Abstract
During dental treatments, patients may experience negative emotions associated with the procedure. This study was conducted with the aim of using functional magnetic resonance imaging (fMRI) to visualize cerebral cortical stimulation among dental patients in response to auditory stimuli produced by ultrasonic scaling and power suction equipment. Subjects (n = 7) aged 23-35 years were recruited for this study. All were right-handed and underwent clinical pure-tone audiometry testing to reveal a normal hearing threshold below 20 dB hearing level (HL). As part of the study, subjects initially underwent a dental calculus removal treatment. During the treatment, subjects were exposed to ultrasonic auditory stimuli originating from the scaling handpiece and salivary suction instruments. After dental treatment, subjects were imaged with fMRI while being exposed to recordings of the noise from the same dental instrument so that cerebral cortical stimulation in response to aversive auditory stimulation could be observed. The independent sample confirmatory t-test was used. Subjects also showed stimulation in the amygdala and prefrontal cortex, indicating that the ultrasonic auditory stimuli elicited an unpleasant response in the subjects. Patients experienced unpleasant sensations caused by contact stimuli in the treatment procedure. In addition, this study has demonstrated that aversive auditory stimuli such as sounds from the ultrasonic scaling handpiece also cause aversive emotions. This study was indicated by observed stimulation of the auditory cortex as well as the amygdala, indicating that noise from the ultrasonic scaling handpiece was perceived as an aversive auditory stimulus by the subjects. Subjects can experience unpleasant sensations caused by the sounds from the ultrasonic scaling handpiece based on their auditory stimuli.
Keywords: Amygdala activation, dental noise, dental treatment, patients’ perception, ultrasonic scaling handpiece
Introduction
During dental treatments, patients may experience negative emotions associated with the procedure.[1] These negative emotions could cause patients to fear and delay dental treatment, and to miss treatment opportunities because of the delay, causing more serious damage. In addition to the negative emotions due to direct nociceptive stimulation, the auditory stimuli from the dental instrument (i.e., ultrasonic scaling handpiece) may induce emotional changes in the patient, but this topic has not yet been explored in the recent literature.
Most research demonstrates that negative emotions are associated with activation of the amygdala[2,3] and that these negative emotions may be due to olfactory,[4] gustatory,[5] visual,[6] or auditory stimulation. Specific to auditory stimulation, noxious stimuli include unpleasant music,[7] high-decibel firing alarms,[8] the sound of fingernails scratching across a blackboard, and rubbing of Styrofoam,[9] all of which have been associated with activation of the amygdala. Other studies have reported the emotional responses caused by unpleasant music to activate the right prefrontal cortex.[10] The effect of dental equipment sounds on cerebral cortex activation has not been previously studied, however, and is therefore being reported on in this current study.
Functional magnetic resonance imaging (fMRI) is an ideal tool for observing cerebral cortical activities[11,12,13] as it allows for the comparison of oxygenated hemoglobin in different cortical regions. Cerebral blood flow varies with the intensity of the stimulus, and stronger stimuli elicit higher levels of oxygenated hemoglobin in activated areas. Therefore, by comparing the distribution of oxygenated hemoglobin at any point in time, we are able to observe metabolically active areas of the brain. This study chose fMRI as the key investigative tool due to its noninvasive and nonradioactive properties. Furthermore, sectional imaging in fMRI allows for the monitoring of metabolic activities from the superficial tissue to the deeper brain structures, providing for localization of the metabolically active areas of the cortex at a particular point in time.
In this study, subjects were exposed to the sound from the ultrasonic scaling handpiece commonly heard in dental calculus removal treatments. Their cortical response was imaged with fMRI in order to provide a picture of the cortical blood oxygen-level-dependent (BOLD) contrast imaging during auditory stimulation and to demonstrate the aversive effect dental equipment sounds had on the cerebral cortex.
Methods
Subjects
Seven male subjects (age range 23~35 years, average 26 ± 4 years, all right-hand-dominant) were recruited for this study. The subjects underwent pure-tone audiometry testing and were confirmed with a hearing threshold of less than 20 dB hearing level (HL). Middle-ear auditory analysis, otoacoustic emission tests (OAE), and auditory brainstem responses (ABR) were also performed to ascertain normal neuroauditory pathways. All test data were appraised by an otolaryngologist. None of the subjects had a history of brain surgery and/or neural pathologies. In addition, the number of previous scalar treatments in the patient's lifetime was more than five times. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, and informed consent was obtained from all participants. The experiment was conducted in accordance with the Declaration of Helsinki. In addition, all subjects were interviewed based on their subjective perception after the routine treatment of dental calculus removal.
