Abstract
The neural correlates of motor inhibition leading to paresis in conversion disorder are not well known. The key question is whether they are different of those of normal subjects feigning the symptoms. Thirteen conversion disorder patients with hemiparesis and twelve healthy controls were investigated using functional magnetic resonance tomography under conditions of passive motor stimulation of the paretic/feigned paretic and the non-paretic hand. Healthy controls were also investigated in a non-feigning condition. During passive movement of the affected right hand conversion disorder patients exhibited activations in the bilateral triangular part of the inferior frontal gyri (IFG), with a left side dominance compared to controls in non-feigning condition. Feigning controls revealed for the same condition a weak unilateral activation in the right triangular part of IFG and an activity decrease in frontal midline areas, which couldn't be observed in patients. The results suggest that motor inhibition in conversion disorder patients is mediated by the IFG that was also involved in inhibition processes in normal subjects. The activity pattern in feigning controls resembled that of conversion disorder patients but with a clear difference in the medial prefrontal cortex. Healthy controls showed decreased activity in this region during feigning compared to non-feigning conditions suggesting a reduced sense of self-agency during feigning. Remarkably, no activity differences could be observed in medial prefrontal cortex for patients vs healthy controls in feigning or non-feigning conditions suggesting self-agency related activity in patients to be in between those of non-feigning and feigning healthy subjects.
Keywords: Conversion disorder, Motor paresis, Feigning, Motor inhibition, fMRI
Highlights
-
•
Motor inhibition was investigated in motor conversion disorder patients and feigners.
-
•
Neural correlates were found in nearby but different lateral inferior frontal regions.
-
•
Altered sense of agency in patients was reflected in medial prefrontal activity level.
1. Introduction
The phenomenon of conversion disorder (CD) is considered to be historically the first described psychic disease (Catonne, 1992). It can mimic every possible neurological symptom such as hyp- and dysaesthesia, visual and auditory defects, motor symptoms as flaccid or spastic-like paresis, coordination or gait disorders, tremor, loss of speech as well as amnesia, pain, fatigue or pseudo-seizures (Stone et al., 2005). In general, the patients present with neurological disease but whose signs show inconsistency and are incongruent with the normal rules of pathology. They are common in clinical practice; their deficits are disabling and can be diagnosed accurately. The mechanisms underpinning such disorders are not well understood as examination demonstrates an intact voluntary motor system which paradoxically cannot be utilized on demand and in which the symptoms are perceived as involuntary.
Even for well-defined symptoms like motor CD the neural correlates are far from being understood. Brain areas in the lateral and medial frontal cortex as well as the supplementary motor area and basal ganglia have been suggested to be involved in this condition (for review see (Bell et al., 2011)). The diversity of the employed study paradigms like motor execution (Spence et al., 2000, Stone et al., 2007, van Beilen et al., 2011), Go/Nogo (Cojan et al., 2009), implicit (de Lange et al., 2007, de Lange et al., 2008) and explicit motor imagery (Burgmer et al., 2013, van Beilen et al., 2011) or vibratory stimulation (Burke et al., 2014, Vuilleumier et al., 2001) have provided a wide range of brain areas that could be involved in the clinical condition but did not isolate a core component. In addition the different and rather small sample sizes, the lack of control in motor imagery and motor execution paradigms in paretic patients as well as the heterogeneity of the included patients probably contributed to the divergent results.
The key question in this regard is whether the neural correlates of inhibition in patients with motor CD are different from those of normal subjects feigning the same symptoms. One study compared motor CD patients and feigning controls and found higher activity for intended execution in the supplementary motor area and a down regulation of the primary motor cortex contralateral in feigning controls (Stone et al., 2007). It remained however unclear whether this finding was due to motor inhibition itself or to the insufficient intention to move. Another study in two CD patients and two feigners found a down regulation of the right prefrontal cortex in feigners (Spence et al., 2000). Motor inhibition in a Go/Nogo-paradigm was also compared in feigners, controls and in one CD patient revealing higher activity in the right IFG in feigners (Cojan et al., 2009). Only one study directly compared motor CD patients, feigners and controls in a bigger sample size (van Beilen et al., 2011). This study employed motor execution und imagery and found during movement of the affected hand a complex pattern of activations including contralateral premotor cortex, anterior cingulate gyrus, superior temporal gyrus, the frontal operculum, dorsolateral frontal cortex, supramarginal gyrus and caudatus in feigners versus controls.
Importantly, no study so far trained the feigners in order to ensure a high quality of the feigning. This is an important aspect, given that the neural correlates of the feigning are to be compared to those of CD patients with a paresis. Therefore in the current approach the subjects took part to a structured training prior to the study. Before scanning two independent observers rated the quality of the feigned paresis without specifically bringing this into the attention of the subjects.
Neural activity was elicited by passive motor stimulation of the “paretic” extremity contrasted versus a rest condition. Passive movement presents a strong proprioceptive-kinaesthetic stimulus that is mostly independent of the concurrent paresis. It typically elicits activity in the sensorimotor network that is also active when the movement is voluntarily executed (Weiller et al., 1996). In this way we were able to use the same well-controlled stimulation setup that elicits robust activity in the network responsible for the control and execution of movements (Hassa et al., 2011). The hemodynamic activity elicited by passive movement of feigning subjects was compared to that of themselves in non-feigning condition and to that of motor CD patients.
2. Methods
2.1. Subjects
2.1.1. Healthy controls
The first measurement (controls in non-feigning condition, CN) of the study in 12 healthy controls was performed 3 years before the second. At that time the mean age of the controls was 39.0 ± 10.7 years with a range between 22 and 56 years. This time the mean age of the subjects was 42.5 ± 10.9 years. During this second measurement (controls in feigning condition, CF) the controls simulated a motor paresis of the right arm. None of the healthy controls had a history of neurological or psychiatric disease or neurological deficits. The controls were recruited across the staff of the rehabilitation hospital.
