Abstract
Background
In the last two decades, mesenchymal stem cells (MSCs) have been pre-clinically utilized in the treatment of a variety of kinds of diseases including chronic obstructive pulmonary disease (COPD). The aim of the current study was to systematically review and conduct a meta-analysis on the published pre-clinical studies of MSC administration in the treatment of COPD in animal models.
Methods and Results
A systematic search of electronic databases was performed. Statistical analysis was performed using the Comprehensive Meta-Analysis software (Version 3). The pooled Hedges’s g with 95% confidence intervals (95% CIs) was adopted to assess the effect size. Random effect model was used due to the heterogeneity between the studies. A total of 20 eligible studies were included in the current systematic review. The overall meta-analysis showed that MSC administration was significantly in favor of attenuating acute lung injury (Hedges’s g = -2.325 ± 0.145 with 95% CI: -2.609 ~ -2.040, P < 0.001 for mean linear intercept, MLI; Hedges’s g = -3.488 ± 0.504 with 95% CI: -4.476 ~ -2.501, P < 0.001 for TUNEL staining), stimulating lung tissue repair (Hedges’s g = 3.249 ± 0.586 with 95% CI: 2.103~ 4.394, P < 0.001) and improving lung function (Hedges’s g = 2.053 ± 0.408 with 95% CI: 1.253 ~ 2.854, P< 0.001). The mechanism of MSC therapy in COPD is through ameliorating airway inflammation (Hedges’s g = -2.956 ± 0.371 with 95% CI: -3.683 ~ -2.229, P< 0.001) and stimulating cytokine synthesis that involves lung tissue repair (Hedges’s g = 3.103 ± 0.734 with 95% CI: 1.664 ~ 4.541, P< 0.001).
Conclusion
This systematic review and meta-analysis suggest a promising role for MSCs in COPD treatment. Although the COPD models may not truly mimic COPD patients, these pre-clinical studies demonstrate that MSC hold promise in the treatment of chronic lung diseases including COPD. The mechanisms of MSCs role in preclinical COPD treatment may be associated with attenuating airway inflammation as well as stimulating lung tissue repair.
Introduction
Over the last two decades, tremendous progress has been made in the field of regenerative medicine and stem cell biology [1]. Mesenchymal stem cells (MSCs) are multi-potent stem cells that have fibroblast-like morphology and the capacity to differentiate into chondrocytes, osteoblasts, adipocytes and muscle cells under different micro-environmental conditions, culture media, and supplements [2, 3]. In addition to their regenerative properties, MSCs have recently been shown to have unique immune-modulatory and anti-inflammatory properties that render the MSCs as potential treatment options for a variety of kinds of inflammatory disorders including chronic obstructive pulmonary disease (COPD).
COPD is the third-leading cause of death in the United States [4, 5]. Despite recent advances in the treatment of symptoms with new pharmaceutical drugs and molecules, there remains no effective treatment to attenuate disease progression or reverse the COPD and emphysematous changes. Over the past decade, MSCs isolated from various tissues including bone marrow, adipose, or cord blood, have been shown to lack immunogenicity and thus, can be used for allogeneic or autologous cellular treatment in a variety of diseases. In this context, studies have demonstrated that MSCs have anti-inflammatory and immune-modulatory effects in diverse types of tissue injury and allergic inflammation [6, 7]. MSCs are now known to have potent beneficial effects in animal models of many types of lung injury including cigarette smoke-induced or elastase-induced COPD/emphysema [8–10], bleomycine-induced fibrosis [11, 12], bronchopulmonary dysplasia [13, 14], ventilator-induced lung injury [15], and bacterial pneumonia [16, 17]. Much of these preclinical data support the therapeutic potential of MSCs in the animal models of human diseases including COPD.
Based on the findings of preclinical studies on MSC administration in COPD animal models, a multicenter double-blind placebo-controlled Phase II trial of allogeneic MSC infusions for patients with moderate to severe COPD (FEV1/FVC < 0.70, 30% < FEV1 < 70%) have recently been completed by Weiss et al [18]. This trial was based on the hypothesis that the immune-modulating actions of MSCs would decrease pulmonary, and perhaps systemic, inflammation associated with COPD, thus improving lung function, dyspnea and quality of life. However, the result of this clinical trial was disappointing and found a lack of even a trend for efficacy of MSC administration in COPD despite significantly reduced serum C-reaction protein (C-RP) levels in the patients who received MSC administration [18].
Therefore, the current study was designed to systematically review pre-clinical studies of MSC administration in the experimental models of COPD and to examine the pooled effect of MSCs in reducing tissue damage and stimulating tissue repair in the animal models of COPD.
Materials and Methods
Data sources
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria [19]. Relevant literature was searched for with the following phrases: “mesenchymal stem cell(s)” and “COPD”, “mesenchymal stem cell(s)” and “emphysema”, “mesenchymal stromal cell(s)” and “COPD”, or “mesenchymal stromal cell(s)” and “emphysema” in the sites of PubMed, Embase and Web of Science. The search was limited to English. Relevant studies were also identified by hand-searching the references of included articles. Literature search was performed by the following authors: Xiangde Liu and Qiuhong Fang
Inclusion and exclusion criteria
Studies were included in the current systematic review and meta-analysis if: 1) Studies examined the relationship between MSCs and COPD or emphysema in animal models, 2) Studies contained full text articles.
