Abstract
More sensitive assays of mouse motor ataxia may provide a better understanding of the pathological profile. Treadmill gait analysis using ventral imaging allows for unhindered access to the ambulating mouse. In contrast to genetic mutations or exogenous brain injury, ethanol (EtOH) allows for the detection of dose dependent changes in motor behavior, which can be used to assess an assay’s detection sensitivity. EtOH induced ataxia was assessed in C57BL/6J (B6) and 129X1/SvJ (129) mice using the DigiGait imaging system. Gait was analyzed across EtOH dosage (1.75, 2.25 and 2.75 g/kg) in each strain using a linear mixed effects model. Overall, 129 mice displayed greater susceptibility to EtOH ataxia than their B6 counterparts. In both strains, hind paws exhibited greater sensitivity to EtOH dosage than fore paws. Across most variables analyzed, only a modest EtOH-induced change in motor behavior was observed in each strain with the 1.75g/kg EtOH doses failing to elicit significant change. These data indicate the ability to detect motor differences between strains, yet only moderate ability to detect change across EtOH dosage using the automated treadmill. Rotarod assays, however, were able to detect motor impairment at lower doses of EtOH. The significant, but opposite changes in paw placement with increasing EtOH doses highlight strain-specific differences in biophysical adaptations in response to acute EtOH intoxication.
Keywords: Ethanol, ataxia, gait, treadmill, DigiGait, C57BL/6J, 129X1/SvJ
1. Introduction
The cerebellum and its connections are crucial for the accurate timing and execution of motor acts (Thach et al., 1992), but its functions extend beyond motor domains (Schmahmann, 2004). Multiple pathologies can affect the cerebellum, ranging from genetic mutations to vascular, inflammatory and autoimmune causes as well as drugs and toxins (Schmahmann, 2004). Quantitative evaluation of cerebellar function in humans or in animals has not been straight forward. Rating scales have been used predominantly in humans (Schmitz-Hubsch et al., 2006), and quantitative measures such as the 9-hole peg board have been introduced to evaluate human progressive cerebellar diseases (Brandt et al., 2004, du Montcel et al., 2008, Takahashi et al., 2008). Quantification of cerebellar dysfunction is important for human trials and for evaluating pre-clinical compounds in rodent model systems. Many movement disorder diseases have their origin in cerebellar dysfunction, such as the inherited Spinocerebellar Ataxias (SCAs) and stroke (Schmahmann et al., 2004). Exogenous perturbation of normal cerebellar functioning may also result in loss of coordination. For instance, ethanol (EtOH) has acute and chronic effects on cerebellar activity (Basile et al., 1983, Deitrich et al., 1989, Mameli et al., 2008). Although EtOH acts at many sites throughout the CNS, part of the observed ataxia is a function of impairment of Purkinje cells (PC) functioning (Yang et al., 2000). Cerebellar dysfunction results in an observable phenotype such as gait and appendicular ataxia. Other features such as foot-hip angle changes and step angle irregularities also serve as tell-tale signs of motor abnormalities (Earhart and Bastian, 2001, Stolze et al., 2002).
In the evaluation of animal models of ataxia, subjects are typically tasked with performing an act of simple coordinated movements such as walking or balancing on a narrow beam (Stanley et al., 2005, Liu et al., 2009). Another task of motor coordination is the rotarod apparatus, which consists of the animal being placed on a rotating cylinder and requiring it to both walk and maintain balance at a constant or changing speed of rotation (Rustay et al., 2003, Liu et al., 2009). The rotarod remains an important behavioral assay for motor coordination in animals. However, it may not detect more subtle alterations in limb coordination that may be present. For example, the rotarod cannot resolve missteps of the fore vs. hind limbs nor can it distinguish amongst other movement pathologies: trunk-torso torsion, step angle or shared limb-stance to name a few. To that end, assays have been developed to service the need for more discrete measures of gait pathology. One example is the inked-paw assay in which each paw of a rodent is marked with a different color of ink. The mouse is then allowed to ambulate across a paper substrate. Paw prints can be subsequently analyzed for stride width and length (Pallier et al., 2009).
The treadmill provides an advantage over traditional over ground gait assays by standardizing speed across. Advances in imaging technology have provided a means to digitally assess each individual limb on a specially modified treadmill. Unlike the traditional inked-paw and runway model assay, researchers may now employ the use of ventral plane video recording to film ambulating animals. Such an assay allows for a more thorough treatment of discrete motor differences in animals. The DigiGait imaging system (Mouse Specifics, Boston MA) may provide a useful tool for quantifying behavioral data due to its ability to simultaneously make recordings of multiple measures of gait. Although not all data gathered from the DigiGait may be germane to every animal model of motor coordination pathologies. For example, stride length disturbance has been reported in animal models of arthritis and pain (Coulthard et al., 2002, Vincelette et al., 2007), and models of delayed onset neurodegenerative ataxia (Yokota et al., 2001). Foot angle and stance width are strongly associated with rat models of ataxia (Hannigan and Riley, 1988, Powell et al., 1999), whereas measurements of paw area in contact with the belt have been associated with models of pain (Coulthard et al., 2002).
