Abstract
Objective
To correlate physical examination findings, central venous pressure, fluid output, and central venous oxygen saturation with pulmonary artery catheter parameters.
Design
Retrospective study.
Setting
Data from the multicenter Fluid and Catheter Treatment Trial of the National Institutes of Health Acute Respiratory Distress Syndrome Network.
Patients
Five hundred thirteen patients with acute lung injury randomized to treatment with a pulmonary artery catheter.
Interventions
Correlation of physical examination findings (capillary refill time >2 secs, knee mottling, or cool extremities), central venous pressure, fluid output, and central venous oxygen saturation with parameters from a pulmonary artery catheter.
Measurements
We determined association of baseline physical examination findings and on-study parameters of central venous pressure and central venous oxygen saturation with cardiac index <2.5 L/min/m2 and mixed venous oxygen saturation <60%. We determined correlation of baseline central venous oxygen saturation and mixed venous oxygen saturation and predictive value of a low central venous oxygen saturation for a low mixed venous oxygen saturation.
Measurements and Main Results
Prevalence of cardiac index <2.5 and mixed venous oxygen saturation <60% was 8.1% and 15.5%, respectively. Baseline presence of all three physical examination findings had low sensitivity (12% and 8%), high specificity (98% and 99%), low positive predictive value (40% and 56%), but high negative predictive value (93% and 86%) for cardiac index <2.5 and mixed venous oxygen saturation <60%, respectively. Central venous oxygen saturation <70% predicted a mixed venous oxygen saturation <60% with a sensitivity 84%, specificity 70%, positive predictive value 31%, and negative predictive value of 96%. Low cardiac index correlated with cool extremities, high central venous pressure, and low 24-hr fluid output; and low mixed venous oxygen saturation correlated with knee mottling and high central venous pressure, but these correlations were not found to be clinically useful.
Conclusions
In this subset of patients with acute lung injury, there is a high prior probability that cardiac index and mixed venous oxygen saturation are normal and physical examination findings of ineffective circulation are not useful for predicting low cardiac index or mixed venous oxygen saturation. Central venous oxygen saturation <70% does not accurately predict mixed venous oxygen saturation <60%, but a central venous oxygen saturation ≥70% may be useful to exclude mixed venous oxygen saturation <60%.
Keywords: acute lung injury, acute respiratory distress syndrome, physical examination, pulmonary artery catheterization, superior vena cava oxygen saturation, mixed venous oxygen saturation
Studies using the pulmonary artery catheter (PAC) in critically ill patients to guide therapy (1–4) or to target specific goals for cardiac index (CI, L/min/m2 body surface area) or mixed venous oxygen saturation (SvO2) (5) have not shown an improved outcome. Physical examination findings and other objective parameters that do not require a PAC may be considered alternatives for assessing circulatory effectiveness and guiding therapy in critically ill patients. These physical examination findings include delayed capillary refill time, knee mottling, and cool skin temperature (6, 7). Objective parameters obtained without a PAC and used to indicate the adequacy of the circulation include fluid and urine output (6, 7), and parameters obtained from a central venous catheter (CVC): central venous pressure and central venous oxygen saturation (ScvO2).
We used data from the Fluid and Catheter Treatment Trial (FACTT) (3, 4) of the National Institutes of Health, National Heart Lung and Blood Institute, Acute Respiratory Distress Syndrome Clinical Trials Network to explore correlations of physical examination findings and three objective parameters (fluid output, central venous pressure, and ScvO2) with CI and SvO2 in patients with acute lung injury (ALI) and acute respiratory distress syndrome. FACTT included 1001 randomized patients in a clinical trial with a four-cell factorial design comparing a fluid-conservative treatment strategy versus a fluid-liberal treatment strategy using parameters from a CVC versus a PAC.
The data collected in the FACTT study included three physical examination findings indicative of ineffective circulation: increased capillary refill time (>2 secs), skin mottling over the knees, and cool skin temperature of the extremities. In patients randomized to treatment guided by a CVC, simultaneous presence of all three of these physical examination findings indicated ineffective circulation and determined protocolized treatment. In patients randomized to treatment guided by a PAC, physical examination findings were not used for treatment decisions, but provided an opportunity to compare physical examination findings reflecting circulation with hemodynamic parameters from a PAC.
We hypothesized that physical examination findings of ineffective circulation and objective parameters obtained without a PAC (24-hr total fluid output, ScvO2, and central venous pressure) are associated with a low CI and SvO2 in patients with ALI and acute respiratory distress syndrome. To test this hypothesis, we analyzed data from patients randomized to a PAC in FACTT with the following three objectives: 1) determine whether physical examination findings at baseline were associated with a CI <2.5 and SvO2 <60%; 2) evaluate data from study days 0 to 7 to determine whether physical examination findings and objective parameters of 24-hr fluid output and central venous pressure were associated with a CI <2.5 and SvO2 <60%; and 3) determine the correlation of ScvO2 and SvO2 and how well a low ScvO2 predicts a low SvO2.
