Abstract
There have been concerns that systemic corticosteroid use is associated with pregnancy-induced hypertension (PIH) and diabetes mellitus. However, the relationship between inhaled corticosteroids (ICSs) and the risk of PIH has not been fully examined, and there was no study investigating the association between ICS use and the development of gestational diabetes mellitus (GDM). The aims of the study are to determine whether the use of ICSs during pregnancy increases the risk of PIH and GDM in women.
We conducted 2 nested case-control studies utilizing the nationwide insurance claims database of the Health Insurance Review and Assessment Service (Seoul, Republic of Korea), in which 1,306,281 pregnant women who delivered between January 1, 2009 and December 31, 2011 were included. Among them, PIH cases and GDM cases were identified and matched controls were included. Conditional logistic regression analyses adjusted by other concomitant drugs use during and before pregnancy and confounding covariates including comorbidities were performed.
Total 43,908 PIH cases and 219,534 controls, and 34,190 GDM cases and 170,934 control subjects were identified. When other concomitant drugs use during pregnancy was adjusted, ICS use was associated with an increased rate of PIH (adjusted odds ratio, 1.40 [95% CI, 1.05–1.87]). ICS medication possession ratios and cumulative doses were associated with an increased risk of PIH. However, the statistical significance was not found in other models. In both unadjusted and adjusted multivariable models, ICSs use was not associated with increase in the risk of GDM.
ICSs use is not associated with an increased risk of PIH and GDM.
INTRODUCTION
Drug safety is an important issue during pregnancy. Corticosteroid is one of the drugs that require a special caution for pregnant women to use. There have been concerns that systemic corticosteroid use is associated with worsened pregnancy outcomes including pregnancy-induced hypertension (PIH).1,2 Hypertension disorders occur in 6% to 8% of pregnancies and contribute significantly to perinatal morbidity and mortality.2,3 The causes of most PIH cases, however, remain unknown.2,3 Systemic administration of glucocorticoids increases insulin resistance by reducing glucose transport 4 expression levels and attenuating cell migration,4 and decreases glycogen synthetase levels,5–8 which leads to glucose intolerance and type 2 diabetes mellitus (DM).9–11 Gestational DM (GDM) is a significant medical problem during pregnancy.12–15 GDM shares not only characteristics with type 2 DM such as insulin resistance but also risk factors, and the majority of women with GDM eventually develop DM after pregnancy.16 There are concerns that the use of systemic corticosteroids is associated with GDM.17
Inhaled corticosteroids (ICSs) are delivered to the airways and lungs through respiratory devices. Delivering corticosteroids through inhalation devices is generally thought to be safe in terms of systemic side effects. However, some of the drugs can reach the pulmonary parenchymal tissue and enter the systemic circulation.18 In fact, studies have reported that long-term use of ICSs may result in adrenal suppression and possibly adrenal crisis when stopped.19,20
For the reasons, there have been concerns that ICS use may be related to PIH development because systemic corticosteroids are associated with PIH.1,2 However, there have been only a few studies investigating the relationship between ICS use and the risk of PIH.1,2,21,22 In addition, these studies have several limitations, including small numbers of cases with PIH22 or only including women with asthma which is a risk factor of PIH,23 which could attenuate the effect of ICSs on PIH. ICSs can be used for other purposes, including as a trial for postinfectious cough24 and treatment of nonasthmatic eosinophilic bronchitis.25 There were several studies reporting that systemic corticosteroid administration in pregnant women at risk of impending preterm delivery to enhance fetal lung maturation increased maternal serum glucose levels,26,27 and that the use of systemic corticosteroids is associated with GDM.17 However, there were few studies that investigated the association between ICS use and GDM.
The purpose of this study was to determine whether or not the use of ICS during pregnancy would increase the risk of PIH and GDM in women with or without asthma through 2 nested case-control studies utilizing a nationwide database.
METHODS
Data Source
We analyzed the database of the Health Insurance Review and Assessment Service (HIRA; Seoul, Republic of Korea), a government agency examining the accuracy of claims for National Health Insurance (NHI) and National Medical Aid in Korea. NHI covers 96.6% of 48.6 million Koreans28 and the reliability and high quality of the HIRA database is highlighted by the large number of articles that have utilized the data.28–32 HIRA database includes demographic variables and all medical services rendered, along with diagnostic codes (International Statistical Classification of Diseases and Related Health Problems, 10th edition, ICD-10) and all prescribed medications. The protocol of this study was approved by the Institute of review Board of the National Evidence-Based Healthcare Collaborating Agency, Seoul, Republic of Korea. Written consent for participants was waived because the study had a retrospective design.