2.1.2. Patients
Thirteen patients (ten women, three men, with a mean age of 38.6 ± 11.0 years ranging from 21 to 51 years) with the symptom of a flaccid hemiparesis or a hemiparesis with an increase in muscle tone were included into the study. All patients were diagnosed with a conversion disorder according to DSM-IV criteria (Diagnostic and Statistical Manual of Mental Disorders, Version IV, 1994). In four patients the paresis was on the left side, in nine patients on the right side. The mean duration of symptoms was 83 weeks, with a range of 12–177 weeks (see Table 1). All patients underwent extensive neurological diagnostic procedures including MRI of brain and spinal cord, somatosensory evoked potentials, motor evoked potentials, peripheral nerve conduction examinations and EMG recordings. All diagnostic procedures did not reveal any pathological result. Patients with severe neurologic or psychiatric disorders including seizures, post-traumatic stress or panic disorder, major depression or other major affective or psychotic disorders were excluded from the study. However, patients with light forms of depression, stress and panic disorders were not excluded since they might be part of the conversion disorder. All patients were recruited in a rehabilitation hospital where they underwent rehabilitation therapy in a special psychosomatic medicine department.
Table 1.
Clinical data.
| Patients | Gender | Age | Side of paresis | Spastic/flaccid | Duration of symptoms (weeks) | Controls | Gender | Age at first fMRI measurement (normal condition) | Age at second fMRI measurement (feigning paresis) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 21 | r | f | 80 | 1 | F | 22 | 25 |
| 2 | F | 28 | l | s | 136 | 2 | F | 39 | 43 |
| 3 | F | 26 | r | f | 19 | 3 | F | 48 | 52 |
| 4 | F | 40 | r | f | 15 | 4 | M | 56 | 60 |
| 5 | F | 48 | l | s | 12 | 5 | F | 40 | 44 |
| 6 | F | 45 | r | f | 177 | 6 | F | 44 | 47 |
| 7 | F | 36 | r | f | 25 | 7 | F | 25 | 28 |
| 8 | F | 41 | r | s | 93 | 8 | M | 42 | 45 |
| 9 | M | 51 | l | f | 129 | 9 | F | 34 | 37 |
| 10 | M | 22 | r | f | 55 | 10 | M | 53 | 57 |
| 11 | M | 46 | r | f | 58 | 11 | M | 38 | 41 |
| 12 | F | 51 | r | s | 108 | 12 | F | 27 | 31 |
| 13 | F | 48 | l | f | 179 |
Notes: F: female, M: male; Age in years.
A board certified neurologist inspected the structural MRI of all subjects ensuring that no subject had any structural brain damage. The Ethical Committee of the University of Constance, approved the study and all participants gave written informed consent.
2.2. Feigning training
Healthy controls trained at least trice per day in a structured video and mental imagery training to feign a right arm paresis and documented the frequency and duration of the training sessions. They were informed about the goals of the study to ensure a convincing simulation. The 6-day training was performed 6–8 days before the fMRI scan.
2.3. Evaluation of the quality of simulation
The subjects maintained the feigned right arm paresis throughout the experiment (from entering the room until the end of the fMRI data acquisition) and were observed during pre-established situations before and in preparation for the MRI. In one situation the testing was explicit (positioning of the simulated paretic arm on a ball in lying position), while in seven other situations it was implicit: (e.g. lying down on the back, grasping the questionnaire). The subjects knew about the rating of the simulation but did not know when this would happen. The rating was performed by two trained investigators and documented on an analogue scale from 1 to 5 points for each of the eight situations. After the fMRI the participants completed two questionnaires. In the first questionnaire they evaluated the training, the second focussed on their estimation of the quality of feigning and the effort to maintain it.
2.4. fMRI design
The paradigm consisted of passive movements of both wrists. Subjects/patients were placed supine on the table of the MRI scanner with their head fixed in the head-holder of the MRI headcoil and their forearms were placed on cushions in comfortable position. Participants were instructed to relax and not to interfere voluntarily with the passive movements. This was trained outside the scanner before the experiment. An investigator performed passive flexion-extension movements of the wrist of 70–90° at a fixed rate of 1 Hz for 16 s paced by a visual signal (invisible to the subjects). The rest condition (rest) was interposed between the blocks and served as baseline for analysis. The 2 conditions (right hand; left hand) were intermixed in a pseudorandomized order and interspersed with the rest condition lasting alternately 8 and 16 s. Six blocks of each condition (24 blocks) were performed in two runs with total duration of 23.5 min. To maintain alertness the subjects were asked to count the number of small red square dots that were superimposed to a face per block and report them after the run. The planed fMRI comparisons were: passive movement of the right hand vs rest and passive movement of the left hand vs rest.
2.5. MRI data acquisition
Images were acquired on a 1.5 Tesla Philips Gyroscan (Philips Medical, Hamburg). Functional T2*-weighted echo echoplanar imaging (EPI) was performed (32 axial slices of 3.1 mm thickness with 1 mm gap, FOV of 230 × 230 mm, 80 × 80 matrix TR = 2.392 ms, TE = 40 ms, flip angle = 90°). A total of 295 volumes were acquired per session. The first five scans were discarded in order to account for T1 saturation effects resulting in 290 scans per session. A FLAIR sequence (21 axial slices of 5 mm thickness with 1 mm gap, FOV 250 × 250 mm, 512 × 512 matrix, TR = 11.000 ms, TE = 140 ms, flip angle = 90°) and a T1 sequence (21 axial slices of 6 mm thickness with no gap, FOV 250 × 250 mm, 512 × 512 matrix, TR = 139.22 ms, TE = 2.3 ms) were also acquired in order to exclude structural lesions.