Methods
The protocol for the multicenter FACTT study has been previously published in detail (3, 4), and only the elements pertinent to this study are described here. Inclusion criteria included patients who were receiving positive pressure ventilation by tracheal tube and had a PaO2/FiO2 ratio <300 (adjusted if altitude >1000 meters) with bilateral infiltrates on chest radiograph consistent with pulmonary edema not thought to be from left atrial hypertension (8). Major exclusion criteria included presence of a PAC after onset of ALI; presence of ALI >48 hrs; inability to obtain consent; presence of chronic conditions that could independently influence survival, impair weaning from mechanical ventilation, or compromise protocol compliance; and irreversible conditions for which 6-month estimated mortality exceeded 50%. The Intermountain Healthcare Institutional Review Board, Salt Lake City, UT, approved this retrospective study of the primary Acute Respiratory Distress Syndrome Network FACTT database with a waiver of informed consent.
The 513 FACTT patients randomized to a PAC constituted our retrospective study sample. The PAC was inserted within 4 hrs of randomization in the FACTT study. Hemodynamic management was started within the next 2 hrs and continued for 7 days or until 12 hrs after achieving unassisted breathing. Physical examination findings used for clinical assessment of circulatory effectiveness included capillary refill time >2 secs, skin mottling over the knees, and cool extremities. In the CVC group of the FACTT study, if all three of these physical examination findings were present, then “ineffective circulation” was judged to be present and influenced protocol directions. In the PAC group, a measured CI <2.5 L/min/m2 was used to indicate ineffective circulation and determine protocol directions. Data on physical examination findings in the PAC group were collected every 4 hrs at the same time that CI was measured, although physical examination findings did not determine protocol directions (in contrast, in the CVC group, an “ineffective circulation” conclusion and appropriate protocol recommendations followed the simultaneous presence of all three of these physical examination findings). We used these data for comparison of CI and SvO2 parameters with physical examination findings in this study. We chose a threshold for low CI of <2.5 because that was used in the FACTT study for protocol decisions. The mean baseline CI in FACTT was 4.2 ± 1.4 L/min/m2 (n = 408). We chose a threshold for low SvO2 of <60% because that was approximately 1 sd below the mean in two studies in which SvO2 was reported in a large number of critically ill patients (5, 9). The mean baseline SvO2 in FACTT was 69 ± 12% (n = 323).
Methods for Objective 1 (Determine Whether Baseline Physical Examination Findings Are Predictive of a CI <2.5 or SvO2 <60%)
We analyzed baseline physical examination data collected after randomization and insertion of the PAC but before initiation of the FACTT fluid protocol. Dichotomous variables included capillary refill time >2 secs, knee mottling, and cold extremities. We used 2 × 2 tables to calculate the sensitivity, specificity, positive predictive value, and negative predictive value of physical examination findings for predicting a CI <2.5 and SvO2 <60%. We determined the predictive value of finding all three findings present (capillary refill >2 secs, knee mottling, and cool extremities), and we determined separately the predictive value of finding only one of three findings present (capillary refill >2 secs, knee mottling, or cool extremities).
Methods for Objective 2 (Determine Whether Physical Examination Findings and Objective Parameters of 24-hr Fluid Output and Central Venous Pressure Predict a CI <2.5 or SvO2 <60%)
We analyzed data collected at baseline and on FACTT study days 0 to 7. We recorded physical examination findings as either present or absent daily. We recorded daily total fluid output (included urine output, nasogastric tube output, stool output, and any drain or tube output) in the preceding 24 hrs and at least one measurement of central venous pressure (closest to 8:00 am). We used for analysis generalized estimating equations model with logit link function and autoregressive correlation structure (PROC GENMOD in SAS; SAS Institute, Cary, NC) to model probability of CI <2.5 and SvO2 <60% using as covariates three physical examination findings: fluid output, and central venous pressure, and adjusting for treatment group. The generalized estimating equations model with logit link function is a generalization of logistic regression capable of handling repeated within-patient measurements for data collected over the first 7 days of the study. In all analyses, we excluded patient records if any of the variables were missing and used only complete patient data records in regression analysis.
Methods for Objective 3 (Determine Correlation Between ScvO2 and SvO2)
We collected baseline data on ScvO2 to analyze the correlation between ScvO2 and SvO2 by median regression. One ScvO2 measurement and one SvO2 measurement were compared for each patient at baseline. The predictive value of a low threshold ScvO2 <70% for a low SvO2 <60% was analyzed using a 2 × 2 table. The threshold value <70% for low ScvO2 was based on a prior study (10) and consensus recommendations (11, 12). Unless otherwise specified, data are presented as means ± sds.
Results
Patients received their first protocol intervention an average of 43 hrs after admission to the intensive care unit and 24 hrs after meeting the criteria for ALI. Low CI and SvO2 were infrequent among the 513 PAC patients. Among those with complete data, only 8.1% (33 of 408) had a baseline CI <2.5, 15.5% (61 of 394) had a baseline SvO2 <60%, and 3.7% (13 of 350) had both a CI <2.5 and SvO2 <60%.
