Abstract
Medication-overuse headache (MOH) is a secondary form of headache related to the overuse of triptans, analgesics and other acute headache medications. It is believed that MOH and substance addiction share some similar pathophysiological mechanisms. In this study we examined the whole brain resting state functional connectivity of the dorsal and ventral striatum in 30 patients (15 MOH and 15 non-MOH patients) to investigate if classification algorithms can successfully discriminate between MOH and non-MOH patients on the basis of the spatial pattern of resting state functional connectivity of the dorsal and ventral striatal region of interest. Our results indicated that both nucleus accumbens and dorsal rostral putamen functional connectivity could discriminate between MOH and non-MOH patients, thereby providing possible support to two interpretations. First, that MOH patients show altered reward functionality in line with drug abusers (alterations in functional connectivity of the nucleus accumbens). Second, that MOH patients show inability to break habitual behavior (alterations in functional connectivity of the dorsal striatum). In conclusion, our data showed that MOH patients were characterized by an altered functional connectivity of motivational circuits at rest. These differences could permit the blind discrimination between the two conditions using classification algorithms. Considered overall, our findings might contribute to the development of novel diagnostic measures.
Highlights
-
•
Nucleus accumbens functional connectivity could discriminate between MOH and non-MOH patients.
-
•
Dorsal rostral putamen functional connectivity could also discriminate between MOH and non-MOH patients.
-
•
Our data provide insights on possible pathophysiological mechanisms of medication abuse.
1. Introduction
Medication-overuse headache (MOH) is a secondary form of headache related to the overuse of triptans, analgesics and other acute headache medications (Russell, 2014, Russell and Lundqvist, 2012). In two thirds of MOH patients the overuse of medication fulfils the criteria for substance abuse (Negro and Martelletti, 2011). For this reason, a number of studies have suggested that MOH might share the same pathophysiological and behavioral mechanisms that intervene in substance abuse and behavioral addictions (Calabresi and Cupini, 2005, Fuh and Wang, 2012). In particular, it has been proposed that some of the behavioral correlates associated with MOH may resemble features of the behavioral sensitization to psychostimulants (Calabresi and Cupini, 2005). This is important, as several studies have shown that substance abusers have functional alterations of the nucleus accumbens in the brain networks both at rest (e.g. Fedota and Stein, 2015, Gu et al., 2010, Hu et al., 2015, Pariyadath et al., 2016, Sutherland et al., 2012, Tomasi et al., 2010, Viswanath et al., 2015a, Viswanath et al., 2015b, Wang et al., 2015, Zhang et al., 2015, reviewed in Gu et al., 2010, Pariyadath et al., 2016, Sutherland et al., 2012) and during reward tasks (Limbrick-Oldfield et al., 2013). The maintenance of compulsive substance use behaviors has been linked to hypofrontality and altered prefrontal-striatal connectivity (Goldstein and Volkow, 2002) and accumbens connectivity is thought to mediate sensation-seeking and substance intake (Weiland et al., 2013). In line with this possibility, imaging activation studies in MOH patients have highlighted metabolic and BOLD alterations at the level of the meso-cortical-limbic structures similar to those exhibited by substance abusers (Ferraro et al., 2012, Fumal et al., 2006). In a previous fMRI study involving reward-related tasks in a decision-making under risk paradigm, MOH patients showed reduced activity in the substantia nigra/ventral tegmental area and increased activity in the ventromedial prefrontal cortex as compared to healthy volunteers (Ferraro et al., 2012). The same research highlighted that during decision-making under risk MOH patients had reduced activity in the substantia nigra in comparison with non-MOH patients affected with chronic headache (Ferraro et al., 2012).
To date, there is limited evidence about changes in large-scale brain networks in MOH patients (Chanraud et al., 2014). Most importantly, no study has investigated the possibility that the spatial pattern of large-scale brain networks might be able to distinguish MOH from non-MOH patients. It is plausible to hypothesize that MOH patients should show a distinct pattern of functional connectivity, different to that of patients without addiction to analgesics, in particular in reward and motivational circuits originating from the nucleus accumbens.
Animal studies have also shown that addictions may result from the impossibility of breaking habitual behaviors (Smith and Graybiel, 2013). It has been shown that over-trained animals undergo a shift from purposeful to habitual behavior. At the functional level, overtraining relies on changes in the activity of the infralimbic cortex (IL) (Smith and Graybiel, 2013), a part of the medial prefrontal cortex and the sensorimotor striatum (dorsal striatum) in rats. In this sense, another possibility is that MOH reflects the impossibility of breaking the habit of taking too many analgesics. This hypothesis, that MOH can be associated with the inability to break learnt behaviors, such as medication overuse, has been underexplored in humans. It could therefore be hypothesized that MOH patients present altered functional connectivity patterns in the dorsal striatum.
In this study we tested these two theoretical possibilities (functional alterations of reward-related circuits vs. habitual behaviors). To investigate the first hypothesis (reward-related) we looked for differential patterns of connectivity in the nucleus accumbens in the two groups. To explore the second hypothesis (habitual learning) we tested the connectivity of two distinct regions of interest (ROIs) in the dorsal striatum, that showed connections with the motor and premotor regions (Di Martino et al., 2008).
Differences were analyzed using a multivariate approach, the Multivoxel Pattern Analysis (MVPA). This method makes use of a classifier to discriminate if two groups (or conditions) are distinguishable on the basis of patterns of activations, in our case the functional connectivity of the striatal ROIs.
2. Methods
2.1. Patients
Patients were recruited from the Neurology Division of Rivoli Hospital. They were assigned either to the MOH (N = 15, 2 men) or non-MOH (N = 15, 2 men) group. The two groups did not differ in terms of age (t = 1.049 p = 0.31, average age: MOH 43.8 ± 10.2 years, non-MOH 38 ± 11.1). The frequency of headache did not differ between the two groups (t = − 2.225 p = 0.14). Table 1 shows the demographic and clinical characteristics of the sample.
Table 1.