Studies were excluded if: 1) Insufficient publications existed to perform a systematic review and meta-analysis, 2) A second publication of similar studies in a different journal from the same research group, 3) Ex vivo or in vitro studies were conducted, 4) Studies utilizing MSC conditioned medium (MSC-CM), 5) Studies lacking measurement data and thus meta-analysis was not able to be performed.
Data extraction
All three authors (XL, QF, HK) were involved in data extraction. Information and data were carefully extracted from all included literature according to the inclusion and exclusion criteria as aforementioned. Data include first author name, publication date, country, source of MSCs, recipient animal species, total number of cases or replication of the experiment, study design and parameters observed.
Statistical analysis
The following forms of data were used for the data entry: 1) Mean, standard deviation (SD), number of animals in control group and number of animals in MSC administration group, 2) Sample size of control or MSC administration group and P value of comparison between the two groups. The strength of MSC effect on COPD or emphysema lung tissue repair or other biological effects was measured by Hedges’s g. A random effect model was applied due to the significant heterogeneity of the data collected. The heterogeneity between studies was assessed by the Q-test and I2 statistics, and P < 0.10 and I2 > 50% was considered as heterogeneous between the studies [20]. All meta-analysis was performed using the Comprehensive Meta-analysis software (Version 3, NJ, USA).
Results
Study features
The process of selecting literature is outlined in Fig 1. After careful review of the abstracts of publications, a total of 36 full-text articles were retrieved. The full-text articles were independently assessed by all three authors. Twenty one articles were included in the systematic review and meta-analysis, as shown in Table 1, including studies of human bone marrow MSC (BM-MSC, n = 1), human adipose stromal cells (ASC, n = 1), human cord blood derived MSC (n = 1) or human tubal MSC (n = 1) [21–24], rat BM-MSC (n = 7) [9, 25–30], rabbit bone marrow derived mesenchymal stem cells (n = 1) [31], rat adipose derived stromal cells (n = 2) [32, 33], guinea pig adipose derived MSCs (n = 2) [34, 35], rat amniotic fluid derived MSC (n = 1) [36], and mice BM-MSC or adipose-derived MSC or lung tissue MSC (n = 4) [8, 10, 37, 38]. Among the 21 articles 8 are from China, 4 are from Japan, 2 are from Korea, 2 are from Brazil, 2 are from Iran, one is from Canada, one from the USA, and one is from Taiwan.
Fig 1. Flow diagram of literature search and eligible publication selection.
Table 1. Characteristics of included twenty one papers.
| First author | Country | Year | MSC source | Recipients | COPD | Delivery | MSC dose & time | Parameters evaluated |
|---|---|---|---|---|---|---|---|---|
| Shigemura N | Japan | 2006 | Rat ASC | Rat | PPE | IV | 5x107/0.2mL | TUNEL, PCNA, RAC index |
| 1 wk after PPE | Factor VII, HGF, PaO2 | |||||||
| Maximum running, | ||||||||
| Yuhgetsu H | Japan | 2006 | Rabbit | Rabbit | PPE | IB | 108/2mL | TUNEL, alveolar space |
| BM-MSC | 24 h after PPE | Ki-67 positivity | ||||||
| BALF total cell, macrophage | ||||||||
| Zhen GH | China | 2008 | Rat | Female rat | Papain | IV | 4x106/0.4mL | MLI, TUNEL |
| BM-MSC | Simultaneously | |||||||
| Zhen GH | China | 2010 | Rat | Female rat | Papain | IV | 4x106/0.4mL | MLI, TUNEL, Caspase-3 |
| BM-MSC | 2 h after papain | VEGF-A | ||||||
| Huh JW | Korea | 2011 | Rat | Rat | CS | IV | 6x105/0.3mL | MLI, TUNEL |
| BM-MSC | 6 m after CS | |||||||
| Katsha AM | Japan | 2011 | C57Blk6 | C57Blk6 | PPE | IT | 5x105/0.2mL | Lm, destructive index |
| BM-MSC | 14 d after PPE | IL-1β, IL-1β mRNA | ||||||
| HGF mRNA, EGF mRNA | ||||||||
| Schweitzer KS | USA | 2011 | Human | Rat | CS | IV | 3x105 cells | Lung macrophage, PMN |
| ASC | 2 m after CS | Caspase3, lung volume | ||||||