Results for Objective 1 (Determine Whether Baseline Physical Examination Findings Are Predictive of a CI <2.5 or SvO2 <60%)
Baseline data on 405 patients were analyzed for the association of physical examination findings with a low CI. All three findings were present in 10 patients (four with CI <2.5 and six with CI ≥2.5), one or two findings were present in 90 patients (13 with CI <2.5 and 77 with CI ≥2.5), and none were present in 305 patients (16 with CI <2.5 and 289 with CI ≥2.5). Baseline data on 392 patients were analyzed for association of physical examination findings with a low SvO2. All three findings were present in nine patients (five with SvO2 <60% and four with SvO2 ≥60%), one or two findings were present in 91 patients (19 with SvO2 <60% and 72 with SvO2 ≥60%), and none were present in 292 patients (36 with SvO2 <60% and 256 with SvO2 ≥60%). Tables 1 through 4 show the association of all three physical examination findings or any one of three physical examination findings with a CI <2.5 and SvO2 <60%.
Table 1. Presence of all three physical examination findings (capillary refill >2 secs, knee mottling, and cool extremities) as predictive of CI <2.5.
| N = 405 | CI <2.5 | CI ≥2.5 | |
|---|---|---|---|
| All 3 physical examination findings present | 4 | 6 | Positive predictive value = 40% |
| All 3 not present | 29 | 366 | Negative predictive value = 93% |
| Sensitivity = 12% | Specificity = 98% |
CI, cardiac index.
Table 4. Presence of any one physical examination finding (capillary refill >2 secs, knee mottling, or cool extremities) as predictive of SvO2 <60%.
| N = 392 | SvO2 <60% | SvO2 ≥60% | |
|---|---|---|---|
| Any 1 physical examination finding present | 24 | 76 | Positive predictive value = 24% |
| No physical examination findings present | 36 | 256 | Negative predictive value = 88% |
| Sensitivity = 40% | Specificity = 77% |
SvO2, mixed venous oxygen saturation.
We also analyzed baseline physical examination findings in those patients receiving intravenous vasopressor drugs (n = 138 patients). Having all three physical examination findings present was associated with a CI <2.5 with a sensitivity of 19%, specificity of 97%, positive predictive value of 43%, and negative predictive value of 90%; and all three physical examination findings were associated with a SvO2 <60% with sensitivity of 17%, specificity of 98%, positive predictive value of 71%, and negative predictive value of 81%.
Results for Objective 2 (Determine Whether Physical Examination Findings And Objective Parameters of 24-hr Fluid Output and Central Venous Pressure Predict a CI <2.5 or SvO2 <60%)
A CI <2.5 occurred in 8% of the measurements on study days 0 to 7. We performed the analysis of study data on days 0 to 7 on 478 patients with complete data. Analysis using the generalized estimating equations model showed that the correlation of CI <2.5 and cool extremities had an odds ratio of 1.9 with 95% confidence interval from 1.0 to 3.5 (p = .10), the correlation of CI <2.5 and high central venous pressure had an odds ratio of 1.06 per 1 cm H2O with 95% confidence interval from 1.03 to 1.09 (p = .002), and the correlation of CI <2.5 and low 24-hr total fluid output had an odds ratio of 0.8 per 1000 mL (p = .001). Low SvO2 <60% occurred in 14% of the measurements on study days 0 to 7. We performed the analysis of study SvO2 data on days 0 to 7 on 217 patients with complete data. Analysis using the generalized estimating equations model showed that SvO2 <60% was significantly correlated with knee mottling (odds ratio 5.0 with 95% confidence interval from 1.8 to 14.4, p = .009) and high central venous pressure (odds ratio 1.09 per 1 cm H2O with 95% confidence interval from 1.04 to 1.14, p = .0004), but this analysis is based on only 16 episodes of knee mottling in 13 patients. We constructed receiver operating curves for these parameters, but none of the statistically significant associations yielded clinically useful results, and those results are not reported.
Results for Objective 3 (Determine Correlation Between ScvO2 and SvO2)
Baseline values of ScvO2 and SvO2 were significantly correlated (Fig. 1) in all patients who had paired comparisons available (n = 218) and in those patients with severe sepsis and ALI who had paired comparisons available (n = 107) (Fig. 2). We also determined the correlation of CI and SvO2 (Fig. 3).
Figure 1.
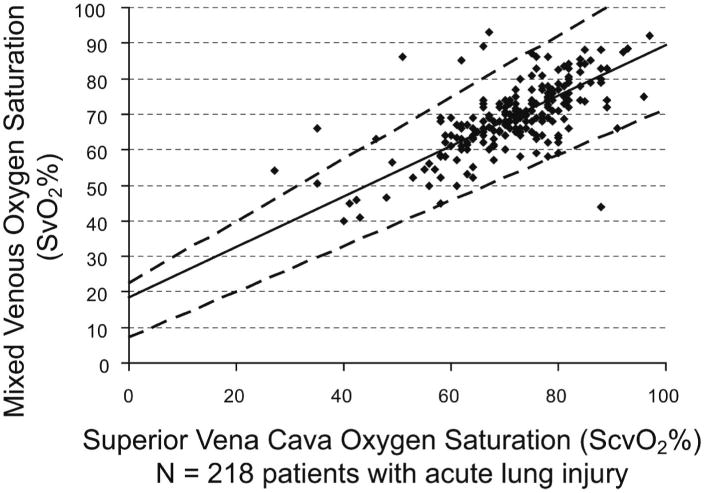
Mixed venous oxygen saturation (SvO2) correlated with central venous oxygen saturation (ScvO2) in 218 patients with acute lung injury at baseline. Analysis by median regression: Spearman's ρ = 0.64 (p < .0001), SvO2 = 0.71*ScvO2 + 18.5 (95% confidence interval for intercept [7.4, 22.5] and slope [0.64, 0.87]). The dashed lines are 95% confidence limits for the regression line. The predicted SvO2 for an ScvO2 of 70% is SvO2 = 68% (95% confidence limits, 52%–83%).
