Abstract
Spiders of the family Nesticidae are members of cave communities around the world with cave-obligate (troglobiotic) species known from North America, Europe, Asia and the Indo-Pacific. A radiation of Nesticus (Araneae: Nesticidae) in the southern Appalachians includes ten troglobiotic species. Many of these species are of conservation interest due to their small ranges, with four species being single-cave endemics. Despite conservation concerns and their important role as predators in cave communities, we know little about reproduction and feeding in this group. We addressed this knowledge gap by examining populations of two species on a monthly basis for one year. We made further observations on several other species and populations, totaling 671 individual spider observations. This more than doubled the reported observations of reproduction and feeding in troglobiotic Nesticus. Female Nesticus carry egg sacs, facilitating the determination of the timing and frequency of reproduction. We found that Nesticus exhibit reproductive seasonality. Females carried egg sacs from May through October, with a peak in frequency in June. These spiders were rarely observed with prey; only 3.3% (22/671) of individuals were observed with prey items. The frequency at which prey items were observed did not vary by season. Common prey items were flies, beetles and millipedes. Troglobiotic species constituted approximately half of all prey items observed. This result represents a greater proportion of troglobiotic prey than has been reported for various troglophilic spiders. Although our findings shed light on the life history of troglobiotic Nesticus and on their role in cave ecosystems, further work is necessary to support effective conservation planning for many of these rare species.
Introduction
Spiders of the family Nesticidae are members of cave communities around the world with cave-obligate (troglobiotic) species known from North America, Europe, Asia and the Indo-Pacific [1–6]. Troglobiotic nesticids have reduced eyes and pigmentation relative to surface species [2]. Two radiations of troglobiotic nesticids are known in the United States, one of Nesticus (Araneae: Nesticidae) in the southern Appalachians and another of Eidmanella (Araneae: Nesticidae) in Texas [2,7–8].
The southwestern Appalachians are a hotspot for cave biodiversity with high levels of troglobiotic species richness and endemism [9–10]. Nesticus spiders are a significant component of this diversity. The radiation of Nesticus in the southeastern United States comprises around thirty described species including ten troglobionts from Tennessee, Alabama and Georgia. Four species are single-cave endemics, and, given their extremely limited ranges, are of significant conservation interest [2,8]. Many other members of this radiation are troglophiles (or ‘eutroglophiles’ after Culver and Pipan [11]). Troglophiles are facultative cave inhabitants; they may complete their entire life cycle underground but can also be found in similar habitats outside of caves [12]. Previous work on these spiders focused on phylogenetics [13–14] and population genetics [15].
Despite the important role of troglobiotic arachnids as predators in cave ecosystems, little is known of the ecology and life history of Nesticus and other troglobiotic arachnids [16]. Most ecological and behavioral observations of troglobiotic Nesticus are anecdotal. Nesticus form tangle webs on the walls and ceilings of caves, from which they hang in an inverted position and wait for prey (Fig 1A). Webs are often constructed along ceilings of stream corridors, in small concavities in rock walls, and in crevices where a mud bank meets the cave wall. A dozen observations of prey items have been reported including troglobiotic millipedes, springtails and beetles as well as troglophilic flies and juvenile cave crickets [17–18].
Fig 1. Photos of troglobiotic Nesticus.
(A) N. barri female in web (The Marlow Holes, Franklin County, Tennessee); (B) N. stygius with egg sac (Obe Lee Cave, Overton County, Tennessee); and (C) N. furtivus with spiderlings (Raccoon Mountain Caverns, Hamilton County, Tennessee). All photos by Alan Cressler.
After laying eggs, females carry them in an egg sac attached to the spinnerettes at the back of the abdomen (Fig 1B). Observations of females carrying egg sacs have been reported in the literature suggesting a trend toward reproduction in the late summer [8,17–18]. With the exception of Mays’ [18] monthly observations of N. barrowsi, these observations were not acquired in a systematic fashion. Egg number is reported to vary from 20 to 58 in troglobiotic Nesticus of the Appalachians [8,17–18]. Once the spiderlings leave the egg sac they can be observed in the mother’s web (Fig 1C).
We addressed knowledge gaps in the biology of troglobiotic Nesticus. This was motivated by their important ecological role as predators in cave communities and the significant conservation interest in the group. We made monthly observations of reproduction and feeding in two troglobiotic Nesticus species–N. barri and N. furtivus–for a year. N. barri is known from caves across four counties in Tennessee and Alabama and N. furtivus is a single-cave endemic from Tennessee. We made further observations of several other poorly known species and populations in Tennessee, Alabama and Georgia. These observations greatly expand the available data on reproduction and feeding in troglobiotic Nesticus from the Appalachians. Our findings shed light on the role of Nesticus in cave ecosystems and on the life history of these spiders.
Materials and Methods
Scientific permits
Work in Tennessee was permitted by the Tennessee Wildlife Resources Agency (permit #1605). Work in Georgia was permitted by the Georgia Department of Natural Resources (permit #8934). Work in Horseskull Cave was permitted by the Southeastern Cave Conservancy.
