Abstract
Perfusion decellularization of cadaveric hearts removes cells and generates a cell-free extracellular matrix scaffold containing acellular vascular conduits, which are theoretically sufficient to perfuse and support tissue-engineered heart constructs. This article contains additional data of our experience decellularizing and testing structural integrity and composition of a large series of human hearts, “Acellular human heart matrix: a critical step toward whole heat grafts” (Sanchez et al., 2015) [1]. Here we provide the information about the heart decellularization technique, the valve competence evaluation of the decellularized scaffolds, the integrity evaluation of epicardial and myocardial coronary circulation, the pressure volume measurements, the primers used to assess cardiac muscle gene expression and, the characteristics of donors, donor hearts, scaffolds and perfusion decellularization process.
Specifications Table
| Subject area |
Biology |
| More specific subject area | Bioengineering human heart matrix |
| Type of data | Table, image, text file, figure |
| How data was acquired | Echocardiography (General Electric), linear mixed-effects models (LME, S-Plus version 8.0, Tibco Software) and angiography (Siemens) |
| Data format | Analyzed, processed |
| Experimental factors | Human hearts used in the study were not suitable for transplantation. |
| Experimental features | Heart decellularization perfusion was performed to remove cells but retain the extracellular matrix scaffold. Characteristics of the scaffold valves, chambers and vasculature were assessed using echocardiography, pressure-volume measurements and coronary angiography. The effect of the human scaffold on the differentiation of human cardiac progenitor cells was also analyzed with different primers |
| Data source location | Madrid, Spain |
| Data accessibility | Within this article |
Value of the data
-
•
The data provides the schematic information of a decellularization heart perfusion technique that could be followed as a standardized technique for additional decellularization studies.
-
•
The data provides the detail information of the characteristics of donors and heart scaffolds. These physiologic data will provide researchers with important age- and sex-specific reference ranges for evaluating experimental results.
-
•
It also provides the basis of different experiments for a clear demonstration of valve competence, coronary angiography assessment and pressure-volume measurements. These novel assays could be useful tools for the in vitro evaluation of decellularized heart scaffolds.
-
•
The data provides the primers used to assess cardiac gene expression in human cardiac progenitor cells grown on human decellularized extracellular matrices. The primers profile data could be used to identify cardiac cell differentiation.
1. Data
Dataset provided in this article shows the perfusion decellularization protocol we used to remove the cells from 39 human hearts while retaining the extracellular matrix [1]. The characteristics of the decellularized valves and anatomical aspects of the decellularized cardiac vessels were assessed by using echocardiography and coronary angiography, respectively. In addition, the passive pressure-volume relationship of the left and right ventricle was measured in 8 human hearts before and after decellularization. Finally, primers used to assess cardiac gene expression in human cardiac progenitor cells grown on human decellularized extracellular matrix are described.
2. Experimental design, materials and methods
2.1. Heart decellularization technique
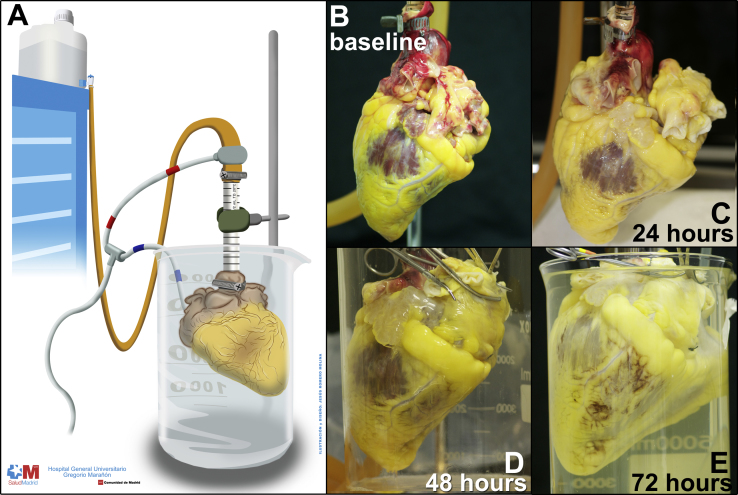
To remove cells but retain the extracellular matrix (ECM) we applied our previously described perfusion decellularization technique, [2] with 1% sodium dodecyl sulfate (SDS) detergent in deionized water via antegrade coronary perfusion of the ascending aorta. Schematic of perfusion decellularization of human heart is shown in Fig. 1, Panel A. Perfusion pressure is monitored to perfuse the heart at ~80–100 mm Hg (normal blood pressure) and is changed by altering the height of the dispensing container that feeds the decellularization or washing reagents into the aorta. During the decellularization period (Fig. 1, Panels B to E) over 72–96 h, there is a loss of color as decellularization proceeds first from the thinnest regions (great vessels and atria) and progresses toward the thickest areas of the heart so that the decellularized parts become more translucent and devoid of most color.
