Abstract
In this experiment, we evaluated the long-term effects of noise by assessing both astrocyte changes in medial prefrontal cortex (mPFC) and mPFC-related alternation/discrimination tasks. Twenty-one-day-old male rats were exposed during a period of 15 days to a standardized rats’ audiogram-fitted adaptation of a human noisy environment. We measured serum corticosterone (CORT) levels at the end of the exposure and periodically registered body weight gain. In order to evaluate the long-term effects of this exposure, we assessed the rats’ performance on the T-maze apparatus 3 months later. Astrocyte numbers and proliferative changes in mPFC were also evaluated at this stage. We found that environmental noise (EN) exposure significantly increased serum CORT levels and negatively affected the body weight gain curve. Accordingly, enduring effects of noise were demonstrated on mPFC. The ability to solve alternation/discrimination tasks was reduced, as well as the number of astroglial cells. We also found reduced cytogenesis among the mPFC areas evaluated. Our results support the idea that early exposure to environmental stressors may have long-lasting consequences affecting complex cognitive processes. These results also suggest that glial changes may become an important element behind the cognitive and morphological alterations accompanying the PFC changes seen in some stress-related pathologies.
Keywords: Cingulate cortex, cytogenesis, development, environmental stress, glia, working memory
Introduction
Noise is a ubiquitous element of the environment that recently has been recognized as a serious health problem. It has been vastly documented that noise exposure induces both auditory and non-auditory damage (Schiff, Barber et al., Seidman and Standring).[1,2,3] Damage to the auditory system is mainly associated to the loss of hearing capacity, a condition affecting 250 million people worldwide (for review see Seidman and Standring).[3] In the other hand, annoyance and the corresponding activation of the stress response are the most frequently reported non-auditory affections (Kryter, Stansfeld and Matheson).[4,5] It has been reported on this regard that high intensity noise may induce serious physiological or psychological disturbances affecting the endocrine, nervous, and cognitive systems (van Raaij et al., Chang and Merzenich, Gonzalez-Perez et al., Jauregui-Huerta et al., Reed et al).[6,7,8,9,10]
It is well-known for example that noise may impair the ability of subjects to solve complex cognitive tasks. The cognitive impairment associated to noise and other environmental stressors may become particularly evident over the processes associated with memory (for review see Sandi and Pinelo-Nava).[11] Working memory is a highly-evolved cognitive function that allows the animal to act upon representations of a cue over a delay period in which the cue is absent. Working memory and its associated functions (alternation/discrimination) are also recognized as crucial stress-vulnerable processes (Dudchenko).[12] The prefrontal cortex (PFC) has been accepted as the main cerebral mediator of this cognitive function. Between other roles, the PFC possess networks of neurons that support the ability of the subjects to maintain information in absence of the stimuli, to protect representations from interferences, to monitor errors, and to promote flexible behavior in order to mend errors (for review, see Arnsten).[13] The PFC is also delicately sensitive to environmental stressors (Manikandan et al., Garrido et al).[14,15] Accordingly, the medial prefrontal cortex (mPFC) expresses a high number of corticosteroid receptors that become activated during the stress response (McDougall et al).[16]
In the recent years, morphometric studies have evidenced that the PFC, as well as the hippocampus, suffers selective volume reduction under stress conditions. Growing data suggest that glial loss may contribute at least in part to this phenomenon and that lower glial numbers might also be associated with the cognitive alterations produced by stress (Barros et al., Rajkowska and Miguel-Hidalgo, Banasr et al., Banasr and Duman).[17,18,19,20] Due to its versatile nature, astrocytes represent the most attractive cell population in order to explain the contribution of glia to the structural volume reduction (for review, see Jauregui-Huerta et al).[21]
It is also known that early development is strongly influenced by environmental factors. Experimental evidence has demonstrated that early adverse experience can modify the conformation of cerebral cytoarchitecture. Cerebral structures whose maturation process conclude at postnatal stages of life (i.e., the PFC) are specially vulnerable (Heim and Nemeroff).[22] Early aversive experiences have been proposed a major epigenetic factor contributing to the beginning of some stress-related pathologies including depression, schizophrenia, and posttraumatic stress disorder. All of these conditions involves crucially PFC structure and function (Moghaddam and Jackson).[23] Since the medial prefrontal cortex (mPFC) represents one of the most vulnerable extra-auditory structures to the damaging effect of environmental stressors (Manikandan et al., Garrido et al),[14,15] we tested here the hypothesis that chronic exposure to environmental noise at the juvenile age that modifies the mPFC in a long-term manner. The objective of this study was then to evaluate the long-term effect of noise on mPFC by assessing both, histochemycal changes in the main subareas of the mPFC, and the related working memory changes by using the alternation/discrimination paradigm of the T-maze apparatus.
Methods
Animals
The subjects were 30 Swiss Wistar male rats obtained from an in-house breeding facility at the Centro de Investigacion Biomedica de Occidente, IMSS, Guadalajara, Mexico. The rats were weaned on postnatal day 21 (PND 21), housed in standard polycarbonate cages, and maintained on a 12 h light-dark cycle, with lights on at 07:00. Standard Purina rat chow pellets and tap water were provided ad libitum. Experimental procedures were approved by the institutional ethics commission and were in accordance to the U.S National Institute of Health Guide for the Care and Use of Laboratory Animals. Body weight was measured routinely on PNDs 21, 24, 27, 30, 34, 37, 41, 45, and 50. Immediately after weaning (21 PND), the animals were randomly assigned to Control (n = 15) or Noise (n = 15) conditions. Control animals remained in the same standard conditions while the Noise group was treated as follows.