Clinical characteristics of the sample.
| Patient | MOH | Age | History of headache or MOH (years) | Frequency of headache (days in a month) | Overused (or used) medication |
|---|---|---|---|---|---|
| 1 | Yes | 36 | 0.5 | 12 | Triptans + Analgesics |
| 2 | Yes | 60 | 4 | 10 | Triptans |
| 3 | Yes | 48 | 10 | 10 | Triptans + Analgesics |
| 4 | Yes | 37 | 3 | 15 | Combination Analgesics |
| 5 | Yes | 63 | 2 | 20 | Analgesics |
| 6 | Yes | 30 | 2 | 15 | Analgesics + Combination analgesics |
| 7 | Yes | 36 | 0.5 | 30 | Analgesics |
| 8 | Yes | 49 | 4 | 15 | Triptans |
| 9 | Yes | 45 | 7 | 20 | Combination analgesics |
| 10 | Yes | 43 | 1 | 18 | Analgesics |
| 11 | Yes | 55 | 5 | 30 | Triptans |
| 12 | Yes | 40 | 3 | 12 | Combination analgesics + opioids |
| 13 | Yes | 49 | 0.6 | 12 | Triptans |
| 14 | Yes | 36 | 10 | 14 | Triptans + FANS |
| 15 | Yes | 30 | 1 | 15 | Triptans |
| 16 | No | 22 | 14 | 15 | Analgesics |
| 17 | No | 37 | 0.4 | 30 | – |
| 18 | No | 51 | 0.6 | 15 | Analgesics |
| 19 | No | 54 | 40 | 4 | – |
| 20 | No | 27 | 20 | 7 | Analgesics |
| 21 | No | 30 | 11 | 6 | Triptans |
| 22 | No | 28 | 18 | 8 | Analgesics |
| 23 | No | 52 | 34 | 13 | Non-steroidal anti-inflammatory drugs |
| 24 | No | 32 | 0.5 | 20 | Triptans |
| 25 | No | 19 | 9 | 20 | – |
| 26 | No | 47 | 20 | 15 | Analgesics |
| 27 | No | 36 | 10 | 12 | Non-steroidal anti-inflammatory drugs |
| 28 | No | 45 | 2 | 20 | Analgesics |
| 29 | No | 39 | 15 | 2 | Analgesics |
| 30 | No | 44 | 20 | 3 | Combination analgesics |
2.2. Psychological questionnaires
Psychological tests were used to assess depression (Beck Depression Inventory, BDI) (Beck et al., 1961), state and trait anxiety (STAI Y1 and Y2) (Spielberger, 1989), Migraine Disability Assessment (MIDAS) (Lipton et al., 2001) and health status (SF-12, with the two subscales Physical and Mental Health Composite Scores, PCS and MCS) (Ware et al., 1996). MIDAS scores reflect the level of disability, with higher MIDAS scores indicating a higher level of disability. Severe disability is defined by scores higher than 21. BDI scores from 0 to 9 indicate minimal depression, from 10 to 18 mild depression, from 19 to 29 moderate depression and from 30 to 63 severe depression. Higher STAI Y1 and 2 scores indicate higher levels of anxiety; scores range from 20 to 80. SF-12 scores range from 0 to 100, where a zero indicates the lowest level of health and 100 the highest level of health.
2.3. Neuropsychological questionnaires
Neuropsychological tasks were used to evaluate the functionality of frontal and prefrontal regions. Specifically, we assessed verbal fluency (phonemic and semantic fluency), cognitive flexibility (Wisconsin Card Sorting Test) (Milner, 1963), planning (Tower of London) (Shallice, 1982). Phonemic verbal fluency was assessed using the FAS, which requires participants to orally produce as many words as possible beginning with the letters F, A, or S within a time frame of 1 min. Semantic fluency requires producing as many words as possible in the time frame of 2 min, using the following semantic categories: cities, fruit, animals and colors. The Wisconsin Card Sorting Test (WCST) assesses participants' ability to display cognitive flexibility. Higher scores reflect a poorer performance. The Tower of London investigates planning abilities, lower scores reflect lower times in the performance of the task, and therefore, a better overall performance.
2.4. Image acquisition and pre-processing
Participants were asked to lay supine in the scanner with their eyes closed and were instructed not to think of anything in particular during the examination. Data acquisition was performed on a 1.5 T INTERA scanner (Philips Medical Systems) with a SENSE high-field, high-resolution (MRIDC) head coil optimized for functional imaging. 3D high-resolution T1-weighted structural images were acquired for each participant using a fast field echo sequence, with a repetition time of 25 ms, the shortest echo time and a 30° flip angle. The acquisition matrix was 256 × 256 and the field of view was 256 mm. The set consisted of 160 sagittal contiguous images covering the whole brain. In-plane resolution was 1 × 1 mm and slice thickness was 1 mm3 (1 × 1 × 1 mm voxels). Functional T2-weighted images were acquired using echoplanar sequences, with a repetition time of 2000 ms, an echo time of 60 ms and a 90° flip angle. The acquisition matrix was 64 × 64 and the field of view was 256 mm. A total of 200 volumes were acquired, with each volume consisting of 19 axial slices, parallel to the anterior–posterior (AC–PC) commissure; slice thickness was 4.5 mm with a 0.5 mm gap. Two scans were added at the beginning of functional scanning and the data discarded to reach steady-state magnetization before acquisition of the experimental data. In the same session, a set of imaging data were analyzed using Brain Voyager QX 2.7 (Brain Innovation, Maastricht, The Netherlands). To each participant's functional data we applied: (i) mean intensity adjustment for the correction of global intensity of the repeatedly measured images of a slice. For each slice, the average intensity across the first image was computed; for each subsequent scan of the same slice, the mean intensity was computed and then scaled to result in the same average slice intensity; this procedure is suggested when “spikes” are detected in the signal (ii) 3D motion correction to adjust small head movements: all volumes were aligned spatially to the first volume by rigid body transformations, using a trilinear interpolation algorithm; iii) spatial smoothing was performed using a Gaussian kernel of 6 mm FWHM; iv) temporal filtering (linear trend removals), and a band pass filter of 0.01–0.08 Hz, used to reduce cardiac and respiratory noise. The 0.08–0.01 Hz frequency range has the greatest power to reveal the underlying connectivity (Biswal et al., 1995, Cauda et al., 2011b, Greicius et al., 2009). After pre-processing, a series of steps were followed in order to allow for precise anatomical locations of brain activity to facilitate inter-subject averaging. First, each subject's slice-based functional scans were co-registered to their 3D high-resolution structural scan. This process involved a mathematical co-registration exploiting slice positioning stored in the headers of the raw data, as well as fine adjustments computed by comparing the data sets on the basis of their intensity values; when needed, manual adjustments were also performed. Second, the 3D structural data set of each subject was transformed into Talairach space (Talairach and Tournoux, 1988): the cerebrum was translated and rotated into the anterior–posterior commissure plane and then the borders of the cerebrum were identified. Third, using the anatomical–functional co-registration matrix and the determined Talairach reference points, the functional time course of each subject was transformed into Talairach space and the volume time course was created.