Figure 2.
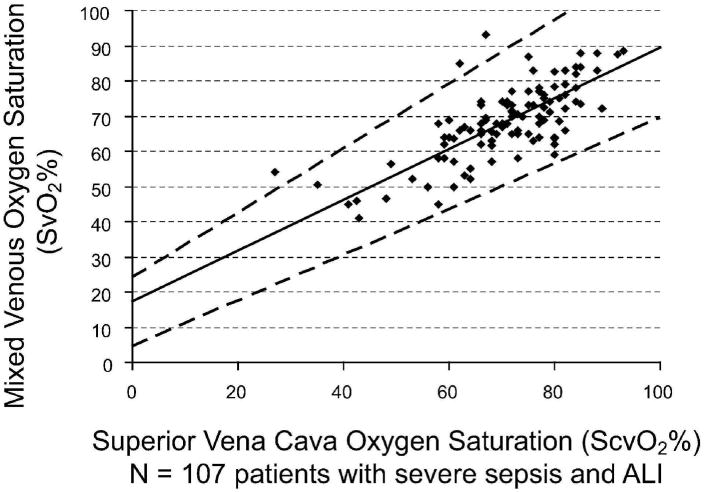
Mixed venous oxygen saturation (SvO2) correlated with central venous oxygen saturation (ScvO2) in 107 patients with acute lung injury and severe sepsis at baseline. Analysis by median regression: Spearman's ρ = 0.63 (p < .0001), SvO2 = 0.72*ScvO2 + 17.5 (95% confidence interval for intercept [4.5, 24.4] and slope [0.65, 0.91]). The dashed lines are 95% confidence limits for the regression line.
Figure 3.
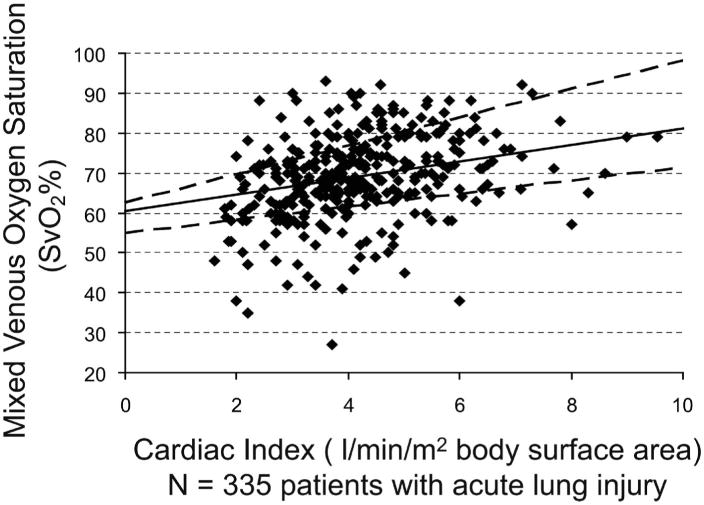
Cardiac index (CI) plotted against mixed venous oxygen saturation (SvO2) in 335 patients with acute lung injury at baseline. Analysis by median regression: SvO2 = 2.07*CI + 60.4 (95% confidence interval for intercept [54.9, 62.5] and slope [1.65, 3.56]); Spearman's ρ = 0.34 (p < .0001). The dashed lines are 95% confidence limits for the regression line. For the correlation of CI and ScvO2, Spearman's ρ = .30, p < .0001 (data not shown).
Mean baseline ScvO2 was 71.5 ± 11.2% and mean baseline SvO2 was 69.3 ± 9.9%. Table 5 shows the value of ScvO2 <70% (10) to predict an SvO2 <60% from baseline paired ScvO2 and SvO2 data (n = 218). In patients with severe sepsis at baseline (n = 107), results were similar for an ScvO2 <70% predicting an SvO2 <60% (sensitivity 95%, specificity 71%, positive predictive value 43%, and negative predictive value 98%).
Table 5. Baseline ScvO2 <70% as predictive of SvO2 <60%.
| N = 218 | SvO2 <60% | SvO2 ≥60% | |
|---|---|---|---|
| ScvO2 <70% | 26 | 57 | Positive predictive value = 31% |
| ScvO2 ≥70% | 5 | 130 | Negative predictive value = 96% |
| Sensitivity = 84% | Specificity = 70% |
ScvO2, central venous oxygen saturation; SvO2, mixed venous oxygen saturation.