Subject
The cohort included 1,306,281 pregnant women who delivered between January 1, 2009, and December 31, 2011.
We performed 2 nested case-control studies using this cohort. For the first study examining the effects of ICS on PIH, we excluded 13,633 women who were prescribed antihypertensive drugs for 30 days or longer periods either within 1 year prior to pregnancy or 4 to 12 months after delivery from analysis.2 Antihypertensive drugs included β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and calcium-channel blockers. Thus, 1,293,648 pregnant women who had no history of hypertension were included in the first case-control study. We defined 43,908 pregnancy-induced hypertension cases and 219,534 matched controls from this cohort.
For the second phase of analysis determining the effects of ICS on GDM, we excluded 37,349 women who were either diagnosed with DM (ICD-10 code E10-14) or prescribed any anti-DM drugs for 30 days or longer either within 1 year before pregnancy or within 1 year after delivery. Anti-DM drugs included sulfonylurea, metformin, meglitinide, α-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase-4(DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) analogues, and insulin. Thus, 1,268,932 pregnant women who had no history of DM were included in the second phase of the analysis. We defined 34,190 GDM cases and 170,934 matched controls (to a ratio of 1:5) from this cohort (Figure 1).
FIGURE 1.
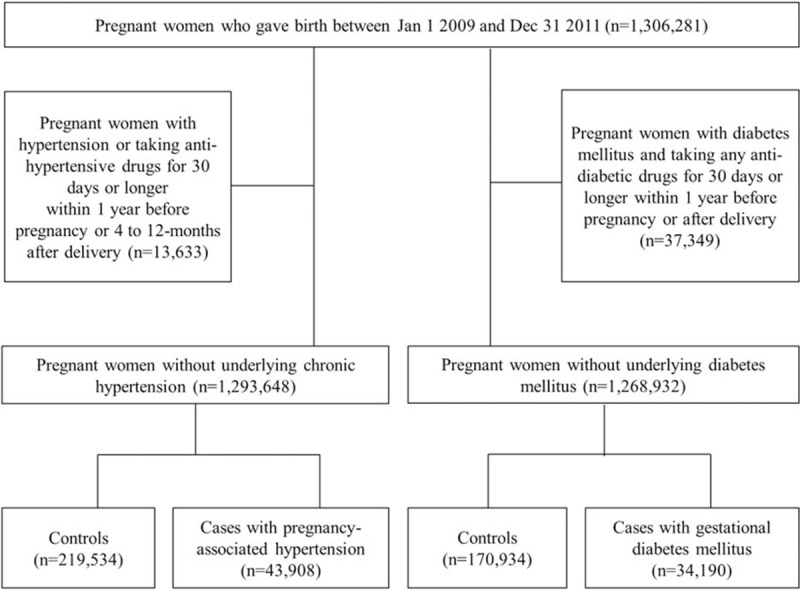
Flowchart of patient selection.
Definition of Cases and Controls
We defined PIH cases as those with ICD-10 code O11–O15 during pregnancy. Controls without PIH were randomly assigned (up to 1:5) matching age (±5 years), baby birth date, ICS use before pregnancy, presence of DM, and presence of asthma during or within 1 year before pregnancy. We defined GDM cases as those with ICD-10 code O24.4 during pregnancy. Controls without GDM matching age (±5 years), baby birth date, ICS use before pregnancy, and/or asthma during or within 1 year before pregnancy were randomly selected up to 5 times of GDM cases. The index date was defined as the date of first assignment of the ICD-10 code. The index date for the controls was defined as the index date for their matching cases.
Exposure to Respiratory Medications Including Inhaled Drugs
Respiratory drugs included ICSs (beclomethasone, budesonide, triamcinolone, ciclesonide, fluticasone, or flunisolide), short-acting inhaled β2-agonists (SABAs; salbutamol, fenoterol, procaterol, or terbutaline), long-acting inhaled β2-agonists (LABAs; salmeterol or formoterol), long-acting inhaled muscarinic antagonists (LAMAs; tiotropium), a combination of ICS and LABA (fluticasone/salmeterol or budesonide/formoterol), systemic corticosteroids (hydrocortisone, cortisone, prednisone, prednisolone, methylprednisolone, triamcinolone, betamethasone, or dexamethasone), and leukotriene receptor antagonists (LTRAs; montelukast, pranlukast, or zafirlukast). Respiratory drug use was defined as a prescription for 30 days or longer period. ICS cumulative doses were calculated as the sum of the prescribed doses converted as fluticasone equivalents. The equivalent doses for inhaled corticosteroids were 100 μg beclomethasone, 50 μg beclomethasone, 80 μg budesonide, 200 μg triamcinolone, 32 μg ciclesonide, 50 μg fluticasone, and 200 μg flunisolide.28,30,33 ICS medication possession ratios (MPRs) were calculated as the total days of prescription for inhaled corticosteroids divided by the 180 days.