Field sites and species studied
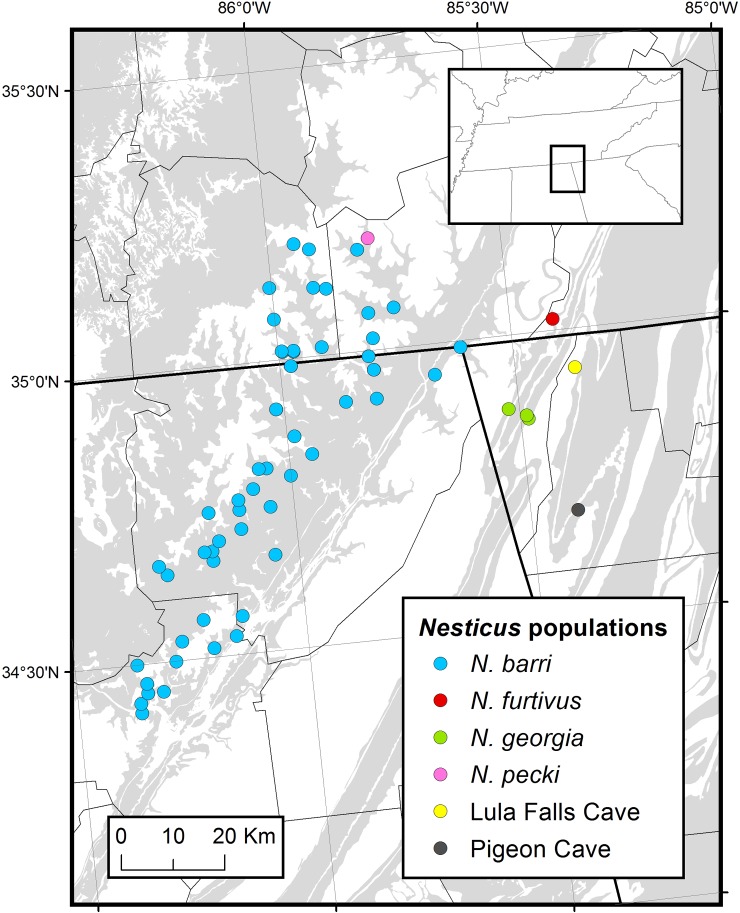
We investigated reproductive seasonality and feeding in Nesticus barri and N. furtivus on a monthly basis for one year. Nesticus barri is known from more than 50 caves in Tennessee and Alabama (Fig 2). Nesticus barri was surveyed in Buckets of Blood Cave (Tennessee Cave Survey (TCS) FR61) in Franklin County, Tennessee. Nesticus furtivus was surveyed in Raccoon Mountain Caverns (TCS HM4) in Hamilton County, Tennessee. This is the type and only known locality for N. furtivus [2,8] (Fig 2). Additional surveys of other Nesticus species and populations were conducted one to three times in Horseskull Cave in Jackson County, Alabama (Alabama Cave Survey AJK613; N. barri), Monteagle Saltpeter Cave in Marion County, Tennessee (TCS MN24, the type and only known locality for N. pecki) [8], Sittons Cave in Dade County, Georgia (Georgia Speleological Survey (GSS) GDD9, N. georgia), Pigeon Cave in Walker County, Georgia (GSS GWK57, home to an undetermined species of Nesticus) and Lula Falls Cave in Walker County, Georgia (GSS GWK617, also home to an undetermined species of Nesticus). As a single species of troglobiotic Nesticus is known from each of these caves we were able to identify Nesticus species by locality.
Fig 2. Troglobiotic Nesticus populations in the vicinity of the Tennessee, Alabama and Georgia junction.
State and county boundaries are outlined. Gray background indicates karst topography, derived from Weary and Doctor [19].
Data collection
We limited our investigation to the transition and deep zones of caves [20]. We searched for spiders using headlamps on all accessible cave surfaces including walls, floor, ceiling and breakdown. The sex and maturity of each spider was noted. Mature males had distinctively enlarged and sclerotized pedipalps. Mature females had a prominent and protruding epigynum. Immature males had enlarged pedipalps that were pale and unsclerotized. Immature females had an incompletely developed and non-protruding epigynum. For spiders that had not yet developed sex-specific characteristics, or in cases where they could not be confidently determined, sex and maturity were recorded as ‘undetermined’. In most cases the sex and maturity of the spiders could be determined without disturbing the spider. In cases where the spider’s position made this difficult, spiders were briefly captured in a shell vial and examined with a 10x magnification hand lens before being released at the point of collection. We also noted whether or not the spider was in a web. The presence of egg sacs or spiderlings was recorded, as was the presence of prey items. All observations were made by one of the authors and all authors had extensive experience observing cave spiders. We did not attempt to estimate population sizes in our surveys; instead we aimed to determine the frequency of reproduction and feeding in these populations throughout the year. The results of each cave trip are presented in Table 1. Egg sacs were collected from N. barri in Buckets of Blood and Horseskull caves and dissected to count eggs. We lost the data from the December 18, 2013 visit to Raccoon Mountain Caverns.
Table 1. All Nesticus observations from this study.