2.5. Seed-based resting state functional connectivity
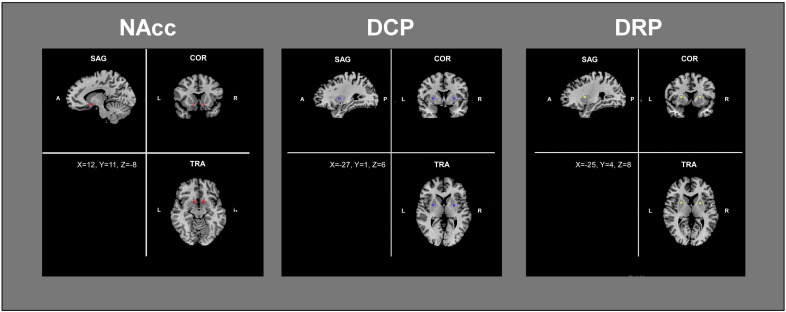
To test the first hypothesis we explored the whole-brain functional connectivity of the ventral striatum (nucleus accumbens) using seed-voxel correlation. The ROI around the bilateral nucleus accumbens was drawn following Cauda et al. (2011a). In detail, we selected two bilateral anatomical ROIs according to the AFNI brain structure atlas (afni.nimh.nih.gov/afni/doc/misc/afni_ttatlas/). The mean ROI volume was 143 mm3. To test the second hypothesis we explored the whole-brain functional connectivity of the dorsal striatum using seed-voxel correlation. More in detail we utilized the dorsal caudal and rostral putamen ROIs (DCP and DRP, respectively) employed by Di Martino et al. (Di Martino et al., 2008). Specifically, bilateral 5 mm spheres were placed at x = ± 28, Y = 1, Z = 3 for the DCP ROIs and x = ± 25; Y = 8; Z = 6 for DRP ROIs (see Fig. 1).
Fig. 1.
Regions of interest (ROIs) used in the study. Functional connectivity was calculated from the nucleus accumbens (NAcc), left panel, the dorsal caudal putamen (DCP), and the dorsal rostral putamen (DRP). Coordinates of the seed are provided in the bottom left square of each figure.
Seed-based functional connectivity (FC) maps were computed according to Margulies et al. (Margulies et al., 2007). BOLD time courses were extracted from each ROI by averaging over voxels within each region. Several nuisance covariates were included in the analyses to reduce the effects of physiological processes such as fluctuations related to cardiac and respiratory cycles, or to motion. To this aim, we included 9 additional covariates that modeled nuisance signals sampled from White Matter (WM), Cerebro-Spinal Fluid (CSF), Global Signal (GS) (Weissenbacher et al., 2009), as well as from 6 motion parameters (3 rotations and 3 translations as saved by the 3D motion correction). We derived the GS/WM/CSF nuisance signals averaging the time courses of the voxels in each subject's whole-brain/WM/CSF masks. These masks are generated by the segmentation process of each subject's brain. All seed-based predictors were z-normalized. A correction (pre-whitening) for autocorrelation (Woolrich et al., 2001) was used. For each subject an FC map was computed on a voxel-wise basis for each previously selected region. For each subject the general linear model (GLM) resulted in an ROI-based t-map, the statistical threshold of p < 0.05 was corrected for multiple comparisons using the FDR (q < 0.05, cluster threshold k > 10 voxels in the native resolution). A global multi-subject random effect map was computed using a one sample t-test. The resulting maps were FDR corrected for multiple comparisons.
2.6. Connectivity-based classification
We applied a pattern classification technique to investigate if the spatial pattern of resting state functional connectivity of our three ROIs was able to discriminate between MOH and non-MOH patients. Functional connectivity maps served as input for classification analyses. To inspect the pattern of connections having the highest discriminative power we employed Multivoxel Pattern Analysis (MVPA). In this multivariate technique multivoxel patterns of foci were analyzed using a method that combines machine learning with an iterative, multivariate voxel selection algorithm: Recursive Feature Elimination (RFE) (De Martino et al., 2008). The method consists of an N-fold cross validation (Bishop and Nasser, 2006). At each fold the examples from fMRI volume are divided into training and test sets. The training set is further divided into several small sets (splits). For each split a support vector machine (SVM) is trained and the weights are calculated. RFE is performed on the subsets. It is based on the ranking of the weights of the training subset. Voxels with the smallest ranking are discarded and high-ranking voxels are used for the successive iteration. Generalizations are performed at each level using the sets of test trials. The final generalizations and discriminative maps are obtained by averaging the results of the N-folds. This method makes it possible to estimate maximally discriminative response patterns without a priori definition of regions of interest. In brief, starting from the entire set of measured voxels, the method uses a training algorithm (least square support vector machine, ls-SVM) iteratively to eliminate irrelevant voxels and to estimate the informative spatial patterns. Correct classification of the test data increases while features/voxels are pruned on the basis of their discriminative ability. MVPA was preferred to a contrast between the functional connectivity of the two groups because: i) mass univariate techniques are less sensitive in detecting differences in complex multivariate data, like functional connectivity data; ii) MVPA investigates differences in the spatial pattern, whereas mass univariate analyses investigate intensity differences at the single voxel level.
3. Results
3.1. Psychological and neuropsychological questionnaires
t-Tests for independent samples were carried out to investigate differences between the groups. All comparisons were at p > 0.05 level, therefore suggesting that the two groups did not differ at the neuropsychological and psychological levels (see Table 2, Table 4 for individual data and Table 3, Table 5 for group results).
Table 2.