Mouse strains vary in their natural motor activation during development and maturation. Between the C57BL/6J (B6) and the 129X1/SvJ (129) strains of mice, the former exhibits greater motor ability in several measures of coordinated movement, yet shows greater age-related declines in coordination and motor fatigue than their 129 counterparts (Serradj and Jamon, 2007; 2009) Furthermore, work from others has indicated difference endogenous motor ability across various strains of mice (Hoit et al, 2002; Kale et al, 2004) as well as difference between strains in response to EtOH (Crabbe et al, 2008).
In this report, we examined motor-coordination in two strains of mice across varying doses of EtOH using the DigiGait imaging system. We examined whether this assay could detect EtOH-induced dose-dependent alterations in gait and whether strain-specific differences existed. In a second experiment we examined the sensitivity of the accelerating rotarod assay to detect motor dysfunction across EtOH dose. The overarching goal of this work was to extend on previous work in order to provide an additional and potentially more efficient and quantitative means of screening animals for motor ataxia using the DigiGait system.
2. Materials and methods
2.1 Animals
Animals were allowed to acclimate to the facility before undergoing any procedures. Mice were housed in a light and temperature controlled room: lights on at 0600, temperature maintained at 20°C. Animals were housed (4–5 per cage) in clear Plexiglas hanging cages (30cm X 18cm X 15cm). HEPA filtered air was forced through barrier protective lids. All animals had ad libitum access to food (Lab Diet 5001) throughout the course of the study except when being tested on the DigiGait machine (approximately 5min per day) or the rotarod (<15 min per day). All animals were matched for age (14–17 weeks) and weight (30g ± 4g). Only trained personal handled the mice and all procedures carried out were in accordance with federal guidelines as well as University of Utah IACUC approved protocols.
2.2 Experiment 1: Gait dynamic assay
Seventeen 129X1/SvJ (n=9 female, n=8 male) and sixteen C57BL/6J (n=8 female, n=8 male) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) for use on the DigiGait instrument. All gait dynamic assays were performed using the DigiGait imaging system along with DigiGait 9.0 analysis software (Mouse Specifics, Inc; Quincy MA). This imaging system has been described in greater detail elsewhere (Kale et al., 2004, Amende et al., 2005). Briefly: the DigiGait apparatus consists of a clear plastic treadmill with a high-speed under-mounted digital camera (Basler Technologies Inc) used for imaging paw prints. Images were collected at a rate of 140 frames/sec. and stored as audio video interleaved (AVI) files for later analysis. Image analysis software digitally encoded animal paw area and position relative to the tread-belt. Each paw of the animal was treated as a unique signature; such that later analysis of foot movement could be performed on separate limbs. An average of 10 sequential strides per paw was collected from each mouse. This number of strides has been validated as being sufficient to analyze treadmill walking behavior in mice (Kale et al., 2004).
Animals were habituated to the machine daily for four days prior to testing. Mice were placed on the treadmill and locked into a clear Plexi-glass chamber (19.5cm x 5cm x 13cm) with the treadmill serving as the floor of the chamber. Habituation consisted of initially allowing the animal to explore the chamber for one-minutes on their first exposure followed by 30 seconds of treadmill activation at a low rpm (10 cm/s). On subsequent habituation trials animals were tasked with walking at a slightly faster pace (15 cm/s). All settings, i.e. camera, lighting, belt speed, were optimized before experimental testing. Work by others has indicated that mice are able to comfortably ambulate at a gait speed of 25 cm/s (Clarke and Still, 1999). On test days mice were administered dosed and returned to their home cage for a minimum of 15min, but not more than 20min, to allow for alcohol diffusion, following which, they were immediately placed in the DigiGait chamber and testing for testing.
2.3 Experiment 2: Accelerating Rotarod
A second cohort of EtOH naïve mice were tested on the rotarod under an accelerating speed paradigm: seven 129X1/SvJ (n=4 female, n=3 male) and eight C57BL/6J (n=4 female, n=4 male) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The rotarod apparatus (Ugo Basile North America Inc., Collegeville, PA) was comprised of a horizontal rod, 3 centimeters in diameter, separated by opaque plastic dividers in order to accommodate up to 5 mice per trial. The trial started at a speed of 4 revolutions per minute (rpm) and slowly accelerated to 40 rpm over the course of 300 seconds. The latency to fall from the rod was recorded as the dependent variable. Data are reported as the average of 3 trials on test day. Rest periods in between trials were limited to < 5 min. On EtOH test days mice were dosed and returned to their home cage for 15min to allow for alcohol diffusion, following which, they were immediately placed on the rotarod to begin testing.