2.6. fMRI data analysis
Statistical parametric mapping software (SPM5; Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/; (Friston et al., 1995) was used for fMRI data analysis. Images were realigned to the first image, spatially normalized using parameters obtained from normalization of the gray matter segments from the flair images to the reference system of the Montreal Neurological Institute's (MNI) segmented reference brain. Finally, the normalized functional data were smoothed with a 3-dimensional isotropic Gaussian kernel (12 mm full-width at half-maximum) to enhance signal-to-noise ratio and to account for residual differences in functional neuroanatomy among participants that persisted after normalization.
Bold effect was modeled with a box-car function convolved with the standard hemodynamic response function for the following block of events: passive movement of the unaffected hand (PMleft) and passive movement of the affected hand (PMright). All other events including emotional and visual stimuli like observing neutral and sad faces solely or in combination with passive movement of the right and left hand were modeled as conditions but were not analyzed. The regressors' coefficients for this voxel-based general linear model were estimated using a least squares approach (Friston et al., 1995) and correction for non-sphericity. The movement parameters obtained from realignment were included in the model as regressors of no interest.
The contrast images of each single subject with the two passive movement conditions (PMleft, PMright) were then entered into a random effects group analysis. The group CN (controls under normal conditions) was compared to CF (controls in feigning condition) using a paired t-test. Two sample t-tests were applied to analyze differences between the groups of conversion disorder patients (CONV) vs controls nonfeigning (CN) and vs controls feigning (CF). The contrast images of patients with paresis on the left side were flipped in that in all patients the hemisphere responsible for the paresis was the right one. Correction for multiple comparisons on the second level was performed using a whole brain (WBC) peak voxel threshold of p < 0.05, family wise error (FWE). In addition, small volume corrections (SVC) were performed in regions related to the supposed motor inhibition and sensorimotor control. In these regions of interest we employed bilateral masks of the IFG, globus pallidus and the supplementary motor area, the prefrontal midline areas and the insula from the automated anatomical labeling atlas (AAL) (Tzourio-Mazoyer et al., 2002). Activations were considered as significant if they survived p < 0.05 FWE corrected (SVC).
A region of interest (ROI) analysis was performed on the activation clusters revealed by the random effects group analysis for the passive movement of the right affected hand (see Table 2). Mean betas for the condition passive movement of the right hand were extracted from single subject data using marsBar (Poldrack, 2007) and submitted to an analysis of variance (ANOVA) with factor group (CN, CF and CONV).
Table 2.
Activation clusters for the comparisons between CD patients, feigners and controls.
| Contrast | Region | MNI coordinates |
kE | t-Values | p-Values (FWE, SVC) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Passive movement of the affected right hand | |||||||
| CF > CN | Right inferior frontal gyrus p. triangularis | 39 | 33 | 3 | 4 | 4.91 | 0.059 (SVC) |
| CN > CF | Left frontal superior med gyrus | − 6 | 54 | 6 | 27 | 7.89 | 0.001 (SVC)⁎⁎ |
| Right frontal superior med gyrus | 3 | 51 | 27 | 28 | 7.27 | 0.001 (SVC)⁎⁎ | |
| CONV > CN | Left inferior frontal gyrus p. triangularis | − 33 | 27 | 12 | 14 | 4.08 | 0.019 (SVC)⁎ |
| Adjacent to right inferior frontal gyrus p. triangularis | 39 | 24 | 21 | 4 | 3.75 | 0.061 (SVC) | |
| CONV > CF | Left inf frontal gyrus pars orbitalis | − 39 | 33 | − 6 | 6 | 4.32 | 0.038 (SVC)⁎ |
| Passive movement of the non-affected left hand | |||||||
| CF > CN | Left inferior frontal gyrus p. triangularis | − 39 | 18 | 21 | 23 | 7.29 | 0.001 (SVC)⁎⁎ |
| Left insula lobe | − 36 | 15 | 0 | 12 | 5.82 | 0.005 (SVC)⁎⁎ | |
CD = conversion disorder; MNI = Montreal Neurologic Institute; kE = cluster extent.
CF = controls feigning; CN = controls non-feigning; CONV = patients with hemilateral paresis; FWE = family wise error; SVC = small volume correction.
Regions were labelled according to the SPM anatomy toolbox; corresponding ROIs from AAL were used for the SVC.
Significant p values, p < 0.05.
Significant p values, p < 0.01.
3. Results
3.1. Behavioral data
3.1.1. Frequency and duration of the training
The mean frequency of training units per subject for the training period showed a high variance: mean 14.58 ± 12.32; range 6–49 (for VT: 4.5 ± 3.15; range 1–14; for MT: 10.08 ± 9.55; range 3–35). The mean duration of both types of training together was 104.09 min ± 31.53 min; range 50–155 per subject (for VT: 55.83 ± 20.98; range 5–80; for MT: 46.36 ± 22.92; range 5–80). 6 of 12 subjects had an overall training period over 100 min, 4 further subjects an overall training period over 75 min.
3.1.2. Quality of simulation
Two investigators rated the simulation quality in eight predefined situations on a scale of 1–5 points with a maximum score of 40 points. All subjects reached at minimum 29 points with a mean of 35.08 ± 3.28, indexing high simulation quality across subjects. After the fMRI session subjects rated the quality and the effort of the feigning in a questionnaire with 4 relevant items using a scale of 1–6 points resulting in a sum score of maximal 24 points (mean 16.42 ± 3.55; the item about maintaining the feigned paresis during the fMRI was rated with 4.42 ± 1.16 on a score of maximal 6 points). The subject's self-estimation was positively correlated with the investigators rating (+ 0.654, p = 0.021).