Psychological questionnaires, individual level. MIDAS (Migraine Disability Assessment), BDI (Beck Depression Inventory), STAI (State Trait Anxiety Inventory), SF-12 (The 12-Item Short Form Survey), PCS (Physical Component Summary), MCS (Mental Component Summary).
| Psychological questionnaires | |||||||
|---|---|---|---|---|---|---|---|
| Patient | MOH | MIDAS | BDI | STAI Y1 | STAI Y2 | SF-12 |
|
| PCS | MCS | ||||||
| 1 | Yes | 0 | 6 | 29 | 30 | 47.7 | 57.46 |
| 2 | Yes | 18 | 8 | 29 | 40 | 42.52 | 46.75 |
| 3 | Yes | 120 | 8 | 58 | 55 | 35.63 | 29.14 |
| 4 | Yes | 32 | 17 | 45 | 49 | 37.94 | 36.34 |
| 5 | Yes | 40 | 8 | 41 | 34 | 42.57 | 40.89 |
| 6 | Yes | 27 | 7 | 41 | 42 | 44.3 | 29.62 |
| 7 | Yes | 36 | 19 | 59 | 51 | 33.04 | 37.03 |
| 8 | Yes | 54 | 8 | 29 | 35 | 40.33 | 51.11 |
| 9 | Yes | 54 | 21 | 68 | 57 | 44.56 | 23.69 |
| 10 | Yes | 60 | 5 | 33 | 42 | 32.77 | 47.23 |
| 11 | Yes | 20 | 0 | 32 | 22 | 48.8 | 53.49 |
| 12 | Yes | 4 | 3 | 34 | 33 | 49.21 | 54.49 |
| 13 | Yes | 29 | 5 | 54 | 42 | 30.04 | 48.66 |
| 14 | Yes | 6 | 11 | 42 | 46 | 41.58 | 37.14 |
| 15 | Yes | 31 | 10 | 36 | 43 | 44.01 | 51.09 |
| 16 | No | 22 | 16 | 51 | 55 | 46.4 | 23.64 |
| 17 | No | 16 | 11 | 56 | 51 | 56.58 | 60.76 |
| 18 | No | 90 | 13 | 44 | 52 | 31.6 | 33.54 |
| 19 | No | 30 | 5 | 32 | 37 | 32.19 | 52.35 |
| 20 | No | 48 | 18 | 44 | 52 | 44.86 | 35.75 |
| 21 | No | 29 | 5 | 35 | 39 | 42.54 | 57.33 |
| 22 | No | 5 | 3 | 33 | 26 | 47.81 | 54.32 |
| 23 | No | 7 | 11 | 30 | 37 | 47.78 | 44.47 |
| 24 | No | 88 | 7 | 43 | 63 | 53.48 | 48.26 |
| 25 | No | 0 | 7 | 25 | 28 | 40.8 | 55.18 |
| 26 | No | 6 | 4 | 32 | 36 | 48.64 | 53.65 |
| 27 | No | 27 | 7 | 30 | 45 | 49.51 | 52.58 |
| 28 | No | 5 | 5 | 30 | 32 | 43.21 | 47.11 |
| 29 | No | 44 | 0 | 31 | 28 | 36.19 | 52.56 |
| 30 | No | 26 | 13 | 30 | 46 | 31.13 | 39.13 |
Table 4.
Neuropsychological profile, individual levels (WCST, Wisconsin Card Sorting Test).
| Neuropsychological questionnaires | |||||
|---|---|---|---|---|---|
| Patient | MOH | Phonemic fluency | Semantic fluency | WCST | Tower of London |
| 1 | Yes | 28 | 15.75 | 33 | 27.75 |
| 2 | Yes | 38 | 19.5 | 9 | 23.25 |
| 3 | Yes | 25 | 18.25 | 11 | 25.5 |
| 4 | Yes | 45 | 21.25 | 9 | 30.25 |
| 5 | Yes | 7 | 15.5 | 5 | 17.75 |
| 6 | Yes | 33 | 16.5 | 29 | 26.5 |
| 7 | Yes | 39 | 19.25 | 4 | 25.25 |
| 8 | Yes | 30 | 13.5 | 17 | 23.25 |
| 9 | Yes | 30 | 22.25 | 8 | 25.5 |
| 10 | Yes | 35 | 24.25 | 22 | 27.5 |
| 11 | Yes | 26 | 20 | 12 | 28.75 |
| 12 | Yes | 39 | 23.25 | 10 | 29.25 |
| 13 | Yes | 34 | 19 | 13 | 20.25 |
| 14 | Yes | 29 | 12.00 | 23 | 28.25 |
| 15 | Yes | 34 | 15 | 8 | 28.25 |
| 16 | No | 31 | 13.5 | 9 | 23.25 |
| 17 | No | 25 | 13.5 | 23 | 21.25 |
| 18 | No | 36 | 27.75 | 10 | 26.25 |
| 19 | No | 30 | 13.5 | 20 | 27.25 |
| 20 | No | 46 | 19.75 | 18 | 21.25 |
| 21 | No | 31 | 12.25 | 8 | 16 |
| 22 | No | 29 | 19 | 17 | 30 |
| 23 | No | 37 | 17.5 | 25 | 30 |
| 24 | No | 32 | 14.25 | 19 | 22.5 |
| 25 | No | 26 | 17 | 9 | 26.25 |
| 26 | No | 39 | 23.5 | 9 | 19.75 |
| 27 | No | 22 | 12.25 | 12 | 24.25 |
| 28 | No | 27 | 19.75 | 25 | 24.25 |
| 29 | No | 28 | 19.75 | 4 | 29.25 |
| 30 | No | 37 | 20 | 3 | 30.5 |
Table 3.
Psychological questionnaires, group level (mean ± standard deviation).
| Group | MIDAS (mean ± sd) | BDI | STAI Y1 | STAI Y2 | SF-12 |
|
|---|---|---|---|---|---|---|
| PCS | MCS | |||||
| MOH | 35.4 ± 29.5 | 9 ± 5.8 | 42 ± 12.4 | 41.4 ± 9.5 | 41 ± 5.9 | 42.9 ± 10.3 |
| Non-MOH | 29.5 ± 28 | 8.3 ± 5.1 | 36.4 ± 8.9 | 41.8 ± 11.2 | 43.5 ± 7.8 | 47.3 ± 10.2 |
Table 5.