2.4 Ethanol
EtOH was diluted in sterile isotonic saline (0.09%). Concentrations were determined to give a volume injection equal to 0.01cc/g body weight. Mice were weighed and then administered EtOH via IP injection using a 1cc syringe with a 26 gauge needle. Following injection animals were immediately returned to their home cage for at least 15 min but no more than 20 min to allow for drug diffusion. Dynamic gait assays and the accelerating rotarod assay were administered on the following schedule: Saline injection followed by 24 hours of recuperation; 1.75g/kg EtOH followed by 48 hours recuperation; 2.25 g/kg EtOH followed by 48 hours rest and finally animals given the largest dosing of EtOH (2.75 g/kg) were allowed 5 days’ rest. This order of dosing follows a similar regimen as reported by Kale and colleagues (Kale et al., 2004).
2.5 Blood Ethanol Concentration
Sixteen EtOH naïve mice (four animals for each of three EtOH doses or saline control) from each strain were used for calculating blood EtOH concentration (BEC). Mice were gently restrained, given an IP EtOH injection and then returned to their home cage; blood was collected fifteen minutes later. Approximately 50 L of blood per animal were collected from the jugular vein into BD Microtainers with K2EDTA (Becton, Dickinson and Company, Franklin Lakes, NJ) and stored at 4°C until the time of processing. Blood EtOH levels were determined using gas chromatography and carried out by the University of Utah department of Human Toxicology. In all cases N = 4, except saline injected 129 and B6 mice, as well as B6 mice dosed with 2.75 g/kg EtOH, where N = 3, due to an inability to collect a sufficient blood volume.
2.6 Statistics
Gait dynamics and accelerating rotarod data were analyzed using mixed-effects models. Mixed models were used to account for repeated measures in each mouse by treating mouse strain and EtOH dose as fixed effects (corresponding to a 2-way analysis of variance) and the individual mice as random effects. The mixed modeling approach naturally accounts for data missing at random, as occurred for 3 mice at the highest alcohol dose in this study (Little et al., 1987; Verbeke et al., 2000). The EtOH dose effect was analyzed based upon a linear contrast across four dose levels (where saline is valued as dose 0). In the event of a significant dose by mouse strain interaction, the dose effect was estimated separately for each individual mouse strain. Comparisons were deemed to be significantly different if P<0.05. Prior to data analysis, right and left limbs were averaged into a single data point representing either the “fore” or “hind paw” across all gait parameters. Initial analysis did not detect a significant interaction of mouse gender and EtOH dose within strain. Therefore we have grouped all data by strain only. Data were analyzed using SAS versions 9.2 and 9.3 and represented as mean ± standard deviation (SD).
3. Results
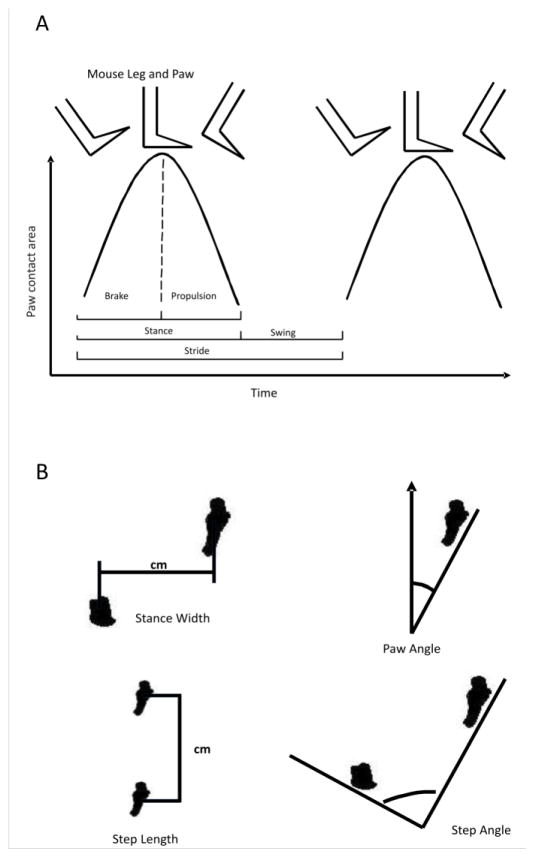
Table 1 provides the average blood EtOH concentrations (BEC), in mg/ml ± standard deviation, obtained from B6 and 129 mice across the three doses of EtOH as well as a saline control. Although a trend towards significant strain differences was detected, this remained non-significant. Gait parameters are visually depicted for measurements of time and space (Figures 1A and 1B respectively). For a more detailed explanation of measurements see Amende et al., 2005, from which these figures were adapted.
Table 1.
Mean ± the standard deviation (SD) of blood ethanol concentrations (mg/ml) from 129 and B6mice across three EtOH doses and saline control.
| Blood Ethanol Concentration (BEC) mg/ml
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Saline | 1.75 | 2.25 | 2.75 | |||||
|
|
||||||||
| mean | ± SD | mean | ± SD | mean | ± SD | mean | ± SD | |
|
|
||||||||
| 129SX1/SvJ | 0 | 0 | 1.31 | 0.69 | 1.94 | 0.37 | 2.57 | 0.51 |
| C57BL/6J | 0 | 0 | 1.68 | 0.42 | 2.34 | 0.42 | 3.12 | 0.62 |
Figure 1. Gait parameters.