| alveolar surface area | ||||||||
| Furuya N | Japan | 2012 | Rat ASC | Rat | PPE | IV | 2.5x106/0.5mL | Lm, PaO2, HGF, CINC-1 |
| 7 d after PPE | IL-1β, | |||||||
| Guan XJ | China | 2013 | Rat | Rat | CS | IT | 6x106/0.15mL | MLI, TUNEL, Caspase3 |
| BM-MSC | 7 wk after CS | Vital capacity, FEV100 | ||||||
| MMP-9, MMP-12, | ||||||||
| TGF-β1, VEGF, | ||||||||
| Antunes MA | Brazil | 2014 | C57Blk6 | C57Blk6 | PPE | IT and IV | 1x105 cells | Normal lung volume (%) |
| BM-MSC | 3 wk after PPE | Hyperinflation (%) | ||||||
| AD-MSC | Lm, TUNEL | |||||||
| L-MSC | Neutrophil | |||||||
| Feizpour A | Iran | 2014 | Guinea pigs | Guinea pigs | CS | IT and IV | 106/0.3mL | EC50 methacholine |
| ASC | Day 1 and 14 | Serum or BALF IL-8 | ||||||
| Blood or BALF WBC | ||||||||
| Ghorbani A | Iran | 2014 | Guinea pigs | Guinea pigs | CS | IT and IV | 106/0.3mL | Emphysema score |
| ASC | Day 1 and 14 | BALF thiol, serum MDA | ||||||
| Blood neutrophil, lymph | ||||||||
| Li X | China | 2014 | Human | Rat | CS | IV | 3x106 cells | Lm, Trichrome |
| iPS-MSC | Day 29 and 43 | |||||||
| BM-MSC | ||||||||
| Li YQ | China | 2014 | Rat | Rat | CS + LPS | IT | 4x105/0.2mL | MLI, TUNEL |
| AFD-MSC | 4th and 8th wk | Mean alveolar area | ||||||
| Tibboel J | Canada | 2014 | C57Blk6 | C57Blk6 | PPE | IT | 5x105/0.2mL | MLI, Dynamic compliance |
| BM-MSC | IV | 1x105/0.1mL | Mean Forced Expiratory | |||||
| Before & after PPE | Flow | |||||||
| Zhang WG | China | 2014 | Rat | Rat | CS | IV | 4x106/0.2mL | MLI, TUNEL, IL-6 |
| BM-MSC | Day 20 and 62 | |||||||
| Zhao YM | China | 2014 | Rat | Rat | CS + LPS | IV | 5x106 cells | Mean alveoli number |
| BM-MSC | Day 36 | Pulmonary alveolar area | ||||||
| Chen YB | Taiwan | 2015 | C57Blk6 | C57Blk6 | PPE | IV | ? | MLI, VEGF mRNA, HSP70 |
| BM-MSC | 14 d after PPE | Whole body plethysmograph | ||||||
| Gu W | China | 2015 | Rat | Rat | CS | IT | 6x106/0.15mL | MLI, COX-2 mRNA, PGE2 |
| BM-MSC | 7 wk after CS | Inflammation score, | ||||||
| IL-6, IL-10 | ||||||||
| Kim YS | Korea | 2015 | Human | C57Blk6 | PPE | IV | Various dose | MLI, VEGF |
| CBD-MSC | 7 d after PPE | |||||||
| Peron JP | Brazil | 2015 | Human | C57Blk6 | CS + | IP or | 1x106 cells | BALF total cell, neutrophil |
| Tubal MSC | Irradiation | Intranasal | Day 60 and 67 | Airway mucus, collagen | ||||
ASC: adipose tissue derived stromal/stem cell; BM-MSC: bone marrow-derived mesenchymal stem cell
AD-MSC: adipose derived mesenchymal stem cell; L-MSC: lung tissue derived mesenchymal stem cell
CBD-MSC: cord blood derived mesenchymal stem cell; CS: cigarette smoke; PPE: porcine pancreatic elastase
IB: intra-bronchial; IV: intravenous; IT: intra-tracheal; IP: intra-peritoneal
Results of overall systematic review
The first study of rodent MSC administration in a COPD model was published by Shigemura et al from Japan in 2006 [25, 32]. However, most studies on COPD therapy with MSC administration were published in 2014 (n = 8). Overall, administration of MSCs demonstrated that MSCs have therapeutic benefit in both structural and functional outcomes in the COPD animal models, which were prepared either by elastase instillation or cigarette smoke exposure. Sources of MSCs were from human, rabbit, rat, guinea pigs or mouse and delivered to the recipients either through intravenous (IV) injection, intra-tracheal (IT) or intra-bronchial (IB) instillation, intra-peritoneal injection or intranasal instillation. One study compared the efficiency of different MSC sources and delivery routes [8]. The authors found that IT administration of BM-MSC was superior to IV injection in terms of reducing alveolar hyperinflation or collagen fiber content in the lung. They also found that IV administration of lung tissue derived MSCs resulted in immediate death of the recipient mice while IV administration of BM-MSC or adipose derived MSC did not [8]. Most recently, Kim et al reported that a minimum number of 5x104 MSCs was required to achieve therapeutic effect [23].
