Abstract
In the present study, a new species Myxobolus dermiscalis n. sp. infecting scales of Labeo rohita, an Indian major carp from Harike Wetland in Punjab, India has been described on the basis of spore morphology and amplification of a part of 18S rDNA gene. The pseudocysts of M. dermiscalis n. sp. are milky white with irregular outline, 0.5–3.6 mm in diameter embedded within the dermal scale in the form of a cavity. The spores 5.84–7.98 × 3.98–5.98 μm in size, having two equal polar capsules 3.98–5.98 × 1.85–3.85 μm in size. The most differentiating feature from closely related species, Myxobolus saugati (Kaur and Singh, 2011) is the presence of two parietal folds at the posterior – lateral margins of the shell valves. The present species is regarded as host, organ and tissue specific in nature. The partial sequence of SSU gene of M. dermiscalis n. sp. clustered with other Myxobolus species infecting cyprinids available in the GenBank. Blast search revealed 98% homogeneity with Myxobolus sp (KM401439) infecting scales of L. rohita in Myanmar (unpubl. data). The present myxobolid parasite has been recorded to cause serious, highly symptomatic disease of the scales, causing their loosening from the skin of L. rohita. It rendered the host fish unsightly giving it cloudy appearance with white patches and mucoid body surface. Scale pseudocyst Index (SPI) has been provided to record the intensity of infection.
Keywords: Myxobolus, Labeo rohita, 18S rDNA, Phylogeny
Graphical abstract
Myxobolus dermiscalis n.sp infecting scales of Indian major Carp in Harike wetland of Ramsar International Importance, Punjab.
Highlights
-
•
A new species, Myxobolus dermiscalis n. sp. infecting scales of Labeo rohita, an Indian major carp from Harike Wetland in Punjab, India has been described on the basis of spore morphology and amplification of a part of 18S rDNA gene. Blast search of partial sequence of SSU gene of M. dermiscalis n. sp. revealed 98% homogeneity with Myxobolus sp (KM401439) infecting scales of L. rohita in Myanmar (unpubl. data).
-
•
The most differentiating feature from closely related species, Myxobolus. saugati (Kaur and Singh, 2011) is the presence of two parietal folds at the posterior – lateral margins of the shell valves.
1. Introduction
Punjab (India) has 3 main wetlands, i.e. Harike, Kanjli and Ropar wetlands which are included in Ramsar list of International importance. Harike wetland (31° 17′ N latitude and 75° 12′ E longitude) is the largest freshwater wetland (in northern India) of 4100 ha area with Beas and Sutlej as primary inflows. It is a habitat for as many as 26 species of fishes which include Catla, Cirrhinus, Channa, Mystus, Chitala chitala, Cyprinus and Ambassis ranga. These wetlands are the major natural fisheries resource for food in whole of the Punjab state. The study indicates that large variety of fishes in these wetlands are infested with myxozoan parasites. Myxozoans include histozoic and coelozoic parasites infecting not only freshwater and marine fishes but have also been detected in molluscs, amphibians, reptiles, waterfowl and mammals (Moncada et al., 2001, Eiras, 2005). As demonstrated firstly by Wolf and Markiw (1984), it has been proven that myxozoan species require an alternate invertebrate host (usually an annelid) to complete the life cycle. Among myxosporeans, the genus Myxobolus includes the highest number of species. Eiras et al. (2005) reported 744 valid species, while Lom and Dykova (2006) counted 792 including 7 amphibian species. Several other reports on Myxobolus species are available from Punjab and West Bengal, India (Basu & Haldar, 2004- Basu et al., 2009, Bandyopadhyay et al., 2006/2007; Kaur and Singh, 2014). About 131 species of Myxobolus have been recorded in India (Kaur and Singh, 2012) and are mainly differentiated by morphological, morphometric characteristics of spores, besides host and organ or tissue specificity. Presently, the molecular analyses have been an important tool in the study of these parasites and this has expanded their taxonomy to the phylogenetic analyses. This has led to correct identification and differentiation of morphologically indistinguishable myxobolid species (Kent et al., 2001, Eszterbauer, 2002, Molnar et al., 2010, Cech et al., 2012, Bartosova et al., 2009). The most revealing aspects of analyses based on SSU rDNA sequences is the incongruence of phylogenetic trees with classification based 63on spore morphology alone (Bartosova et al., 2009). So far, there are 14 sequences i.e caudatus KC865607; Myxobolus cuttacki 465682; M. orrissae KF448527; Myxobolus basuhaldari KM029974, KM029975, KM029976; M. kalavatiae KM029973; M. meerutensis KM029977;M. bhadrensis KM029968, KM029969, KM029970, KM029971, KM029972 and M. catlae KM029967 (Mondal et al., 2014, Szekely et al., 2014, Rajesh et al., 2014, Abraham et al., 2015) from India are available in the Genbank. In this study, we present morphological and molecular characterization of M. dermiscalis n. sp. infecting the scales of L. rohita collected from Harike wetland, Punjab, India along with 18S rDNA based phylogenetic analysis with Myxobolus group and other related texa. In future, the molecular methods can be implied on the diagnostics of the economically important myxozoan parasites in this part of the world.