Measurements pictorially represented for time variables (A) and spatial variables (B) as measured by the DigiGait instrument.
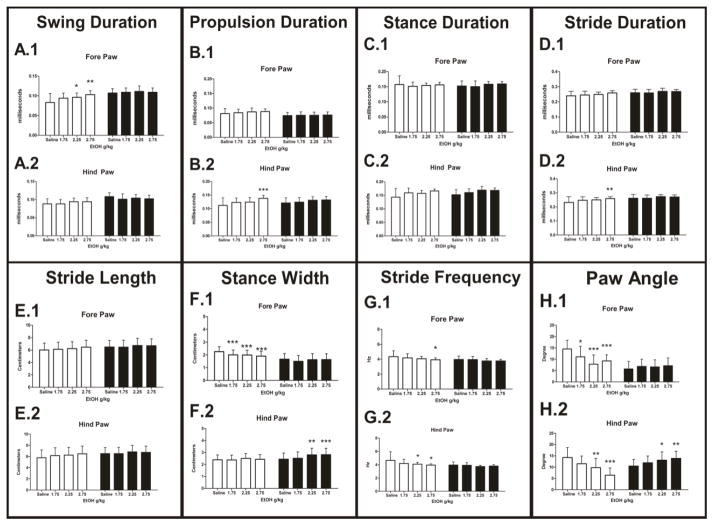
Swing duration is defined and calculated as the amount of time (ms) from the end of the propulsion phase to the beginning of braking phase. Data collected for swing duration suggested an overall significant main effect of mouse strain (P<0.001), a main effect of dose (P<0.001) and a significant dose by strain interaction (P<0.001) in the fore paw (Figure 2A.1). 129 mice showed a significant linear does effect (P<0.001); compared to saline injection, EtOH doses of 2.25 and 2.75 g/kg were significant (P<0.05 and P<0.01 respectively). No linear dose effect was observed in the B6 line of animals. A simple main effect of strain (P<0.001), EtOH dose (P<0.05) as well as a strain by dose interaction (P<0.01) existed in hind paw performance. However, no linear dose effect for either strain was detected (Figure 2A.2).
Figure 2. Dynamic measurements of gait detected by the DigiGait apparatus.
A) Swing duration (ms), 129 mice exhibited a significant increase in their fore paw swing time (A.1). B) An increase in propulsion time was detected in the hind paws of 129 mice (B.2). C) Stance duration (ms), no effect of EtOH dose. D) Stride duration time (ms) increased in the fore (D.1) and hind paws (D.2) of 129 mice. No significant difference was detected in B6 mice. E) Stride length (cm) increased in the fore (E.1) and hind paws (E.2) of 129 mice. B6 mice did not exhibit a detectable increase in stride length. F) Stance width (cm) decreased in the fore paws of the 129 mice (F.1) and increased in the hind paws of B6 mcice (F.2). G) Stride frequency (Hz) significantly decreased in the fore and hind paws of the 129 mouse. H) Paw angle measurements (degree) saw a significant overall decrease in 129 mice fore paws. A significant decrease in paw angle was detected in the hind paws of 129 mice. A significant increase in paw angle was identified in the hind paws of the B6 mice (H.2). White bars = 129X1/SvJ (129), Black bars = C57BL/6J (B6) mice. *= P<0.05, **=P<0.01 ***=P<0.001. Error bars represent ± SD.
Conversely propulsion duration, time (ms) of the downward slope of the stance curve, showed significant main effect of dose (P<0.001) and strain (P<0.05) and an interaction between strain and dose (P<0.05) in the hind paw measurement (Figure 2B.2). In 129 mice a linear dose effect was detected (P<0.01). Post-tests determined the highest dose of EtOH, 2.75 g/kg, was significantly different compared to saline injection (P<0.001). No EtOH dose effect was present in the hind paws of B6 mice. A main effect of strain was detected in fore paw measurements (P<0.01) but not EtOH dose, nor a strain by dose interaction (Figure 2B.1).
Brake duration, characterized by the upward slope of the stance curve, indicated no main effect of strain, EtOH dose or a strain by dose interaction in either the fore or hind paws (data not shown).
Stance duration, defined as the total time (ms) that the paw is in contact with the belt, is the summation of both the braking and propulsion phases of the stance (Figure 2C). We observed an overall significant main effect of EtOH dose in the hind paws (P<0.01) and a significant main effect of strain (P<0.05). No Strain by dose interaction was detected. A simple main effect of dose was detected in the fore paws (P<0.01) as well, yet no other events were significant (Figure 2C.1).