Effect of MSC administration on lung injury and repair in COPD animal models
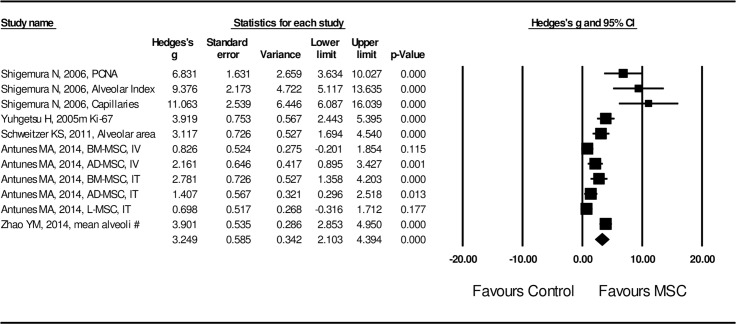
Effect size of MSC administration on lung structural injury was examined by length of mean linear interception (MLI) and positivity of TUNEL staining in the lung tissue. A random effect model was adopted in assessing the effect size of MSCs on MLI length and TUNEL positivity due to the high heterogeneity of the studies (I2 = 87.5 for MLI length and I2 = 82.7 for TUNEL positivity, P < 0.01). Effect size of MSC transplantation on MLI was significantly (Hedges’s g = -2.325 ± 0.145 with 95% CI: -2.609 ~ -2.040, P < 0.001, Fig 2) in favor of MSC treatment. Effect size of MSC administration on TUNEL positivity was also significant (Hedges’s g = -3.488 ± 0.504 with 95% CI: -4.478 ~ -2.501, P < 0.001, Fig 3) by random model of assessment in favor of MSC treatment. Effect size of MSC on lung tissue repair was evaluated by PCNA positivity, Ki-67 positivity, radial alveolar count index, factor VII for capillary assessment, alveolar surface area, and percent of normal lung. As shown in Fig 4, effect of MSC administration on lung tissue repair was significantly (Hedges’s g = 3.249 ± 0.586 with 95% CI: 2.103 ~ 4.394, P < 0.001) in favor of MSC treatment.
Fig 2. Forest plot for the MSC effect on mean linear interception (MLI).
A random effect model was used due to significant heterogeneity of publications (I2 = 87.5, P < 0.01). Effect size was assessed by Hedges’s g and 95% CI, and the effect on MLI reduction was in favor of MSC treatment (Hedges’s g = -2.325 ± 0.145, 95% CI: -2.609~-2.040, P < 0.001) compared to control, which was the COPD model without MSC treatment.
Fig 3. Forest plot for the effect of MSCs on TUNEL positivity.
A random effect model was used due to significant heterogeneity of publications (I2 = 82.7, P < 0.01). Effect size was assessed by Hedges’s g and 95% CI, and the inhibitory effect on TUNEL positivity was in favor of MSC treatment (Hedges’s g = -3.488 ± 0.504, 95% CI: -4.478~-2.501, P < 0.001) compared to control group, which was the COPD model without MSC treatment.
Fig 4. Forest plot for the effect of MSCs on lung tissue repair parameters.
A random effect model was used due to significant heterogeneity of publications was observed (I2 = 83.2, P <0.01). Effect size was assessed by Hedges’s g and 95% CI, and the stimulatory effect on lung tissue repair was in favor of MSC administration (Hedges’s g = 3.249 ± 0.586, 95% CI: 2.103~4.394, P < 0.001). Control group was the COPD model without MSC treatment.
Next, effect size of MSCs on pulmonary functions was examined. Specifically, effect on vital capacity (VC), FEV at 100 milliseconds (FEV100), dynamic compliance (Cdyn), mean forced expiratory flow, EC50 methacholine (the effective concentration causing 50% of maximum contraction response to a methacholine challenge test), and plethysmograph (Peth) were evaluated. Again, a random effect model was applied in assessing the effect size of MSCs on lung function improvement (I2 = 80.1, P < 0.01), which was statistically significant in favor of MSC administration (Hedge’s g = 2.053 ± 0.408 with 95% CI: 1.253 ~ 2.854, P < 0.001, Fig 5).
Fig 5. Forest plot for the effect of MSCs on lung function in the COPD models.
A random effect model was used due to significant heterogeneity of publications (I2 = 80.1, P < 0.01). Effect size was assessed by Hedges’s g and 95% CI, and the effect on lung function improvement was in favor of MSC administration (Hedges’s g = 2.053 ± 0.408, 95% CI: 1.253~2.854, P < 0.001). Control group was the COPD model without MSC treatment.
Lastly, effect size of MSC administration on inflammation and production of anti-inflammatory cytokines or growth factors stimulating tissue repair was also evaluated. Inflammation was evaluated by the following parameters: infiltration of neutrophils or macrophages, IL-6 release, cyclooxygenase-2 (COX-2) expression, PGE2 release, and production of matrix metalloproteinases (MMP-9 and MMP-12) etc. In addition, the effect size of MSCs on the release of IL-10, VEGF, HGF, EGF and TGF-β1 was assessed to evaluate the potential mechanism of MSC on lung tissue repair. MSC administration resulted in inhibition of airway inflammation, and the effect size was statistically significant (Hedge’s g = -2.956 ± 0.371 with 95% CI: -3.683 ~ -2.229, P < 0.001, Fig 6,) with significant heterogeneity (I2 = 84.8, P < 0.01). In contrast, MSC administration resulted in up-regulation of anti-inflammatory cytokine (IL-10) and growth factors (VEGF, HGF, EGF and TGF-β). The effect size was also statistically significant (Hedge’s g = 3.103 ± 0.734 with 95% CI: 1.664 ~ 4.541, P < 0.001, Fig 7) with high heterogeneity (I2 = 88.0, P < 0.01).
Fig 6. Forest plot for the effect of MSCs on airway infiltration of inflammatory cells or release of pro-inflammatory cytokines in lung or blood.