3.2. fMRI data
3.2.1. Feigning versus non-feigning in controls
The passive movement of the affected right hand of the feigning group (CF) was contrasted against the passive movement of the right hand of non-feigning controls (CN). Higher activation in the non-feigning compared to feigning condition (PMright: CN > CF) was observed in bilateral frontal midline areas (frontal superior medial gyrus, see Fig. 1; for MNI coordinates and t-values see Table 2). The inverse contrast for passive movement of the right hand (PMright: CF > CN) revealed an activation trend in the triangular part of the right inferior frontal gyrus (p = 0.059, see Table 2 and Fig. 2b).
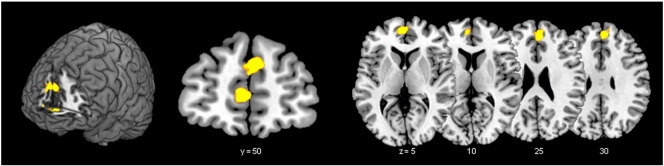
Fig. 1.
Activation during passive movement of the right hand in controls for the comparison non-feigning vs feigning condition [CN > CF]; (for the reverse contrast [CF > CN] see Fig. 2b). The activation cluster bilateral in medial prefrontal cortex showed a decrease of activation in the feigning condition.
Left superior medial frontal gyrus (SmFG): pFWE = 0.001 (SVC), t-value = 7.89; Right SmFG: pFWE = 0.001 (SVC), t-value = 7.27 (pFWE = family wise error corrected; SVC = small volume corrected). The numbers below the axial slices are the z-axis coordinates, the number below the coronal slices are y-axis coordinates in the MNI space.
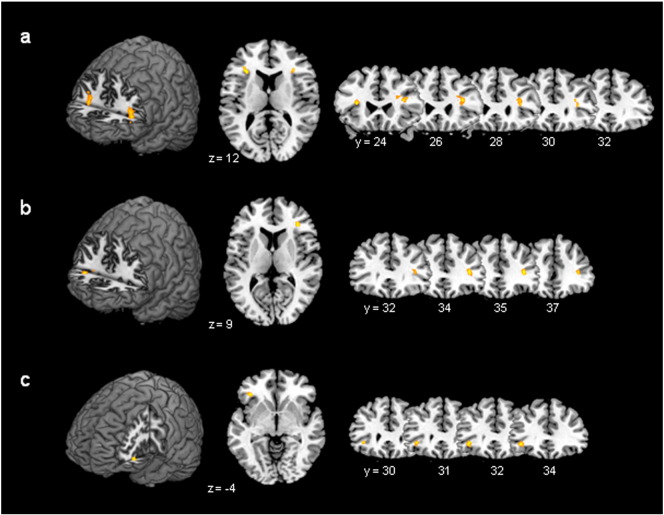
Fig. 2.
Activation during passive movement of the right hand in
a) CD patients vs controls non-feigning [CONV > CN]: bilateral cluster in the pars triangularis of the inferior fontal gyrus (IFG). Left IFG: pFWE = 0.019 (SVC), t-value = 4.08; Right IFG: pFWE = 0.061 (SVC), t-value = 3.75 (pFWE = family wise error corrected; SVC = small volume corrected).
b) Controls feigning vs non-feigning [CF > CN]: activation in the pars triangularis of the right IFG, pFWE = 0.059 (SVC), t-value = 4.91.
c) CD patients vs controls feigning [CONV > CF]: activation in the pars orbitalis of the left IFG, pFWE = 0.038 (SVC), t-value = 4.32.
The numbers below the axial slices are the z-axis coordinates, the number below the coronal slices are y-axis coordinates in the MNI space.
In response to the passive movement of the left (unaffected) hand, feigning subjects exhibited activations in the left insula and the pars triangularis of the left frontal inferior gyrus compared to the non-feigning condition (PMleft: CF > CN) (see Table 2). No activation was observed for the inverse contrast (PMleft: CN > CF).
3.2.2. Conversion disorder patients versus CF and CN
The hemodynamic activity of conversion disorder patients (CONV) was contrasted against that of CF and CN for passive movement of the affected right and the left non-affected hand (note that due to the flipping procedure in all patients the hemisphere responsible for the affected hand was the left hemisphere). The contrast of passive movement of the affected right hand in patients against non-feigning controls (PMright: CONV > CN) revealed an activation cluster in the triangular part of the left frontal inferior gyrus expanding to the left insula and an activation trend in the triangular part of the right inferior frontal gyrus (p = 0.061, see Fig. 2a and Table 2). The same contrast in patients against feigning controls (PMright: CONV > CF) also revealed activity in the left frontal inferior gyrus located more ventrally in the opercular part of the IFG (see Fig. 2c and Table 2). All other possible contrasts (PMright: CN > CONV and CF > CONV; PMleft: CONV > CN, CONV > CF, CN > CONV, CN > CF and CF > CONV) did not reveal any significant activation.