Fig. 1.
Heart decellularization technique.
2.2. Characteristics of donors and heart scaffolds
Characteristics of donors, donor hearts, scaffolds and perfusion decellularization process are shown in Table 1. Thirty-nine human hearts were decellularized and 13 human hearts were used as controls.
Table 1.
Characteristics of donors, donor hearts, scaffolds, and perfusion decellularization process.
| Sex | Age | Heart weight | Scaffold weight | Time to start of decellularization | Days of decellularization |
|---|---|---|---|---|---|
| Male | 50 | 558 | 452 | 9 h | 4 |
| Male | 73 | 466 | 436 | 12 h | 7 |
| Female | 58 | 332 | 224 | 24 h | 4 |
| Female | 50 | 369 | 292 | 24 h | 7 |
| Male | 80 | 398 | 327 | 24 h | 7 |
| Female | 67 | 488 | 457 | 24 h | 8 |
| Female | 87 | 358 | Control | Control | Control |
| Female | 35 | 267 | Control | Control | Control |
| Female | 54 | 587 | 522 | 9 h | 7 |
| Male | 59 | 550 | 500 | 13 h | 8 |
| Male | 49 | 380 | 351 | 8 h | 4 |
| Male | 76 | 343 | 288 | 24 h | 4 |
| Female | 68 | 526 | 432 | 3 days | 5 |
| Female | 65 | 314 | 283 | 3 days | 4 |
| Female | 17 | 231 | 189 | 8 h | 5 |
| Male | 57 | 671 | 657 | 5 h | 5 |
| Male | 66 | 435 | 344 | 24 h | 4 |
| Male | 61 | 512 | Control | Control | Control |
| Male | 41 | 398 | Control | Control | Control |
| Male | 41 | 680 | 572 | 12 h | 7 |
| Female | 72 | 284 | Control | Control | Control |
| Male | 43 | 353 | 300 | 24 h | 4 |
| Male | 58 | 376 | 331 | 4 days | 4 |
| Male | 59 | 432 | 376 | 24 h | 4 |
| Male | 57 | 326 | Control | Control | Control |
| Male | 46 | 358 | Control | Control | Control |
| Male | 30 | 291 | Control | Control | Control |
| Male | 78 | 298 | Control | Control | Control |
| Male | 70 | 644 | 528 | 10 h | 6 |
| Male | 78 | 430 | 354 | 5 days | 4 |
| Male | 55 | 252 | 214 | 12 h | 7 |
| Female | 32 | 271 | 171 | 12 h | 3 |
| Male | 56 | 303 | 249 | 2 days | 7 |
| Female | 60 | 404 | 334 | 60 h | 5 |
| Female | 66 | 335 | Control | Control | Control |
| Female | 80 | 464 | 361 | 12 h | 7 |
| Male | 58 | 419 | 279 | 10 h | 6 |
| Male | 73 | 515 | 439 | 12 h | 4 |
| Male | 52 | 457 | 435 | 18 h | 8 |
| Male | 70 | 464 | 393 | 12 h | 8 |
| Male | 52 | 565 | 373 | 24 h | 6 |
| Male | 54 | 362 | Control | Control | Control |
| Male | 59 | 410 | Control | Control | Control |
| Male | 67 | 373 | 306 | 12 h | 8 |
| Female | 64 | 305 | 244 | 12 h | 8 |
| Female | 64 | 321 | 238 | 18 h | 8 |
| Female | 63 | 526 | 418 | 18 h | 4 |
| Female | 38 | 343 | Control | Control | Control |
| Female | 56 | 265 | 214 | 15 h | 8 |
| Female | 65 | 327 | 295 | 18 h | 8 |
| Male | 52 | 682 | 532 | 12 h | 8 |
| Male | 70 | 677 | 525 | 12 h | 8 |
2.3. Valve competence evaluation
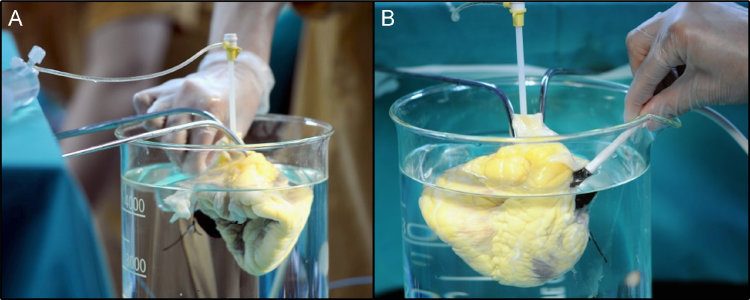
Macroscopic valve inspection was performed by cardiac surgeons and by echocardiography. Valve obstruction was easily excluded by macroscopic valve inspection of the valves. The semilunar valves (pulmonary and aortic) prevented retrograde flow back into the ventricles by a simply maneuver of filling with saline the ascending aorta and the pulmonary arteries. The atrioventricular valves (tricuspid and mitral) were evaluated with echocardiography filling the LV and RV through a 5-French intravascular sheath, inserted across the hermetically closed sutured aortic or pulmonary valves, with isotonic saline using 60 ml syringes (Fig. 2). Systole and diastole was modified by aspiration or injection of saline through the syringes helped by an external compression of the ventricles with the hand. During echocardiography evaluation, the scaffolds were submerged in deionized water. We obtained images with a broadband 14 MHz transducer (General Electric).