Auditory stimuli
Auditory stimuli used in the study were recorded in the dental clinic of Chang Gung Hospital during a routine treatment of dental calculus removal. The dental chair (Kavo, Biberach, Germany) was used in this study. The hardware used to record the noises produced by working dental instruments (i.e., ultrasonic scaling handpiece) in this study was a National Instruments PXI 1042 (National Instruments, Austin, TX, USA) with data acquisition cards PXI 4461 and PXI 4462 (National Instruments) as well as one G.R.A.S. microphone (Type 46AE, National Instruments). During recording of the instrument-generated noises, a dentist operated an instrument and sat at the same position as he would during an actual operation. A microphone was set up 10 cm away from the subject's ear to record the instrument-generated noises in the operating room, as illustrated in Figure 1. The average distance between the microphone next to the subject's ear and the operating dental instrument was 20.2 ± 3.9 cm. It included sound from an ultrasonic scaling hand piece [14564 Hz, 80 dB sound pressure level (SPL), 15 s] and power suction (1604 Hz, 80 dB SPL, 15 s). In addition, the study also utilized a pure-tone stimulus of 1000 Hz, duration 15 s, produced via Audition 2.0 software (Adobe Systems, San Jose, CA, USA). Each pure note had a wavelength of 800 ms with an interval of 200 ms. The auditory stimulation protocol utilized a “block” design, where each of the three recordings was played in a random nonrepetitive fashion to the subjects, as shown in Figure 2.
Figure 1.
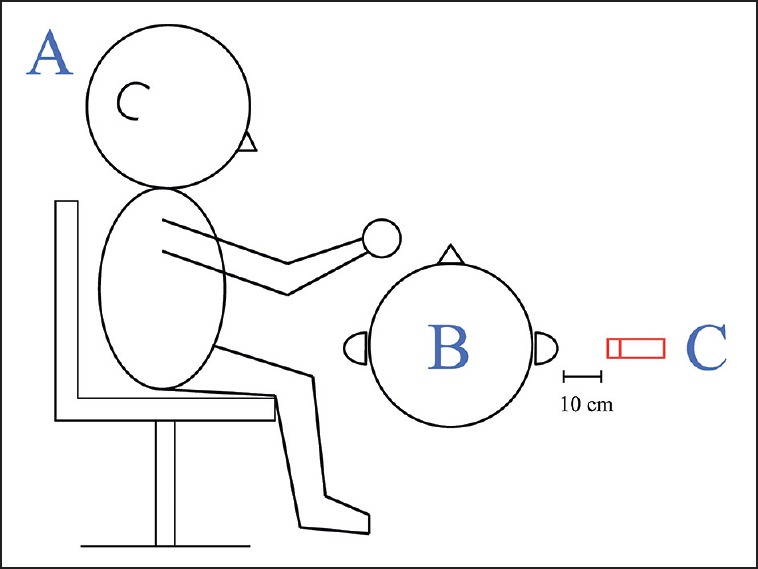
Positions of the dentist, the subject, and microphone during noise recording: (a) dentist, (b) subject, (c) microphone 10 cm away from subject's ear (unit: cm)
Figure 2.
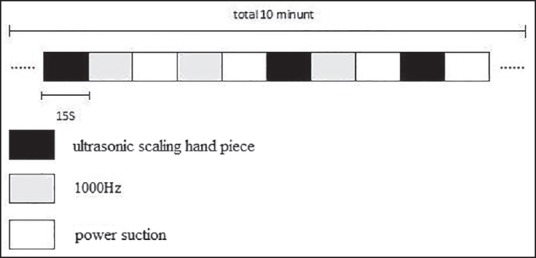
Auditory stimuli were presented in a “block” design, where the three recordings were played in a random but nonrepetitive fashion to the subjects
Experimental procedure
The subjects underwent complete dental calculus removal treatment at Chang Gung Hospital Dental Clinic prior to the start of the study. Within 1 h of completing the treatment, subjects presented to the Chang Gung Hospital Radiology department for fMRI imaging. The equipment used was a 3 Tesla MRI system (Trio a TIM system, Magnetom, Siemens, Erlangen, Germany). The subjects were exposed to 10 min of the recorded auditory stimulus during imaging, with the three recordings played in a random, nonrepetitive fashion. Subjects were advised to indicate when they heard each change of recording type by pressing down a buzzer in their hands. This allowed for comparison between the cortical images and the particular auditory stimuli being played at the time.