2. Material and methods
2.1. Collection
Fresh specimens of Indian major carp, rohu, L. rohita were collected from the fisher-man at Harike wetland during the period June 2013 to July 2014. The infected scales were removed with the help of forceps in a petridish containing 0.9% saline. The pseudocysts were visible with the naked eye and appeared as creamish white patches on the scale. The plasmodium were teased on a clean slide to liberate spores and were examined under the microscope. Fresh spores were treated with 8% KOH solution for the extrusion of polar filaments. For permanent preparation, air-dried smears were stained with Ziehl–Neelsen and Iron-haematoxylin. Spores were measured with the help of a calibrated ocular micrometre. All measurements were recorded in microns (μm).
2.2. Prevalence
The prevalence (in percentage) of M. dermiscalis infecting scales of L. rohita was calculated according to Bush et al. (1997). The intensity of infection was determined by following the index proposed by Kaur and Attri (2015) for infection on the scales. 0 = no infection; 1 = one pseudocyst per scale in 10% of total scales (indicating light infection); 2 = two pseudocysts per scale in 15–20% of total scales (moderate infection); 3 = three to four pseudocysts per scale in 50% of total scales (heavy infection); 4 = four to five pseudocysts per scale in 100% of scales (severe infection).
2.3. Molecular analysis
2.3.1. DNA extraction, polymerase chain reaction
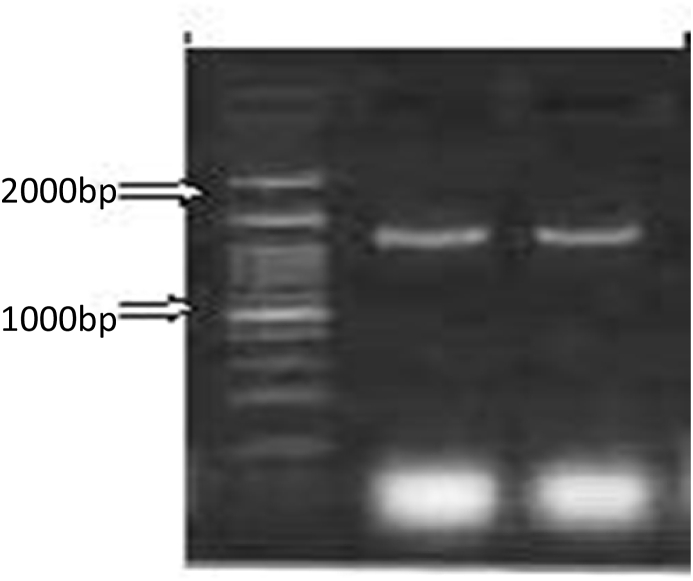
The pseudocysts (50 in numbers) present on ethanol fixed scales were ruptured with the help of a sharp needle in a watch glass having double distilled water. The contents in the watch glass containing spores were collected in 1.5 ml micro centrifuge tubes. The DNA was Scientific, Wilmington, USA) spectrophotometer at 175 ng/μl. Polymerase chain reaction (PCR) was carried out according to the Andree et al. (1999) at the final volume of 25 μl using the primers MX5-MX3 which amplified the fragments of 1597 bp respectively of the 18S rDNA gene. The amplification reactions were conducted with 30–75 ng of genomic DNA, 12.5 μl of 1 × reaction buffer (Hi media), 1.0 μl of each primer, 1.0 μl of total DNA and 10.5 μl of double purified water. Amplification was done by initial denaturation at 95 °C for 3 min, followed by 33 cycles of denaturation at 95 °C for 30 s, annealing of primers at 58 °C for 30 s, extension at 72 °C for 1 s. The final extension was at 72 °C for 10 s. The PCRproducts were analysed agarose gel electrophoresis, and size was estimated by comparison with the1Kb Plus DNA ladder (Fig. 1).
Fig. 1.
Agarose gel (1.8%) showing amplified 18S rDNA gene of M. dermiscalis n. sp. infecting scales of Labeo rohita.