Fig. 2.
Valve competence evaluation during systole and diastole using echocardiography from the epicardium.
2.4. Integrity of epicardial and myocardial coronary circulation
Simultaneous angiography of the left and right coronary artery was assed to test the integrity of epicardial and myocardial coronary circulation of the whole decellularized human hearts. Controls and decellularized hearts were positioned in a foam mold, with a similar height to the anteroposterior thorax length, where the heart shape was dug (Fig. 3, Panel A and B). To record the coronary angiographic sequences we used an Artis Zee biplane system (Siemens Healthcare) (Fig. 3, Panel B). Both coronary ostium were cannulated with a conventional irrigating DeBakey heparing cannula (3 mm tip, 2 ½", 6.3 cm length, Pilling) and simultaneous angiography injections were performed using a conventional mechanical injector pump (Fig. 3, Panel C-D). We also cannulated the coronary sinus with a conventional retrograde cannula (Medtronic) in order to simultaneously drain the injected contrast media (iodixanol, VisipaqueTM, General Electric).
Fig. 3.
Angiographic coronary vascular study.
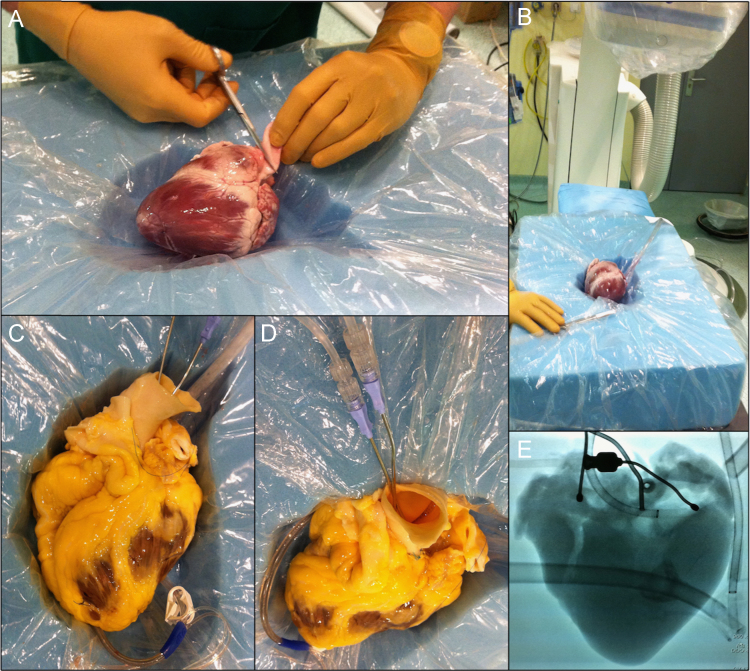
2.5. Pressure–volume measurements
We studied the relative contributions of the ECM to overall passive mechanical global properties of the left ventricle (LV) and right ventricle (RV) chambers. Mechanical properties of the decellularized human hearts were compared to the intact heart before decellularization both for the LV and RV, slightly modifying previously reported methods. [3], [4], [5] LV and RV are hermetically closed suturing aortic (Fig. 4, Panel A), pulmonary, mitral and tricuspid valves in a fully closed position. A 5-French intravascular sheath is inserted across the aortic (Fig. 4, Panel B) and pulmonary valve depending whether LV or RV pressure-volume measurements are calculated. Non- compliant polyethylene balloons are inserted across the aortic or pulmonary valves and hermetically attached to the 5-French intravascular sheath because saline leakage was observed in scaffolds (Fig. 4, panel C). A 5-French high-fidelity micromanometer (Millar-Instrument) was placed inside the balloon and connected to a 3-port manifold (Fig. 4, Panel D). Pressure and volume are then modified by aspiration followed by slow injection of isotonic saline using 60 ml syringes attached to the manifold (Fig. 4, Panel E). Differences in repated measures obtained during pre- and post-decellularization of each specimen are assessed by linear mixed-effects models (LME, S-Plus version 8.0, Tibco Software)(Fig. 2, Panel F).
Fig. 4.
Pressure–volume measurement to assess global chamber mechanical passive properties.
2.6. Cardiac gene expression
Primers used to assess cardiac gene expression in human cardiac progenitor cells grown on human decellularized ECM for 21 days are shown in Table 2.