SvO2 <60% was significantly associated with higher mortality and fewer ventilator-free days. In patients with a baseline SvO2 <60% (n = 62), survival to day 60 was 62% (95% confidence interval, 52%–74%) as compared with patients with a baseline SvO2 ≥60% (n = 326) who had a survival to day 60 of 76% (95% confidence interval, 71%–81%). There was a 14% difference in mortality at 60 days between groups (95% confidence interval, 2%–26%) (p = .03). In patients with a baseline SvO2 <60% (n = 62), median ventilator-free days was 6 and in patients with an SvO2 ≥60% (n = 326), median ventilator-free days was 17 (p = .004, Wilcoxon's test).
Discussion
Our results suggest that physical examination findings of an ineffective circulation (capillary refill time >2 secs, knee mottling, and cool extremities) are not useful predictors of a low CI or low SvO2 in a population of patients with ALI, including those in shock receiving intravenous vasopressor medications. In those patients with a CI <2.5, only 52% had at least one physical examination finding (low sensitivity), and only 17% of those patients with at least one physical exam finding had a CI <2.5 (low positive predictive value). In those patients with a SvO2 <60%, only 40% had at least one physical examination finding (low sensitivity), and only 24% of those patients with at least one physical examination finding had a SvO2 <60% (low positive predictive value).
Even the absence of physical examination findings has no demonstrable clinical usefulness. Despite the high specificity and negative predictive value, the low prevalence of a CI <2.5 (8.1% at baseline) and SvO2 <60% (15.5% at baseline) in patients with ALI make guessing that CI is >2.5 and SvO2 >60% just as accurate as if absence of physical examination findings are used to make these predictions. Because of the high prior probability of normal values, our study is underpowered to show usefulness in negative physical findings.
The low prevalence of cardiac insufficiency in this study population of patients with ALI is partly the result of exclusion of patients with clinical evidence of left atrial hypertension or an acute myocardial infarction within the prior 30 days. Even with these exclusion criteria, 29% of the patients in the PAC group had an initial pulmonary artery occlusion pressure that exceeded 18 mm Hg, although the majority of these were 19 or 20 mm Hg, and only 3% of patients with a pulmonary artery occlusion pressure greater than 18 mm Hg had a CI <2.5 (3). Inclusion of patients in this study with an initial pulmonary artery occlusion pressure that exceeded 18 mm Hg, however, raises the question of the homogeneity of the study population, because 29% of patients included in FACTT would not have been classified as having acute respiratory distress syndrome if the diagnosis of acute respiratory distress syndrome was made at the time the PAC was placed. This diversity of patients included in the study may have contributed to the lack of association of clinical examination findings with ineffective circulation.
The findings in this study are consistent with previous reports in which clinician judgment using physical examination findings and objective parameters did not correlate with parameters obtained from a PAC. Clinicians' bedside evaluations predict cardiac output, at most, 50% of the time (13–16). Previous studies evaluating physical examination findings to predict the effectiveness of the arterial circulation have had mixed results. Cool skin temperature was found to significantly correlate with a lower cardiac output, CI, SvO2, pH, and elevated lactate in a study of 264 patients in a surgical intensive care unit (17). We also found a significant correlation between cool extremities and low CI with regression analysis, but the receiver operating curve did not show clinical usefulness. Differences in characteristics of patients in a surgical intensive care unit and patients with ALI in FACTT might account for differences in the predictive value of physical examination findings. Cool extremities may be more predictive of hypovolemic shock, rather than septic shock, that may occur more often in a critically ill surgical patient population.
We observed a significant correlation between low SvO2 and knee mottling, but these observations were based on low numbers of cases and require further study for confirmation. In the previous study of surgical patients (17), only cool skin temperature was evaluated and not knee mottling, and we found no other previous studies evaluating knee mottling as a clinical sign of inadequate perfusion.
We did not find that increased capillary refill had clinical usefulness for predicting low CI or SvO2. This is consistent with a review of previous studies in which capillary refill time was determined to have no diagnostic value in adults with hypovolemia (18). A similar review of previous studies in pediatric patients, however, found that capillary refill time was a useful physical examination finding indicative of ineffective circulation resulting from hypovolemia (19).
We found a correlation between ScvO2 and SvO2 in patients with ALI but with wide confidence intervals making clinical usefulness uncertain. The SvO2 provides information about the balance between oxygen delivery and metabolic demand (although SvO2 may also be decreased because of low hemoglobin and increased oxygen extraction or low arterial oxygen saturation with normal oxygen extraction). In our study, a low SvO2 was associated with increased mortality. This association has been reported previously (20, 21), and a low SvO2 has been associated with a greater inflammatory response and increased mortality (22). Therapy directed at increasing SvO2 to normal levels may improve survival (23). Using ScvO2 as a surrogate for SvO2 is appealing because it does not require placement of a PAC. Previous studies have shown a correlation between ScvO2 and SvO2, but the clinical usefulness of the correlation is controversial (24–28). Our study reports a greater number of paired measurements of ScvO2 and SvO2 than any previously published study and shows a significant correlation between ScvO2 and SvO2, but with a great deal of variation that makes clinical usefulness uncertain.