Setting of fMRI parameters
The images obtained in this study were produced with single-shot T2-weighted echo planar imaging. Imaging areas included the cerebellum and the cerebral cortex, consisting of 44 slices with a sectional thickness of 3 mm each. Image parameters were repetition time (TR)/echo time (TE)/flip angle = 3000 ms/35 ms/90°, matrix size = 64*64, field of view (FOV) = 192 mm, for each subject; T1-weighted images were also used for comparison. Image parameters for T1-weighted images were TR/TE/flip angle = 9 ms/4.2 ms/90°, spatial resolution 0.86 mm * 0.86 mm * 1.0 mm.
fMRI data analysis
In order to quantify activation of metabolically active regions, fMRI images have to be analyzed, and the mode of analysis can determine accuracy of interpretation. Recent studies of similar topics utilized three major forms of analysis:
Fixed-effect analysis,
Random-effect analysis, and
Mixed-effect analysis.
The analytical procedures are shown in Figure 3. Differences in study design and result clusters have a direct influence on the analytical method used. In the present study, mixed-effect analysis was used for image interpretation. The analytical software for fMRI is SPM5 (Wellcome Trust Functional Imaging Laboratory, London, UK) under the main command platform MATLAB 7.1 (MathWorks, Inc., Natick, MA, USA). Images were standardized with SPM5 internal reference parameters and subsequent minor curve smoothing. The images were analyzed with the independent sample confirmatory t-test via mixed-effect analysis. The final result was subjected to “xjview” in the SPM5 software to determine the areas of metabolic activity and degree of stimulation. All results had a P value < 0.05.
Figure 3.
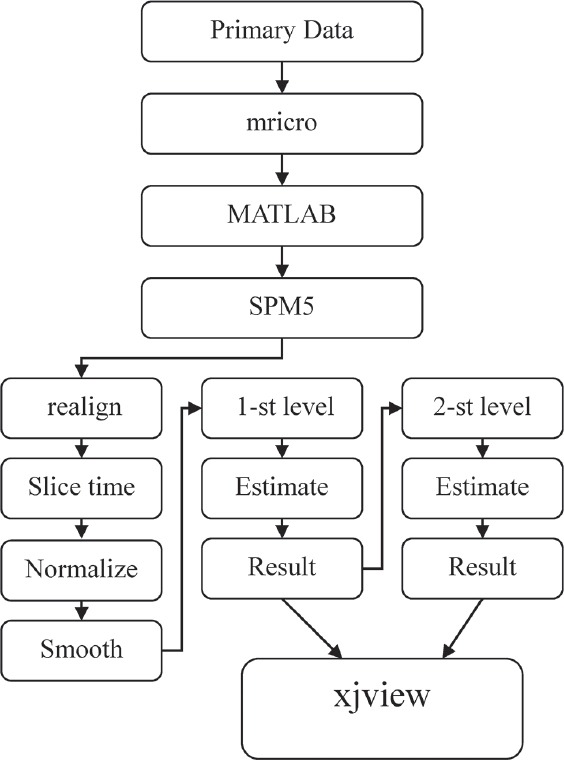
fMRI data analysis logarithm
Results
Analysis with SPM5 of subjects exposed to the auditory stimuli from an ultrasonic scaling handpiece [Table 1] revealed an area of activity in the superior temporal gyrus, with the right hemisphere demonstrating a Voxel-level T value of 4.96 and a Z value 2.93, and the coordinates were X = 68, Y = −44, Z = 20; the left-hemisphere Voxel-level T value was 3.36, Z value 2.38, and the coordinates were X = −68, Y = −44, and Z = 16. According to Brodmann's area classification, both the left and right coordinates were within area 22. The area of activity in the middle frontal gyrus was predominantly in the right hemisphere, its Voxel-level T value was 5.13 and Z value 3.07, and its coordinates were X = 36, Y = 62, and Z = 0; it was located in Brodmann's area 10. In addition, activity was also identified in the amygdala area, Voxel-level T value 2.71 and Z value 2.12, and its coordinates were X = 28, Y = −6, and Z = −22.