The stride duration variable is calculated as the time (ms) from the beginning of one stance slope to the beginning of the next stance slope. Fore paw performance exhibited main effects of strain (P<0.001), but not EtOH dose or a strain by dose interaction (Figure 2D.1). In the hind paws a significant main effect of strain was detected (P<0.001), EtOH dose (P<0.001) and an interaction of strain by dose (P<0.01). 129 mice demonstrated a significant overall linear dose effect (P<0.001). Once again the 2.75 g/kg dose of EtOH produced a significant response in 129 mice (P<0.01) (Figure 2D.2). No significant EtOH dose effects were observed in the hind paws of B6 mice (P>0.05).
Data from Stride length, measured in centimeters (cm), is given in Figure 2E. The two strains of mice exhibited a significant main effect of strain in the fore paw (P<0.001) and hind paw performance (P<0.001). Additionally, main effects of EtOH dose were present in the hind paw (P<0.05). No strain by dose interaction was apparent in either for or hind paws.
The stance width, measured in (cm) calculated the distance from the centroid of left and right paws. A main effect of strain (P<0.001), EtOH dose (P<0.001) and a significant strain by dose interaction (P<0.01) was detected in fore paws. In hind paws a main effect of strain (P<0.001), EtOH dose (P<0.001) and a strain by dose interaction was detected as well (P<0.05). Fore paws of the 129 mice displayed a robust negative dose linear response (P<0.001), in which each dose was significantly different than saline injection (P<0.001 for all doses; Figure 2F.1). However, hind paws of the 129 mice did not indicate any significant relationship to EtOH dosage (P>0.05). Conversely, no EtOH dose effect was detected in fore paw measure of B6 mice (P>0.05). Yet, the hind paws of B6 animals exhibited an overall linear dose response (P<0.001), by increasing the stance width at the 2.25 (P<0.01) and 2.75 (P<0.001) g/kg dose of EtOH. Thus, increasing EtOH dose resulted in an increased stance width in the hind paws of B6 mice (Figure 2F.2).
Figure 2G represents EtOH dose response data collected for the stride frequency variable, which reflects the number of steps taken in 4 second time period. This length of time has been validated to provide viable stride frequency data (Amende et. al., 2005). In the fore paws main effects of strain (P<0.001) and EtOH dose (P<0.001) as well as a significant strain by dose interaction were detected (P<0.05). 129 mice decreased their stride frequency at the 2.75 g/kg dose EtOH (P<0.05). Analysis of hind paw data revealed main effects of strain (P<0.001), dose (P<0.001) and a significant strain by dose interaction (P<0.01). Overall significant linear dose effect was detected in the hind paws of the 129 mice (P<0.001) with decreased stride frequency at 2.25 and 2.75 g/kg of EtOH (P<0.05 in both instances) (Figure 2G.2). The B6 strain of mice did not exhibit any significant change as a function of EtOH dosing in the hind paws (P>0.05).
Step angle, formed by the total angle of the left and right paws, data was not significantly different between strains, nor did EtOH dose have a main effect on step angle, and no strain by dose interaction was present. This was the case in both the fore and hind paws (data not shown).
Perhaps, most surprising were data obtained from analysis of the absolute paw angle, in which strain differences emerged as a function of EtOH. Increasing doses of EtOH induced an opposite response in the two strains. In the fore paws, an overall effect of strain (P<0.001), EtOH dose (P<0.001), and a strain by dose interaction (P<0.001) was present (Figure 2H.1). A linear dose effect in the 129 strain (P<0.001) detected EtOH having a significant effect on paw angle placement at 1.75 (P<0.05), 2.25 (P<0.001) and 2.75 (P<0.001) g/kg of EtOH. In hind paws main effects of strain (P<0.001) EtOH dose (P<0.001) and an interaction of strain by dose (P<0.001) (Figure 2H.2) were also detected. Hind paws of the 129 mice demonstrated linear dose effect (P<0.001), decreases in paw angle were observed at 2.25 (P<0.01) and 2.75 (P<0.001) g/kg EtOH. Hind paws of B6 mice showed a significant overall linear dose effect (P<0.001) with increase in the paw angle at 2.25 and 2.75 g/kg EtOH (P<0.05; P<0.01 respectively).
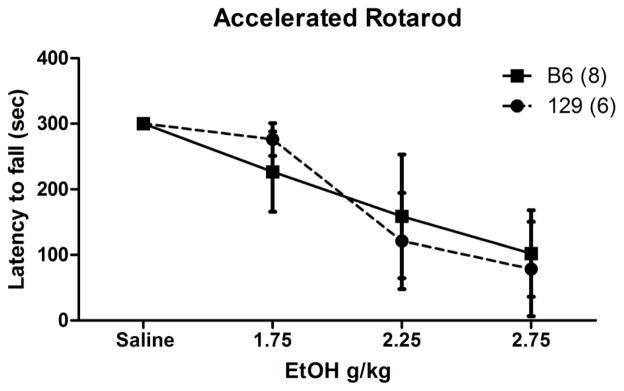
We compared performance on the DigiGait against a standard assay of motor behavior, the accelerating rotarod (Figure 3). These experiments indicated that EtOH dosage had a significant effect on performance in both genotypes. Mixed effects model analysis of performance indicated a significant main effect of EtOH dose (P<0.001).
