing using fluorescent protein guide stars. Opt. Lett. 2011;36:3389–3391. doi: 10.1364/OL.36.003389. [DOI] [PubMed] [Google Scholar]
- 83.Wang K, et al. Rapid adaptive optical recovery of optimal resolution over large volumes. Nature Methods. 2014;11:625–628. doi: 10.1038/nmeth.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rueckel M, Mack-Bucher JA, Denk W. Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing. Proc. Natl Acad. Sci. USA. 2006;103:17137–17142. doi: 10.1073/pnas.0604791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hermann B, et al. Adaptive-optics ultrahigh-resolution optical coherence tomography. Opt. Lett. 2004;29:2142–2144. doi: 10.1364/ol.29.002142. [DOI] [PubMed] [Google Scholar]
- 86.Lev A, Sfez B. In vivo demonstration of the ultrasound-modulated light technique. J. Opt. Soc. Am. A. 2003;20:2347–2354. doi: 10.1364/josaa.20.002347. [DOI] [PubMed] [Google Scholar]
- 87.Jang M, Ruan H, Judkewitz B, Yang C. Model for estimating the penetration depth limit of the time-reversed ultrasonically encoded optical focusing technique. Opt. Express. 2014;22:5787–5807. doi: 10.1364/OE.22.005787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Si K, Fiolka R, Cui M. Breaking the spatial resolution barrier via iterative sound-light interaction in deep tissue microscopy. Sci. Rep. 2012;2:748. doi: 10.1038/srep00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki Y, Tay JW, Yang Q, Wang LV. Continuous scanning of a time-reversed ultrasonically encoded optical focus by reflection-mode digital phase conjugation. Opt. Lett. 2014;39:3441–3444. doi: 10.1364/OL.39.003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruan H, Jang M, Judkewitz B, Yang C. Iterative time-reversed ultrasonically encoded light focusing in backscattering mode. Sci. Rep. 2014;4:7156. doi: 10.1038/srep07156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Judkewitz B, Wang YM, Horstmeyer R, Mathy A, Yang C. Speckle-scale focusing in the diffusive regime with time reversal of variance-encoded light (TROVE) Nature Photon. 2013;7:300–305. doi: 10.1038/nphoton.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y, et al. Optical focusing deep inside dynamic scattering media with near-infrared time-reversed ultrasonically encoded (TRUE) light. Nature Commun. 2015;6:5904. doi: 10.1038/ncomms6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Newman JA, Webb KJ. Imaging optical fields through heavily scattering media. Phys. Rev. Lett. 2014;113:263903. doi: 10.1103/PhysRevLett.113.263903. [DOI] [PubMed] [Google Scholar]
- 94.Laforest T, et al. A 4000 Hz CMOS image sensor with in-pixel processing for light measurement and modulation; New Circuits and Systems Conf. (NEWCAS), IEEE 11th Intl; 2013. pp. 1–4. [Google Scholar]
- 95.Vellekoop IM, Aegerter CM. Focusing light through living tissue. Proc. SPIE. 2010;7554:755430. [Google Scholar]
- 96.Stockbridge C, et al. Focusing through dynamic scattering media. Opt. Express. 2012;20:15086–15092. doi: 10.1364/OE.20.015086. [DOI] [PubMed] [Google Scholar]
- 97.Nixon M, et al. Real-time wavefront shaping through scattering media by all-optical feedback. Nature Photon. 2013;7:919–924. [Google Scholar]
- 98.Hollmann JL, Horstmeyer R, Yang C, DiMarzio CA. Diffusion model for ultrasound-modulated light. J. Biomed. Opt. 2014;19:035005. doi: 10.1117/1.JBO.19.3.035005. [DOI] [PubMed] [Google Scholar]
- 99.Dremeau A, et al. Reference-less measurement of the transmission matrix of a highly scattering material using a DMD and phase retrieval techniques. Opt. Express. 2015;23:11898–11911. doi: 10.1364/OE.23.011898. [DOI] [PubMed] [Google Scholar]
- 100.Yilmaz H, Vos WL, Mosk AP. Optimal control of light propagation through multiple-scattering media in the presence of noise. Biomed. Opt. Express. 2013;4:1759–1768. doi: 10.1364/BOE.4.001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Beijnum F, van Putten EG, Lagendijk A, Mosk AP. Frequency bandwidth of light focused through turbid media. Opt. Lett. 2011;36:373–375. doi: 10.1364/OL.36.000373. [DOI] [PubMed] [Google Scholar]
- 102.Kohlgraf-Owens TW, Dogariu A. Transmission matrices of random media: means for spectral polarimetric measurements. Opt. Lett. 2010;35:2236–2238. doi: 10.1364/OL.35.002236. [DOI] [PubMed] [Google Scholar]
- 103.Fink M. Time reversed acoustics. Phys. Today. 1997;50(3):34–40. [Google Scholar]
- 104.Lerosey G, de Rosny J, Tourin A, Fink M. Focusing beyond the diffraction limit with far-field time reversal. Science. 2007;315:1120–1122. doi: 10.1126/science.1134824. [DOI] [PubMed] [Google Scholar]
- 105.Aulbach J, Gjonaj B, Johnson PM, Mosk AP, Lagendijk A. Control of light transmission through opaque scattering media in space and time. Phys. Rev. Lett. 2011;106:103901. doi: 10.1103/PhysRevLett.106.103901. [DOI] [PubMed] [Google Scholar]
- 106.McCabe DJ, et al. Spatio-temporal focusing of an ultrafast pulse through a multiply scattering medium. Nature Commun. 2011;2:447. doi: 10.1038/ncomms1434. [DOI] [PubMed] [Google Scholar]
- 107.Katz O, Small E, Bromberg Y, Silberberg Y. Focusing and compression of ultrashort pulses through scattering media. Nature Photon. 2011;5:372–377. [Google Scholar]
- 108.Paudel HP, Stockbridge C, Mertz J, Bifano T. Focusing polychromatic light through strongly scattering media. Opt. Express. 2013;21:17299–17308. doi: 10.1364/OE.21.017299. [DOI] [PubMed] [Google Scholar]
- 109.Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003;103:577–644. doi: 10.1021/cr010379n. [DOI] [PubMed] [Google Scholar]
- 110.Goto K, Nakagawa T, Nakamura O, Kawata S. An implantable power supply with an optically rechargeable lithium battery. IEEE Trans. Biomed. Eng. 2001;48:830–833. doi: 10.1109/10.930908. [DOI] [PubMed] [Google Scholar]
- 111.Williams JC, Denison T. From optogenetic technologies to neuromodulation therapies. Sci. Transl. Med. 2013;5:177ps6. doi: 10.1126/scitranslmed.3003100. [DOI] [PubMed] [Google Scholar]
- 112.Yoon J, et al. Optogenetic signaling-pathway regulation through scattering skull using wavefront shaping. 2015 doi: 10.1038/srep13289. Preprint at http://arxiv.org/abs/1502.04826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Papagiakoumou E, et al. Functional patterned multiphoton excitation deep inside scattering tissue. Nature Photon. 2013;7:274–278. [Google Scholar]
- 114.van Putten EG, Lagendijk A, Mosk AP. Optimal concentration of light in turbid materials. J. Opt. Soc. Am. B. 2011;28:1200–1203. [Google Scholar]
- 115.Volpe G, Kurz L, Callegari A, Volpe G, Gigan S. Speckle optical tweezers: micromanipulation with random light fields. Opt. Express. 2014;22:18159–18167. doi: 10.1364/OE.22.018159. [DOI] [PubMed] [Google Scholar]