3.2.3. Activity in medial prefrontal cortex
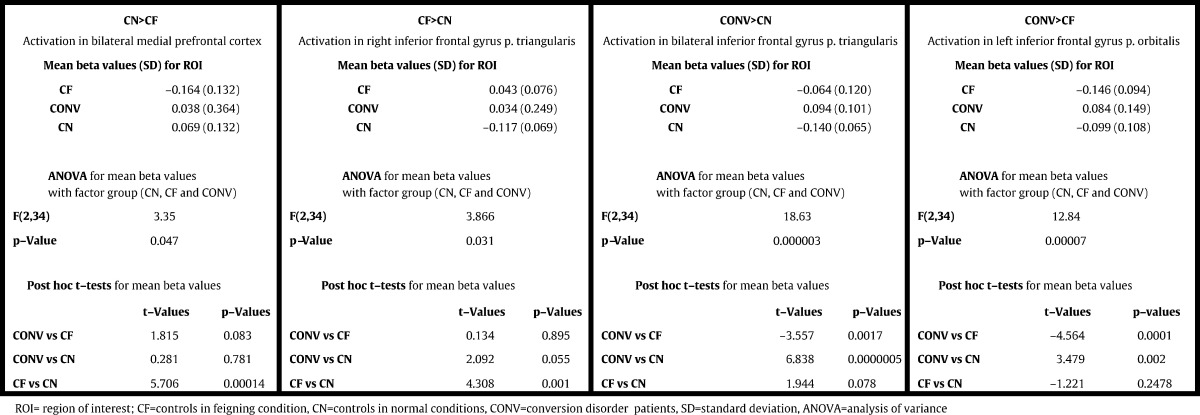
Healthy controls showed decreased activity in this region during feigning compared to non-feigning conditions. Remarkably, no significant activity differences could be observed in frontal midline regions for the comparisons patients vs healthy controls in feigning or non-feigning conditions. It was therefore reasonable to assume that activity in the medial prefrontal cortex in patients is in between those of non-feigning and feigning subjects. To substantiate this assumption we performed a region-of-interest analysis in this region. We observed a group effect for the cluster (F(2.34) = 3.35; p = 0.047). Subsequent post hoc t-tests revealed a significant difference between controls in feigning vs non-feigning conditions (T = 5.706, p = 0.00014). A t-test for patients vs controls in feigning condition (CONV vs CF) revealed a trend towards significance (T = 1.815, p = 0.083), while no group difference (T = 0.281, p = 0.781) was observed between patients and controls in non-feigning condition (CONV vs CN). In sum, the activity level of the patients in medial prefrontal cortex was higher than that of controls in feigning and lower than that in non-feigning conditions (see Fig. 3a and Table 3). The inverse contrast (CF > CN) revealed an activation trend in the triangular part of the right IFG; the region-of-interest analysis of this cluster revealed a comparable pattern: the group effect was significant (F(2.34) = 3.866; p = 0.031); in the subsequent post hoc t-tests we observed a significant difference for CF vs CN (T = 4.308, p = 0.001) and a strong trend for CONV vs CN (T = 2.092, p = 0.055) (for details see Fig. 3c and Table 3).
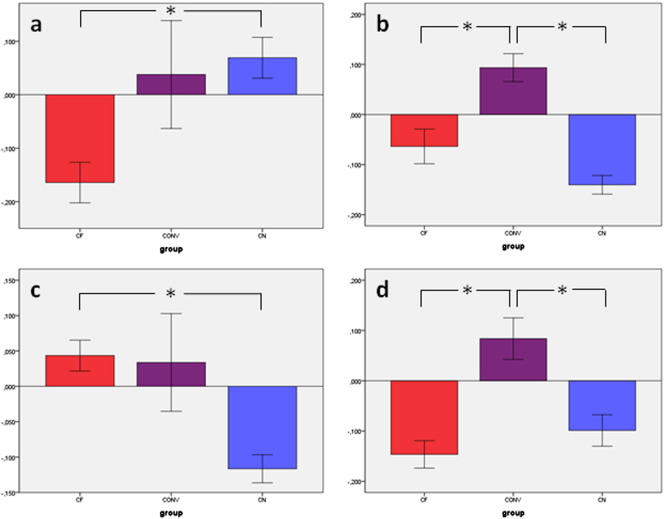
Fig. 3.
a) Mean beta values of region of interest (ROI) analysis for the activation cluster in medial prefrontal cortex (mPFC) resulting from the contrast passive movement of the affected right hand: controls for non-feigning vs feigning condition [CN > CF; see also Fig. 1]. Mean beta values for the groups: controls feigning (CF), conversion disorder patients (CONV) and non-feigning controls (CN) with standard error of mean (SEM)
b) Mean beta values of the region of interest (ROI) analysis for the activation clusters in bilateral IFG [CONV > CN, see also Fig. 2 a] during passive movement of the (affected) right hand: controls feigning (CF), conversion disorder patients (CONV) and non-feigning controls (CN) with standard error of mean (SEM)
c) Mean beta values of the region of interest (ROI) analysis for the activation cluster in the right IFG [CF > CN, see also Fig. 2 b] during passive movement of the (affected) right hand: controls feigning (CF), conversion disorder patients (CONV) and non-feigning controls (CN) with standard error of mean (SEM)
d) Mean beta values of the region of interest (ROI) analysis for the activation clusters in left IFG [CONV > CF, see also Fig. 2 c] during passive movement of the (affected) right hand: controls feigning (CF), conversion disorder patients (CONV) and non-feigning controls (CN) with standard error of mean (SEM).
Table 3.
ROI analysis
ROI analysis of activation clusters for the comparisons between CD patients, feigners and controls (passive movement of the affected right hand)
The region-of-interest analysis of the activation clusters for passive movement of the right hand for CD patients versus controls in non-feigning (bilateral inferior frontal gyrus for CONV > CN; see Figs. 2a and 3b) and feigning conditions (left inferior frontal gyrus pars orbitalis for CONV > CF; see Figs. 2c and 3d) revealed positive mean beta values for the patients and negative mean beta values for both control groups. We observed a group effect for both clusters (CONV > CN: F(2.34) = 18.63; p = 0.000003; for CONV > CF: F(2.34) = 12.84; p = 0.00007). Subsequent post hoc t-tests showed a significant difference for patients versus controls in feigning or non-feigning conditions (for details see Table 3).