Lane 1: 1kb DNA Ladder
Lane 2, 3: M. dermiscalis n. sp. (1597bp)
2.3.2. DNA sequencing, sequence alignment and phylogenetic analysis
Extracted from spores using the DNeasy Blood & Tissue Kit (Qiagen) following the manufacturer's instructions. The product was then quantified in a Nanodrop (Thermocycler). In the present study, the PCR amplified products were sequenced at the Molecular Diagnostics & Research Laboratories (MDRL) Pvt. Ltd. Chandigarh, India. 1597 bp of 18S rDNA sequences of M. dermiscalis n. sp. were deposited in GenBank with accession number KM092529. Phylogenetic analysis involving 23 nucleotide sequences was performed using the Bayesian, Maximum likelihood (ML), Maximum parsimony (MP) and Neighbour joining (NJ) methods. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates). The evolutionary distances were computed using the Kimura 2-parameter method Kimura (1980) and units of the number of base substitutions per site. The rate variation among sites was modelled with a gamma distribution. The best fit model for analysis for the current data was GTR + G with lowest BIS (Bayesian Information Criterion) was estimated by using model test tool in Mega 6.06.
3. Results
3.1. Morphological characteristics of Myxobolus dermiscalis n. sp.
3.1.1. Pseudocyst (Fig. 2)
Fig. 2.
Infected scales of L. rohita showing creamish white pseudocysts of M. dermiscalis n. sp scale bar = 1 cm.
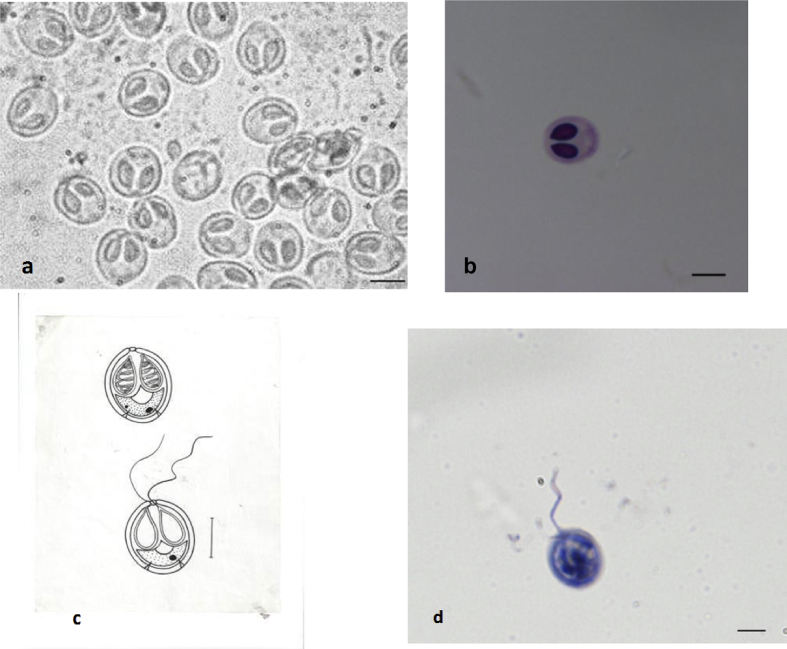
Round to irregular, white, 4-5pseudocysts per scale, histozoic, present within a cavity and measure 0.5–3.6 mm in diameter. 400–500 of spores were present per pseudocyst.
3.1.2. Spore description (Fig. 3)
Fig. 3.
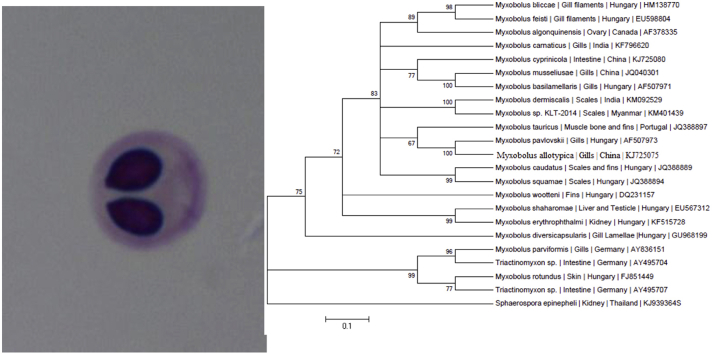
Photomicrographs of myxospores of M. dermiscalis n. sp. a) fresh under phase contrast microscope b) stained with Ziehl–Neelson c) Line drawing d) stained with Iron-haematoxylin Scale bar = 10 μm.
(Measurements based on 10 spores in frontal view).
The spores measure 5.84–7.84 × 3.98–5.98 μm, oval to spherical in frontal view having rounded anterior and posterior ends. Both the shell valves are thick, symmetrical and 0.5 μm in thickness. Parietal folds two, present on the posterio-lateral margins of the shell valves. Polar capsules are two, equal, measure 3.98–5.98 × 1.85–3.85 μm and are pyriform with distinct neck at the anterior end. They converge anteriorly and are placed at a distance posteriorly. Polar filaments form 5–6 coils and are arranged obliquely to the polar capsule axis, 7.2 μm in length when extruded. An intercapsular process is absent. Two capsulogenic nuclei are present beneath each of the polar capsule. Sporoplasm agranular, homogenous occupying whole of the extracapsular space behind the polar capsules and contain one nucleus and a large iodinophilous vacuole.