A random effect model was used due to significant heterogeneity of publications (I2 = 84.8, P < 0.01). Effect size was assessed by Hedges’s g and 95% CI, and the inhibitory effect on airway inflammation and systemic inflammation was in favor of MSC administration (Hedges’s g = -2.956 ± 0.371, 95% CI: -3.683~-2.229, P < 0.001). Control group was the COPD model without MSC treatment.
Fig 7. Forest plot for the effect of MSCs on growth factors and anti-inflammatory cytokines.
A random effect model was used due to significant heterogeneity of publications (I2 = 88.0, P < 0.01). Effect size was assessed by Hedges’s g and 95% CI, and the stimulatory effect on growth factors and anti-inflammatory cytokines was in favor of MSC administration (Hedges’s g = 3.103 ± 0.734, 95% CI: 1.664~4.541, P < 0.001). Control group was the COPD model without MSC treatment.
Publication bias
Publication bias was originally defined as the publication or non-publication of studies depending on the direction and statistical significance of the results [39]. Publication bias was examined by the funnel plot of standard error versus Hedges’s g. As shown in S1–S6 Figs, distribution of the funnel plot was nearly symmetric in MLI, tissue repair parameters, and lung function assay parameters, but it was asymmetric in the remainder of the plots.
Discussion
Despite recent advances in the treatment of symptoms in COPD patients, the treatment of severe COPD continues to be very challenging and there remains no effective therapy that has been shown to reduce progression of emphysema [40]. Over the past decade, increasing number of preclinical studies have suggested that administration of mesenchymal stem/stromal cells (MSCs) can prevent or have a therapeutic effect in COPD animal models. Based on the preclinical findings, a multi-center clinical trial of MSC administration in the treatment of COPD patients had been conducted, although the results in human studies were less promising compared to the findings of preclinical studies [18]. We systematically reviewed 21 publications of preclinical studies of MSC administration in the treatment of COPD in animal models and further performed a meta-analysis to examine the combined effect of MSC in COPD therapy. The current meta-analysis indicated that MSC administration either by intravenous injection or intra-tracheal instillation resulted in significant reduction of MLI and TUNEL positivity in the animal models of COPD, significant stimulation of lung tissue repair, and significant improvement of lung function. MSCs significantly attenuated airway infiltration of neutrophils and macrophages and production of pro-inflammatory cytokines including IL-1β and IL-6, but significantly stimulated anti-inflammatory cytokine IL-10 and growth factors including VEGF, HGF, EGF, and TGF-β, suggesting MSC administration is an effective approach to treat COPD/emphysema in the animal models and hold promise of future application of MSC administration in COPD patients.
While initial interest of MSC administration in a variety of diseases was centered on the capacity for multi-lineage differentiation of the cells, recently MSCs have been considered potent modulators of disease-associated tissue microenvironments such as milieu of chronic inflammation and autoimmune reaction [41]. Thus, in the past decade, studies on MSCs have been focused on not only direct tissue and organ regeneration but also modulatory effects on damaged and diseased tissues [42]. The anti-inflammatory and immunomodulatory properties of MSCs [6, 7, 43] has been the focus of many of the recently published literature reports. COPD is characterized by chronic airway inflammation and insufficient tissue repair [44]. Therefore, MSC administration could be an effective cellular therapy for COPD[8, 18, 32]. Although there are 8 clinical trials currently ongoing to examine safety and efficacy of MSC administration in COPD patients (Clinicaltrials.gov: NCT02645305, NCT01849159, NCT02348060, NCT02412332, NCT02161744, NCT02041000, NCT02216630, NCT01559051), only two clinical trials of MSC administration in COPD patients have been completed. In this content, Ribeiro-Paes et al from Brazil examined the effects of intravenous infusion of autologous bone marrow mononuclear cells in the treatment of advanced COPD patients (4 cases total) and 12-month follow-up showed a significant improvement in the quality of life as well as a clinically stable condition [45]. The result of a clinical trial conducted by Weiss et al from the USA, however, was disappointing, and found lack of even a trend for efficacy of MSC administration in COPD [18]. Therefore, the current study was designed to systematically review and analyze recent publications of preclinical studies of MSCs and COPD, but not clinical trials.
The preclinical studies provide important evidence of MSC safety, toxicity, therapeutic efficacy and mechanism of MSC action for future human clinical use. In this regard, studies on rodent animal models of COPD have demonstrated that intravenous injection or intra-tracheal instillation of rodent bone marrow MSCs (BM-MSCs) or adipose derived MSCs (AD-MSCs) were safe and effective in attenuating airway injury by ameliorating airway inflammation and apoptosis [8, 28, 38]. In contrast, intravenous injection of mice lung tissue derived MSCs (L-MSCs) resulted in immediate death of the recipient mice, which may be associated with the larger size of the L-MSCs or with cellular clumping resulting in pulmonary embolism [8]. In addition, intra-tracheal instillation of BM-MSC seemed to be superior to intravenous injection in reducing alveolar hyperinflation and collagen fiber content in the elastase-induced emphysema models [8]. These findings suggested that intra-tracheal or intra-bronchial instillation is a preferred and safer way of MSC administration for the treatment of airway diseases.