4. Discussion
The present study investigated the neural basis for motor inhibition in patients with motor conversion disorder causing a paresis and compared these to those of healthy controls who did or did not feign a similar paresis. Importantly, conversion disorder and feigning subjects exhibited motor-inhibition related neural activity in neighboring but different lateral inferior frontal regions. The activations were bilateral, stronger and somewhat more dorsal in patients compared to feigning controls arguing that the neural correlates of conversion disorder-related motor inhibition of the paretic hand arise from similar but not from exactly the same neural ensembles of the IFG. An important decrease in the hemodynamic activity of the bilateral superior frontal medial gyrus was observed when feigning were compared to non-feigning subjects. No differences in these medial frontal regions were observed when CD patients were compared to feigning and to non-feigning subjects indicating that patient's activation level in these regions were in-between the levels of feigning and non-feigning controls.
In comparison to non-feigning controls CD patients exhibited higher activity in the triangular part of the left inferior frontal gyrus (IFG) reaching into the insula and a trend towards higher activity in the triangular part of the right IFG during passive movement of the paretic hand. These areas were previously shown to be strongly associated with motor inhibition in several tasks (Brass and Haggard, 2007, Chikazoe et al., 2009, Forstmann et al., 2008, Hampshire et al., 2010, Leung and Cai, 2007, Verbruggen and Logan, 2009). In the context of Go/Nogo-tasks the IFG was suggested to be also related to attentional processes rather than to the motor inhibition process itself (for reviews see (Chambers et al., 2009, Criaud and Boulinguez, 2013, Simmonds et al., 2008)). The IFG is part of the ventral attention system (Corbetta and Shulman, 2002) but also part of the motor inhibition network (Aron et al., 2014). Both attention and motor inhibition processes are triggered during passive movement of the hand. The movement itself might capture attention in an exogenous way and motion inhibition processes are required in order to prevent movement execution processes triggered by the unexpected proprioceptive input. It is important to note that in the direct contrast between CD patients and feigning controls passive movement of the affected right hand also evoked activity in left IFG, but this activity was located more ventral in the orbital part. This finding underlines the importance of the IFG in these conditions and argues for a role of this brain area in monitoring motor inhibition. Furthermore, the findings of the present study suggest that the neural correlates of motor inhibition in CD patients and feigning controls are located in the same brain regions, namely in the IFG with slight differences in location and laterality. This is well in line with the wide body of literature on motor inhibition (Aron and Poldrack, 2006, Chambers et al., 2006, Garavan et al., 1999, Hirose et al., 2009, Menon et al., 2001, Rubia et al., 2001, Swick et al., 2008).
A further important aspect of motor inhibition in the current study was that the motor inhibition in CD patients as well as in feigning subjects was maintained over a long period of time and control subjects especially trained in order to gain this ability. In contrast to several studies investigating mostly phasic response inhibition, the motor inhibition in this study had to be of tonic nature and was maintained by an unconscious process in CD patients or a voluntary process in controls. Recently (Aron et al., 2014) it was suggested that the inhibition function of the right inferior cortex can also be triggered by unconscious stimuli (van Gaal et al., 2010) and that the IFG could provide tonic motor suppression (Berman et al., 2012), which would be in full agreement with the current results.
However, not all studies in CD patients agree on the role of the IFG in motor inhibition. Cojan and colleagues used Go/Nogo-Task to examine a motor CD patient and compared the results with those of controls feigning and non-feigning a paresis (Cojan et al., 2009). In contrast to controls, who activated the IFG during inhibition the CD patient rather exhibited hemodynamic activity in left superior frontal gyrus and the precuneus. On the other hand, several imaging studies consistently observed activity in the IFG of CD patients with motor symptoms (Stone et al., 2007), sensory (Mailis-Gagnon et al., 2003), visual loss (Werring et al., 2004) or amnestic disorders (Kanaan et al., 2007, Markowitsch et al., 1997). Depending on the employed experimental paradigms such as attempted movement execution (Marshall et al., 1997), movement observation (Burgmer et al., 2006), movement inhibition (Cojan et al., 2009), mental rotation (de Lange et al., 2010) or vibratory stimulation (Burke et al., 2014, Vuilleumier et al., 2001) studies have described several different neural structures to be involved or abnormally active in CD. Some of the differences could also steam from the type of paresis (flaccid or with muscle tone increase) of the CD patients that might have different neural correlates due to different levels of inhibition. The current study focused on CD patients with motor paresis and employed passive movement stimulation in a larger group of patients. Since at the level of motor excitability similar patterns of results were found independent of the type of paresis (flaccid or spastic) (Liepert et al., 2009) we included all patients into the study. The current results are well in line with the literature and provide strong evidence for a major role of the IFG in motor inhibition in this patient group.
Another important brain area in this context is the medial frontal cortex. The activity in this area was modulated as a function of whether the controls were feigning a paresis or not. Importantly, in the feigning condition the activity was lower than in the non-feigning one when the affected hand was passively moved. Previous work has shown that the medial prefrontal cortex distinguishes mental state from physical state representations (Frith and Frith, 2003) and is also part of the default network for self-referential processing (Zhang and Li, 2012). In a meta-analysis of 104 imaging studies activations in this region belonging to Brodmann Area 10 were associated with mentalizing (Gilbert et al., 2006). The medial prefrontal cortex (mPFC), however, also mediates internal directed cognition (Dixon et al., 2014) and has a role in self referencing (Philippi et al., 2012). This region is involved in processing of self-generated vs externally generated items and in the discrimination of imagined vs perceived stimuli. One study (Simons et al., 2008) described activity in the ventral mPFC related to the recollection of self-status. The same region exhibited activity when subjects were required to recollect source information for self-generated stimuli (Turner et al., 2008). Furthermore the mPFC mediates the attribution of emotions to self, self-judgements (Ochsner et al., 2004), processes related to self-reflections (Johnson et al., 2002) and the perception of self-agency (Amodio and Frith, 2006, Miele et al., 2011, Renes et al., 2015). Taking all this evidence together, the mPFC appears to monitor self-referential processes and reflects the experience of self-agency (David et al., 2006). In the context of the current study the activity in this region would most likely be referred to self-agency in healthy subjects with higher activity during the non-feigning condition. Importantly, no differences in activity could be found between controls and CD patients.