Neuropsychological profile, group level (mean ± standard deviation) (WCST, Wisconsin Card Sorting Test).
| Group | Phonemic fluency (mean ± sd) | Semantic fluency | WCST | Tower of London |
|---|---|---|---|---|
| MOH | 31.4 ± 8.7 | 18.3 ± 3.5 | 14.2 ± 8.7 | 25.8 ± 3.4 |
| Non-MOH | 31.7 ± 6.2 | 17.5 ± 4.4 | 14 ± 7.3 | 24.8 ± 4.2 |
3.2. Functional connectivity
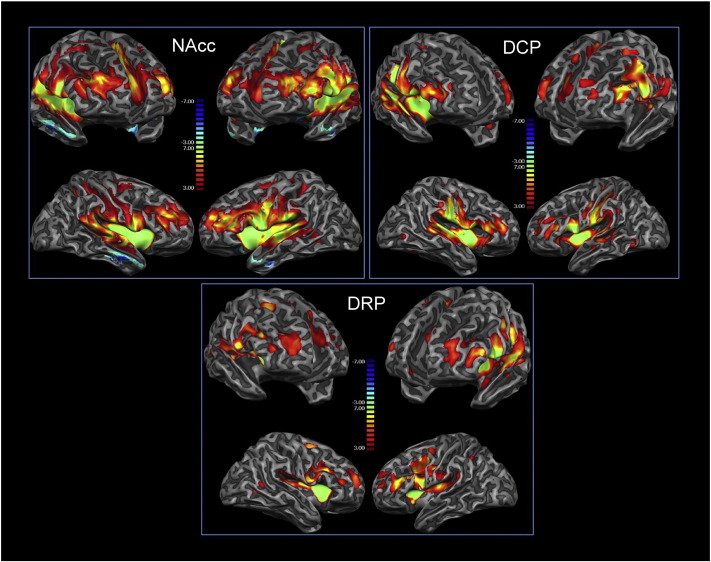
The observed functional connectivity of the dorsal and ventral striatum was in line with previous reports (Cauda et al., 2011a). For the NAcc, ROI connections were found with the insula bilaterally, orbitofrontal and cingulate cortices, thalamus, basal ganglia, amygdala. We also observed connections between the accumbens and sensorimotor cortices. DCP and DRP ROIs showed a prevalently premotor-sensorimotor connectivity with the DCP connectivity predominantly sensorimotor and the DRP more premotor. Both ROIs also showed prefrontal-anterior cingulate connectivity. These results were consistent with previously reported ones (Di Martino et al., 2008) (see Fig. 2, and Supplementary Fig. 1 for the connectivity of the two groups separately and Supplementary Fig. 2 for the connectivity of a control group of healthy subjects).
Fig. 2.
Resting state functional connectivity of the nucleus accumbens (NAcc), dorsal caudal putamen (DCP) and dorsal rostral putamen (DRP). Areas in red represent positive correlations, those in blue represent negative correlations among voxels. This figure shows functional connectivity of all groups together, separate figures per group can be found in the supplementary material. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Connectivity-based classification
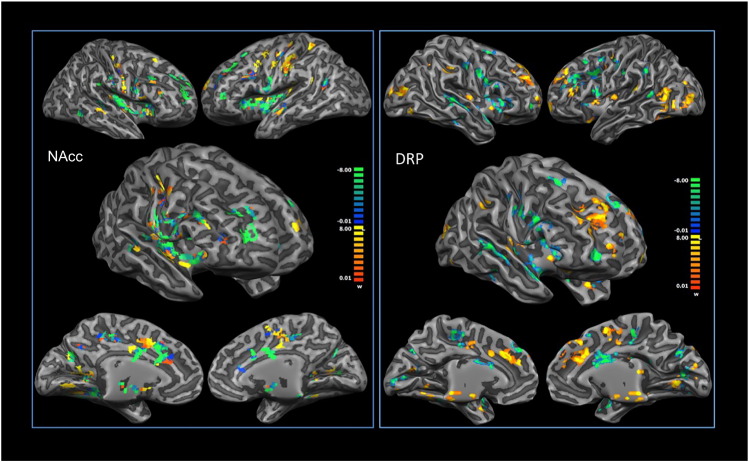
NAcc resting state functional connectivity could efficiently discriminate between the two groups of MOH and non-MOH patients. Training accuracy was 93%, test accuracy 75%. The discriminative voxels are visualized in Fig. 3. Areas that showed the highest discriminative power for MOH patients were prevalently placed in the bilateral anterior and posterior insulae, dorsolateral prefrontal cortices, midcingulate cortices, precuneus, secondary sensorimotor cortices and thalami. Areas that showed the highest discriminative power for non-MOH patients were prevalently placed in the sensorimotor and premotor brain areas.
Fig. 3.
Results of the MVPA for the nucleus accumbens (NAcc) (left) and the dorsal rostral putamen (DRP) (right). Yellow areas show greater predictive values for patients with medication overuse (MOH). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In addition, DRP connectivity (but not DCP connectivity) could discriminate between the two groups. Training accuracy was 90%, test accuracy 66% (DCP results: training 88%; test 50%). The areas that showed the highest discriminative power for MOH patients were prevalently located in the sensorimotor, premotor, anterior insular and mid-cingulate cortices. The areas that showed the highest discriminative power for non-MOH patients were prevalently located in the prefrontal, visual, insular, cingulate, lingual and posterior temporal cortices. The results are summarized in Fig. 3. Supplementary Fig. 3 shows the same analysis with voxels grouped into large-scale brain networks.
4. Discussion
It is becoming increasingly clear that addictions can modify the dynamics of large-scale brain networks (Sutherland et al., 2012). Several studies have shown that both substance and behavioral addictions are associated with altered patterns of resting state connectivity (Fedota and Stein, 2015, Gu et al., 2010, Hu et al., 2015, Pariyadath et al., 2016, Sutherland et al., 2012, Tomasi et al., 2010, Viswanath et al., 2015a, Viswanath et al., 2015b, Wang et al., 2015, Zhang et al., 2015), reviewed in Limbrick-Oldfield et al., (2013) and Sutherland et al., (2012). These functional changes mainly involve reward-related circuits (such as the accumbens-orbitofrontal one), although, to date, the study of resting state mesocorticolimbic connections in cocaine abusers and heroine abusers has revealed both increased and reduced accumbens-prefrontal cortices connectivity (Gu et al., 2010, Hu et al., 2015, Jasinska et al., 2014, Ma et al., 2010, Tomasi et al., 2010, Wilcox et al., 2011, Zhang et al., 2011).