Studies on pharmacokinetics of MSCs in vivo demonstrated that both allogeneic and autologous MSCs appeared to distribute in a similar manner [46]. BM-MSCs distributed mostly in lungs, liver and spleen at early stages (hours) of intravenous injection regardless the injury located in the brain or heart [47, 48]. Consistent with animal model studies, a study on the fate of MSCs examined autopsy materials from 18 patients who had received human leukocyte antigen (HLA)-mismatched MSCs and found that MSC donor DNA was detectable in the lungs, lymph nodes and intestine [49]. Furthermore, no signs of ectopic tissue formation or malignant tumors of MSC-donor origin were found on macroscopic or histological examination [49]. These findings indicate the lung is one of the organs where MSCs initially distribute following systemic administration.
Preclinical models have also provided important opportunities for testing and evaluating the immune response induced by allogeneic MSCs under varying conditions [43]. Allogeneic MSCs are considered to be poorly immunogenic in comparison with other cells and tissues, and thus, human BM-MSCs, AD-MSCs, iPSCs, and cord blood derived MSCs (CBD-MSCs) had been tested and evaluated in the experimental models of emphysema [21–24]. These human MSCs were delivered to rodent recipients either by intravenous injection or by intra-peritoneal injection. Administration of these MSCs significantly reduced airway inflammation, parenchymal lung cell apoptosis and peri-bronchial collagen deposition in the recipient animals of cigarette smoke- or elastase-induced emphysema. These findings suggest that the preclinical studies provide valuable information regarding mechanisms of MSC action, safety, immunogenicity, and in vivo kinetics of therapeutically administered MSCs. Moreover, these pre-clinical studies demonstrate that xenogeneic MSC administration is safe and effective, and thus, in addition to autologous MSC, administration of allogeneic human MSC is safe and plausible in clinical trials.
Substantial progress has been made recently in our understanding of the mechanisms of interactions between MSCs and the recipient tissue microenvironment. As a result of such studies MSCs are now known to have anti-inflammatory and immune modulatory effects. In this regard, MSCs are adapted to their microenvironment through either the release of soluble factors such as PGE2, kyneurnine, IL-10, TNF-stimulated gene 6 protein (TSG-6), NO, and TGF-β1 [50–55], or context-dependent modification of T helper (Th1/Th2) balance or pro-inflammatory Th17 cell differentiation [55–57]. Consistent with these reports, in the experimental models of COPD, MSCs could also modulate release of inflammation-associated factors, that is, inhibiting pro-inflammatory cytokines or mediators such as IL-1β, TNF-α, IL-6, and PGE2 [29, 30, 33, 37], stimulating anti-inflammatory cytokine IL-10 [29], and up-regulating synthesis of growth factors associated with tissue repair such as VEGF, HGF, EGF and TGF-β1 [10, 23, 26, 28, 33, 37].
While the current meta-analysis was carried out on a rigorous systematic review that could avoid publication bias, potential publication bias may exist in the current study [58, 59]. In this regard, funnel plots of MLI, lung tissue repair parameters and lung function assay parameters were nearly symmetrically distributed, suggesting no publication bias might exist in these observations. However, distribution in the funnel plots of TUNEL positivity, inflammation, and growth factors was asymmetric, indicating publication bias may exist in these analyses.
There are several limitations in the current systematic review and meta-analysis. First, studies used different sources of stem cells including bone marrow, adipose tissue, lung tissue, umbilical cord blood, tubal tissue, and amniotic fluid MSCs from human as well as rodents. Second, various routes of MSC administration were used by different investigators, i.e., delivered through intravenous injection, intra-tracheal or intra-nasal instillation, or intra-peritoneal injection. Third, the protocol of assessment of emphysematous lung damage and repair was not standardized in terms of time of MSC delivery and period of observation etc. Due to the aforementioned limitations, publication bias may exist in the current review. Additionally, a random model was used to examine the effect size of MSC therapeutic effects on COPD due to the data heterogeneity. Fourth, all of the animal models of COPD were in acute phase or sub-acute phase of lung tissue injury, which varies greatly from the chronic inflammation-induced lung tissue damage and insufficient repair observed in clinical COPD patients. Fifth, the publication bias was examined only by funnel plot and not further examined by other methods such as Egger’s regression.
Following the completion of the clinical trial by Weiss et al [18], administration of MSCs for COPD treatment is being further tested in eight clinical trials (clinicaltrials.gov: NCT02645305, NCT01849159, NCT02348060, NCT02412332, NCT02161744, NCT02041000, NCT02216630, NCT01559051). These clinical trials were designed to further evaluate efficacy and safety of systemic administration of autologous or allogeneic MSCs in the treatment of COPD. Researchers of the clinical trials anticipate that MSCs will inhibit chronic inflammation in airway, alveoli and endothelium, promote tissue repair through releasing growth factors, and improve patient’s quality of life. We expect that systemic administration of MSCs in COPD patients is safe and will become an effective cellular therapy for COPD in near future.