State, county and cave survey numbers for caves are presented in the methods.
| Species | Locality | Date | Total observed | Sex and Maturity | Egg sacs | Prey items | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Undetermined | ||||||||
| Mature | Immature | Mature | Immature | |||||||
| Nesticus barri | Buckets of Blood Cave | 30-Jan-13 | 14 | 1 | 1 | 8 | 1 | 3 | 0 | 0 |
| Buckets of Blood Cave | 13-Feb-13 | 37 | 5 | 3 | 20 | 1 | 8 | 0 | 1 | |
| Buckets of Blood Cave | 27-Mar-13 | 24 | 6 | 0 | 12 | 0 | 6 | 0 | 0 | |
| Buckets of Blood Cave | 24-Apr-13 | 40 | 4 | 4 | 15 | 0 | 17 | 0 | 1 | |
| Buckets of Blood Cave | 25-May-13 | 24 | 2 | 1 | 11 | 0 | 10 | 1 | 1 | |
| Buckets of Blood Cave | 26-Jun-13 | 26 | 0 | 0 | 15 | 0 | 11 | 9 | 1 | |
| Buckets of Blood Cave | 17-Jul-13 | 31 | 3 | 4 | 13 | 1 | 10 | 6 | 0 | |
| Buckets of Blood Cave | 30-Aug-13 | 56 | 6 | 7 | 24 | 2 | 17 | 7 | 2 | |
| Buckets of Blood Cave | 25-Sep-13 | 50 | 2 | 11 | 10 | 5 | 22 | 2 | 0 | |
| Buckets of Blood Cave | 31-Oct-13 | 35 | 6 | 10 | 10 | 2 | 7 | 0 | 2 | |
| Buckets of Blood Cave | 18-Nov-13 | 42 | 8 | 4 | 15 | 3 | 12 | 0 | 0 | |
| Buckets of Blood Cave | 24-Dec-13 | 20 | 7 | 1 | 6 | 0 | 6 | 0 | 1 | |
| Buckets of Blood Cave | 27-Jan-14 | 24 | 6 | 5 | 8 | 2 | 3 | 0 | 0 | |
| Horseskull Cave | 20-Feb-13 | 9 | 0 | 0 | 5 | 0 | 4 | 0 | 0 | |
| Horseskull Cave | 14-Jul-13 | 34 | 1 | 2 | 19 | 1 | 11 | 9 | 2 | |
| Horseskull Cave | 17-Sep-13 | 22 | 0 | 2 | 14 | 0 | 6 | 7 | 1 | |
| N. furtivus | Raccoon Mountain Caverns | 24-Feb-13 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| Raccoon Mountain Caverns | 31-Mar-13 | 5 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | |
| Raccoon Mountain Caverns | 28-Apr-13 | 17 | 0 | 6 | 6 | 0 | 5 | 0 | 2 | |
| Raccoon Mountain Caverns | 24-May-13 | 13 | 0 | 2 | 6 | 0 | 5 | 0 | 0 | |
| Raccoon Mountain Caverns | 28-Jun-13 | 9 | 2 | 1 | 4 | 0 | 2 | 1 | 0 | |
| Raccoon Mountain Caverns | 18-Jul-13 | 15 | 1 | 4 | 5 | 0 | 5 | 1 | 3 | |
| Raccoon Mountain Caverns | 29-Aug-13 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Raccoon Mountain Caverns | 20-Sep-13 | 14 | 2 | 3 | 6 | 1 | 2 | 1 | 3 | |
| Raccoon Mountain Caverns | 30-Oct-13 | 8 | 0 | 0 | 1 | 0 | 7 | 1 | 0 | |
| Raccoon Mountain Caverns | 17-Nov-13 | 36 | 0 | 1 | 7 | 0 | 28 | 0 | 1 | |
| Raccoon Mountain Caverns | 18-Dec-13 | No data | No data | No data | No data | No data | No data | No data | No data | |
| Raccoon Mountain Caverns | 28-Jan-13 | 4 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | |
| N. georgia | Sittons Cave | 28-Apr-13 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| N. pecki | Monteagle Saltpeter Cave | 1-Sep-13 | 6 | 0 | 0 | 2 | 0 | 4 | 1 | 0 |
| N. sp. | Lula Falls Cave | 8-Aug-13 | 35 | 4 | 8 | 8 | 6 | 9 | 0 | 0 |
| N. sp. | Pigeon Cave | 8-Aug-13 | 14 | 0 | 0 | 9 | 2 | 3 | 8 | 0 |
Other sources of data
When possible we incorporated previously published information into our analyses. Observations of troglobiotic Nesticus with egg sacs are reported from several previous studies [8,17–18]. Only two studies detailed the number of mature females, mature males and immatures. Mays [18] used the same methods as this study in surveying a troglobiotic Nesticus species on a monthly basis for a year, totaling 430 observations of N. barrowsi. Mays [18] also recorded prey items. Hedin and Dellinger [8] conducted four surveys totaling 56 observations of N. furtivus. These surveys were done in different years, once in April and July and twice in August. Observations of N. barri, N. furtivus and N. barrowsi taken on a monthly basis for a year constituted 85% of all spider observations considered, limiting any effect of seasonality on the results. The number of eggs per egg sac has been reported for several troglobiotic Nesticus species from the southern Appalachians [8,17–18]. Observer bias in these studies is limited as the information collected about each spider (sex, presence/absence of egg sac, presence/absence of prey) is straightforward.
Results
General observations
We made 32 cave visits between January 2013 and January 2014 resulting in 671 Nesticus observations (Table 1). The majority of our observations were of N. barri (N = 488 spiders observed) and N. furtivus (N = 126). Additional observations were made of several other Nesticus species and populations (N = 57). Across all populations we observed mature females (N = 267) nearly four times as often as mature males (N = 69).