Figure 3. Latency to fall from an accelerated rotarod.
Data obtained from B6 and 129 mouse strains under conditions of increasing EtOH dosing. Solid line, black square = B6; perforated line, solid circle = 129. Error bars represent ± SD.
4. Discussion
Here we report that a video-based gait analysis system (DigiGait) was able to detect changes in movement patterns induced by alcohol using the C57BL/6J (B6) and 129X1/SvJ (129) strains. Overall, 129 mice were more susceptible to the effects of EtOH than were B6 mice. Furthermore, measures of hind limb incoordination were more likely to display ataxic perturbation than fore limbs. Only a few measures of gait showed a similar significant direction of change in both strains. Surprisingly, for some measures the direction of change was opposite in the two strains, indicating EtOHs intoxicating effects may have unpredictable results on biomechanics depending on genetic background. Our data are in agreement with the work of others suggesting a general disparity in both behavioral and physiological response to EtOH between mouse strains (Crabbe et al., 2008). In a second experiment using an accelerating rotarod paradigm both strains showed a near equal deterioration in motor performance as a result of increasing doses of EtOH.
Responses to the motor depressant effects of EtOH are genetically modifiable in mouse lines selectively bred for sensitivity or resistance to alcohol (Schafer and Crabbe, 1996). It is well established that 129 and B6 mouse strains diverge considerably on physiological and behavioral measures induced by EtOH (Crabbe et al., 2008). For example, B6 mice have a higher preference for voluntary consumption of EtOH than 129 mice and show a considerably greater tolerance to its intoxicating effects (Belknap et al., 1993).
The possibility remains that B6 mice did not absorb EtOH as efficiently or metabolized it more quickly than their 129 counterparts. However, measurements of blood alcohol concentrations did not support this notion. BECs were not significantly different between strains 15 min after an IP injection of the drug. This is in general agreement with research from others, which indicated that 129 mice and B6 mice exhibit a similar BEC 120 min after a single injection of 3 g/kg EtOH (Crabbe et al., 2006). Furthermore, both strains exhibited open cage behavior generally associated with EtOH intoxication, i.e. lethargy, an attenuation of the righting reflex and ataxic gait. Finally, performance on the accelerating rotarod assay was equally poor for both strains with increasing EtOH doses. Our findings now extend these observations by demonstrating that the DigiGait system was able to detect effects of EtOH intoxication with some measures showing a linear dose response whereas others exhibited an opposite strain specific. These observations have important implications when testing mice with mixed genetic backgrounds as strain-specific counter acting adaptations may cancel each other out in mice of mixed genetic background.
Amende and colleagues (2005) have reported that they were able to detect differences in mouse performance following treatment of MPTP or 3NP using the DigiGait technology. Although in this study the DigiGait system was able to detect differences between mouse strains as well as EtOH induced gait pathology, it is important to note that treadmill assays do not provide the same vestibular, proprioceptive or visual input as over ground ambulation and therefore may not truly represent natural gait (Vogt et al., 2002, Pereira et al., 2006, Herbin et al., 2007). Others have reported a disparity in stride length, stance width, propulsion and brake duration measurements obtained from treadmill as compared with over ground assays in rodents (Guillot et al., 2008, Pallier et al., 2009). Recent reports from Cendelin and colleagues (2010) also highlight the complexities relating to motor performance in mice by pointing to gait speed as a confounding variable that is difficult to address. In their report Lurcher mice exhibited detectable motor deficits when allowed to run at an individually comfortable speed. When speed was normalized, however, differences in motor deficits between Lurcher and wild-type mice were undetectable. Yet more intriguing was the fact that the rotarod apparatus was able to detect gait abnormalities between mutant and wild-type animals regardless of speed (Cendelin et al., 2010). Other experimental work suggests that treadmill walking in mice, more so than over ground movement is significantly influenced both by genetic background as well as experience and learning (Wooley et al., 2009). Our data taken together with those described above strongly imply that detection of gait pathology may be task and practice dependent. It should also be noted that many gait parameters are highly related. For example in the work presented here stride length and swing time increased as stride frequency decreased. Interestingly, we found that our two strains of mice employed different strategies for maintaining balance. 129 mice decreased their fore paw stance width, whereas the B6 mice increased their hind paw stance width. This may be attributed to the need for increasing stability (Bolton and Misiaszek, 2009).
In addition to strain-specific difference in EtOH sensitivity and differences between B6 and 129 mice in gait kinematics during free movement (Riley et al., 2008, Serradj and Jamon, 2009), data presented here suggest that B6 mice also employed a different biomechanical strategy for maintaining gait stability on the treadmill assay when exposed to EtOH. Notably, B6 mice increased their paw angle, an ambulation behavior more in line with stereotypical traits of human ataxia (Stolze et al., 2002) as an increase in foot angle would provide greater stability. Whereas ataxic gait is characterized by a decrease in stride length and stride frequency in humans, 129 mice showed increases (Earhart and Bastian, 2001). Human locomotion, however, is bipedal and more prone to imbalance. Therefore, these comparisons should be viewed in their proper context and not taken to be exact replications of the human ataxic condition.