We also determined if a low threshold ScvO2 <70% could predict a low SvO2 <60%. We chose a threshold ScvO2 of <70% because a previous prospective randomized study comparing two algorithms for early goal-directed therapy in patients with severe sepsis and septic shock showed that maintenance of continuously measured ScvO2 >70%, using cardiac inotropes if necessary (in addition to maintaining central venous pressure >8, mean arterial pressure >65 mm Hg, and urine output >0.5 mL/kg/hr), resulted in improved survival (10). This threshold ScvO2 of 70% has been recommended by the Surviving Sepsis Campaign (11, 12). In our study, a low threshold ScvO2 <70% predicted a low SvO2 <60% with a high sensitivity of 84% but a low positive predictive value of 31% because of the high false-positive rate (patients whose ScvO2 <70% but SvO2 >60%). We obtained similar results when patients with ALI and sepsis were evaluated (n = 111) for association of ScvO2 <70% with an SvO2 <60% (sensitivity 90%, specificity 70%, positive predictive value 41%, and negative predictive value 97%). In our study, a normal ScvO2 ≥70% was significantly associated with an SvO2 ≥60% in 96% of measurements. This suggests that the clinical usefulness of a ScvO2 ≥70% is to exclude SvO2 <60%. The true value of ScvO2, however, is when it is below 70% and associated with an increased lactate. A limitation of our study is that we did not measure lactate levels and we had a predominance of increased ScvO2 and SvO2 values and relatively few low values. Only 26 patients had ScvO2 values less than 70% paired with SvO2 values less than 60%. Thus, any statements regarding the relationship of ScvO2 and SvO2 do not have adequate power and should be considered observations.
The association of baseline ScvO2 with SvO2 <65% is about the same as with SvO2 <60%. An SvO2 <65% is a threshold value recommended by the Surviving Sepsis Campaign (11, 12). Baseline ScvO2 <70% is associated with SvO2 <65% with a sensitivity of 78%, specificity of 78%, positive predictive value of 59%, and negative predictive value of 90%.
A limitation of our study is that we evaluated only one time point of correlation between ScvO2 and SvO2. Previous studies suggest that following trends in ScvO2 with continuous monitoring provides a more accurate correlation with SvO2 in critically ill patients (24, 27), but with the disadvantage of requiring placement of a specialized CVC.
It is important to recognize that our study evaluated parameters of circulatory effectiveness 24 hrs after onset of ALI and 48 hrs after intensive care unit admission and only one third of the patients in our study had shock and/or required vasopressors and approximately one fourth had severe sepsis. Our study does not reflect acute resuscitation of patients in shock. This is in contrast to the study of Rivers and colleagues (10) of early, goal-directed resuscitation of severe sepsis and septic shock in the emergency department before admission to the intensive care unit. Additionally, an unavoidable weakness of the retrospective nature of our study is that the physical examination data and the PAC measurements were not collected blindly. Knowledge of the patient's CI may have influenced the clinician's perception of the physical examination signs. Another limitation of this study is observing knee mottling in patients with pigmented or dark skin, and approximately 36% of the patients in this study were classified as “nonwhite” for race or ethnicity (4). We also did not evaluate interrater reliability, which may have introduced a strong bias into this study, and those who evaluated the physical examination findings were not blinded to the hemodynamic data, further promoting potential bias.
Conclusions
Our results do not support the usefulness of looking for increased capillary refill, cool skin, or knee mottling as a proxy for low CI or low SvO2, because the prior probability of a normal CI and SvO2 is high, and the predictive value of a positive clinical examination finding is low.
ScvO2 significantly correlates with SvO2 in patients with ALI, but the confidence intervals are wide and the clinical usefulness of this relationship is uncertain. Our study does suggest, however, that a high ScvO2 ≥70% in this group of patients excludes a SvO2 of <60%. If ScvO2 <70%, then other clinical parameters should be evaluated to confirm ineffective circulation. These results require further validation in a prospective study.
Table 2. Presence of any one physical examination finding (capillary refill >2 secs, knee mottling, or cool extremities) as predictive of CI <2.5.
| N = 405 | CI <2.5 | CI ≥2.5 | |
|---|---|---|---|
| Any 1 physical examination finding present | 17 | 83 | Positive predictive value = 17% |
| No physical examination findings present | 16 | 289 | Negative predictive value = 95% |
| Sensitivity = 52% | Specificity = 78% |
CI, cardiac index.
Table 3. Presence of all three physical examination findings (capillary refill >2 secs, knee mottling, cool extremities) as predictive of SvO2 <60%.
| N = 393 | SvO2 <60% | SvO2 ≥60% | |
|---|---|---|---|
| All 3 physical examination findings present | 5 | 4 | Positive predictive value = 56% |
| All 3 not present | 55 | 329 | Negative predictive value = 86% |
| Sensitivity = 8% | Specificity = 99% |
SvO2, mixed venous oxygen saturation.