Table 1.
Properties of the three auditory stimuli as obtained by fMRI
| Condition | L/R | Z | Region of activation | Brodmann’s area | X, Y, Z | P |
|---|---|---|---|---|---|---|
| Ultrasonic scaling handpiece — base | R | 2.93 | STG | 22 | 68, −44, 20 | 0.01 |
| L | 2.38 | STG | 22 | −68, −44, 16 | ||
| R | 3.07 | MFG | 10 | 36, 62, 0 | ||
| R | 2.12 | Amygdala | 28, −6, −22 | |||
| Power suction — base | R | 2.23 | STG | 22 | 62, −28, 2 | 0.05 |
| L | 1.71 | STG | 22 | −66, −50, 10 | ||
| R | 2.02 | MFG | 10 | 46, 52, 2 | ||
| 1000 Hz — base | R | 2.62 | STG | 22 | 64, −20, 0 | 0.01 |
| L | 2.20 | STG | 22 | −56, −4, −6 | ||
| R | 2.77 | MFG | 10 | 34, 58, −2 |
STG = Superior temporal gyrus, MFG = Middle frontal gyrus
Analysis of subjects exposed to auditory stimuli from power-suction equipment [Table 1] revealed an area of activity in the superior temporal gyrus. In the right hemisphere, the Voxel-level T value was 2.93 and Z value 2.23, and its coordinates were X = 62, Y = −28, and Z = −2; in the left hemisphere, the Voxel-level T value was 2.06 and Z value 1.71, and the coordinates were X = −66, Y = −50, and Z = 10. Both the left and right coordinates corresponded to Brodmann's area 22. Area of activity in the middle frontal gyrus was located predominantly in the right hemisphere. Voxel-level T value was 2.58 and Z value 2.02, and its coordinates were X = 46, Y = 52, and Z = 2, located in Brodmann's area 10. There were no areas of activity associated with the auditory stimuli in the amygdala.
Exposure to pure tone [Table 1] indicated an area of activity in the superior temporal gyrus. The right hemisphere Voxel-level T value was 3.82 and Z value 2.62, and the coordinates were X = 64, Y = –20, and Z = 0; and left hemisphere Voxel-level T value was 2.89 and Z value 2.20, and the coordinates were X = –56, Y = −4, and Z = −6. Both coordinate sets corresponded with Brodmann area 22. Activities from the middle frontal gyrus demonstrated a predominantly right-sided area of activity. Its Voxel-level T value was 4.22 and Z value 2.77, and its coordinates were X = 34, Y = 58, and Z = −2, classifying it into Brodmann's area 10. There was no region of activity associated with the amygdala.
Two-sample-test analysis between auditory stimuli from ultrasonic scaling equipment and pure tone [Table 2] revealed that the ultrasonic scaling stimulus induces comparatively higher levels of stimulation. Active areas in the superior temporal gyrus (right hemisphere) had a Voxel-level T value of 4.72 and Z value 3.48 with coordinate X = 68, Y = −20, and Z = 4; left hemisphere Voxel-level T value was 3.93 and Z value 3.09, and its coordinates were X = −54, Y = −6, and Z = −8; both coordinate sets corresponded with Brodmann's area 22. The area of activity in the middle frontal gyrus was located in the right hemisphere with a Voxel-level T value of 3.05 and Z value of 2.58, and coordinates of X = 40, Y = 58, and Z = 0, placing it in Brodmann's area 10. The area of activity in the amygdala [Figure 4] had a Voxel-level T value of 3.18 and Z value of 2.72, and coordinates X = 26, Y = −6, and Z = −14.
Table 2.
Results of two-sample t-test between ultrasonic scaling handpiece vs power suction, and ultrasonic hand piece vs 1000-Hz pure sound
| Condition | L/R | Z | Region of activation | Brodmann’s area | X, Y, Z | P |
|---|---|---|---|---|---|---|
| Ultrasonic scaling handpiece vs 1000 Hz | R | 3.48 | STG | 22 | 68, −20, 4 | 0.05 |
| L | 3.09 | STG | 22 | −54, −6, −8 | ||
| R | 2.58 | MFG | 10 | 40, 58, 0 | ||
| R | 2.72 | Amygdala | 26, −6, −14 | |||
| Ultrasonic scaling handpiece vs power suction | R | 2.31 | STG | 22 | 52, −60, 14 | 0.01 |
| L | 1.74 | STG | 22 | −60, −40, 4 | ||
| R | 3.29 | MFG | 10 | 38, 40, 14 | ||
| R | 2.58 | Amygdala | 24, −2, −22 |
STG = Superior temporal gyrus, MFG = Middle frontal gyrus
Figure 4.