Reproduction
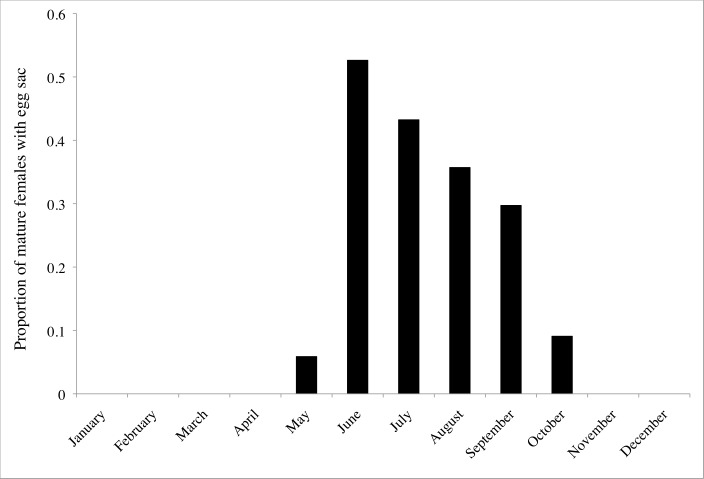
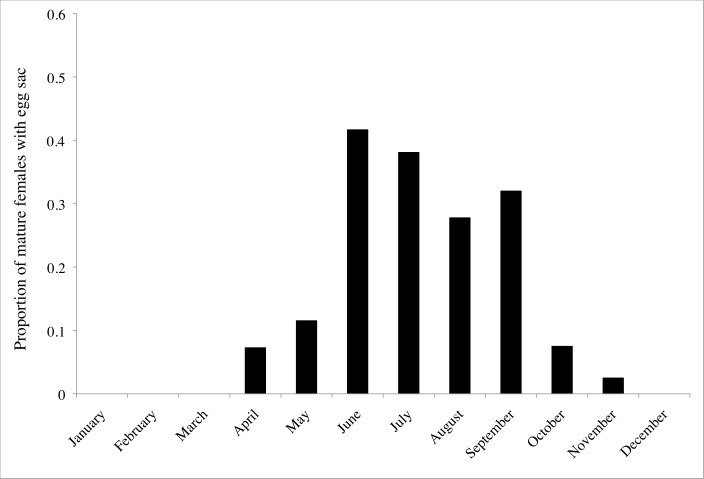
Across all the observed populations, 20.2% (54/267) of mature females were observed with egg sacs. Troglobiotic Nesticus exhibited reproductive seasonality in the species and populations studied. We observed egg sacs from May through October. The frequency of egg sacs peaked in June, when more than 50% of mature females carried egg sacs (Fig 3). When we combined our data with previous observations from the literature [8,18] a similar pattern of reproductive periodicity was present with egg sacs first observed in April, peaking in frequency in June, and continuing to be present at low frequency into November (Fig 4). On three occasions we observed mature male and female Nesticus in the same web. We observed a pair of N. barri mating on April 24, 2013 in Buckets of Blood Cave. We observed 26 spiderlings in a N. furtivus web on November 17, 2013 and an undetermined number of spiderlings in a N. barri web on July 13, 2013.
Fig 3. Proportion of mature Nesticus females observed with egg sacs each month.
Data collected in this study for N. barri, N. furtivus, N. georgia, N. pecki and Nesticus sp. from Lula Falls Cave and Pigeon Cave.
Fig 4. Proportion of mature Nesticus females observed with egg sacs each month.
Reproduction in three troglobiotic Nesticus species has been surveyed in a single cave on a monthly basis for a year–N. barri (this study), N. furtivus (this study) and N. barrowsi [18]. Of those species, mature females were observed with egg sacs at a higher frequency in N. barri (15.0%, 25/167) than in either N. furtivus (9.8%, 4/41) or N. barrowsi (10.1%, 19/188), but this difference was not significant (X2 (2, N = 396) = 2.2, p = 0.33).
The mean number of eggs in N. barri egg sacs collected during this study was 39, with a range of 22 to 66 (N = 7; Table 2). The mean number of eggs per egg sac previously reported in the literature for five troglobiotic Nesticus species from the southern Appalachians was 38, with a range from 20 to 58 (N = 13; Table 2) [8,17–18].
Table 2. Number of eggs per egg sac for troglobiotic Nesticus from the southern Appalachians.