The region-of-interest analysis confirmed that CD patients exert a mid-level activity that is lower than that of controls during non-feigning but higher than during feigning. Although controls extensively trained to feign and maintain the paresis the patients can be regarded as experts in this matter. While healthy subjects show higher activity in the mPFC during passive movement in the non-feigning condition indexing a high sense of agency that decreases during feigning, CD patients displayed a mid-level activation most likely suggesting a minor degree of change concerning the sense of agency due to their increased and longer-lasting experience with the paresis.
In summary, passive movement of the paretic hand elicited activity in similar but different regions of the inferior frontal gyrus associated with motor inhibition in feigning controls and conversion disorder patients. Furthermore we observed a mid-level medial prefrontal cortex activation in patients compared to feigning and non-feigning healthy subjects arguing for an altered sense of agency in conversion disorder patients.
Acknowledgments
Funding/Support: This study was funded by the Stiftung Schmieder für Wissenschaft und Forschung.
Contributors
The study was designed by TH, OT, RS and MAS. Literature search and drafting of the report were performed by TH, RS and MAS. Data collection was performed by TH, EdJ, OT and RS. Data analysis and/or interpretation were performed by TH, EdJ, OT, RS and MAS. Statistical analysis was performed by TH, OT, RS and MAS. All authors reviewed the final version of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethical Committee of the University of Constance.
Conflict of financial disclosures: None.
References
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. 2014. Inhibition and the Right Inferior Frontal Cortex: One Decade on. (Trends Cogn Sci) [DOI] [PubMed] [Google Scholar]
- Bell V., Oakley D.A., Halligan P.W., Deeley Q. Dissociation in hysteria and hypnosis: evidence from cognitive neuroscience. J. Neurol. Neurosurg. Psychiatry. 2011;82:332–339. doi: 10.1136/jnnp.2009.199158. [DOI] [PubMed] [Google Scholar]
- Berman B.D., Horovitz S.G., Morel B., Hallett M. Neural correlates of blink suppression and the buildup of a natural bodily urge. NeuroImage. 2012;59:1441–1450. doi: 10.1016/j.neuroimage.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M., Haggard P. To do or not to do: the neural signature of self-control. J. Neurosci. 2007;27:9141–9145. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmer M., Konrad C., Jansen A., Kugel H., Sommer J., Heindel W., Ringelstein E.B., Heuft G., Knecht S. Abnormal brain activation during movement observation in patients with conversion paralysis. NeuroImage. 2006;29:1336–1343. doi: 10.1016/j.neuroimage.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Burgmer M., Kugel H., Pfleiderer B., Ewert A., Lenzen T., Pioch R., Pyka M., Sommer J., Arolt V., Heuft G., Konrad C. The mirror neuron system under hypnosis — brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437–445. doi: 10.1016/j.cortex.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Burke M.J., Ghaffar O., Staines W.R., Downar J., Feinstein A. Functional neuroimaging of conversion disorder: the role of ancillary activation. Neuroimage Clin. 2014;6:333–339. doi: 10.1016/j.nicl.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catonne J.P. Hippocratic concept of hysteria. Ann. Med. Psychol. (Paris) 1992;150:705–719. [PubMed] [Google Scholar]
- Chambers C.D., Bellgrove M.A., Stokes M.G., Henderson T.R., Garavan H., Robertson I.H., Morris A.P., Mattingley J.B. Executive "brake failure" following deactivation of human frontal lobe. J. Cogn. Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Chambers C.D., Garavan H., Bellgrove M.A. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci. Biobehav. Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chikazoe J., Jimura K., Asari T., Yamashita K., Morimoto H., Hirose S., Miyashita Y., Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb. Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Cojan Y., Waber L., Carruzzo A., Vuilleumier P. Motor inhibition in hysterical conversion paralysis. NeuroImage. 2009;47:1026–1037. doi: 10.1016/j.neuroimage.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Criaud M., Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci. Biobehav. Rev. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- David N., Bewernick B.H., Cohen M.X., Newen A., Lux S., Fink G.R., Shah N.J., Vogeley K. Neural representations of self versus other: visual-spatial perspective taking and agency in a virtual ball-tossing game. J. Cogn. Neurosci. 2006;18:898–910. doi: 10.1162/jocn.2006.18.6.898. [DOI] [PubMed] [Google Scholar]
- de Lange F.P., Roelofs K., Toni I. Increased self-monitoring during imagined movements in conversion paralysis. Neuropsychologia. 2007;45:2051–2058. doi: 10.1016/j.neuropsychologia.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lange F.P., Roelofs K., Toni I. Motor imagery: a window into the mechanisms and alterations of the motor system. Cortex. 2008;44:494–506. doi: 10.1016/j.cortex.2007.09.002. [DOI] [PubMed] [Google Scholar]
- de Lange F.P., Toni I., Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 2010;48:1782–1788. doi: 10.1016/j.neuropsychologia.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Dixon M.L., Fox K.C., Christoff K. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia. 2014;62:321–330. doi: 10.1016/j.neuropsychologia.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Forstmann B.U., Jahfari S., Scholte H.S., Wolfensteller U., van den Wildenberg W.P., Ridderinkhof K.R. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J. Neurosci. 2008;28:9790–9796. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Poline J.B., Grasby P.J., Williams S.C., Frackowiak R.S., Turner R. Analysis of fMRI time-series revisited. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Stein E.A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.J., Spengler S., Simons J.S., Steele J.D., Lawrie S.M., Frith C.D., Burgess P.W. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J. Cogn. Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa T., Schoenfeld M.A., Dettmers C., Stoppel C.M., Weiller C., Lange R. Neural correlates of somatosensory processing in patients with neglect. Restor. Neurol. Neurosci. 2011;29:253–263. doi: 10.3233/RNN-2011-596. [DOI] [PubMed] [Google Scholar]