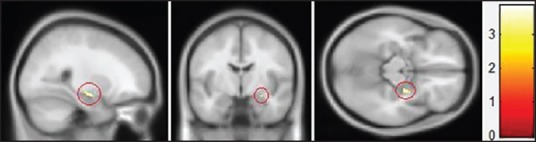
Region of amygdala activity obtained by subtracting areas of activities between ultrasonic scaling handpiece and 1000-Hz pure sound. (Voxel-level T value = 3.18, Z value = 2.72, P value <0.5)
Two-sample-test analysis of auditory stimuli between ultrasonic scaling and power suction equipment was also conducted. Comparison of stimulated areas between the two sources indicated that stimulation was comparatively more intense with auditory stimuli from ultrasonic scaling equipment [Table 2]. The area of stimulation on the right hemisphere superior temporal gyrus had a Voxel-level T value of 2.68 and Z value of 2.31, and coordinates X = 52, Y = −60, and Z = 14; left hemisphere Voxel-level T value was 1.88 and Z value 1.74, and its coordinates were X = –60, Y = −40, and Z = −4. Both areas of activity were located in Brodmann's area 22. The area of stimulation in the middle frontal gyrus was again located on the right hemisphere, with a Voxel-level T value of 4.32 and Z value 3.29, and coordinates X = 38, Y = 40, and Z = 14, placing it in Brodmann's area 10. The area of activity in the amygdala [Figure 5] had a Voxel-level T value of 3.05 and Z value 2.58, and coordinates of X = 24, Y = −2, and Z = −22. All values were statistically significantly with a P value <0.005. In addition, the results of the subjective perception interview revealed that all subjects had unpleasant feelings during dental treatments.
Figure 5.
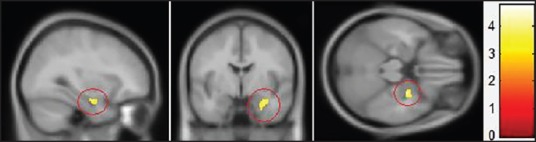
Region of amygdala activity obtained by subtracting areas of cortical activities between ultrasonic scaling handpiece and power suction. (Voxel-level T value = 3.05, Z value = 2.58, P value <0.5)
Discussion
From our results in Table 1 it can be observed that Brodmann's area 22 was activated in subjects exposed to auditory stimuli from ultrasonic scaling, power suction equipment, and 1000 Hz pure sound. Brodmann's area 22 is known as the auditory association area, with functions relating to auditory memories and language comprehension, so it was not surprising to observe activity in this region upon auditory stimuli from the dental equipment.
Brodmann's area 10 was also stimulated by the dental equipment sounds. The main function of this brain region is cognition and memory consolidation. We speculated that upon hearing the auditory stimulus the subjects searched through existing memories for similar experiences,[14] and the presence of past experience further enhanced recognition of the particular sound. The medial and lateral parietal lobes also possess similar functions in memory consolidation; however, no activities were detected during our studies in these areas in response to the auditory stimulation. Possible reasons include the fact that the subjects were advised prior to the study to concentrate on the auditory stimuli during the session, and this priming prevented subjects from processing the auditory stimuli in comparison to existing memories. Thus, the low levels of activity in the lateral[15] and medial aspects of the parietal lobes may have been undetectable by imaging.
The biggest observed difference was in the amygdala, which was activated during exposure to auditory stimuli from the ultrasonic scaling machine but not from the power suction or 1000-Hz pure sound. The amygdala is an integral component of the limbic system. In humans, it mostly processes negative emotions;[8,16] its activation in this instance indicated that the auditory stimulus from the ultrasonic scaling machine is perceived as aversive by the subjects and induces an unpleasant feeling. The results observed in our fMRI study are consistent with the results of the interview after routine treatment of dental calculus removal.