3.1.3. Taxonomic summary of M. dermiscalis n. sp.
Host: L. rohita (Ham.) vern. Rohu.
Locality: Harike Wetland, Punjab, India.
Site of infection: Scales.
Type specimen: Paratype are spores stained with Ziehl–Neelsen and Iron-Haematoxylin, deposited in the Parasitology Laboratory, Department of Zoology & Environmental Sciences, Punjabi University, Patiala (India). Slide no. M/ZN/02.11.2014 and M/IH/02.11.2014.
Frequency of parasite species (%): 59.7% (52/87).
Intensity of infection with parasite species (Index): 4 (Severe infection).
Clinical Symptomatology: Highly symptomatic; creamish white patches and mucous laden body surface. Loosening of scales.
Etymology: The specific epithet dermiscalis has been given on the basis of location of pseudocysts within the layers of dermal scales.
3.2. Molecular data
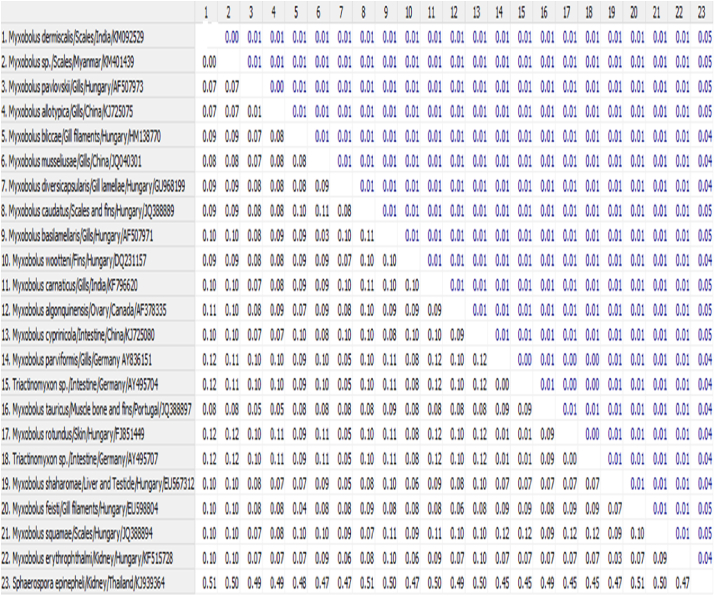
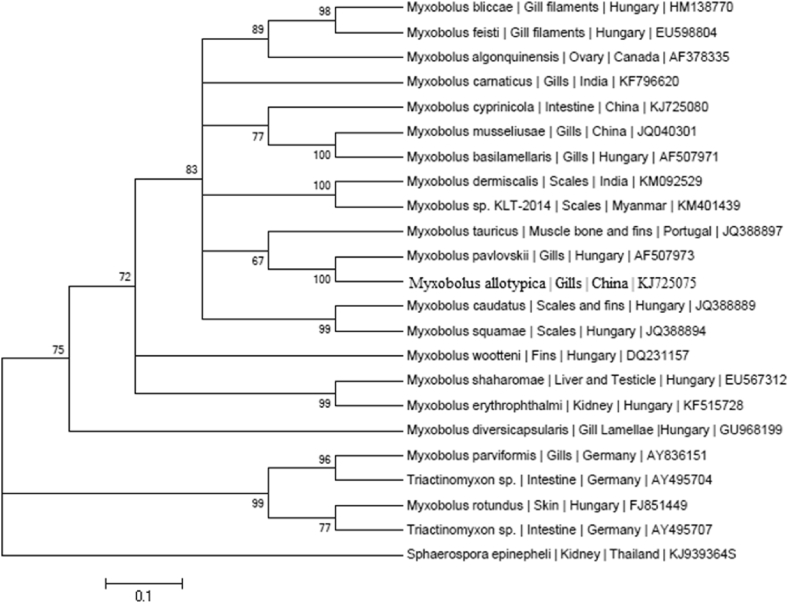
The edited nucleotide sequence was 1597 bp of M. dermiscalis n. sp. which was deposited in Gen-Bank under the accession number of KM092529. BLASTN search (www.ncbi.nlm.gov/BLAST) with 21 Myxobolus exhibited 90–93% sequence homology and maximum homology of 98% recorded was recorded with Myxobolus sp. (KM401439 unpubl) infecting scales of a cyprinid, L. rohita in Myanmar available in the GenBank (Table 1). In the phylogenetic tree based on the final edited alignment with neighbour–joining analysis (Fig. 5), M. dermiscalis was placed with the M. sp. with highest bootstrap value and confirmed the clustering pattern of maximum likelihood and maximum parsimony analysis (100% in ML, 100% MP).
Table 1.