Early-life exposure to noise (from PND 21 to PND 35)
In order to better adjust to the objective of our experiment (i.e., the extra-auditory effects of noise) and to avoid unnecessary interferences with the rats’ auditory system whose development is critical during weaning (0-21 PN) (Bures et al),[24] we chose the rats’ juvenile period of life (21-35 PN). The juvenile-prepubescent period is a stress-sensitive stage that resembles the human childhood (Horovitz et al., Horovitz et al).[25,26] Beyond the fact that auditory structures are relatively mature, we also attended the role of this stage as a critical period for the maturation of executive functions and their underlying neural circuitries (i.e., medial prefrontal cortex circuitries) (Van Den Berg et al., Tsoory et al., Fuentes et al., Horovitz et al).[26,27,28,29] Then, we handled the rat in a time window previously validated for the study of enduring effects of early-life stress (Chen et al., Ishikawa et al., Kaffman)[30,31,32] while avoid other non-desirable situations (i.e., causing additional stress by handling animals during weaning, affecting hearing structures, etc.). To this end, the rats’ audiogram-fitted adaptation of a noisy environment (kindly provided by Dr. A. Rabat) was employed (Rabat et al., Jauregui-Huerta et al., Jauregui-Huerta et al).[9,33,34] Figure 1 illustrates the general procedure followed in this experiment. Briefly, urban audio files containing unpredictable noise events with a duration ranging from 18-39 s and spaced by silent intervals ranging from 20-165 s were randomly presented to rats in a 24 h fashion throughout the 15 days post-weaning (i.e., PNDs 21-35). Animals were housed in a special sound-isolated acoustic stress chamber afforded with professional tweeters (Steren 80-1088) suspended 60 cm above the solid grid cages and connected to an amplifier (Mackie M1400; freq. 20 Hz--70 kHz; 300 W-8 Ω) equipment with mixer software that delivered the acoustic signal at levels ranging from 70 dB for the background noise to 85-103 dB for the noisy events. To make sure that the sound intensity was homogeneous at all places in the cage, noise intensity was measured by placing a sound-level meter (Radio Shack, Mexico) (Bohbot et al).[35]
Figure 1.
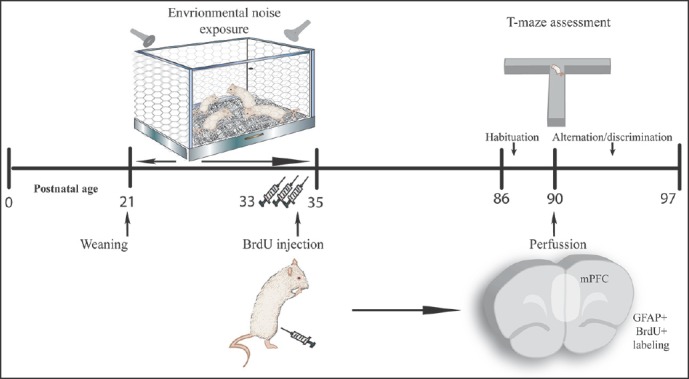
General procedure: Illustrates the general procedure followed in our experiment. Experimental procedures are chronologically depicted above and below the line. Postnatal age is written below. Control animals followed the same procedure except for the noise exposition occurring between PND 21 to 35. Immunohistochemical and cognitive assessments were carried out on different rats (n = 5 for immunohistochemistry and n = 10 for T-maze procedure)
BrdU injections
To label newly born cells at the mPFC of the early-life exposed rats, we injected the thymidine analog Bromodeoxiuridine (BrdU) (Sigma-Aldrich, St. Louis, Missoruri) in each of the last 3 days of exposition [Figure 1]. 5 animals per group were injected at 07:00 h using a single daily dose of 50 mg/kg i.p. (Cameron and McKay).[36] So, we labeled mitotic cells at the time of the exposure (21-35 PND) and quantified these newborn-surviving cells corresponding to astrocytes 3 months later when the animals were sacrificed for hystochemical analysis (90 PND).
Corticosterone assays
Immediately after the noise exposure, another five rats per group were randomly selected in order to obtain serum via the quick tail vein sampling method. The quick tail method was selected in order to avoid additional stress for animals and facilitate the use of these animals on behavioral assessments (Fluttert et al).[37] Blood samples were obtained immediately after the noise was ended at PND 36 (between 07:00 and 08:00, in order to avoid circadian variation). Plasma corticosterone levels were measured using an enzyme immunoassay kit (Correlate-EIA from Assay Designs Inc., USA).
Long-term assessments
Once the animals completed the noise protocol (35 PND), we kept them in standard conditions until 90 PND and then proceed to evaluate the long-term effects of exposure. At the age of 90 PN, 2 subgroups of rats were created in order to evaluate cognitive and hystochemical changes for each condition. From the 15 rats belonging to each condition (control and noise), 10 rats were assigned to the T-maze assessment, and the remaining 5 BrdU-injected rats were assigned to histochemical analysis as follows:
T-maze procedure
As stated before, 10 rats per condition were assessed when reached the age of 90 PND in order to estimate the remaining effects of the previous early-life exposure on cognition. To this end, we employed a modified version of the classical T-Maze that accurately evaluates mPFC-related functions (Shoji et al).[38] The followed protocol included:
-
a.
Apparatus setting (we used a partitioned T-maze with automatic sliding doors and pellet dispenser),
-
b.
Animal preparation (we housed the animals in recommended conditions and transported them always at the same time period with at least 30 min before the first trial),
-
c.
Food restriction (animals were maintained at 80% to 85% of their free-feeding body weight),
-
d.
Habituation-pre-training (animals were allowed free exploration with open doors for habituation followed by a daily/5 trials pre-training period with closed doors),
-
e.
Forced alternation task (animals executed 10 consecutive trials in a session per day, each trial consisted of one forced choice followed by one free choice run in order to detect working memory changes), and
-
f.
Left-right discrimination task (animals executed 10-20 consecutive trials in one session per day consisting of one free choice run in order to appreciate reference memory changes) (See Figure 2 explanation for a better understanding).
Figure 2.