- Hirose S., Chikazoe J., Jimura K., Yamashita K., Miyashita Y., Konishi S. Sub-centimeter scale functional organization in human inferior frontal gyrus. NeuroImage. 2009;47:442–450. doi: 10.1016/j.neuroimage.2009.04.094. [DOI] [PubMed] [Google Scholar]
- Johnson S.C., Baxter L.C., Wilder L.S., Pipe J.G., Heiserman J.E., Prigatano G.P. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kanaan R.A., Craig T.K., Wessely S.C., David A.S. Imaging repressed memories in motor conversion disorder. Psychosom. Med. 2007;69:202–205. doi: 10.1097/PSY.0b013e31802e4297. [DOI] [PubMed] [Google Scholar]
- Leung H.C., Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J. Neurosci. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J., Hassa T., Tuscher O., Schmidt R. Abnormal motor excitability in patients with psychogenic paresis. A TMS study. J. Neurol. 2009;256:121–126. doi: 10.1007/s00415-009-0090-4. [DOI] [PubMed] [Google Scholar]
- Mailis-Gagnon A., Giannoylis I., Downar J., Kwan C.L., Mikulis D.J., Crawley A.P., Nicholson K., Davis K.D. Altered central somatosensory processing in chronic pain patients with "hysterical" anesthesia. Neurology. 2003;60:1501–1507. doi: 10.1212/wnl.60.9.1501. [DOI] [PubMed] [Google Scholar]
- Markowitsch H.J., Calabrese P., Fink G.R., Durwen H.F., Kessler J., Harting C., Konig M., Mirzaian E.B., Heiss W.D., Heuser L., Gehlen W. Impaired episodic memory retrieval in a case of probable psychogenic amnesia. Psychiatry Res. 1997;74:119–126. doi: 10.1016/s0925-4927(97)03041-2. [DOI] [PubMed] [Google Scholar]
- Marshall J.C., Halligan P.W., Fink G.R., Wade D.T., Frackowiak R.S. The functional anatomy of a hysterical paralysis. Cognition. 1997;64:B1–B8. doi: 10.1016/s0010-0277(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. Error-related brain activation during a Go/No-Go response inhibition task. Hum. Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele D.B., Wager T.D., Mitchell J.P., Metcalfe J. Dissociating neural correlates of action monitoring and metacognition of agency. J. Cogn. Neurosci. 2011;23:3620–3636. doi: 10.1162/jocn_a_00052. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Knierim K., Ludlow D.H., Hanelin J., Ramachandran T., Glover G., Mackey S.C. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Philippi C.L., Duff M.C., Denburg N.L., Tranel D., Rudrauf D. Medial PFC damage abolishes the self-reference effect. J. Cogn. Neurosci. 2012;24:475–481. doi: 10.1162/jocn_a_00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renes R.A., van Haren N.E., Aarts H., Vink M. An exploratory fMRI study into inferences of self-agency. Soc. Cogn. Affect. Neurosci. 2015;10:708–712. doi: 10.1093/scan/nsu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., Simmons A., Williams S.C., Giampietro V., Andrew C.M., Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Pekar J.J., Mostofsky S.H. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J.S., Henson R.N., Gilbert S.J., Fletcher P.C. Separable forms of reality monitoring supported by anterior prefrontal cortex. J. Cogn. Neurosci. 2008;20:447–457. doi: 10.1162/jocn.2008.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S.A., Crimlisk H.L., Cope H., Ron M.A., Grasby P.M. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000;355:1243–1244. doi: 10.1016/S0140-6736(00)02096-1. [DOI] [PubMed] [Google Scholar]
- Stone J., Carson A., Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J. Neurol. Neurosurg. Psychiatry. 2005;76(Suppl. 1):i2–12. doi: 10.1136/jnnp.2004.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Zeman A., Simonotto E., Meyer M., Azuma R., Flett S., Sharpe M. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom. Med. 2007;69:961–969. doi: 10.1097/PSY.0b013e31815b6c14. [DOI] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.S., Simons J.S., Gilbert S.J., Frith C.D., Burgess P.W. Distinct roles for lateral and medial rostral prefrontal cortex in source monitoring of perceived and imagined events. Neuropsychologia. 2008;46:1442–1453. doi: 10.1016/j.neuropsychologia.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Beilen M., de Jong B.M., Gieteling E.W., Renken R., Leenders K.L. Abnormal parietal function in conversion paresis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaal S., Ridderinkhof K.R., Scholte H.S., Lamme V.A. Unconscious activation of the prefrontal no-go network. J. Neurosci. 2010;30:4143–4150. doi: 10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Chicherio C., Assal F., Schwartz S., Slosman D., Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–1090. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- Weiller C., Juptner M., Fellows S., Rijntjes M., Leonhardt G., Kiebel S., Muller S., Diener H.C., Thilmann A.F. Brain representation of active and passive movements. NeuroImage. 1996;4:105–110. doi: 10.1006/nimg.1996.0034. [DOI] [PubMed] [Google Scholar]
- Werring D.J., Weston L., Bullmore E.T., Plant G.T., Ron M.A. Functional magnetic resonance imaging of the cerebral response to visual stimulation in medically unexplained visual loss. Psychol. Med. 2004;34:583–589. doi: 10.1017/S0033291703008985. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li C.S. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum. Brain Mapp. 2012;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]