Homogeneity of 18S rRNA gene sequences of Myxobolus dermiscalis (Accession number KM092529) and other myxobolids available in NCBI GenBank.
| Myxozoan species | Host fish | Site of infection | Accession number | Country | DNA sequence homogeneity to M. dermiscalis n. sp (KM029925) |
|---|---|---|---|---|---|
| Myxobolus sp | Labeo rohita | Scale | KM401439 | Myanmar | 2771/2771(98) |
| M. pavlovskii | Aristichthys nobilis | Gills | AF507973 | Hungry | 1796/1796(93) |
| M. allotypica | Hypothalmichthys molitrix | Gills | KJ725075 | China | 1779/1779(93) |
| Myxobolus bliccae | Blicca bjoerkna | Gills | HM138770 | Hungry | 1725/1725 (92) |
| M. mussaliusae | Cyprinus carpio | Gills | JQ040301 | China | 1707/1707(93) |
| M. diversicapsularis | Rutilus rutilius | Gills | GU968199 | Hungry | 1690/2037(92) |
| M. caudatus | Barbus bynni | Tail fin | JQ388889 | Egypt | 1679/1679(91) |
| M. basilamellaris | Cyprinus carpio | Gills | AF507971 | Hungary | 1676/1676(91) |
| M. wooteni | Rutilus rutilus | Fins | DQ231157 | Hungary | 1670/1670(91) |
| M. carnaticus | Cirrhinus mrigala | Gills | KF796620 | India | 1663/1663(91) |
| M. algonquinensis | Notemigonus crysoleucas | Ovary | AF378335 | Canada | 1657/1961(91) |
| M. cyprinicola | Leuciscus waleckii | Intestine | KJ725080 | Russia | 1635/1635(91) |
| M. parviformis | Abramis brama | Gill lamellae | AY836151 | Germany | 1609/1609(90) |
| Triactinomyxon sp. | Tubifex tubifex | AY495704 | Germany | 1609/1609(90) | |
| M. tauricus | Barbus tauricus | Gills, fins, muscles | JQ388897 | Ukraine | 1572/1572(92) |
| M. rotundus | Abramis brama | Gills | FJ851449 | Germany | 1570/1902(90) |
| Triactinomyxon sp. | Tubifex tubifex | AY495707 | Germany | 1568/1568(90) | |
| M. shaharomae | Alburnus alburnus | Liver, kidney, testis, gut | EU567312 | Hungary | 1559/1559(91) |
| M. feisti | Rutilus rutilus | Gill filament | EU598804 | Hungary | 1552/1552(91) |
| M. squamae | Barbus barbus | Scales | JQ388894 | Hungary | 1504/1504(91) |
| M. erythrophthalmi | Scardinius erythrophthalmus | Liver | KF515728 | Hungary | 1411/1411(91) |
| Sphaerospora epinepheli | Epinephelus coioides | Kidney | KJ939364 | China | Outgroup |
Fig. 5.
Estimates of evolutionary divergence between the sequences of M. dermiscalis and other Myxosporea available in GenBank.
4. Discussion
4.1. Morphological comparison
The present species has been compared with M. squamae (Keysseltz, 1908) from scales of Abramis brama; M. mrigalae (Chakravarty, 1939) from scales of Cirrhinus mrigala; M. yogendrai (Tripathi, 1952), M. rewensis (Srivastava, 1979) and M. vanivillasae (Seenappa and Manohar, 1980) from scales of Cirrhinus mrigala; M. rohitae (Haldar et al., 1981) from scales of L. rohita, Labeo calbasu, Labeo bata and Puntius sarana; M. indirae (Gupta and Khera, 1988) from scale, tail, fin, cartilage and head of Cirrhinus mrigala M. episquamae (Egusa et al., 1990) from scales of Mugil cephalus; M. squamaphilus (Molnar, 1997) from scales of A. brama; M. dermatis (Haldar et al., 1983) from scales of L. rohita; and M. saugati (Kaur and Singh, 2011) from scales and gill lamellae of Catla catla and L. rohita. The present species, M. dermiscalis n. sp. was compared with all the above mentioned species and was found different in spore morphology and morphometrics. The spores of the present species are characterized in having oval to spherical shape with rounded anterior end without intercapsular process and posterior end with two parietal folds. Polar capsules are two, equal, pyriform with distinct neck. In this respect, the present species differed from M. squamae, M. yogendrai, M. rewensis, M. vanivillasae, M. rohitae, M. indirae,M.episquamae, M. squamaphilus, M. dermatis, and M. saugati in lacking the intercapsular process. Furthermore, it differed from M. saugati, M. rohitae, M. dermatis and M. indirae in having two parietal folds placed posterio-laterally on the shell valves. The present species is also different from M. mrigalae in which several triangular markings are present, in addition to unequal polar capsules. In view of the above differences in morphology and morphometrics, the present species under study is proposed as new to the science and named as M. dermiscalis n. sp.