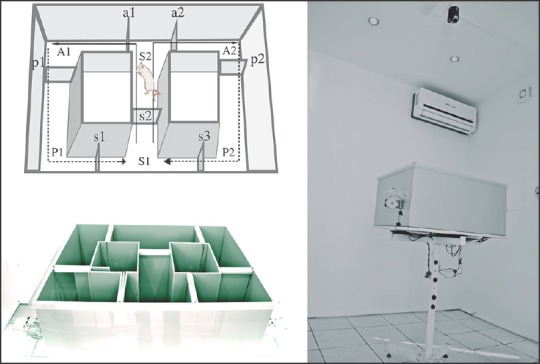
T-maze Apparatus. Diagrams on the left part illustrate the mechanics of this protocol. The apparatus was divided into 6 areas (A1, A2, S1, S2, P1, P2) by sliding doors (s1, s2, s3, a1, a2, p1, p2) that the animals explored during the training sessions. Animals were placed in the star box (area s1) and allowed to explore the corresponding area according the task (forced alternation or left-right discrimination). The forced-choice run started with the doors s2, a1 and a2 in the open position. The sucrose pellet was delivered to the food compartment of the open area. The animal consumed the pellet and returned to the start box because doors p1 or p2 were closed while s1 or s3 were opened. Once completed the forced-choice run, a free choice run begun with doors s2, a1 and a2 in the open position. Animals then choose between the two arms. If the animal choice was the same than the forced choice, it was then considered and reported as “correct” choice. This procedure was repeated for 10 consecutive trials in a session per day. Once the animals completed the 80% correct response criterion, they were tested introducing a delayed alternation period of 10, 30 and 60 s between the forced and the free-choice runs. In the left-right discrimination task the animals were allowed to freely choose between left or right arms. The pellet was delivered onto the goal arm. If the animal choice was this arm, it was considered a correct response. Once the animal completed this part, it was allowed to return to the start box. The sucrose pellet was always delivered on the same arm (the goal arm). Assessment was conducted in a soundproof room provided with a close circuit camera (depicted in the right part of the figure)
Animal behavior in the T-Maze was video-recorded and analyzed using the Image TM software. We analyzed the percentage of correct responses and the latency to complete a session.
Astrocyte/proliferative analysis
For an estimation of mPFC hystochemical changes associated to the early-life experience, the animals (n = 5/group) that received the BrdU injections when juveniles were perfused at 90 PND using a standard protocol (Hernandez et al).[39] We performed a double immunofluorescence staining method to confocally co-localize cells that went through mitosis (BrdU+) and co-localize with the filamentary protein GFAP expressed throughout the cytoskeleton of astrocytes (astrogenesis). The total number of BrdU+ cells was reported as cytogenesis while the BrdU+ cells that co-localize with GFAP+ was reported as astrogenesis. Cells that expressed the GFAP marker were also counted and reported as the total astrocyte numbers. We considered that BrdU and GFAP co-localized when green nuclear fluorescence (BrdU) coincided with red soma fluorescence (GFAP) over consecutive 1 μm z-stacks, and when co-localization was confirmed in x-y, x-z, and y-z cross-sections. We assessed BrdU+GFAP changes using a confocal laser-scanning microscope (Leica TCS SP2) connected to a PC running the Leica confocal software (LCS). For every section, the percentage of co-localization of BrdU+ cells was calculated as the fraction of the number of BrdU+ cells that co-expressed GFAP/the total number of BrdU+ cells per section multiplied by 100. Figure 3 illustrates the anatomic location of regions and staining methods used for this purpose.
Figure 3.
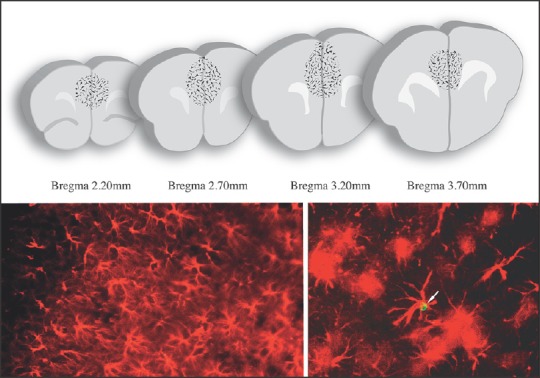
mPFC immunohistochemical analyses. Counting of BrdU (green cell close to the arrow highlighted in micrograph posted in the inferior right part) and GFAP (red star-like cells showed in inferior micrographs) positive cells carried out in the mPFC. Regions of interest are displayed on a series of slices depicted in the top of the figure
Immunofluorescence protocols were conducted as follows: A series of free-floating sections obtained from bregma 3.7 mm to 2.2 mm (Paxinos and Watson)[40] were first pre-treated to denature DNA, sections were incubated in 2N HCL for 30 min at 37° C and rinsed with 0.1M borate buffer (pH 8.5). Then, sections were washed with phosphate buffer (PB) and incubated with blocking solution (10% normal goat serum in 0.1 phosphate buffer) for 60 min at room temperature. Tissues were then incubated for 12 h at 4° C with the monoclonal antibody against BrdU (rat anti-BrdU, Accurate Scientific, OBT003, dilution 1:1000) and the polyclonal antibody against GFAP (rabbit anti-GFAP, Dako, dilution 1:500). After rinsing in PB, sections were incubated for 2 h with the secondary antibodies (Alexa fluor 488 goat anti-rat IgG and Alexa fluor 594 goat anti-rabbit from Molecular Probes in a dilution 1:1000). Sections were mounted on slides and cover-slipped using fluorescent mounting media (Vectashield Vector Labas, Burlingame, CA).
Series of systematically selected brain sections (40 μm-thick every 120-μm starting on bregma –3.7 and ending on bregma –2.2) were manually counted using a X400 magnification for a) BrdU+ cells, b) GFAP+ cells, and c) BrdU+GFAP+ cells. An additional optical density analysis was conducted over selected (10 cells/area) GFAP-positive cells in order to elucidate morphological differences between groups. Individual cells were delineated using the «lasso» tool (Apple inc.), extracted and placed on Image J (NIH free software) where we obtained optical density for every extracted cell [Figure 3].
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). For astrocyte/BrdU+ cells we used the Student t test to compare groups. Behavioral data was analyzed by analysis of variance (ANOVA) comparing condition x session or condition x delay. In all cases, a value of P ≤ .05 was chosen to establish significant differences. SPSS version 19 was used to run all statistics.
Results
Stress-like effects of early life environmental noise exposure
To determine whether environmental noise produces stress-like effects on exposed rats, we assessed serum corticosterone (CORT) levels at PND 36 and weighed rats from PND 21 to PND 50.
Serum CORT levels obtained from tail veins at the morning of PND 36 were significantly higher in environmental noise (EN)-exposed rats (7.31 ± 1.0) than those found in control rats (2.67 ± 1.20) (F .210) t = –2.952/–2.952 (P £0.05). Figure 4 illustrates the mean ± SEM CORT levels on each group. Stressing effects of EN were confirmed by body weight measure since exposed rats gain weight in a less pronounced curve than controls (Jauregui-Huerta et al).[34] Differences were particularly marked at days 34 (137.60 ± 3.53 vs 155.04 ± 2.72) (F .455) t = 3.954/3.885 (**P < 0.001) and 37 (157.48 ± 4.82 vs 172.52 ± 2.51) (F .446) t = 2.831/2.764 (*P < 0.01). Figure 4 right side illustrates the mean ± SEM body weight gain.