In agreement with the view that reward dysregulation is related to emotional dysregulation in addicted individuals, some studies have reported reductions in the connectivity of the amygdala with prefrontal regions (Gu et al., 2010, Upadhyay et al., 2010).
MOH has been conceptualized as a form of addiction (Calabresi and Cupini, 2005, Fuh and Wang, 2012) and in line with this and with the evidence on substance abuse, previous fMRI studies have shown that MOH patients have reduced activity in the striatal-prefrontal areas in comparison to non-MOH patients (Ferraro et al., 2012, Fumal et al., 2006).
Animal studies have also proposed that addictions may be related to the impossibility of breaking habitual behaviors (Smith and Graybiel, 2014). Research has shown that when animals become over-trained on a task, they continue to work at it, and persist in seeking reinforcement despite its devaluation. In addition, to learn habitual behaviors, it is important that animals become insensitive to outcome values or to new associations between action-outcome contingency (Smith and Graybiel, 2014). Importantly, it has also been proposed that the transition from voluntary to compulsive substance intake is reflected, at the central level, by a transition from the prefrontal to the limbic areas, and from the ventral to the dorsal striatum (Everitt and Robbins, 2005).
In the present study, we experimentally tested i) the possibility that MOH are characterized by altered functional connectivity of the ventral striatum in order to explore the hypothesis of ‘dysfunctional’ reward-related circuits; and ii) the connectivity of the dorsal striatum, in line with the hypothesis of overtraining behaviors. Our experimental approach did not take into account the possible direction of the strength of the functional connectivity, but rather how well the spatial pattern of functional connectivity was able to discriminate between the two clinical conditions. This choice was motivated by the existence of contradictory findings in the substance abuse research, as previously outlined.
Our findings seem to suggest that MOH patients present a specific spatial pattern of connectivity of the ventral and dorsal striatum that can differentiate them from other forms of headache. We observed that functional connectivity of the NAcc was able to discriminate between the two groups above chance level. In this sense, we can conclude that connectivity of reward networks does show differences between the two groups. Interestingly, we also found that the connectivity of the DRC, the one characterized by greater premotor connectivity, can discriminate between the two groups. This finding also supports the suggestion that medication abuse can be related to the inability to break habits, even if the behavior-reward contingency has changed in time (Everitt and Robbins, 2005).
More specifically, we have shown that the voxels discriminating MOH patients (ventral striatum connectivity) were prevalently placed in the bilateral anterior and posterior insulae, dorsolateral prefrontal cortices, midcingulate cortices, precuneus, secondary sensory motor cortices and thalami. Moreover, we also found that areas showing the highest discriminative power for MOH patients (dorsal striatum connectivity) were prevalently located in the sensorimotor, premotor, anterior insular and mid-cingulate cortices.
For a better understanding of these results in terms of brain network dynamics and considering the complex pattern of connectivity of the NAcc (Cauda et al., 2011a), we have considered discriminative voxels as part of large-scale brain networks (see Supplementary Fig. 3). Indeed, several studies have pointed to how pathological conditions are characterized by altered brain network dynamics (Cauda et al., 2014, Farmer et al., 2012, Seeley et al., 2009). We observed that MOH could be discriminated by the functional connectivity of the nucleus accumbens to the orbitofrontal cortex, the dorsal attentional network, the default mode network and the saliency network and of the DRP to the sensorimotor circuits. The first group of networks can be considered as part of the greater associative network as proposed by the dual intertwined rings theory (Mesmoudi et al., 2013). In this view, a possible interpretation of the greater ability of the associative ring to discriminate abusers may be related to difficulties abusers have in integrating homeostatic and motivational information into a coherent construct. Indeed, theories of addiction postulate that abusers can either have altered incentive salience for drugs and drug-associated stimuli (Berridge and Robinson, 1998) or increased activation of anti-reward systems (Le Moal and Koob, 2007). The insula would appear to intervene in the conscious urge to take drugs, due to its role in integrating homeostatic feelings and conscious decision-making (Cisler et al., 2013, Naqvi et al., 2014). The greater ability of the insula, as part of the saliency network, to predict the abuser group, may thus support the interpretation of MOH patients as characterized by a failure to integrate motivational information in the long term future, or, alternatively, by an enhanced awareness of homeostatic feelings.
In addition to altered sensitivity to the ‘reward’, patients may also fail to update the habitual behavior of drug intake. This possibility is supported by our results of specific altered connectivity of the dorsal striatum with the motor and premotor networks. Habitual behaviors are not modified by changes in contingency between the action and the reward (Dickinson, 1985), but, at least in animals, they can be stopped by inactivating the infralimbic cortex online (Smith et al., 2012) or even after the habit has been established (Coutureau and Killcross, 2003). Detoxification is the elective treatment for MOH (for a review on the topic see Kristoffersen and Lundqvist, 2014). It is possible that detoxification interrupts the habitual behavior and therefore re-establishes pre-MOH functional characteristics. Indeed, fMRI studies have shown that, after withdrawal, glucose metabolism can be restored to almost pre-overuse levels (Fumal et al., 2006). This finding would suggest that altered functional connectivity is related to drug intake, although at present we cannot exclude that altered functional connectivity predisposes to excessive drug intake.
We also observed that non-MOH patients were better predicted by the connectivity of the nucleus accumbens to the motor and thalamic-basal ganglia circuits. This may be a distinctive feature of migraine. Previous studies have reported that patients suffering from migraine show increased functional connectivity between the anterior insula and the thalami, and the anterior insula and the periaqueductal grey (PAG) (Schwedt et al., 2013). In addition, some authors have also observed increases between the somatosensory regions (Yuan et al., 2013) and a relationship between disease duration and increased functional connectivity between the nucleus accumbens and the anterior cingulate cortex (Hadjikhani et al., 2013). A part of these functional alterations is probably shared by MOH and non-MOH patients and, as such, does not appear as a distinctive feature in the discriminative process.
In this study, we have also conducted an extensive psychological and neuropsychological examination. We did not observe any significant difference between groups in terms of psychological and neuropsychological variables. Previous studies reported mixed findings regarding psychological and neuropsychological results (Biagianti et al., 2012, Radat et al., 2013, Usai et al., 2009), with differences present mainly in the anxiety domain (Kristoffersen et al., 2016, Sarchielli et al., 2016).