5. Conclusions
The use of digital footprint analysis may be an important means of quantifying gait dysfunction in mouse models of movement disorders. However, as the data presented here strongly suggest, strain-specific biomechanical adaptations to EtOH induced ataxia exist. Thus, caution should be exercised when extrapolating these results to other strains of mice. Although differences were detected amongst strain and EtOH dose, a more sensitive measure of overall ataxic performance is still left wanting. To our surprise, the variability in gait measures detected in the DigiGait assay remained small across all EtOH doses tested, despite visible signs of motor impairment in open cage behavior and poor performance on the accelerating rotarod.
Highlights.
We characterized a treadmill based assay of ethanol induced ataxic motor performance in mice
Mouse strain affects response to ethanol induced ataxia.
We observed disparate biomechanical response to ethanol between mouse strains.
Accelerating rotarod assay was more sensitive at detecting ethanol induced ataxia.
Treadmill based assays may be of limited utility in detecting ataxia in mixed genetic strains.
Acknowledgments
This work was funded by grants RO1 NS33123 and RC4NS073009 from the National Institutes of Health (SMP) and by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764). The authors would like to thank Dr. Thomas Greene and Hsing-Yi Weng for help with statistical modeling and Drs. David Andrenyak and Diana Wilkins for help in measuring BECs.
Abbreviations
- EtOH
Ethanol
- B6
C57BL/6J
- 129
129X1/SvJ
- SCAs
Spinocerebellar ataxias
- BEC
Blood ethanol concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J Neuroeng Rehabil. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A, Hoffer B, Dunwiddie T. Differential sensitivity of cerebellar Purkinje neurons to ethanol in selectively outbred lines of mice: maintenance in vitro independent of synaptic transmission. Brain Res. 1983;264:69–78. doi: 10.1016/0006-8993(83)91121-6. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–10. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bolton DAE, Misiaszek JE. Contribution of hindpaw cutaneous inputs to the control of lateral stability during walking in the cat. J Neurophys. 2009;102:1711–24. doi: 10.1152/jn.00445.2009. [DOI] [PubMed] [Google Scholar]
- Brandt J, Leroi I, O'Hearn E, Rosenblatt A, Margolis RL. Cognitive impairments in cerebellar degeneration: a comparison with Huntington's disease. J Neuropsychiatry Clin Neurosci. 2004;16:176–84. doi: 10.1176/jnp.16.2.176. [DOI] [PubMed] [Google Scholar]
- Cendelin J, Voller J, Vozeh F. Ataxic gait analysis in a mouse model of the olivocerebellar degeneration. Behav Brain Res. 2010;210:8–15. doi: 10.1016/j.bbr.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Clarke KA, Still J. Gait analysis in the mouse. Physiol Behav. 1999;66:723–29. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Coulthard P, Pleuvry BJ, Brewster M, Wilson KL, Macfarlane TV. Gait analysis as an objective measure in a chronic pain model. J Neurosci Methods. 2002;116:197–213. doi: 10.1016/s0165-0270(02)00042-0. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Cameron AJ, Munn E, Bunning M, Wahlsten D. Overview of mouse assays of ethanol intoxication. Curr Protoc Neurosci. 2008;Chapter 9(Unit 9):26. doi: 10.1002/0471142301.ns0926s42. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Ponomarev I, Prescott CA, Wahlsten D. Effects of Genetic and Procedural Variation on Measurement of Alcohol Sensitivity in Mouse Inbred Strains. Behavior Genetics. 2006;36:536–52. doi: 10.1007/s10519-006-9067-6. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- du Montcel ST, Charles P, Ribai P, Goizet C, Le Bayon A, Labauge P, et al. Composite cerebellar functional severity score: validation of a quantitative score of cerebellar impairment. Brain. 2008;131:1352–61. doi: 10.1093/brain/awn059. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Bastian AJ. Selection and coordination of human locomotor forms following cerebellar damage. J Neurophysiol. 2001;85:759–69. doi: 10.1152/jn.2001.85.2.759. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Asress SA, Richardson JR, Glass JD, Miller GW. Treadmill gait analysis does not detect motor deficits in animal models of Parkinson's disease or amyotrophic lateral sclerosis. J Mot Behav. 2008;40:568–77. doi: 10.3200/JMBR.40.6.568-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan JH, Riley EP. Prenatal ethanol alters gait in rats. Alcohol. 1998;5:451–54. doi: 10.1016/0741-8329(88)90081-x. [DOI] [PubMed] [Google Scholar]
- Herbin M, Hackert R, Gasc JP, Renous S. Gait parameters of treadmill versus overground locomotion in mouse. Behav Brain Res. 2007;181:173–79. doi: 10.1016/j.bbr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, et al. Naturally occurring variations in cardiovascular traits among inbred mouse strains. Genomics. 2002;79:679–85. doi: 10.1006/geno.2002.6754. [DOI] [PubMed] [Google Scholar]