Figure 4.
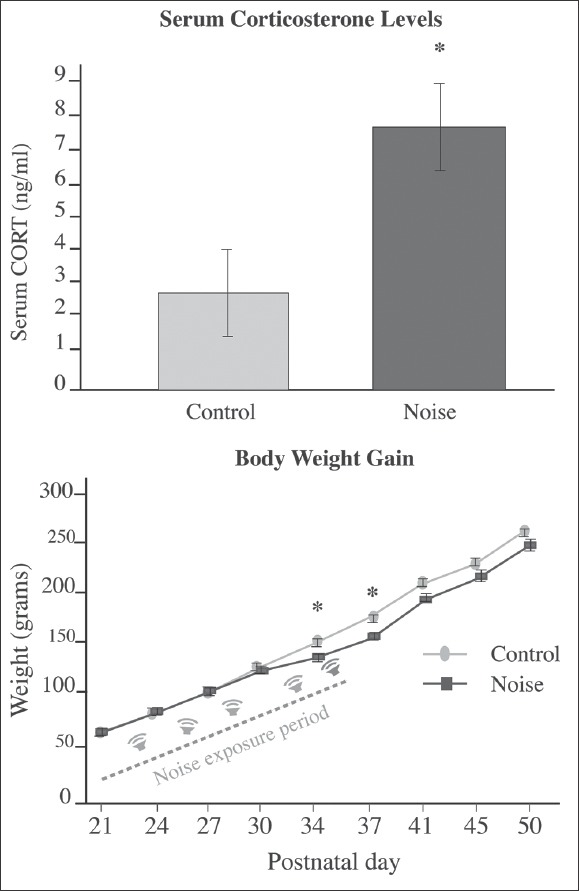
Stress-like effects of noise exposure. Left: Mean ± S.E.M plasma levels of corticosterone obtained after exposure to environmental noise. Increased CORT levels were found immediately after exposure to noise (*P < 0.05). Right: Body weight gain during the first 7 weeks of the experiment. The rat's body weight gain is expressed in grams from 21 to 50 PND. The noise-exposed rats gained less weight as compared to the control rats (statistically significant only on 34 and 37 PND). *P < 0.05. Bars represent the mean ± SEM
T-Maze execution
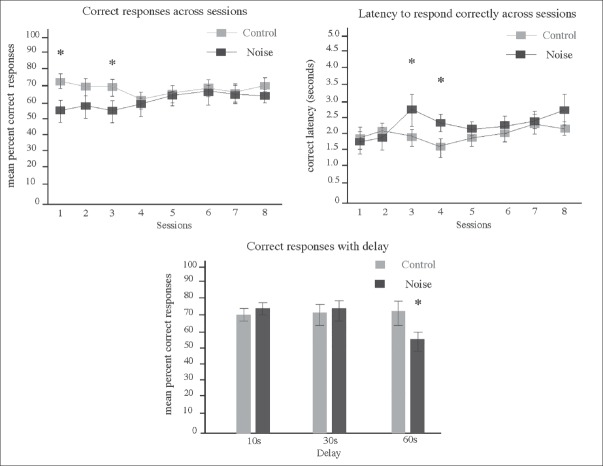
As established before, animals exposed to EN when juveniles were evaluated on the T-maze apparatus 3 months later. We analyzed with this design the long-term effects of this exposition on mPFC-associated cognition. As seen in Figure 5, performance of noise exposed and non-exposed rats was different between groups. EN exposed rats showed a significant lower percentage of correct responses than control rats during the first 3 days of execution. Such difference was statistically significant on days 1 (39.44 ± 11.7 vs 82.22 ± 9.0) (F 2.109), t = -2.872/-2.872 (*P < 0.05), and 3 (52.72 ± 5.89 vs 83.20 ± 7.0) (F 0.996) t = -3.327/3.301 (*P < 0.05).
Figure 5.
T-Maze execution on adult rats exposed to environmental noise when juveniles. Top Chart shows results of correct responses across sessions illustrating Mean ± SEM. Significant interactions were found on mean percent of correct responses for sessions 1 and 3 (*P < 0.05) where EN exposed animals showed a reduced number of correct responses. Middle chart shows results of correct latency quantification. Significant interactions were found on sessions 3 and 4 where EN exposed animals increased the latency to respond correctly (*P < 0.05). Bottom chart shows mean percent of correct responses (10 trials/day) on the forced alternation task with delays of 10 (day 9), 30 (day 10), and 60 sec (day 11). Exposed rats showed less percent of correct responses when a 60 sec delay period was introduced (*P < 0.05)
We also registered the time that the animals employed to reach the correct goal. We report this observation as correct latency. Figure 5 (middle chart) shows the mean ± SEM values obtained here. EN-exposed rats showed higher latencies than control rats. Such differences were statistically significant on days 3 (22.57 ± 5.25 vs 9.59 ± 0.75) (F 5.312), t = 2.935/2.448 (*P < 0.05), and 4 (7.84 ± 1.31 vs 3.30 ± 0.25) (F 10.989) t = 3.391/3.391 (*P < 0.05).
Finally, in the delayed alternation task, the correct choice percentages of EN-exposed rats (58.00 ± 9.0) were significantly lower than those of the control group (59.44 ± 4.13) when we introduced the 60 sec delay period (F 4.512) t = 0.145/0.145 (*P < 0.05) (Figure 5 at bottom chart illustrates this values).
mPFC immunohistochemical analysis
In this part of the experiment, we assessed the long-term effects of early life exposure to noise on mPFC astrocytes. To this end, we counted the total number of GFAP+ cells on prelimbic, infralimbic (8 sections, coordinates: 4.20-2.20 mm from bregma), and cingulate cortices (6 sections, coordinates: 2.7-1.2 mm from bregma). By adding the BrdU proliferative marker, we also obtained a general view of changes affecting cytogenesis. Figure 6 shows the mean ± SEM GFAP-positive cells (left-top), the mean ± SEM BrdU-positive cells (left middle-top), the percent of double BrdU+GFAP+ -astrogenesis- labeled cells (left middle-bottom), and the mean ± SEM optical density measurement (left-bottom) of individual astrocytes extracted from corresponding areas.