4.2. Molecular comparison
The universal primer sets MX5 and MX3 successfully amplified approximately 1600 bp fragments of the 18srDNAgene from the present sample of Myxobolus (Fig. 3). The edited nucleotide sequence was 1597 bp of M. dermiscalis n. sp. which was deposited in GenBank under the accession number of KM092529. The DNA sequences of M. dermiscalis n. sp. was closest to Myxobolus sp. infecting scales of a cyprinid, L. rohita in Myanmar available in the GenBank (unpubl), which showed 98% homogeneity (Table 1). In the phylogenetic tree based on the final edited alignment with neighbour–joining analysis (Fig. 5), M. dermiscalis was placed with the M. sp. with highest bootstrap value and confirmed the clustering pattern of maximum likelihood and maximum parsimony analysis (100% in ML, 100% MP). The present study indicated close similarity based on 18S rDNA suggesting sister species, however, the morphological comparisons could not be made due to lack of published record. Minimum evolutionary divergence between the two species was estimated using Bayesian Information Criterion (BIS scores) using best model test tool in Mega 6.06 (Fig. 4). In order to estimate the pattern of nucleotide substitution involving 23 nucleotide sequences using GTR + G model in (Table 2).
Fig. 4.
Neighbour-Joining analysis of small subunit ribosomal DNA sequence of M. dermiscalis n.sp.in relation to 23 other sequenced members of the genus.
Table 2.
Showing the transitional and transversion substitutions obtained by maximum likelihood estimate of substitution matrix in Mega 6.0 (Rates of different transitional substitutions are shown in bold and those of transversion substitutions are shown in italics).
| A | T/U | C | G | |
|---|---|---|---|---|
| A | – | 5.31 | 3.82 | 13.21 |
| T/U | 5.26 | – | 14.38 | 5.51 |
| C | 5.26 | 19.99 | - | 5.51 |
| G | 12.62 | 5.31 | 3.82 | – |
Rates of different nucleotide substitution(r) from one base (transitional -in bold) and to another base(transversional-in italics) were assessed. The rates of transition were more than transversion for all base substitutions. Maximum transition was C→ G having 19.99 probability value followed by T→C 14.38, A→G 13.21, G→A 12.62. As for trasversions A→T, G→T 5.31; T→A, C→A 5.26, T→G, C→G 247 5.51, A→C, G→C 3.82. The nucleotide frequencies were 26.44%(A), 26.69%(T/U), 248(G) 19.19%(C) and 27.68%(G). Furthermore, the information generated confirmed the importance of features such as host, tissue tropism, geographical location of the host and correlation with the results of molecular data based on comparison of 18S rDNA sequence (Kent et al., 2001, Seo et al., 2012, Zhu et al., 2012, Shin et al., 2013). Index of intensity of infection by M. dermiscalis n.sp was recorded as 4 indicated by the presence of four to five pseudocysts per scale in 100% of scale. The intensity of infection was recorded as ‘severe’ as per to the index number 4 of pseudocysts per scale.
The prevalence of M. dermiscalis n. sp. infesting scales of L. rohita was 59.7%. This study is the first report the molecular and phylogenetic characterization of scale infecting M. dermiscalis n. sp. from Indian subcontinent. As the list of Indian species of the Genus Myxobolus have increased over the years (Basu et al., 2009, Bandyopadhyay et al., 2006/2007; Singh & Kaur, 2012–2014) thus the correct identification in future studies must focus on molecular and phylogenetic characterization together with morphological characteristics, host, organ and tissue specificity.
5. Conclusions
The present study describe a myxobolid species commonly found infecting scales of an Indian major carp, L. rohita in freshwater habitat. The intensity of infection was severe as indicated by the Scale Pseudocyst Index and highly symptomatic to the fish host. Further investigations are being carried out on the presence of gill infecting myxozoan parasites among major Indian carps in polycultured conditions. This is important to have assessment of the impact on these parasites on the carp fishes. Beside morphological features, genetic data has been provided in the present manuscript which can form basis for development of diagnostics of this species.
Acknowledgements
The authors are grateful to Dr. Sukhbir Kaur, Professor in the Department of Zoology, Panjab University, Chandigarh for providing the infrastructure in her laboratory and special thanks are due to Dr. Jyoti Joshi, research scholar in the Department of Zoology, Panjab University, Chandigarh for the help rendered during the experimental work.