- Kale A, Amende I, Meyer P, Crabbe JC, Hampton TG. Ethanol's effects on gait dynamics in mice investigated by ventral plane videography. Alcohol Clin Exp Res. 2004;28:1839–48. doi: 10.1097/01.alc.0000148103.09378.81. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. Wiley; New York: 1987. [Google Scholar]
- Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29:9148–62. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Botta P, Zamudio PA, Zucca S, Valenzuela CF. Ethanol decreases Purkinje neuron excitability by increasing GABA release in rat cerebellar slices. J Pharmacol Exp Ther. 2008;327:910–17. doi: 10.1124/jpet.108.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallier PN, Drew CJ, Morton AJ. The detection and measurement of locomotor deficits in a transgenic mouse model of Huntington's disease are task-and protocol-dependent: influence of non-motor factors on locomotor function. Brain Res Bull. 2009;78:347–55. doi: 10.1016/j.brainresbull.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Pereira JE, Cabrita AM, Filipe VM, Bulas-Cruz J, Couto PA, Melo-Pinto P, et al. A comparison analysis of hindlimb kinematics during overground and treadmill locomotion in rats. Behav Brain Res. 2006;172:212–18. doi: 10.1016/j.bbr.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Powell E, Anch AM, Dyche J, Bloom C, Richtert RR. The splay angle: A new measure for assessing neuromuscular dysfunction in rats. Physiol Behav. 1999;67:819–21. doi: 10.1016/s0031-9384(99)00127-4. [DOI] [PubMed] [Google Scholar]
- Riley PO, Dicharry J, Franz J, Della Croce U, Wilder RP, Kerrigan DC. A kinematics and kinetic comparison of overground and treadmill running. Med Sci Sports Exerc. 2008;40:1093–1100. doi: 10.1249/MSS.0b013e3181677530. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci U S A. 2003;100:2917–22. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer GL, Crabbe JC. Sensitivity to ethanol-induced ataxia in HOT and COLD selected lines of mice. Alcohol Clin Exp Res. 1996;20:1604–12. doi: 10.1111/j.1530-0277.1996.tb01705.x. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Rosene DL, Pandya DN. Ataxia after pontine stroke: insights from pontocerebellar fibers in monkey. Ann Neurol. 2004;55:585–89. doi: 10.1002/ana.20060. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- Serradj N, Jamon M. Age-related changes in the motricity of the inbred mice strains 129/sv and C57BL/6j. Behav Brain Res. 2007;177:80–89. doi: 10.1016/j.bbr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Serradj N, Jamon M. The adaptation of limb kinematics to increasing walking speeds in freely moving mice 129/Sv and C57BL/6. Behav Brain Res. 2009;201:59–65. doi: 10.1016/j.bbr.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Stanley JL, Lincoln RJ, Brown TA, McDonald LM, Dawson GR, Reynolds DS. The mouse beam walking assay offers improved sensitivity over the mouse rotarod in determining motor coordination deficits induced by benzodiazepines. J Psychopharmacol. 2005;19:221–27. doi: 10.1177/0269881105051524. [DOI] [PubMed] [Google Scholar]
- Stolze H, Klebe S, Petersen G, Raethjen J, Wenzelburger R, Witt K, et al. Typical features of cerebellar ataxic gait. J Neurol Neurosurg Psychiatry. 2002;73:310–312. doi: 10.1136/jnnp.73.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008;131:425–37. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–42. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer; New York: 2000. [Google Scholar]
- Vincelette J, Xu Y, Zhang LN, Schaefer CJ, Vergona R, Sullivan ME, et al. Gait analysis in a murine model of collagen-induced arthritis. Arthritis Res Ther. 2007;9:R123. doi: 10.1186/ar2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt L, Pfeifer K, Banzer W. Comparison of angular lumbar spine and pelvis kinematics during treadmill and overground locomotion. Clin Biomech. 2002;17:162–65. doi: 10.1016/s0268-0033(01)00111-5. [DOI] [PubMed] [Google Scholar]
- Wooley CM, Xing S, Burgess RW, Cox GA, Seburn KL. Age, experience and genetic background influence treadmill walking in mice. Physiol Behav. 2009;96:350–61. doi: 10.1016/j.physbeh.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Ethanol modulation of gamma-aminobutyric acid (GABA)-mediated inhibition of cerebellar Purkinje neurons: relationship to GABAb receptor input. Alcohol Clin Exp Res. 2000;24:682–90. [PubMed] [Google Scholar]
- Yokota T, Igarashi K, Uchihara T, Jishage K, Tomita H, Inaba A, et al. Delayed-onset ataxia in mice lacking alpha -tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci U S A. 2001;98:15185–90. doi: 10.1073/pnas.261456098. [DOI] [PMC free article] [PubMed] [Google Scholar]