Figure 6.
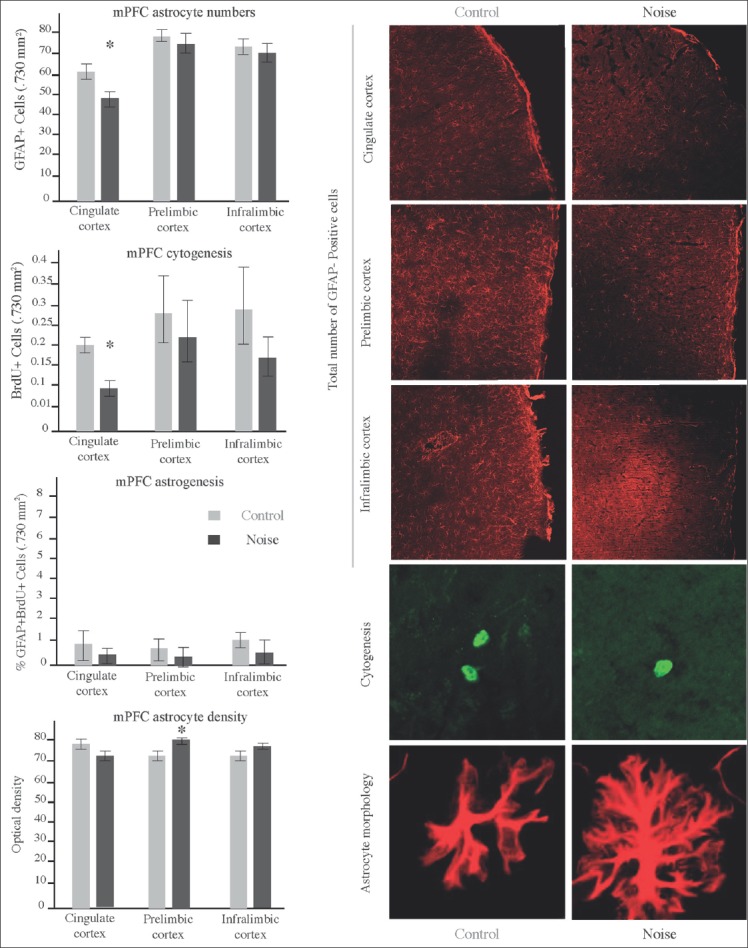
Effects of early-life noise exposure on mPFC cytogenesis and astrogenesis. Left Panel contains graphics illustrating mean ± SEM of GFAP-positive cells (Top), BrdU-positive cells (middle-top), double labeled cells (middle bottom), and individual astrocyte optical density (bottom) in cingulate cortex, prelimbic cortex and infralimbic cortex. Cingulate cortex of EN exposed rats exhibited a significant reduction of GFAP and BrdU positive cells (*P < 0.05) but not of double-labeled cells. Astrocytes from infralimbic area exhibited a more robust morphology evidenced by higher optical densities (*P < 0.05). Right panel illustrates differences among groups including staining for GFAP (red upper slices 730μ × 730μ), BrdU (stylized green cells in the middle-bottom pictures) and individual astrocyte density (stylized red cells in the bottom pictures)
Analysis of the different mPFC regions demonstrated that EN affected cytogenesis by reducing the number of BrdU labeled cells. Such phenomenon was statistically significant in cingulate cortex (0.1250 ± 0.035 vs 0.1942 ± 0.055) (F 4.431), t = 1.103/1.1054) (P < 0.05). Both, prelimbic and infralimbic regions evidenced nonsignificant decrease in BrdU-labeled cells.
Quantification of the total astrocyte numbers revealed that EN affected astroglial cells in a long-term mode. Again, cingulate cortex resulted the most affected region exhibiting a significant reduction in the number of GFAP+ cells (49.04 ± 2.62 vs 61.17 ± 2.89) (F 1.024) t = 3.079/3.103, (P < 0.05). If so, astrocyte numbers were also reduced in prelimbic and infralimbic regions, such reduction was not statistically significant.
Confocal microscopic analysis revealed no changes in astrogenesis of the EN exposed rats. Percent of colocalization of BrdU with the GFAP marker of astrocytes was in general reduced for control and exposed rats (the number of BrdU cells that colocalize with GFAP was less than 10%). From the limited double-labeled cells detected, no differences were found between groups.
Analysis of individual astrocyte morphology revealed enlargement of the cytoplasmic arborizations. Hipertrophic changes were statistically significant in prelimbic cortex (81.11 ± 1.16 vs 72.02 ± 1.20) (F 3.744) t = 2.729/2.717 (P < 0.05).
Discussion
The aim of the present study was to determine the long-term effects of noise exposure on the mPFC structure and function. We choose for this purpose the most abundant and versatile cell population in brain named astrocytes, and one of the most strongly associated cognitive task to the mPFC named working memory (Pontecorvo et al., Shoji et al., Ishikawa et al).[31,38,41] We found decreased numbers of astrocytes and proliferative cells among mPFC of the exposed rats. We also found an impairing effect of this early noise exposure over the cognitive skills undergoing alternation/discrimination tasks at adulthood. These non-auditory effects of environmental noise were associated to a marked activation of the stress response system.
The PFC has long been proposed as the main cerebral structure mediating the most evolved cognitive skills (i.e., working memory, inhibitory behavior, behavioral flexibility) and one of the most important structures mediating adaptation to aversive conditions. The PFC has also been extensively studied in the context of psychiatric illness where early life experience and PFC cytoarchitecture have converged to offer complementary explanations to the onset and/or evolution of depression, addiction, and post-traumatic stress disorder. Previous studies have in fact demonstrated that aversive experience negatively affects cytogenesis in the mPFC of rats (Czeh et al. Banasr and Duman, Banasr et al).[20,42,43] Our experiment extends these results providing for the first time evidence that these cytogenic changes may persist over long periods of time. As showed on the results section, the numbers of BrdU+ cells were reduced about 30% in the noise-exposed rats. Considering the fact that these cells were marked at early stages and visualized 3 months later, our results reflects in fact the long-term survival of these newborn cells. Thereafter, we provide here arguments to believe that some cytogenic changes reported on adult “stressed” brains might be explained at least in part by early-life experiences affecting proliferation.