References
- Abraham T.H., Banerjee S., Patra A., Sarkar A., Adikesavalu H., Dash G. Molecular phylogeny of Myxobolus orissae(Myxosporea:Myxobolidae) infecting the gill lamellae of mrigal carp Cirrhinus mrigala(Actinopterygii:Cyprinidae) Mol. Biol. Res. Comm. 2015;4(1):15–24. [PMC free article] [PubMed] [Google Scholar]
- Andree K.B., Shekels C., Molnar K., Gresoviac S.J., Hedrick R.P. Relationship among members of the genus Myxobolus (Myxozoa: Bivalvulida) based on small subunit ribosomal DNA sequences. J. Parasitol. 1999;85:68–74. [PubMed] [Google Scholar]
- Bandyopadhyay P.K., Hemananda T., Mitra A.K., Mohilal N. Myxobolus dhanachandi sp. N. (Myxozoa, Myxosporea, Bivalvulida) from an Indian freshwater fish, Channa orientalis (Bloch–Schneider) Protistology. 2006/2007;14(4):353–356. [Google Scholar]
- Bartosova P., Fiala I., Hypsa V. Concatenated SSU and LSU rdna data confirm the main evolutionary trends within myxosporeans (Myxozoa: Myxosporea) and provide an effective tool for their molecular phylogenetics. Mol. Phylogenet. Evol. 2009;53:81–93. doi: 10.1016/j.ympev.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Basu S., Haldar D.P. Description of three new species (Myxozoa: Myxosporea: Bivalvulida) of the genera Myxobilatus Davis, 1944 and Myxobolus Butschli, 1882. Acta Protozool. 2004;43(4):337–343. [Google Scholar]
- Basu S., Modak B.K., Haldar D.P. Two new species of Myxobolus Butschli, 1882(Myxozoa: Mysporea: Bivalvulida) from food fishes of West Bengal, India-a light and scanning electron microscopy study. Acta Protozool. 2009;48(83):89. [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997;83(4):575–583. [PubMed] [Google Scholar]
- Cech G., Molnar K., Szekely C. Molecular genetic studies on morphologically distinguishable Myxobolus spp. infecting cyprinid fishes, with the description of three new species, M. alvarezae sp. nov., M. sitjae sp. nov. and M. eirasianus sp. nov. Acta Parasitol. 2012;57(4):354–366. doi: 10.2478/s11686-012-0045-2. [DOI] [PubMed] [Google Scholar]
- Chakravarty M. Studies on Myxosporidia from the fishes of Bengal, with a note on the myxosporidea infection in aquarium fishes. Arch. Protistenkd. 1939;92:169–178. [Google Scholar]
- Egusa S., Maeno Y., Sorimachi M. A new species of myxozoa, Myxobolusepisquamalis sp. nov., infecting the scales of the mullet, Mugil cephalus. L. Fish. Pathol. 1990;25(2):87–91. [Google Scholar]
- Eiras J.C. An overview on the myxosporean parasites in amphibians and reptiles. Acta Parasitol. 2005;50:267–275. [Google Scholar]
- Eiras J.C., Molnar K., Lu Y.S. Synopsis of the species of Myxobolus Butschli, 1882 (Myxozoa: Myxosporea: Myxobolidae) Syst. Parasitol. 2005;61:1–46. doi: 10.1007/s11230-004-6343-9. [DOI] [PubMed] [Google Scholar]
- Eszterbauer E. Molecular biology can differentiate morphologically indistinguishable Myxosporean species: Myxobolus elegans and M. Hungaricus. Acta Vet. Hung. 2002;50:59–62. doi: 10.1556/AVet.50.2002.1.8. [DOI] [PubMed] [Google Scholar]
- Gupta S., Khera S. On a new myxozoan parasite (Myxozoa) Lomosporusindicus gen. sp. n. From fresh water fishes, Labeo calbasu(Ham.) Acta Protozool. 1988;27:171–175. [Google Scholar]
- Haldar D.P., Mukherjee M., Kundu T.K. Observations on two new species of Myxosoma Thelohan, 1892 (Myxozoa: Myxosomatidae) from fresh water teleost fishes. Arch. Protistenkd. 1981;124:244–251. [Google Scholar]
- Haldar D.P., Das M.K., Sharma B.K. Studies on protozoan parasites from fishes. Four new species of the genera henneguya thelohan, 1892, Thelohanellus Kudo, 1933 and Myxobolus Butschli, 1892. Arch. Protistenkd. 1983;127:283–296. [Google Scholar]
- Kaur H., Attri R. Prevalence and tissue specificity of Myxobolus saugati Kaur and Singh, 2011 (Myxozoa; Myxosporea; Bivalvulida)Causing Dermal myxoboliosis in wild and cultured Indian major carps in Punjab. Species. 2015;15(47):14–18. [Google Scholar]
- Kaur H., Singh R. Two new species of Myxobolus (Myxozoa: Myxosporea: Bivalvulida) from freshwater fishes of Punjab wetlands (India) J. Parasit. Dis. 2011;35(1):33–41. doi: 10.1007/s12639-011-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Singh R. A synopsis of the species Myxobolus Butschli, 1882(Myxozoa:Bivalvulida) parasitizing Indian fishes and a revised key to myxosporean genera. Syst. Parasitol. 2012;81:17–37. doi: 10.1007/s11230-011-9321-z. [DOI] [PubMed] [Google Scholar]