| Species | Locality | Eggs | Source |
|---|---|---|---|
| Nesticus barri | Buckets of Blood Cave | 22, 29, 36, 37, 40, 66 | This study |
| Horseskull Cave | 43 | This study | |
| Moody Cave | 35 | [17] | |
| N. barrowsi | Gregorys Cave | 28, 32, 34, 37, 48 | [18] |
| N. dilutus | Grassy Creek Cave | 20, 41 | [8] |
| N. georgia | Sittons Cave | 41, 44, 54, 58 | [17] |
| N. stygius | Raven Bluff Cave | 22 | [8] |
Feeding
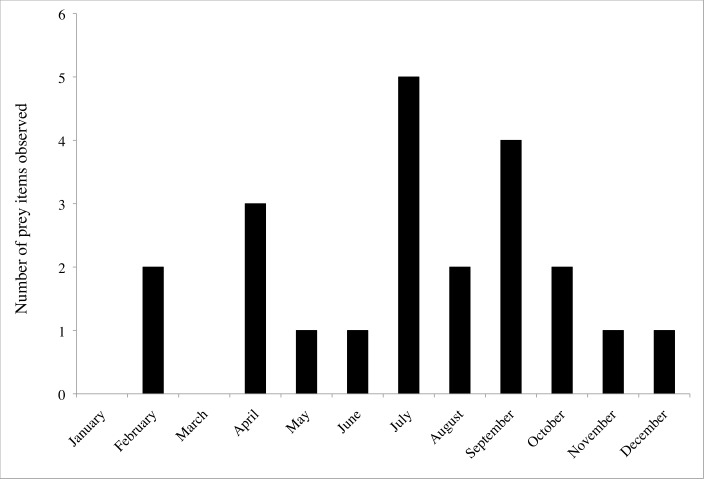
3.3% (22/671) of spiders were observed with prey. At least one spider with prey was observed each month with the exception of January and March (Fig 5). Troglobiotic Nesticus fed on flies (Heleomyzidae and other families), beetles (Ptomaphagus hatchi and other unidentified species) and millipedes (Pseudotremia sp. and Scoterpes sp.) (Table 3). Dipterans represented 50% (11/22) of prey items observed. When the two populations were compared, N. furtivus was observed with prey at a higher frequency (8.7%, 11/126) than N. barri (1.9%, 8/423) (X2 (1, N = 549) = 11.62, p = 0.0007).
Fig 5. Number of prey items observed monthly for all Nesticus populations and species observed.
Table 3. Prey observations for troglobiotic Nesticus from the southern Appalachians.
| Species | Locality | Prey | Source |
|---|---|---|---|
| Nesticus barri | Buckets of Blood Cave | Pseudotremia minos (Diplopoda, Cleidogonidae) (N = 2), Ptomaphagus hatchi (Coleoptera, Leiodidae) (N = 1), Heleomyzidae (Diptera) (N = 4), unknown (N = 1) | This study |
| Horseskull Cave | Scoterpes stewartpecki (Diplopoda, Trichopetalidae) (N = 1), unidentified beetle (N = 2) | This study | |
| N. barrowsi | Gregorys Cave | Scoterpes blountensis (Diplopoda, Trichopetalidae) (N = 6), dipterans (N = 2), unknown (N = 1) | [18] |
| N. furtivus | Raccoon Mountain Cave | Pseudotremia sp. (N = 2), Heleomyzidae (Diptera) (N = 5), other dipterans (N = 2), unidentified beetle (N = 1), unknown (N = 1) | This study |
| N. georgia | Sittons Cave | Ptomaphagus whiteselli (Coleoptera, Leiodidae), Pseudosinella hirsuta (Collembola, Entomobryidae), Gryllacrididae (Orthoptera) | [17] |
Although more prey items were present in the summer and fall (June through November) than in the winter and spring (December to May) (Fig 5), the frequency that we observed prey items did not differ between summer-fall and winter-spring (X2 (1, N = 671) = 0.01, p = 0.92). During the summer and fall, 3.4% (15/435) of spiders had prey items. In the winter and spring, 3.0% (7/236) of spiders had prey items.
Discussion
Reproduction
We observed reproductive seasonality with troglobiotic Nesticus reproducing in the summer and fall (Fig 3). This was consistent with previous studies that reported the number of egg sacs and mature females (Fig 4; [8,18]). This was also consistent with published and unpublished observations noting the presence of egg sacs, all of which were observed from May to November ([8,17]; P. Perlaky and A. Cressler unpublished observations). Although reproductive seasonality was evident, we may have overestimated the frequency of females with egg sacs. The white egg sacs are conspicuous (Fig 1B) and this may lead to a sampling bias due to increased detection of females with egg sacs relative to individuals without egg sacs.
Our observations of a mating pair of N. barri in April, N. barri spiderlings in a web in July and N. furtivus spiderlings in a web in November are also consistent with a reproductive cycle running through the summer and fall. Other reports of N. furtivus spiderlings are from July, August and September (P. Perlaky and A. Cressler unpublished observations). Our observations suggest females carry egg sacs for four to six weeks. Similarly, Ives [21] reported that females of troglophilic N. carteri carried egg sacs for slightly more than a month until spiderlings emerged. Across five troglobiotic Nesticus species, there was a range of 20 to 66 eggs per egg sac (Table 2) with a mean of 38 eggs per egg sac.
From the data collected in this study and by Mays [18] we can estimate how often troglobiotic Nesticus reproduce. With 12.1% (48/396) of mature females observed with egg sacs in monthly surveys, females carrying egg sacs for four to six weeks, and a sampling bias favoring the detection of females with egg sacs, it appears that mature females produce around one egg sac per year. As we did not track individual spiders it is possible that some mature females produce no egg sacs in a year whereas others produce more than one egg sac in a year. With an average of 38 eggs per egg sac (Table 2), we estimate a mature female produces ~40 eggs per year.