Glial cells were included in this context (for review, see (Smialowska et al)[44] when former postmortem studies reported decreased glial cell density in prefrontal and cingular areas of depressed patients. As chronic stress has been considered a hallmark of depressive illness, these data may in fact reflect the glial vulnerability to stressing conditions (Rajkowska, Rajkowska and Miguel-Hidalgo).[18,45] Outstanding studies preceding ours confirmed that some aversive experiences might affect the PFC integrity by reducing the glial cell numbers (Czeh et al., Banasr and Duman, Banasr et al).[20,42,43] Given the fact that the astrocyte lineage represents the most abundant and versatile population over the brain, it would be admissible that astrocytes should be affected under stress conditions. That was our hypothesis and that was in fact what we found. Accordingly, we planned our experiment having in mind that astrocytes could change its proliferative rate early in life, and that this condition could negatively impact the total numbers at later stages. However, we fail in our effort to explain this reduction under astrogenic reasons. A careful review of the papers previously cited allows us to believe that even when the total numbers were reduced at the end of the experiment, there is not enough evidence to sustain that this reduction obeyed to a proliferative depletion. As Banasr et al. pointed, gliogenesis in PFC is markedly scarce (Banasr et al).[46] Then, it is highly possible that reductions on astrocyte density reported in this and other stress-related studies must be due to non-proliferative changes (i.e., differentiation, apoptotic changes, etc.). Additional experiments should then clarify the particular contribution of astrocytes and other glial cells (i.e., NG2+ or microglia) to the long-term proliferative depletion seen in this experiment. It must be noted at this point that the reduction on astrocyte numbers produced by noise was at least in part compensated by morphological changes. Astrocytes belonging to the noise group exhibited a more ramified and intensely stained morphology as we qualitatively appreciated on infra and prelimbic areas. Considering the crucial role of protoplasmatic astrocytes in synaptic transmission and neural tissue protection and regeneration, it is not surprising that astrocytes generate this morphological change in order to compensate their numerical declive. According to this, it has been demonstrated that changes in GFAP synthesis become one of the earliest astrocyte responses against environmental threats and that some specific epigenetic changes may affect its expression on selected stress pathologies (Nagy et al).[47] So far, even when other studies suggest that astrocytic changes fade rapidly once experience is discontinued, at least for acute experiences (Braun et al),[48] we evidenced here a permanent change on the intermediate filaments arrangement whose functional correlate should be elucidated. We may speculate that glial impairment lies behind some other physiological changes associated to stress. It has been reported for example that glutamate concentrations are frequently increased in the prefrontal cortex of stressed subjects (Martin and Wellman).[49] It is also known that astrocytes contribute crucially to the supply and cleaning of glutamate for neurons (Popoli et al).[50] So, astrocyte malfunction may become a crucial factor explaining the neurotransmitter imbalance seen in subjects exposed to aversive conditions. Moreover, in accordance with a contemporary experiment, our results also support the idea that glial damage may underlie as a former step, the cognitive impairment seen in subjects who fail when execute tasks that depend on the integrity of this brain region (Lima et al).[51]
Interestingly, cytological changes produced by noise in our experiment were particularly pronounced over the cingulate cortex. This result is consistent with Cohen et al., which reported smaller anterior cingulate cortex volumes on people who have experienced significant early-life stressing events (Cohen et al).[52] So, this data suggest that the developing cingulate cortex may be a key structure mediating the long-term effects of early aversive circumstances. Future studies should focus this structure.
It is well-known that the cingulate cortex as a privileged part of the mPFC, is crucial for the intentional control of behavior and also participates in the superior control of the stress response (Cohen, Cohen et al).[53,54] It has also been demonstrated that the entire mPFC supports some of the most complex cognitive functions including response inhibition, temporal organization of behavior and working memory (Cerqueira et al).[55] Having this in mind, our experiment included a behavioral correlate of mPFC function. We choose the T-maze alternation/discrimination paradigm due to its proved ability to exhibit changes on the rats’ mPFC integrity (Shoji et al).[38] We confirm with our results that these executive functions are sensible to the effects of environmental stressors.
Using an eight-arm water maze protocol, we previously found that the long-term effects of early life-noise mostly become evident when the animal must to relearn the task (Jauregui-Huerta et al).[34] So, trying to get a better understand of these results, we perform this new experiment with focus on the mPFC alternation/discrimination related tasks. Subjects solving this kind of paradigms must employ recent information to make correct choices. This process implies indeed abilities like behavioral flexibility, monitoring, and mending of errors (Arnsten).[13] Viewed as a whole, our results support the idea that aversive stimuli when is presented at early stages of life promote inflexibility. As the reader might note, effects of early exposure on adult T-maze seems transient so execution profiles become equal at the end of the assessment. It must be noted, however, that the assessment was conducted 3 months after the end of the exposure and that relevant changes remained until the adult stage. Even when damage seems not catastrophic for working memory, it is evident that the noise-exposed rats exhibited a delayed curve of learning. This delayed curve of learning coincides with the perseverative behavior profile reported in other experiments (El Massioui et al. Clinton et al. Jauregui-Huerta et al., Guariglia and Chadman).[34,56,57,58] Then, we believe on this basis that environmental noise may represent an important source of stress that silently exacerbates the behavioral inflexibility frequently seen in stress-derived pathologies (Nikiforuk and Popik).[59] So, inflexibility/perseveration are in fact the underlying sequela behind alternation/discrimination. Support for this affirmation may be extracted from studies showing that juvenile stress has powerful associations with the onset of mood and/or anxiety disorders frequently characterized for perseveration and other cognitive changes (Horovitz et al., Horovitz et al).[25,26] Specifically, working memory impairment has been proposed a reliable predictor for the development of posttraumatic stress disorder in subjects suffering continuous traumatic events (Roncone et al).[60] Recent experiments in rats also confirmed that working memory is particularly sensible to the long-term effects of early aversive circumstances (Jin et al).[61] It must not be disregarded that the aversive condition used in this experiment belongs to a class of environmental stressors, whose presence in modern societies is often excessive and frequently disregulated. Thereafter, besides the short-term proved damaging effect of noise over cognitive skills and other auditory functions (Chang and Merzenich, Reed et al),[7,10] it must now be attended the long-term effect proposed in this experiment.