In Table 2, we show analysis of a “two-sample-test” between reactions to the ultrasonic scaling machine and the power suction tool, and between the ultrasonic scaling machine and 1000-Hz pure sound. A “two-sample-test” was not conducted between the power suction and pure sound due to the fact that neither stimulus elicited stimulation in the amygdala area. Therefore, we were unable to compare their effect on the amygdala. From the “two-sample-test” analysis, it appeared that the ultrasonic scaling machine stimulus induced a higher degree of activation in the auditory area compared to the power suction tool and the 1000-Hz pure sound. This higher level of activation[9,17] also enhanced neurotransmission between the auditory cortex and the associated auditory cortex.
From the results in Table 2, it can be seen that we were able to demonstrate that in the right parietal cortex area, subject exposure to ultrasonic scaling machine stimuli led to a greater degree of stimulation compared to the power suction tool and the 1000-Hz pure sound stimuli. The right parietal cortex is activated in response to negative emotional stimuli,[7] so this observation further confirms that auditory stimuli from the ultrasonic scaling machine was perceived by the test subjects as an unpleasant, aversive auditory stimulus.
Patients often experience a sense of fear and anxiety during dental treatments. This is largely due to not knowing when the instruments will come in contact with the teeth due to the operation site being inside the oral cavity. In addition, the pain due to the treatment procedure is also associated with a sense of unpleasantness and fear. However, in addition to the nociceptive stimuli, this study demonstrated that aversive auditory stimuli such as sounds from the ultrasonic scaling machine also cause aversive emotions. This was indicated by observed stimulation of the auditory cortex as well as the amygdala, indicating that noise from the ultrasonic scaling machine was perceived as an aversive auditory stimulus by the patients. Perhaps playing pleasant music or a neutral sound to shift the patient's attention away from the machine sounds could reduce the aversive emotions.
Financial support and sponsorship
Chang Gung Memorial Hospital, Taiwan.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Support for this project was provided by Chang Gung Memorial Hospital (CMRPG371023). Facilities were provided by the Biomedical Engineering Center in Chang Gung University and the Molecular Imaging Center at Chang Gung Memorial Hospital.
References
- 1.Doebling S, Rowe MM. Negative perceptions of dental stimuli and their effects on dental fear. J Dent Hyg. 2000;74:110–6. [PubMed] [Google Scholar]
- 2.Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–44. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 3.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–91. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94:4119–24. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121:1143–54. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- 6.Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA. Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport. 1996;7:1765–9. doi: 10.1097/00001756-199607290-00014. [DOI] [PubMed] [Google Scholar]
- 7.Flores-Gutiérrez EO, Díaz JL, Barrios FA, Favila-Humara R, Guevara MA, del Río-Portilla Y, et al. Metabolic and electric brain patterns during pleasant and unpleasant emotions induced by music masterpieces. Int J Psychophysiol. 2007;65:69–84. doi: 10.1016/j.ijpsycho.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Hirano Y, Fujita M, Watanabe K, Niwa M, Takahashi T, Kanematsu M, et al. Effect of unpleasant loud noise on hippocampal activities during picture encoding: An fMRI study. Brain Cogn. 2006;61:280–5. doi: 10.1016/j.bandc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. Neuroimage. 2002;16:746–53. doi: 10.1006/nimg.2002.1115. [DOI] [PubMed] [Google Scholar]
- 10.Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–7. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- 11.Yu JF, Chen CK, Wang SR, Wu CM, Ng SH, Ying L, et al. Activation of the auditory cortex in subjects with unilateral sensorineural hearing impairment in response to hearing their own names. J Med Biol Eng. 2010;30:221–6. [Google Scholar]
- 12.Huang CI, Huang YP, Ling CC, Sun YN. Using independent component analysis to detect active regions in brain fMRI for tactile stimulation. J Med Biol Eng. 2008;28:147–54. [Google Scholar]
- 13.Lin PY, Lin SI, Penney T, Chen JJ. Review: Applications of near infrared spectroscopy and imaging for motor rehabilitation in stroke patients. J Med Biol Eng. 2009;29:210–21. [Google Scholar]
- 14.Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–86. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- 15.Nolde SF, Johnson MK, D’Esposito M. Left prefrontal activation during episodic remembering: An event-related fMRI study. Neuroreport. 1998;9:3509–14. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- 16.Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, et al. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J Cogn Neurosci. 2001;13:1035–47. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- 17.Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–9. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]