Conclusion
Taken together, the recent literature of preclinical studies of MSCs administration in COPD animal models provides a wealth of potentially valuable information regarding in vivo safety, immunogenicity, pharmacokinetics, and mechanisms of action of therapeutically administered MSCs. These preclinical studies demonstrated that intravenous injection or intra-tracheal delivery of MSCs (regardless BM-MSC, AD-MSC, or CBD-MSC) is safe and effective in the therapy of COPD experimental models. The current systematic review and meta-analysis suggest a promising role for MSC administration in COPD treatment. The mechanisms of MSCs in pre-clinical COPD treatment may be associated with attenuating airway inflammation as well as stimulating lung tissue repair.
Supporting Information
(DOC)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Acknowledgments
This study was supported by WonKwang University Fund in the year 2013. The authors appreciate Ms. Amy Nelson for English editorial assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by WonKwang University Fund in the year 2013. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Armstrong L, Lako M, Buckley N, Lappin TR, Murphy MJ, Nolta JA, et al. Editorial: Our top 10 developments in stem cell biology over the last 30 years. Stem Cells. 2012;30(1):2–9. 10.1002/stem.1007 . [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7. 10.1002/jcp.21200 . [DOI] [PubMed] [Google Scholar]
- 3.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301. 10.1634/stemcells.2005-0342 . [DOI] [PubMed] [Google Scholar]
- 4.Minino AM. Death in the United States, 2009. NCHS Data Brief. 2011;(64):1–8. . [PubMed] [Google Scholar]
- 5.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011;59(4):1–51. . [PubMed] [Google Scholar]
- 6.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. 10.1038/mt.2011.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96. 10.1038/nri3209 . [DOI] [PubMed] [Google Scholar]
- 8.Antunes MA, Abreu SC, Cruz FF, Teixeira AC, Lopes-Pacheco M, Bandeira E, et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res. 2014;15:118 10.1186/s12931-014-0118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Xu A, Xu Q, Zhao W, Li D, Fang X, et al. Bone marrow mesenchymal stem cell transplantation for treatment of emphysemic rats. Int J Clin Exp Med. 2014;7(4):968–72. [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YB, Lan YW, Chen LG, Huang TT, Choo KB, Cheng WT, et al. Mesenchymal stem cell-based HSP70 promoter-driven VEGFA induction by resveratrol alleviates elastase-induced emphysema in a mouse model. Cell Stress Chaperones. 2015;20(6):979–89. 10.1007/s12192-015-0627-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–11. 10.1073/pnas.1432929100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175(1):303–13. 10.2353/ajpath.2009.080629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302(9):L829–37. 10.1152/ajplung.00347.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122–30. 10.1164/rccm.200902-0242OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, et al. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67(6):496–501. 10.1136/thoraxjnl-2011-201059 . [DOI] [PubMed] [Google Scholar]
- 16.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302(10):L1003–13. 10.1152/ajplung.00180.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67(6):533–9. 10.1136/thoraxjnl-2011-201176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–8. 10.1378/chest.12-2094 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. 10.1016/j.jclinepi.2009.06.005 . [DOI] [PubMed] [Google Scholar]
- 20.Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. 2002;7(1):51–61. . [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer KS, Johnstone BH, Garrison J, Rush NI, Cooper S, Traktuev DO, et al. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011;183(2):215–25. 10.1164/rccm.201001-0126OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, et al. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51(3):455–65. 10.1165/rcmb.2013-0529OC . [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Kim JY, Huh JW, Lee SW, Choi SJ, Oh YM. The Therapeutic Effects of Optimal Dose of Mesenchymal Stem Cells in a Murine Model of an Elastase Induced-Emphysema. Tuberc Respir Dis (Seoul). 2015;78(3):239–45. 10.4046/trd.2015.78.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peron JP, de Brito AA, Pelatti M, Brandao WN, Vitoretti LB, Greiffo FR, et al. Human Tubal-Derived Mesenchymal Stromal Cells Associated with Low Level Laser Therapy Significantly Reduces Cigarette Smoke-Induced COPD in C57BL/6 mice. PLoS One. 2015;10(8):e0136942 10.1371/journal.pone.0136942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhen G, Liu H, Gu N, Zhang H, Xu Y, Zhang Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–22. . [DOI] [PubMed] [Google Scholar]
- 26.Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu Y, et al. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy. 2010;12(5):605–14. 10.3109/14653241003745888 . [DOI] [PubMed] [Google Scholar]
- 27.Huh JW, Kim SY, Lee JH, Lee JS, Van Ta Q, Kim M, et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011;301(3):L255–66. 10.1152/ajplung.00253.2010 . [DOI] [PubMed] [Google Scholar]
- 28.Guan XJ, Song L, Han FF, Cui ZL, Chen X, Guo XJ, et al. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem. 2013;114(2):323–35. 10.1002/jcb.24377 . [DOI] [PubMed] [Google Scholar]