Conclusion
In conclusion, these results support the idea that early exposure to environmental noise may have long-lasting consequences affecting cognitive processes behind working memory, decision-making and/or error correction. These results also suggest that glial loss may be an important element underlying the cognitive and cytoarchitectural alterations accompanying the PFC changes frequently seen in some stress-related pathologies.
Acknowledgments
We thank Dr. Arnoud Rabat for the donation of noise recordings. We also thank to Pamela Hernandez, Griselda Yañez, Gustavo Chipres, and Tania Morales for their generous dedication to the lab's projects as volunteers. To Dr. Limei Zhang and Vito Hernandez for their helpful discussion.
Footnotes
Source of Support: This work was supported by research grants: CONACyT 221092 to FJH, CONACyT 238313 to YRD, PROMEP red UDG CA-63 UCOL CA-5 and IMSS followship to YRD 2011032.
Conflicts of Interest: The authors declare that they have no competing interests.
References
- 1.Schiff M. Nonauditory of effects of noise. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:ORL384–98. [PubMed] [Google Scholar]
- 2.Barber JR, Crooks KR, Fristrup KM. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol Evol. 2010;25:180–9. doi: 10.1016/j.tree.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Seidman MD, Standring RT. Noise and quality of life. Int J Environ Res Public Health. 2010;7:3730–8. doi: 10.3390/ijerph7103730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kryter KD. Non-auditory effects of environmental noise. Am J Public Health. 1972;62:389–98. doi: 10.2105/ajph.62.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stansfeld SA, Matheson MP. Noise pollution: Non-auditory effects on health. Br Med Bull. 2003;68:243–57. doi: 10.1093/bmb/ldg033. [DOI] [PubMed] [Google Scholar]
- 6.van Raaij MT, Dobbe CJ, Elvers B, Timmerman A, Schenk E, Oortigiesen M, et al. Hormonal status and the neuroendocrine response to a novel heterotypic stressor involving subchronic noise exposure. Neuroendocrinology. 1997;65:200–9. doi: 10.1159/000127273. [DOI] [PubMed] [Google Scholar]
- 7.Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Perez O, Chavez-Casillas O, Jauregui-Huerta F, Lopez-Virgen V, Guzman-Muniz J, Moy-Lopez N, et al. Stress by noise produces differential effects on the proliferation rate of radial astrocytes and survival of neuroblasts in the adult subgranular zone. Neurosci Res. 2011;70:243–50. doi: 10.1016/j.neures.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Jáuregui-Huerta F, Garcia-Estrada J, Ruvalcaba-Delgadillo Y, Trujillo X, Huerta M, Feria-Velasco A, et al. Chronic exposure of juvenile rats to environmental noise impairs hippocampal cell proliferation in adulthood. Noise Health. 2011;13:286–91. doi: 10.4103/1463-1741.82961. [DOI] [PubMed] [Google Scholar]
- 10.Reed AC, Centanni TM, Borland MS, Matney CJ, Engineer CT, Kilgard MP. Behavioral and neural discrimination of speech sounds after moderate or intense noise exposure in rats. Ear Hear. 2014;35:e248–61. doi: 10.1097/AUD.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast 2007. 2007 doi: 10.1155/2007/78970. 78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manikandan S, Padma MK, Srikumar R, Jeya Parthasarathy N, Muthuvel A, Sheela Devi R. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neuroscience Lett. 2006;399:17–22. doi: 10.1016/j.neulet.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Garrido P, De Blas M, Ronzoni G, Cordero I, Antón M, Giné E, et al. Differential effects of environmental enrichment and isolation housing on the hormonal and neurochemical responses to stress in the prefrontal cortex of the adult rat: Relationship to working and emotional memories. J Neural Transm. 2013;120:829–43. doi: 10.1007/s00702-012-0935-3. [DOI] [PubMed] [Google Scholar]
- 16.McDougall SJ, Widdop RE, Lawrence AJ. Medial prefrontal cortical integration of psychological stress in rats. Eur J Neurosci. 2004;20:2430–40. doi: 10.1111/j.1460-9568.2004.03707.x. [DOI] [PubMed] [Google Scholar]
- 17.Barros VG, Duhalde-Vega M, Caltana L, Brusco A, Antonelli MC. Astrocyte-neuron vulnerability to prenatal stress in the adult rat brain. J Neurosci Res. 2006;83:787–800. doi: 10.1002/jnr.20758. [DOI] [PubMed] [Google Scholar]
- 18.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–33. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: Reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–70. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jauregui-Huerta F, Ruvalcaba-Delgadillo Y, Gonzalez-Castañeda R, Garcia-Estrada J, Gonzalez-Perez O, Luquin S. Responses of glial cells to stress and glucocorticoids. Curr Immunol Rev. 2010;6:195–204. doi: 10.2174/157339510791823790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–22. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 23.Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotoxicity Res. 2004;6:73–8. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- 24.Bures Z, Grécová J, Popelár J, Syka J. Noise exposure during early development impairs the processing of sound intensity in adult rats. Eur J Neurosci. 2010;32:155–64. doi: 10.1111/j.1460-9568.2010.07280.x. [DOI] [PubMed] [Google Scholar]
- 25.Horovitz O, Tsoory MM, Hall J, Jacobson-Pick S, Richter-Levin G. Post-weaning to pre-pubertal (‹juvenile›) stress: A model of induced predisposition to stress-related disorders. Neuroendocrinology. 2012;95:56–64. doi: 10.1159/000331393. [DOI] [PubMed] [Google Scholar]
- 26.Horovitz O, Tsoory MM, Yovell Y, Richter-Levin G. A rat model of pre-puberty (juvenile) stress-induced predisposition to stress-related disorders: Sex similarities and sex differences in effects and symptoms. World J Biol Psychiatry. 2014;15:36–48. doi: 10.3109/15622975.2012.745604. [DOI] [PubMed] [Google Scholar]
- 27.Van Den Berg CL, Van Ree JM, Spruijt BM. Sequential analysis of juvenile isolation-induced decreased social behavior in the adult rat. Physiol Behav. 1999;67:483–8. doi: 10.1016/s0031-9384(99)00062-1. [DOI] [PubMed] [Google Scholar]