Covariates
To investigate the association between ICSs and the development of PIH or GDM, we adjusted age, each disease-associated comorbidity, other concomitant respiratory medication uses during and before pregnancy and healthcare utilization. PIH-associated comorbidities included PIH in the prior pregnancy, chronic kidney disease (ICD-10 code N18 or undergoing dialysis), antiphospholipid antibody syndrome or inherited thrombophilia (ICD-10 code D68.5 or D68.6), vascular or connective tissue diseases (ICD-10 code M05–60 or M32), hydrops fetalis (ICD-10 code P56, P83.2, or O36.2), unexplained fetal growth restriction (ICD-10 code P05–08), hydatidiform mole (ICD-10 code Z35.1, D39.2, or O01 or procedure for hydatidiform mole), and multiple gestation. GDM-associated comorbidities were preeclampsia in the prior pregnancy, prior history of pregnancy, history of GDM(O24) during a previous pregnancy, history of pregnancy-induced hypertension (O11–O15) during a previous pregnancy, history of impaired glucose metabolism (R73), history of hypertension (I10–I15), history of antihypertensive drug use, history of dyslipidemi (E780, 786, 789), and history of polycystic ovary syndrome (PCOS). Concomitant medication use included LTRAs, systemic corticosteroids, and SABAs used during (within 180 days prior to the index date) and before pregnancy (within 180 days prior to pregnancy). Healthcare utilization included visits to obstetricians and visits to pulmonologists during pregnancy.
Statistical Methods
To describe the baseline characteristics of cases and controls, statistics including proportions, medians, and interquartile ranges were used. We also summarized continuous variables into the appropriate categorical variables based on their distributions. Statistical significances were derived from independent t tests for continuous variables and X2 tests for categorical variables. To investigate the association between ICSs and the development of PIH and GDM, conditional logistic regression analyses were performed. Confounding covariates that showed statistically significant differences between cases and controls and the use of respiratory medications were also included in the multivariable models. Likelihood ratio tests were used to examine the goodness of fit of the model, and no significant lack of fit was found. To explore the dose–response relationship, we initially divided the distribution of the cumulative inhaled corticosteroid dose into <15,000 μg or ≥15,000 μg. Adjusted odds ratios (aOR) were presented with 95% confidence intervals (CI) and P values. A P value of less than 0.05 was regarded as statistically significant, and all statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC).
RESULTS
Effects of ICSs on PIH
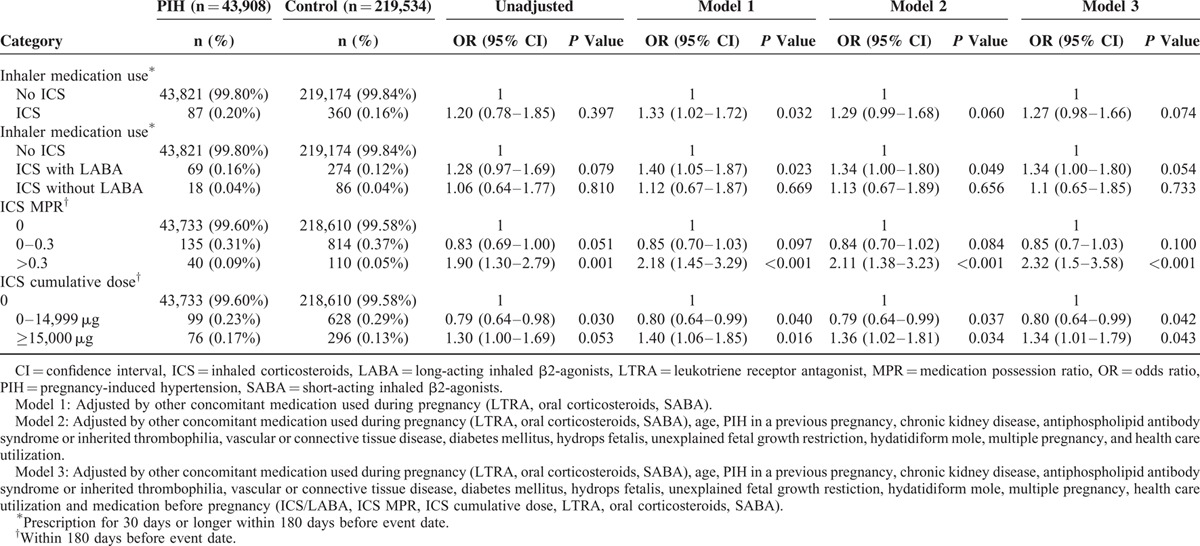
Table 1 shows the characteristics of cases and controls. Table 2 shows the respiratory medications used by cases and controls. Eighty-seven pregnant women were prescribed respiratory drugs. PIH patients exhibited higher ICS MPRs, higher ICS cumulative dose during pregnancy, and higher ICS cumulative dose within 6 months before pregnancy compared with controls. ICS use within 180 days before the index date was significantly associated with increased odds of PIH (model 1: aOR, 1.33% [CI, 1.02–1.72]) after adjustment for other concomitant respiratory medication during pregnancy (Table 3). The statistical significance was no longer present in the analysis adjusting for other concomitant respiratory medication use during pregnancy, age, associated comorbidities, healthcare utilization, and respiratory medication use before pregnancy (model 3). ICS MPRs >0.3 and ICS cumulative doses over 15,000 μg were also significantly associated with an increased risk of PIH, which remained statistically significant in model 3.