The physical environment and food supply in temperate caves vary seasonally due to changes in surface temperature and precipitation [12,22]. This seasonal variation is thought to influence reproduction in troglobionts. Several examples of reproductive seasonality in troglobionts from the eastern United States are known. Kane et al. [23] found larvae and pupae of a predatory cave beetle from the Mammoth Cave system were most common in the early spring. They ascribed this pattern to seasonal variation in food availability to adult beetles (in the form of cricket eggs). Similarly, a troglobiotic crayfish and several species of cave fish from the eastern United States maintain annual reproductive cycles and release young in the summer, when food availability may be greatest [24–26]. However, not all troglobionts from the eastern United States exhibit reproductive seasonality. Year-round reproduction has been reported for several species of round fungus beetles (Coleoptera: Leodidae) [27] and aquatic isopod species show no consistent pattern of annual reproductive seasonality (summarized in [28]).
Work conducted by Ives [21,29] on N. carteri presents an important contrast to the reproductive seasonality we observed in troglobiotic Nesticus. Ives [21] monitored reproduction in a population of N. carteri in Three Springs Cave (Hamblen County, Tennessee) on a monthly basis for six years. N. carteri ranges from Indiana to Virginia. It is troglophilic and can be found in cave and surface habitats (Gertsch 1984). Ives [21] found that N. carteri in Three Springs Cave reproduced year-round. The proportion of spiders with an egg sac varied from 19% to 38% per month. Assuming some of the spiders he observed were male and/or immature, the proportion of mature females carrying an egg sac would have been higher. Thus, reproduction in this population of troglophilic N. carteri differed from what we observed in troglobiotic Nesticus species, as they reproduced more frequently and lacked reproductive seasonality. Unfortunately, Three Springs Cave was flooded after the construction of a hydroelectric dam in 1942, precluding further study of this population.
Feeding
Despite their important role as predators in cave ecosystems, little is known about the diet of troglobiotic spiders, including Nesticus. Previous observations of N. barrowsi and N. georgia identified a range of prey items including millipedes, flies, beetles, springtails and juvenile cave crickets [17–18]. Consistent with those reports, we observed millipedes (Scoterpes and Pseudotremia), flies (family Heleomyzidae and others) and beetles (Ptomaphagus and others) in webs of N. barri and N. furtivus (Table 3). While food availability has been suggested to drive reproductive seasonality in some troglobionts, we did not find support for that pattern, as the frequency of prey in webs did not differ by season. However, the small number of prey items observed (22 total) limited our ability to detect seasonal differences.
The diet of troglobiotic Nesticus differed significantly from that of the troglophilic spiders Meta ovalis, M. menardi, M. bourneti and Metellina merianae. In caves, the troglophilic spiders fed largely on trogloxenic and troglophilic prey [30–32], only rarely capturing troglobiotic prey [33]. In contrast, approximately half of the observed prey of troglobiotic Nesticus was troglobiotic (beetles, millipedes and springtails). The other half of the observed prey was troglophilic flies and crickets (Table 3). Similarly, Mays [18] observed that N. barrowsi also fed predominantly on troglobiotic prey (six of nine observed prey items were troglobiotic millipedes, Table 3). Troglobiotic Nesticus are thus more deeply integrated into cave-specific food webs than troglophilic spiders. This observation highlights how cave food webs may differ with distance from a cave entrance. Troglobiotic spiders (and troglobiotic prey) are typically encountered deeper in a cave, whereas troglophilic spiders (and troglophilic prey) are more likely to be encountered close to an entrance.
Diversity and Endemism
The Nesticus radiation in the southeastern United States includes some of the rarest spiders in North America. Of the ten troglobiotic species described from Tennessee, Alabama and Georgia, four are single-cave endemics. Several other species are known from fewer than five caves. We surveyed two undescribed populations which may represent new species and other undescribed populations are known. Short-range endemic invertebrate species such as these troglobiotic Nesticus are typically of great conservation interest [34–35].
In the course of this study we observed two single-cave endemic Nesticus species. We made repeated observations of N. furtivus at Raccoon Mountain Caverns, a commercial cave in Hamilton County, Tennessee. Of the single-cave endemic Nesticus species it is undoubtedly the best known. Although known only from this cave, and never observed in large numbers, the cave’s large size (> 8 km of passage, much of which is rarely visited) and the careful attention of the cave manager (P. Perlaky) and the cave owner confer a significant degree of protection to the species. We made a single observation of N. pecki, a single-cave endemic from Marion County, Tennessee. In contrast to N. furtivus, N. pecki is extremely poorly known. Our observation of N. pecki in September 2013 was, to our knowledge, the first observation of the species in more than twenty years [8]. We observed six spiders, including one female carrying an egg sac, in the vicinity of the small second entrance. Similar to our observations, Hedin and Dellinger [8] reported seeing fewer than ten spiders on visits in 1991 and 1992, suggesting N. pecki is rare even within its only known locality. As suggested by Hedin and Dellinger [8], further study of caves and similar habitats nearby could clarify whether the range of N. pecki extends beyond this cave.
We observed two Nesticus populations that may represent new species. Nesticus populations were reported from two caves on Pigeon Mountain in Walker County, Georgia [36]. We visited Pigeon Cave in August 2013 and observed 14 spiders, including eight females with egg sacs. With no other Nesticus known from Pigeon Mountain, it is likely these eyeless spiders represent an undescribed species. The second unidentified Nesticus population was discovered by one of the authors (A. Cressler) in Lula Falls Cave on Lookout Mountain in Walker County, Georgia. We observed 35 individuals during a visit in August 2013. Although located less than 15 km from populations of N. georgia and N. furtivus, the Lula Falls Cave population is clearly distinct, as they have eyes whereas N. georgia and N. furtivus are eyeless. Searches of several caves in the immediate vicinity of Lula Falls Cave have not identified other Nesticus populations. As with the Pigeon Mountain populations, we suspect the Lula Falls Cave population represents an undescribed Nesticus species. Specimens from both populations were collected and shared with spider systematists to facilitate the determination and/or description of these species.