Acknowledgments
The following persons and institutions participated in the Fluid and Catheter Treatment Trial: Steering Committee Chair—G.R. Bernard; Clinical Coordinating Center—D.A. Schoenfeld, B.T. Thompson, N. Ringwood, C. Oldmixon, F. Molay, A. Korpak, R. Morse, D. Hayden, M. Ancukiewicz, A. Minihan; Protocol Review Committee—J.G.N. Garcia, R. Balk, S. Emerson, M. Shasby, W. Sibbald; Data Safety and Monitoring Board—R. Spragg, G. Corbie-Smith, J. Kelley, K. Leeper, A.S. Slutsky, B. Turnbull, C. Vreim; National Heart, Lung, and Blood Institute—A.L. Harabin, D. Gail, P. Lew, M. Waclawiw; ARDS Clinical Trials Network Consultant—P. Parsons; Clinical Centers—University of Washington, Harborview— L. Hudson, K. Steinberg, M. Neff, R. Maier, K. Sims, C. Cooper, T. Berry-Bell, G. Carter, L. Andersson; University of Michigan—G.B. Toews, R.H. Bartlett, C. Watts, R. Hyzy, D. Arnoldi, R. Dechert, M. Purple; University of Maryland—H. Silverman, C. Shanholtz, A. Moore, L. Heinrich, W. Corral; Johns Hopkins University—R. Brower, D. Thompson, H. Fessler, S. Murray, A. Sculley; Cleveland Clinic Foundation—H.P. Wiedemann, A.C. Arroliga, J. Komara, T. Isabella, M. Ferrari; University Hospitals of Cleveland—J. Kern, R. Hejal, D. Haney; Metro Health Medical Center—A.F. Connors; University of Colorado Health Sciences Center—E. Abraham, R. McIntyre, F. Piedalue; Denver Veterans Affairs Medical Center—C. Welsh; Denver Health Medical Center—I. Douglas, R. Wolkin; St. Anthony Hospital—T. Bost, B. Sagel, A. Hawkes; Duke University—N. MacIntyre, J. Govert, W. Fulkerson, L. Mallatrat, L. Brown, S. Everett, E. VanDyne, N. Knudsen, M. Gentile; University of North Carolina—P. Rock, S. Carson, C. Schuler, L. Baker, V. Salo; Vanderbilt University—A.P. Wheeler, G. Bernard, T. Rice, B. Christman, S. Bozeman, T. Welch; University of Pennsylvania—P. Lanken, J. Christie, B. Fuchs, B Finkel, S. Kaplan, V. Gracias, C.W. Hanson, P. Reilly, M.B. Shapiro, R. Burke, E. O'Connor, D. Wolfe; Jefferson Medical College—J. Gottlieb, P. Park, D.M. Dillon, A. Girod, J. Furlong; LDS Hospital—A. Morris, C. Grissom, L. Weaver, J. Orme, T. Clemmer, R. Davis, J. Gleed, S. Pies, T. Graydon, S. Anderson, K. Bennion, P. Skinner; McKay-Dee Hospital—C. Lawton, J. d'Hulst, D. Hanselman; Utah Valley Regional Medical Center—K. Sundar, T. Hill, K. Ludwig, D. Nielson; University of California, San Francisco—M.A. Matthay, M. Eisner, B. Daniel, O. Garcia; San Francisco General—J. Luce, R. Kallet; University of California, San Francisco, Fresno—M. Peterson, J. Lanford; Baylor College of Medicine—K. Guntupalli, V. Bandi, C. Pope; Baystate Medical Center—J. Steingrub, M. Tidswell, L. Kozikowski; Louisiana State University Health Sciences Center—B. deBoisblanc, J. Hunt, C. Glynn, P. Lauto, G. Meyaski, C. Romaine; Louisiana State University Earl K. Long Center—S. Brierre, C. LeBlanc, K. Reed; Alton-Ochsner Clinic Foundation—D. Taylor, C. Thompson; Tulane University Medical Center—F. Simeone, M. Johnston, M. Wright; University of Chicago—G. Schmidt, J. Hall, S. Hemmann, B. Gehlbach, A. Vinayak, W. Schweickert; Northwestern University—J. Dematte D'Amico, H. Donnelly; University of Texas Health Sciences Center—A. Anzueto, J. McCarthy, S. Kucera, J. Peters, T. Houlihan, R. Steward, D. Vines; University of Virginia—J. Truwit, A.F. Connors, M. Marshall, W. Matsumura, R. Brett; University of Pittsburgh—M. Donahoe, P. Linden, J. Puyana, L. Lucht, A. Verno; Wake Forest University—R.D. Hite, P. Morris, A. Howard, A. Nesser, S. Perez; Moses Cone Memorial Hospital—P. Wright, C. Carter-Cole, J. McLean; St. Paul's Hospital, Vancouver—J. Russell, L. Lazowski, K. Foley; Vancouver General Hospital—D. Chittock, L. Grandolfo; Mayo Foundation—M. Murray.
Supported, in part, by National Heart, Lung and Blood Institute NO1-HR46054-64 and NO1-HR-16146-54.
Dr. Lanken received grant support from the National Institutes of Health, National Heart, Lung and Blood Institute.