It is also important to mention that not all MOH patients fulfill the criteria to be considered substance abusers according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) (Fuh and Wang, 2012, Fuh et al., 2005, Radat et al., 2008). In this view, it will be interesting, in future studies, to address differences across subgroups of patients at the connectivity level.
We acknowledge that our study is not free from possible methodological confounds. First, the patients in our study were taking various analgesic drugs and mostly when needed, therefore the exact impact of medication on resting state connectivity could not be ascertained. In addition, the patients had different disease duration, which has been shown to have an impact on resting state networks (Chanraud et al., 2014, Liu et al., 2015). These limitations are often observed in clinical pain studies comparing different populations (Baliki et al., 2014).
In conclusion, our data suggest that MOH patients are characterized by a specific spatial pattern of functional connectivity of the striatum at rest. Our findings support both theoretical possibilities, namely that patients with MOH have altered nucleus accumbens connectivity, in line with the hypothesis of reward system dysregulation; and that they have altered dorsal striatum connectivity in line with the habit learning hypothesis. Importantly, these functional alterations are sufficiently marked to allow the blind discrimination between MOH and non-MOH patients above chance level. Considered as whole, these findings provide important suggestions for the possible development of novel complementary diagnostic measures.
Acknowledgements
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.05.007.
Appendix A. Supplementary data
Supplementary figures S1, S2, and S3.
References
- Baliki M.N., Mansour A.R., Baria A.T., Apkarian A.V. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 2014;9:e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Biagianti B., Grazzi L., Gambini O., Usai S., Muffatti R., Scarone S., Bussone G. Orbitofrontal dysfunction and medication overuse in patients with migraine. Headache. 2012;52:1511–1519. doi: 10.1111/j.1526-4610.2012.02277.x. [DOI] [PubMed] [Google Scholar]
- Bishop C., Nasser M. vol. 1. Springer; New York: 2006. (Pattern Recognition and Machine Learning). [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Cupini L.M. Medication-overuse headache: similarities with drug addiction. Trends Pharmacol. Sci. 2005;26:62–68. doi: 10.1016/j.tips.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Cauda F., Cavanna A.E., D'Agata F., Sacco K., Duca S., Geminiani G.C. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J. Cogn. Neurosci. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- Cauda F., D'Agata F., Sacco K., Duca S., Geminiani G., Vercelli A. Functional connectivity of the insula in the resting brain. NeuroImage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cauda F., Palermo S., Costa T., Torta R., Duca S., Vercelli U., Geminiani G., Torta D.M. Gray matter alterations in chronic pain: a network-oriented meta-analytic approach. Neurol. Clin. 2014;4:676–686. doi: 10.1016/j.nicl.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S., Di Scala G., Dilharreguy B., Schoenen J., Allard M., Radat F. Brain functional connectivity and morphology changes in medication-overuse headache: clue for dependence-related processes? Cephalalgia. 2014;34:605–615. doi: 10.1177/0333102413519514. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Elton A., Kennedy A.P., Young J., Smitherman S., Andrew James G., Kilts C.D. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res. 2013;213:39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E., Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav. Brain Res. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- De Martino F., Valente G., Staeren N., Ashburner J., Goebel R., Formisano E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. NeuroImage. 2008;43:44–58. doi: 10.1016/j.neuroimage.2008.06.037. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M., Uddin L.Q., Shehzad Z., Biswal B., Walters J.R., Castellanos F.X., Milham M.P. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: the development of behavioral autonomy. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1985;308:67–78. [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Farmer M.A., Baliki M.N., Apkarian A.V. A dynamic network perspective of chronic pain. Neurosci. Lett. 2012 doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota J.R., Stein E.A. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann. N. Y. Acad. Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro S., Grazzi L., Muffatti R., Nava S., Ghielmetti F., Bertolino N., Mandelli M.L., Visintin E., Bruzzone M.G., Nigri A., Epifani F., Bussone G., Chiapparini L. In medication-overuse headache, fMRI shows long-lasting dysfunction in midbrain areas. Headache. 2012;52:1520–1534. doi: 10.1111/j.1526-4610.2012.02276.x. [DOI] [PubMed] [Google Scholar]
- Fuh J.L., Wang S.J. Dependent behavior in patients with medication-overuse headache. Curr. Pain Headache Rep. 2012;16:73–79. doi: 10.1007/s11916-011-0240-0. [DOI] [PubMed] [Google Scholar]
- Fuh J.L., Wang S.J., Lu S.R., Juang K.D. Does medication overuse headache represent a behavior of dependence? Pain. 2005;119:49–55. doi: 10.1016/j.pain.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Fumal A., Laureys S., Di Clemente L., Boly M., Bohotin V., Vandenheede M., Coppola G., Salmon E., Kupers R., Schoenen J. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain. 2006;129:543–550. doi: 10.1093/brain/awh691. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Salmeron B.J., Ross T.J., Geng X., Zhan W., Stein E.A., Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N., Ward N., Boshyan J., Napadow V., Maeda Y., Truini A., Caramia F., Tinelli E., Mainero C. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013 doi: 10.1177/0333102413490344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Salmeron B.J., Gu H., Stein E.A., Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- Jasinska A.J., Stein E.A., Kaiser J., Naumer M.J., Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen E.S., Lundqvist C. Medication-overuse headache: epidemiology, diagnosis and treatment. Ther. Adv. Drug Saf. 2014;5:87–99. doi: 10.1177/2042098614522683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen E.S., Straand J., Vetvik K.G., Benth J.S., Russell M.B., Lundqvist C. Brief intervention by general practitioners for medication-overuse headache, follow-up after 6 months: a pragmatic cluster-randomised controlled trial. J. Neurol. 2016;263:344–353. doi: 10.1007/s00415-015-7975-1. [DOI] [PubMed] [Google Scholar]