Like most short-range endemic species, troglobiotic Nesticus have limited dispersal abilities and are confined to discontinuous habitats. This has resulted in remarkable diversification within Nesticus of the southern Appalachian region with six species from Tennessee, Alabama and Georgia known from five or fewer caves. Little is know about most of these species. This is exemplified in this study by N. pecki, which to our knowledge has been observed only once since it was described [8]. Basic information on population sizes, prey items, reproduction and habitat threats is lacking for most of these species. For species known from more than one cave, we lack information about connectivity between cave populations (see [15] for an exception). Surveys of caves in the vicinity of known populations might uncover new populations of known species. All of this information would inform conservation assessments of these species. As indicated by this study, all of these projects are feasible as the spiders are reasonably conspicuous members of cave communities.
Conclusion
With ten described troglobiotic species and numerous troglophilic species, Nesticus spiders are part of many cave communities in the southern Appalachians. While surface and troglophilic Nesticus species generally have large ranges, many troglobiotic Nesticus are short-range endemics, with numerous species known from one or a few caves; several cave populations of Nesticus that likely represent undescribed species are also known. We found that troglobiotic Nesticus exhibit reproductive seasonality, reproducing during the summer and fall with mature females producing an average of one egg sac per year. Troglobiotic Nesticus feed on beetles, millipedes, flies and other invertebrates. In contrast to cave-inhabiting troglophilic spiders which rarely capture troglobiotic prey, around half of observed prey items were troglobiotic, likely a result of troglobiotic Nesticus being part of food webs deeper in caves than troglophilic spiders. Despite this contribution to our understanding of the ecology of cave spiders, further study of Nesticus, in particular on the short-range endemic members of the genus, is critical to ensuring the proper management of these rare species.
Acknowledgments
We thank S. Perlaky for permitting our work in Raccoon Mountain Caverns. The Southeastern Cave Conservancy permitted our work in Horseskull Cave. We thank J. Mays and D. Culver for advice. Three reviewers provided helpful comments. We thank J. Young and C. Van de Ven for assistance with GIS. We also thank W. Coleman, J. Grimes, S. Haynes, Z. Loehle, J. Mulloy and J. Wallace for assistance in the field.
Data Availability
All relevant data are within the paper.
Funding Statement
LMC received a Fellowship in Karst Studies from the Cave Conservancy Foundation (http://www.caveconservancyfoundation.org/). The Cave Conservancy Foundation had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. KSZ received research support from Sewanee: The University of the South (http://www.sewanee.edu/). The University of the South had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Raccoon Mountain Caverns provided support in the form of salary for one author [PP], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nakamura H, Kuramoto T. On the mode of life in cavernicolous spider, Nesticus akiyoshiensis (Uyemura). Bulletin of the Akiyoshi-dai Science Museum 1973;9: 29–37. [Google Scholar]
- 2.Gertsch WJ. The spider family Nesticidae (Araneae) in North America, Central America and the West Indies. Texas Memorial Museum Bulletin 1984;31: 1–91. [Google Scholar]
- 3.Benjamin SP. Nesticella marapu sp. n., a blind nesticid (Araneae: Nesticidae) from Sumba, Indonesia. Revue Suisse de Zoologie 3004;111: 303–307. [Google Scholar]
- 4.Lopez-Pancorbo A, Ribera C. Nesticus baeticus sp. n., a new troglobiotic spider species from south-west Europe (Araneae, Nesticidae). ZooKeys 2011;89: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Li S. Ancient lineage, young troglobites: recent colonization of caves by Nesticella spiders. BMC Evolutionary Biology 2013;13: 183 10.1186/1471-2148-13-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribera C, Elverici M, Kunt BG, Ozkutuk RS. Typhlonesticus gocmeni sp. n., a new cave-dwelling blind spider species from the Aegean region of Turkey (Araneae, Nesticidae). ZooKeys 2014;419: 87–102. 10.3897/zookeys.419.5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cokendolpher JC, Reddell JR. New and rare nesticid spiders from Texas caves (Araneae: Nesticidae) Texas Memorial Museum Speleological Monographs 2001;5: 25–34. [Google Scholar]
- 8.Hedin M, Dellinger B. Descriptions of a new species and previously unknown males of Nesticus (Araneae: Nesticidae) from caves in eastern North America, with comments on species rarity. Zootaxa 2005;904: 1–19. [Google Scholar]
- 9.Culver DC, Master LL, Christman MC, Hobbs HH III. Obligate cave fauna of the 48 contiguous United States. Conservation Biology 2000;14: 386–401. [Google Scholar]
- 10.Niemiller ML, Zigler KS. Patterns of cave biodiversity and endemism in the Appalachians and Interior Plateau of Tennessee, USA. PLoS ONE 2013;8: e64177 10.1371/journal.pone.0064177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culver DC, Pipan T. The Biology of Caves and Other Subterranean Habitats. Oxford University Press; 2009. 256 pp. [Google Scholar]
- 12.Barr TC. Observations on the ecology of caves. The American Naturalist 1967;101: 475–491. [Google Scholar]
- 13.Hedin MC. Speciational history in a diverse clade of habitat-specialized spiders (Araneae: Nesticidae: Nesticus): inferences from geographic-based sampling. Evolution 1997a;51: 1929–1945. [DOI] [PubMed] [Google Scholar]
- 14.Hedin MC. Molecular phylogenetics at the population/species interface in cave spiders of the southern Appalachians (Araneae: Nesticidae: Nesticus). Molecular Biology and Evolution 1997b;14: 309–324. [DOI] [PubMed] [Google Scholar]
- 15.Snowman CV, Zigler KS, Hedin M. Caves as islands: mitochondrial phylogeography of the cave-obligate spider species Nesticus barri (Araneae: Nesticidae). Journal of Arachnology 2010;38: 49–56. [Google Scholar]
- 16.Reddell JR. Arachnids In: White WB, Culver DC, editors. Encyclopedia of caves. 2nd edition Amsterdam: Academic Press; 2012. pp. 787–797. [Google Scholar]
- 17.Reeves W. Cave-dwelling Nesticidae (Araneae) in the southeastern United States: New distribution records and notes on their bionomics. Insecta Mundi 1999;13: 93–94. [Google Scholar]
- 18.Mays JD. A systematic approach to sampling the arthropod assemblage of Gregorys Cave, Great Smoky Mountains National Park. M. Sc. Thesis, Western Carolina University. 2002.