- Kent M.L., Andree K.B., Bartholomew J.L., Matbouli M., Desser S.S., Delvin R.H., Feist S.W., Hedrick R.P., Hoffmann R.W., Khattra J., Hallet S.L., Lester R.J.G., Longshaw M., Palenzeula O., Siddal M.E., Xiao C. Recent advances in our knowledge of the Myxozoa. J. Eukaryot. Microbiol. 2001;48:395–413. doi: 10.1111/j.1550-7408.2001.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Kimura M.A. Simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–112. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Lom J., Dykova I. Myxozoan genera: definition and notes on taxonomy, life cycle terminology and pathogenic species. Folia Parasitol. 2006;53:1–36. [PubMed] [Google Scholar]
- Molnar K. Myxobolus squamaphilus sp. n. (Myxozoa: Myxosporea), a common parasite of the scales of bream (Abramis brama L. Acta Protozool. 1997;36:221–226. [Google Scholar]
- Molnar K., Marton Sz, Szekely C., Eszterbauer E. Differentiation of Myxobolusspp. (Myxozoa: Myxobolidae) infecting roach (Rutilus rutilus) in Hungary. Parasitol. Res. 2010;107:1137–1150. doi: 10.1007/s00436-010-1982-z. [DOI] [PubMed] [Google Scholar]
- Moncada L.I., Lopez M.C., Murcia M.I., Nicholis S.F., Guio O.L. Myxobolus sp.,another opportunistic parasite in immunosuppressed patients. J. Clin. Microb. 2001;39(5):1938–1940. doi: 10.1128/JCM.39.5.1938-1940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal A., Banerjee S., Patra A., Adikesavalu H., Ramudu R.K. Molecular and morphometric characterization of Thelohanellus caudatus (Myxosporea:Myxobolidae) infecting the caudal fin of Labeo rohita (Hamilton) Parasitology. 2014;8(2):41–52. [Google Scholar]
- Rajesh S.C., Banerjee S., Patra A., Dash G., Abraham T.H. Molecular characterization if Myxobolus cuttacki (Myxozoa: Myxosporea, Bivalvulida) infecting gill lamellae of minor carp Labeo bata (Ham.) Mol. Biol. Res. Comm. 2014;3(4):231–234. [PMC free article] [PubMed] [Google Scholar]
- Seenappa D., Manohar L. Myxobolus vanivilasae n. sp. parasitic in Cirrhinamrigala (Ham.) Proc. Indian Acad. Sci. Anim. Sci.) 1980;89:485–491. [Google Scholar]
- Seo J.S., Jeon E.J., Kim M.S., Woo S.H., Kim J.D., Jung S.H., Park M.A., Jee B.Y., Kim J.W., Kim Y.C., Lee E.H. Molecular identification and real time quantification PCR (qpcr) for rapid detection of Thelohanellus kitauei, a myxozoan parasite causing intestinal giant cystic disease in the Israel carp. Korean J. Parasitol. 2012;50:103. doi: 10.3347/kjp.2012.50.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S.P., Kim J.H., Choresca C.H., Jr., Han J.E., Jun J.W., Kim J.H., Park S.C. Molecular identification and phylogenetic characterisation of Thelohanellus kitauei. Acta Vet. Hung. 2013;61:30–35. doi: 10.1556/AVet.2012.055. [DOI] [PubMed] [Google Scholar]
- Srivastava S.P. A new species of Myxobolusfrom scales of Cirrhina mrigala(Hamilton) Sci. Cult. 1979;45:444–445. [Google Scholar]
- Szekely C., Cech G., Chaudhary A., Borzak R., Singh H.S., Molnar K. Myxozoan infection of the three Indian major carps in fish ponds around Meerut, UP, India, with descriptions of three new species, Myxobolus basuhaldari sp.n.,M.kalavatiae sp.n. andM. meerutensis sp. N., and the redescription of M. catlae andM. bhadrensis. Parasitol. Res. 2014 doi: 10.1007/s00436-014-4307-9. [DOI] [PubMed] [Google Scholar]
- Tripathi Y.R. Studies on the parasites of Indian fishes. І. Protozoa. Myxosporidia together with a checklist of parasitic protozoa described from Indian fishes. Rec. Indian Mus. 1952;50:63–88. [Google Scholar]
- Wolf K., Markiw M.E. Biology contravenes taxonomy in the Myxozoa- new discoveries show alternation of invertebrates and vertebrates hosts. Science. 1984;225:1449–1452. doi: 10.1126/science.225.4669.1449. [DOI] [PubMed] [Google Scholar]
- Zhu Y.T., Lu H.D., Cai S.J. Redescription of Thelohanellus wuhanensis Xiao etchen (Myxozoa: Myxosporea) infecting allogyno genetic crusian carp (Carassius auratus gibelio) and phylogenetic analysis based on 18s rdna sequence. Acta Zootax Sin. 2012;37:681–686. [Google Scholar]