- 29.Zhang WG, He L, Shi XM, Wu SS, Zhang B, Mei L, et al. Regulation of transplanted mesenchymal stem cells by the lung progenitor niche in rats with chronic obstructive pulmonary disease. Respir Res. 2014;15:33 10.1186/1465-9921-15-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu W, Song L, Li XM, Wang D, Guo XJ, Xu WG. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci Rep. 2015;5:8733 10.1038/srep08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuhgetsu H, Ohno Y, Funaguchi N, Asai T, Sawada M, Takemura G, et al. Beneficial effects of autologous bone marrow mononuclear cell transplantation against elastase-induced emphysema in rabbits. Exp Lung Res. 2006;32(9):413–26. 10.1080/01902140601047633 . [DOI] [PubMed] [Google Scholar]
- 32.Shigemura N, Okumura M, Mizuno S, Imanishi Y, Nakamura T, Sawa Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am J Transplant. 2006;6(11):2592–600. 10.1111/j.1600-6143.2006.01522.x . [DOI] [PubMed] [Google Scholar]
- 33.Furuya N, Takenaga M, Ohta Y, Tokura Y, Hamaguchi A, Sakamaki A, et al. Cell therapy with adipose tissue-derived stem/stromal cells for elastase-induced pulmonary emphysema in rats. Regen Med. 2012;7(4):503–12. 10.2217/rme.12.25 . [DOI] [PubMed] [Google Scholar]
- 34.Feizpour A, Boskabady MH, Ghorbani A. Adipose-derived stromal cell therapy affects lung inflammation and tracheal responsiveness in guinea pig model of COPD. PLoS One. 2014;9(10):e108974 10.1371/journal.pone.0108974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghorbani A, Feizpour A, Hashemzahi M, Gholami L, Hosseini M, Soukhtanloo M, et al. The effect of adipose derived stromal cells on oxidative stress level, lung emphysema and white blood cells of guinea pigs model of chronic obstructive pulmonary disease. Daru. 2014;22(1):26 10.1186/2008-2231-22-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Gu C, Xu W, Yan J, Xia Y, Ma Y, et al. Therapeutic effects of amniotic fluid-derived mesenchymal stromal cells on lung injury in rats with emphysema. Respir Res. 2014;15:120 10.1186/s12931-014-0120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19(1):196–203. 10.1038/mt.2010.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tibboel J, Keijzer R, Reiss I, de Jongste JC, Post M. Intravenous and intratracheal mesenchymal stromal cell injection in a mouse model of pulmonary emphysema. COPD. 2014;11(3):310–8. 10.3109/15412555.2013.854322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothstein HR, Sutton AJ, Borenstein M. Publication Bias in Meta-Analysis In: Rothstein H.R.; Sutton AB, M, editor. Publication Bias in Meta-Analysis—Prevention, Assessment and Adjustments. John Wiley & Sons, Ltd: Wiley; 2005. p. 1–7. [Google Scholar]
- 40.Mulhall AM, Droege CA, Ernst NE, Panos RJ, Zafar MA. Phosphodiesterase 4 inhibitors for the treatment of chronic obstructive pulmonary disease: a review of current and developing drugs. Expert Opin Investig Drugs. 2015:1–15. 10.1517/13543784.2015.1094054 . [DOI] [PubMed] [Google Scholar]
- 41.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–24. 10.1002/path.2469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. 10.1038/nm.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91(1):40–51. 10.1038/icb.2012.67 . [DOI] [PubMed] [Google Scholar]
- 44.Rennard SI, Wachenfeldt K. Rationale and emerging approaches for targeting lung repair and regeneration in the treatment of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011;8(4):368–75. 10.1513/pats.201102-019RM . [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro-Paes JT, Bilaqui A, Greco OT, Ruiz MA, Marcelino MY, Stessuk T, et al. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J Chron Obstruct Pulmon Dis. 2011;6:63–71. 10.2147/COPD.S15292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. 10.1182/blood-2002-06-1830 . [DOI] [PubMed] [Google Scholar]
- 47.Park BN, Shim W, Lee G, Bang OY, An YS, Yoon JK, et al. Early distribution of intravenously injected mesenchymal stem cells in rats with acute brain trauma evaluated by (99m)Tc-HMPAO labeling. Nucl Med Biol. 2011;38(8):1175–82. 10.1016/j.nucmedbio.2011.05.009 . [DOI] [PubMed] [Google Scholar]
- 48.Jasmin, Jelicks LA, Koba W, Tanowitz HB, Mendez-Otero R, Campos de Carvalho AC, et al. Mesenchymal bone marrow cell therapy in a mouse model of chagas disease. Where do the cells go? PLoS Negl Trop Dis. 2012;6(12):e1971 10.1371/journal.pntd.0001971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–8. 10.1002/stem.1118 . [DOI] [PubMed] [Google Scholar]
- 50.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118(2):330–8. 10.1182/blood-2010-12-327353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149–60. 10.1111/j.1365-2249.2009.03874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312–20. 10.1097/TP.0b013e3181fed001 . [DOI] [PubMed] [Google Scholar]
- 53.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66(4):523–31. 10.1111/j.1398-9995.2010.02509.x . [DOI] [PubMed] [Google Scholar]
- 54.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9. 10.1038/nm.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107(12):5652–7. 10.1073/pnas.0910720107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghannam S, Pene J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185(1):302–12. 10.4049/jimmunol.0902007 . [DOI] [PubMed] [Google Scholar]
- 57.Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41(10):2840–51. 10.1002/eji.201141499 . [DOI] [PubMed] [Google Scholar]
- 58.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55. . [DOI] [PubMed] [Google Scholar]
- 59.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002 10.1136/bmj.d4002 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.