- Le Moal M., Koob G.F. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur. Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Limbrick-Oldfield E.H., van Holst R.J., Clark L. Fronto-striatal dysregulation in drug addiction and pathological gambling: consistent inconsistencies? Neurol. Clin. 2013;2:385–393. doi: 10.1016/j.nicl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton R.B., Stewart W.F., Sawyer J., Edmeads J.G. Clinical utility of an instrument assessing migraine disability: the Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41:854–861. [PubMed] [Google Scholar]
- Liu J., Zhao L., Lei F., Zhang Y., Yuan K., Gong Q., Liang F., Tian J. Disrupted resting-state functional connectivity and its changing trend in migraine suffers. Hum. Brain Mapp. 2015 doi: 10.1002/hbm.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Liu Y., Li N., Wang C.X., Zhang H., Jiang X.F., Xu H.S., Fu X.M., Hu X., Zhang D.R. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D.S., Kelly A.M., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mesmoudi S., Perlbarg V., Rudrauf D., Messe A., Pinsard B., Hasboun D., Cioli C., Marrelec G., Toro R., Benali H., Burnod Y. Resting state networks' corticotopy: the dual intertwined rings architecture. PLoS One. 2013;8:e67444. doi: 10.1371/journal.pone.0067444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Effects of brain lesions on card sorting. Arch. Neurol. 1963;9:90–100. [Google Scholar]
- Naqvi N.H., Gaznick N., Tranel D., Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann. N. Y. Acad. Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro A., Martelletti P. Chronic migraine plus medication overuse headache: two entities or not? J. Headache Pain. 2011;12:593–601. doi: 10.1007/s10194-011-0388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariyadath V., Gowin J.L., Stein E.A. Resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks. Prog. Brain Res. 2016;224:155–173. doi: 10.1016/bs.pbr.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Radat F., Creac'h C., Guegan-Massardier E., Mick G., Guy N., Fabre N., Giraud P., Nachit-Ouinekh F., Lanteri-Minet M. Behavioral dependence in patients with medication overuse headache: a cross-sectional study in consulting patients using the DSM-IV criteria. Headache. 2008;48:1026–1036. doi: 10.1111/j.1526-4610.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- Radat F., Chanraud S., Di Scala G., Dousset V., Allard M. Psychological and neuropsychological correlates of dependence-related behaviour in medication overuse headaches: a one year follow-up study. J. Headache Pain. 2013;14:59. doi: 10.1186/1129-2377-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M.B. Headache: medication overuse headache—seeking a management consensus. Nat. Rev. Neurol. 2014;10:309–310. doi: 10.1038/nrneurol.2014.85. [DOI] [PubMed] [Google Scholar]
- Russell M.B., Lundqvist C. Prevention and management of medication overuse headache. Curr. Opin. Neurol. 2012;25:290–295. doi: 10.1097/WCO.0b013e328352c431. [DOI] [PubMed] [Google Scholar]
- Sarchielli P., Corbelli I., Messina P., Cupini L.M., Bernardi G., Bono G., Di Piero V., Petolicchio B., Livrea P., Prudenzano M.P., Pini L.A., Sandrini G., Allena M., Tedeschi G., Russo A., Caproni S., Beghi E., Calabresi P., Group, S.S. Psychopathological comorbidities in medication-overuse headache: a multicentre clinical study. Eur. J. Neurol. 2016;23:85–91. doi: 10.1111/ene.12794. [DOI] [PubMed] [Google Scholar]
- Schwedt T.J., Schlaggar B.L., Mar S., Nolan T., Coalson R.S., Nardos B., Benzinger T., Larson-Prior L.J. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. 2013;53:737–751. doi: 10.1111/head.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Smith K.S., Graybiel A.M. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79:361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.S., Graybiel A.M. Investigating habits: strategies, technologies and models. Front. Behav. Neurosci. 2014;8:39. doi: 10.3389/fnbeh.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.S., Virkud A., Deisseroth K., Graybiel A.M. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. second ed. Consulting Psychologists Press; Palo Alto, CA: 1989. State-Trait Anxiety Inventory: Bibliography. [Google Scholar]
- Sutherland M.T., McHugh M.J., Pariyadath V., Stein E.A. Resting state functional connectivity in addiction: lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Tomasi D., Volkow N.D., Wang R., Carrillo J.H., Maloney T., Alia-Klein N., Woicik P.A., Telang F., Goldstein R.Z. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J., Maleki N., Potter J., Elman I., Rudrauf D., Knudsen J., Wallin D., Pendse G., McDonald L., Griffin M., Anderson J., Nutile L., Renshaw P., Weiss R., Becerra L., Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usai S., Grazzi L., D'Amico D., Andrasik F., Bussone G. Psychological variables in chronic migraine with medication overuse before and after inpatient withdrawal: results at 1-year follow-up. Neurol. Sci. 2009;30(Suppl. 1):S125–S127. doi: 10.1007/s10072-009-0066-2. [DOI] [PubMed] [Google Scholar]
- Viswanath H., Velasquez K.M., Savjani R., Molfese D.L., Curtis K., Molfese P.J., Eagleman D.M., Baldwin P.R., Frueh B.C., Fowler J.C., Salas R. Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology. 2015;92:63–68. doi: 10.1016/j.neuropharm.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Viswanath H., Velasquez K.M., Thompson-Lake D.G., Savjani R., Carter A.Q., Eagleman D., Baldwin P.R., De La Garza R., II, Salas R. Alterations in interhemispheric functional and anatomical connectivity are associated with tobacco smoking in humans. Front. Hum. Neurosci. 2015;9:116. doi: 10.3389/fnhum.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Suh J., Li Z., Li Y., Franklin T., O'Brien C., Childress A.R. A hyper-connected but less efficient small-world network in the substance-dependent brain. Drug Alcohol Depend. 2015;152:102–108. doi: 10.1016/j.drugalcdep.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Weiland B.J., Welsh R.C., Yau W.Y., Zucker R.A., Zubieta J.K., Heitzeg M.M. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128:130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wilcox C.E., Teshiba T.M., Merideth F., Ling J., Mayer A.R. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Yuan K., Zhao L., Cheng P., Yu D., Dong T., Xing L., Bi Y., Yang X., von Deneen K.M., Liang F., Gong Q., Qin W., Tian J. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J. Pain. 2013;14:836–844. doi: 10.1016/j.jpain.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tian J., Yuan K., Liu P., Zhuo L., Qin W., Zhao L., Liu J., von Deneen K.M., Klahr N.J., Gold M.S., Liu Y. Distinct resting-state brain activities in heroin-dependent individuals. Brain Res. 2011;1402:46–53. doi: 10.1016/j.brainres.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Gong J., Xie C., Ye E.M., Jin X., Song H., Yang Z., Shao Y. Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: evidence from resting state fMRI. Neuroscience. 2015;284:998–1010. doi: 10.1016/j.neuroscience.2014.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures S1, S2, and S3.