Footnotes
See also p. 2846.
The remaining authors have not disclosed any potential conflicts of interest.
For information regarding this article, colin.grissom@imail.org
References
- 1.Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): A randomised controlled trial. Lancet. 2005;366:472–477. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 2.Richard C, Warszawski J, Anguel N, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: A randomized controlled trial. JAMA. 2003;290:2713–2720. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler AP, Bernard GR, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 4.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid—Management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med. 1995;333:1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- 6.Maier RV. Shock. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's 15th Edition Principles of Internal Medicine. New York: McGraw-Hill; 2001. pp. 222–228. [Google Scholar]
- 7.Walley KR. Shock. In: Hall JB, Schmidt GA, Wood LDH, editors. Principles of Critical Care. 3rd. New York: McGraw-Hill; 2005. pp. 249–265. [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, et al. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 9.Chawla LS, Zia H, Gutierrez G, et al. Lack of equivalence between central and mixed venous oxygen saturation. Chest. 2004;126:1891–1896. doi: 10.1378/chest.126.6.1891. [DOI] [PubMed] [Google Scholar]
- 10.Rivers E, Nguyen B, Havstad S, et al. the Early Goal-Directed Therapy Collaborative. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 11.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 13.Celoria G, Steingrub JS, Vickers-Lahti M, et al. Clinical assessment of hemodynamic values in two surgical intensive care units. Effects on therapy. Arch Surg. 1990;125:1036–1039. doi: 10.1001/archsurg.1990.01410200100016. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg PR, Jaffe AS, Schuster DP. Clinical evaluation compared to pulmonary artery catheterization in the hemodynamic assessment of critically ill patients. Crit Care Med. 1984;12:549–553. doi: 10.1097/00003246-198407000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mimoz O, Rauss A, Rekik N, et al. Pulmonary artery catheterization in critically ill patients: A prospective analysis of outcome changes associated with catheter-prompted changes in therapy. Crit Care Med. 1994;22:573–579. doi: 10.1097/00003246-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Connors AF, Jr, McCaffree DR, Gray BA. Evaluation of right-heart catheterization in the critically ill patient without acute myocardial infarction. N Engl J Med. 1983;308:263–267. doi: 10.1056/NEJM198302033080508. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan LJ, McPartland K, Santora TA, et al. Start with a subjective assessment of skin temperature to identify hypoperfusion in intensive care unit patients. J Trauma. 2001;50:620–627. doi: 10.1097/00005373-200104000-00005. discussion 627-628. [DOI] [PubMed] [Google Scholar]
- 18.McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022–1029. doi: 10.1001/jama.281.11.1022. [DOI] [PubMed] [Google Scholar]
- 19.Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291:2746–2754. doi: 10.1001/jama.291.22.2746. [DOI] [PubMed] [Google Scholar]
- 20.Kasnitz P, Druger GL, Yorra F, et al. Mixed venous oxygen tension and hyperlactatemia. Survival in severe cardiopulmonary disease. JAMA. 1976;236:570–574. [PubMed] [Google Scholar]
- 21.Varpula M, Tallgren M, Saukkonen K, et al. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31:1066–1071. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]
- 22.Rivers EP, Kruse JA, Jacobsen G, et al. The influence of early hemodynamic optimization on biomarker patterns of severe sepsis and septic shock. Crit Care Med. 2007;35:2016–2024. doi: 10.1097/01.ccm.0000281637.08984.6e. [DOI] [PubMed] [Google Scholar]
- 23.Yu M, Burchell S, Hasaniya NW, et al. Relationship of mortality to increasing oxygen delivery in patients > or = 50 years of age: a prospective, randomized trial. Crit Care Med. 1998;26:1011–1019. doi: 10.1097/00003246-199806000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Dueck MH, Klimek M, Appenrodt S, et al. Trends but not individual values of central venous oxygen saturation agree with mixed venous oxygen saturation during varying hemodynamic conditions. Anesthesiology. 2005;103:249–257. doi: 10.1097/00000542-200508000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Krafft P, Steltzer H, Hiesmayr M, et al. Mixed venous oxygen saturation in critically ill septic shock patients. The role of defined events. Chest. 1993;103:900–906. doi: 10.1378/chest.103.3.900. [DOI] [PubMed] [Google Scholar]
- 26.Ladakis C, Myrianthefs P, Karabinis A, et al. Central venous and mixed venous oxygen saturation in critically ill patients. Respiration. 2001;68:279–285. doi: 10.1159/000050511. [DOI] [PubMed] [Google Scholar]
- 27.Reinhart K, Kuhn HJ, Hartog C, et al. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Intensive Care Med. 2004;30:1572–1578. doi: 10.1007/s00134-004-2337-y. [DOI] [PubMed] [Google Scholar]
- 28.Varpula M, Karlsson S, Ruokonen E, et al. Mixed venous oxygen saturation cannot be estimated by central venous oxygen saturation in septic shock. Intensive Care Med. 2006;32:1336–1343. doi: 10.1007/s00134-006-0270-y. [DOI] [PubMed] [Google Scholar]