- 28.Tsoory M, Guterman A, Richter-Levin G. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–93. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- 29.Fuentes S, Carrasco J, Armario A, Nadal R. Behavioral and neuroendocrine consequences of juvenile stress combined with adult immobilization in male rats. Horm Behav. 2014;66:475–86. doi: 10.1016/j.yhbeh.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Chen LJ, Shen BQ, Liu DD, Li ST. The effects of early-life predator stress on anxiety-and depression-like behaviors of adult rats. Neural Plast 2014. 2014 doi: 10.1155/2014/163908. 163908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa J, Nishimura R, Ishikawa A. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. Eur J Neurosci. 2015;41:442–53. doi: 10.1111/ejn.12825. [DOI] [PubMed] [Google Scholar]
- 32.Kaffman A. Early-life stress restricts the capacity of adult progenitor cells to differentiate into neurons. Biol Psychiatry. 2015;77:307–9. doi: 10.1016/j.biopsych.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabat A, Bouyer JJ, Aran JM, Courtiere A, Mayo W, Le Moal M. Deleterious effects of an environmental noise on sleep and contribution of its physical components in a rat model. Brain Res. 2004;1009:88–97. doi: 10.1016/j.brainres.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 34.Jauregui-Huerta F, Ruvalcaba-Delgadillo Y, Garcia-Estrada J, Feria-Velasco A, Ramos-Zuñiga R, Gonzalez-Perez O, et al. Early exposure to noise followed by predator stress in adulthood impairs the rat's re-learning flexibility in Radial Arm Water Maze. Neuro Endocrinol Lett. 2010;31:538–48. [PubMed] [Google Scholar]
- 35.Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with les. 1998;36:1217–38. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 36.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 37.Fluttert M, Dalm S, Oitzl MS. A refined method for sequential blood sampling by tail incision in rats. Lab Anim. 2000;34:372–8. doi: 10.1258/002367700780387714. [DOI] [PubMed] [Google Scholar]
- 38.Shoji H, Hagihara H, Takao K, Hattori S, Miyakawa T. T-maze forced alternation and left-right discrimination tasks for assessing working and reference memory in mice. J Vis Exp. 2012 doi: 10.3791/3300. pii: 3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández VS, Luquín S, Jáuregui-Huerta F, Corona-Morales AA, Medina MP, Ruíz-Velasco S, et al. Dopamine receptor dysregulation in hippocampus of aged rats underlies chronic pulsatile L-Dopa treatment induced cognitive and emotional alterations. Neuropharmacology. 2014;82:88–100. doi: 10.1016/j.neuropharm.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson CR. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [DOI] [PubMed] [Google Scholar]
- 41.Pontecorvo MJ, Sahgal A, Steckler T. Further developments in the measurement of working memory in rodents. Brain Res Cogn Brain Res. 1996;3:205–13. doi: 10.1016/0926-6410(96)00007-9. [DOI] [PubMed] [Google Scholar]
- 42.Czéh B, Müller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: Hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 43.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, et al. Glial pathology in an animal model of depression: Reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry. 2010;15:501–11. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smialowska M, Szewczyk B, Woźniak M, Wawrzak-Wlecial A, Domin H. Glial degeneration as a model of depression. Pharmacol Rep. 2013;65:1572–9. doi: 10.1016/s1734-1140(13)71518-4. [DOI] [PubMed] [Google Scholar]
- 45.Rajkowska G. Depression: What we can learn from postmortem studies. Neuroscientist. 2003;9:273–84. doi: 10.1177/1073858403252773. [DOI] [PubMed] [Google Scholar]
- 46.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015;20:320–8. doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun K, Antemano R, Helmeke C, Büchner M, Poeggel G. Juvenile separation stress induces rapid region- and layer-specific changes in S100ss- and glial fibrillary acidic protein-immunoreactivity in astrocytes of the rodent medial prefrontal cortex. Neuroscience. 2009;160:629–38. doi: 10.1016/j.neuroscience.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 49.Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011;21:2366–73. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lima A, Sardinha VM, Oliveira AF, Reis M, Mota C, Silva MA, et al. Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Mol Psychiatry. 2014;19:834–41. doi: 10.1038/mp.2013.182. [DOI] [PubMed] [Google Scholar]
- 52.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–82. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Cohen RA. Neural Mechanisms of Attention. In: Cohen R, editor. The Neuropsychology of attention. US: Springer; 1993. pp. 211–64. [Google Scholar]
- 54.Cohen RA, Kaplan RF, Meadows ME, Wilkinson H. Habituation and sensitization of the orienting response following bilateral anterior cingulotomy. Neuropsychologia. 1994;32:609–17. doi: 10.1016/0028-3932(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 55.Cerqueira J, Pêgo JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Massioui N, Ouary S, Chéruel F, Hantraye P, Brouillet E. Perseverative behavior underlying attentional set-shifting deficits in rats chronically treated with the neurotoxin 3-nitropropionic acid. Exp Neurol. 2001;172:172–81. doi: 10.1006/exnr.2001.7766. [DOI] [PubMed] [Google Scholar]
- 57.Clinton SM, Sucharski IL, Finlay JM. Desipramine attenuates working memory impairments induced by partial loss of catecholamines in the rat medial prefrontal cortex. Psychopharmacology (Berl) 2006;183:404–12. doi: 10.1007/s00213-005-0221-2. [DOI] [PubMed] [Google Scholar]
- 58.Guariglia SR, Chadman KK. Water T-maze: A useful assay for determination of repetitive behaviors in mice. J Neurosci Methods. 2013;220:24–9. doi: 10.1016/j.jneumeth.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Nikiforuk A, Popik P. Ketamine prevents stress-induced cognitive inflexibility in rats. Psychoneuroendocrinology. 2014;40:119–22. doi: 10.1016/j.psyneuen.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Roncone R, Giusti L, Mazza M, Bianchini V, Ussorio D, Pollice R, et al. Persistent fear of aftershocks, impairment of working memory, and acute stress disorder predict post-traumatic stress disorder: 6-month follow-up of help seekers following the L’Aquila earthquake. Springerplus. 2013;2:636. doi: 10.1186/2193-1801-2-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin F, Li L, Shi M, Li Z, Zhou J, Chen L. The longitudinal study of rat hippocampus influenced by stress: Early adverse experience enhances hippocampal vulnerability and working memory deficit in adult rats. Behav Brain Res. 2013;246:116–24. doi: 10.1016/j.bbr.2013.02.029. [DOI] [PubMed] [Google Scholar]