- 19.Weary DJ, Doctor DH. Karst in the United States: A digital map compilation and database. U.S. Geological Survey Open-File Report 2014. –1156: 1–23. [Google Scholar]
- 20.Howarth FG. Ecology of cave arthropods. Annual Review of Entomology 1983;28: 365–389. [Google Scholar]
- 21.Ives JD. Breeding habits of a cave spider, Nesticus carteri Emerton. Elisha Mitchell Scientific Society. Chapel Hill, NC. 1947;63.2: 215. [PubMed]
- 22.Barr TC, Kuehne RA. Ecological studies in the Mammoth Cave system of Kentucky. II The ecosystem. Annales de Speleologie 1971;26: 47–96. [Google Scholar]
- 23.Kane TC, Norton RM, Poulson TL. The ecology of a predaceous troglobiotic beetle, Neaphaenops tellkampfii (Coleoptera: Carabidae, Trechinae). I. Seasonality of food input and early life history stages. International Journal of Speleology 1975;7: 45–54. [Google Scholar]
- 24.Jegla TC. Reproductive and molting cycles in cave crayfish. Biological Bulletin 1966;130: 345–358. [Google Scholar]
- 25.Jegla TC, Poulson TL. Circannian rhythms–I. Reproduction in the cave crayfish, Orconectes pellucidus inermis. Comparative Biochemistry and Physiology 1970;33: 347–355. [Google Scholar]
- 26.Niemiller ML, Poulson TL. Subterranean fishes of North America: Amblyopsidae In: Trajano E, Bichuette ME, Kapoor BG, editors. Biology of Subterranean Fishes. CRC Press; 2010. pp. 169–280. [Google Scholar]
- 27.Peck SB. Evolution of adult morphology and life-history characters in cavernicolous Ptomaphagus beetles. Evolution 1986;40: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 28.Zigler KS, Cooper GM. Brood size of the stygobiotic asellid isopod Caecidotea bicrenata bicrenata from Franklin County, Tennessee, USA. Speleobiology Notes 2011;3: 1–3. [Google Scholar]
- 29.Ives JD. A study of the cave spider, Nesticus pallidus Emerton, to determine whether it breeds seasonally or otherwise. Journal of the Elisha Mitchell Scientific Society 1935;51: 297–299. [Google Scholar]
- 30.Smithers P. The diet of the cave spider Meta menardi (Latreille 1904) (Araneae: Tetragnathidae). Journal or Arachnology 2005;33: 243–246. [Google Scholar]
- 31.Novak T, Tkavc T, Kuntner M, Arnett AE, Delakorda SL, Perc M, et al. Niche partitioning in orbweaving spiders Meta menardi and Metellina merianae (Tetragnathidae). Acta Oecologica 2010;36: 522–529. [Google Scholar]
- 32.Mammola S, Isaia M. Niche differentiation in Meta bourneti and M. menardi (Araneae, Tetragnathidae) with notes on life history. International Journal of Speleology 2014;43: 343–353. [Google Scholar]
- 33.Slay ME, Fong DW, Kottmyer MD. Meta ovalis (Araneae: Tetragnathidae) observed preying on a troglobiotic milliped, Causeyella (Chordeumatida: Trichopetalidae). Speleobiology Notes 2009;1: 3–5. [Google Scholar]
- 34.Harvey MS. Short-range endemism among the Australian fauna: some examples from non-marine environments. Invertebrate Systematics 2002;16: 555–570. [Google Scholar]
- 35.Harvey MS, Rix MG, Framenau VW, Hamilton ZR, Johnson MS, Teale RJ, et al. Protecting the innocent: studying short-range endemic taxa enhances conservation outcomes. Invertebrate Systematics 2011;25: 1–10. [Google Scholar]
- 36.Buhlmann KA. A biological inventory of eight caves in northwestern Georgia with conservation implications. Journal of Cave and Karst Studies 2001;63: 91–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.