Table 2.
Primers used to assess cardiac muscle gene expression in hCPCs grown on human dECM for 21 days.
| Gene | Sense | Anti-Sense |
|---|---|---|
| bMHC | 5′-GCTGGGGCTGATGCGCCTAT-3′ | 5′-TGGGGATGGGTGGAGCGCAA-3′ |
| cActin | 5′-GACGAGGAGACCACCGCCCT-3′ | 5′-ACCATAACTCCCTGGTGCCGC-3′ |
| GATA4 | 5′-AGCAGCTTCTGCGCCTGTGG-3′ | 5′-TGGGGGCAGAAGACGGAGGG-3′ |
| MEF2C | 5′- TCCTGCAAATATGGCCCTAG-3′ | 5′- CCTGACACACCGGGATTGTT-3′ |
| Nkx2.5 | 5′-TCACCGGCCAAGTGTGCGTC-3′ | 5′-GCAGCGCGCACAGCTCTTTC-3′ |
| Troponin T | 5′-GGAGCAGGAAGAAGCAGCTGTTGA-3′ | 5′-TTCTGCCCTGGTCTCCTCGGTC-3′ |
| GusB | 5′-CAACGAGCCTGCGTCCCACC-3′ | 5′-ACGGAGCCCCCTTGTCTGCT-3′ |
2.7. Summarizing table
Table 3 summarizes critical steps and troubleshooting, number of samples analyzed and homogeneity results of the aforementioned principal protocols.
Table 3.
Critical steps and troubleshooting of decellularization technique and valve competence, coronary circulation and pressure-volume assessments.
| Principal steps | Trouble shooting | Samples | Homogeneity | |
|---|---|---|---|---|
| Decellularization technique |
|
|
N=39 | Yes |
| Valve competence | • Competency of valves was assed by macroscopic inspection, by filling with saline the ascending aorta and pulmonary arteries in the case of the semilunar valves (aortic and pulmonary), and by echocardiography. | • To obtain adequate echocardiography images make sure the heart scaffold is fully submerged in 36 °C deionized water. | N=5 | Yes |
| Coronary circulation |
|
|
N=5 | Yes |
| Pressure-volume assesment |
|
|
N=8 | Yes |
Acknowledgment
This work was supported by grants from the Spanish Ministry of Science and Innovation (PLE2009–0152, PLE2009-147 and PLE2009-109) and the Instituto de Salud Carlos III (Ministry of Economy and Competitiveness, Spain: PI10–00141 and PI10-02038), Red de Investigación Cardiovacular (RIC) and Red de Terapia Celular (TerCel) from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness, Spain) and Comunidad Autónoma de Madrid (CAM: S2010/BMD–2420). Salvatore Costanza was supported by PI10–00141; Mª Ángeles González de Nicolás were supported by PLE2009-0152; and Judith R Acebes was supported by PLE2009-0109.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.04.069.
Contributor Information
Doris A Taylor, Email: dtaylor@texasheart.org.
Francisco Fernández-Avilés, Email: faviles@secardiologia.es.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Sanchez P.L., Fernandez-Santos M.E., Costanza S., Climent A.M., Moscoso I., Gonzalez-Nicolas M.A., San-Ruiz R., Rodriguez H., Kren S.M., Garrido G., Escalante J.L., Bermejo J., Elizaga J., Menarguez J., Yotti R., Perez del Villar C., Espinosa M.A., Guillem M.S., Willerson J.T., Bernad A., Matesanz R., Taylor D.A., Fernandez-Aviles F. Acellular human heart matrix: a critical step toward whole heat grafts. Biomaterials. 2015;62:279–289. doi: 10.1016/j.biomaterials.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 2.Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I., Taylor D.A. Perfusion-decellularized matrix: using nature׳s platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 3.Diamond G., Forrester J.S., Hargis J., Parmley W.W., Danzig R., Swan H.J. Diastolic pressure-volume relationship in the canine left ventricle. Circ. Res. 1971;29:267–275. doi: 10.1161/01.res.29.3.267. [DOI] [PubMed] [Google Scholar]
- 4.MacKenna D.A., Omens J.H., McCulloch A.D., Covell J.W. Contribution of collagen matrix to passive left ventricular mechanics in isolated rat hearts. Am. J. Physiol. 1994;266:H1007–H1018. doi: 10.1152/ajpheart.1994.266.3.H1007. [DOI] [PubMed] [Google Scholar]
- 5.Jobsis P.D., Ashikaga H., Wen H., Rothstein E.C., Horvath K.A., McVeigh E.R., Balaban R.S. The visceral pericardium: macromolecular structure and contribution to passive mechanical properties of the left ventricle. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3379–H3387. doi: 10.1152/ajpheart.00967.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material
Supplementary material