TABLE 1.
Baseline Characteristics of Cases and Matched Controls
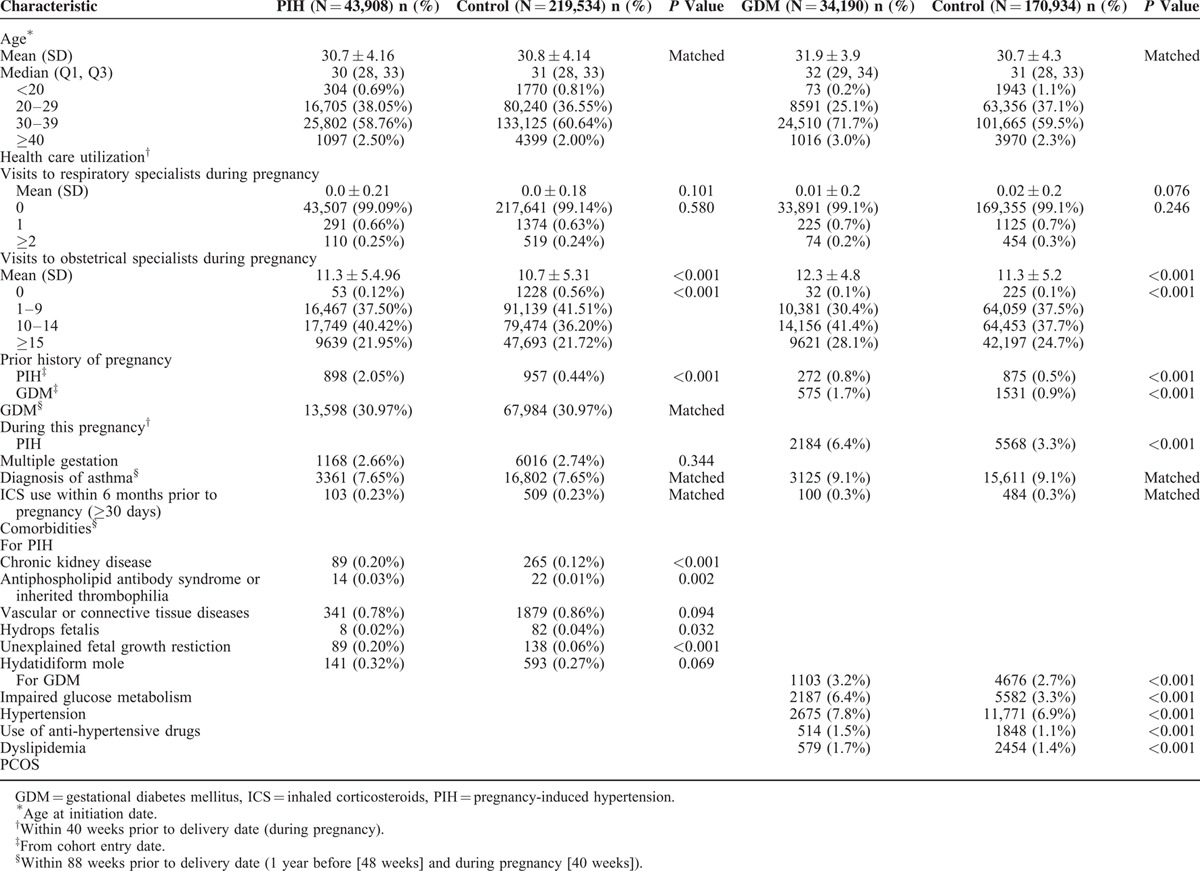
TABLE 2.
Comparison of Respiratory Medication Use Between Cases and Matched Controls
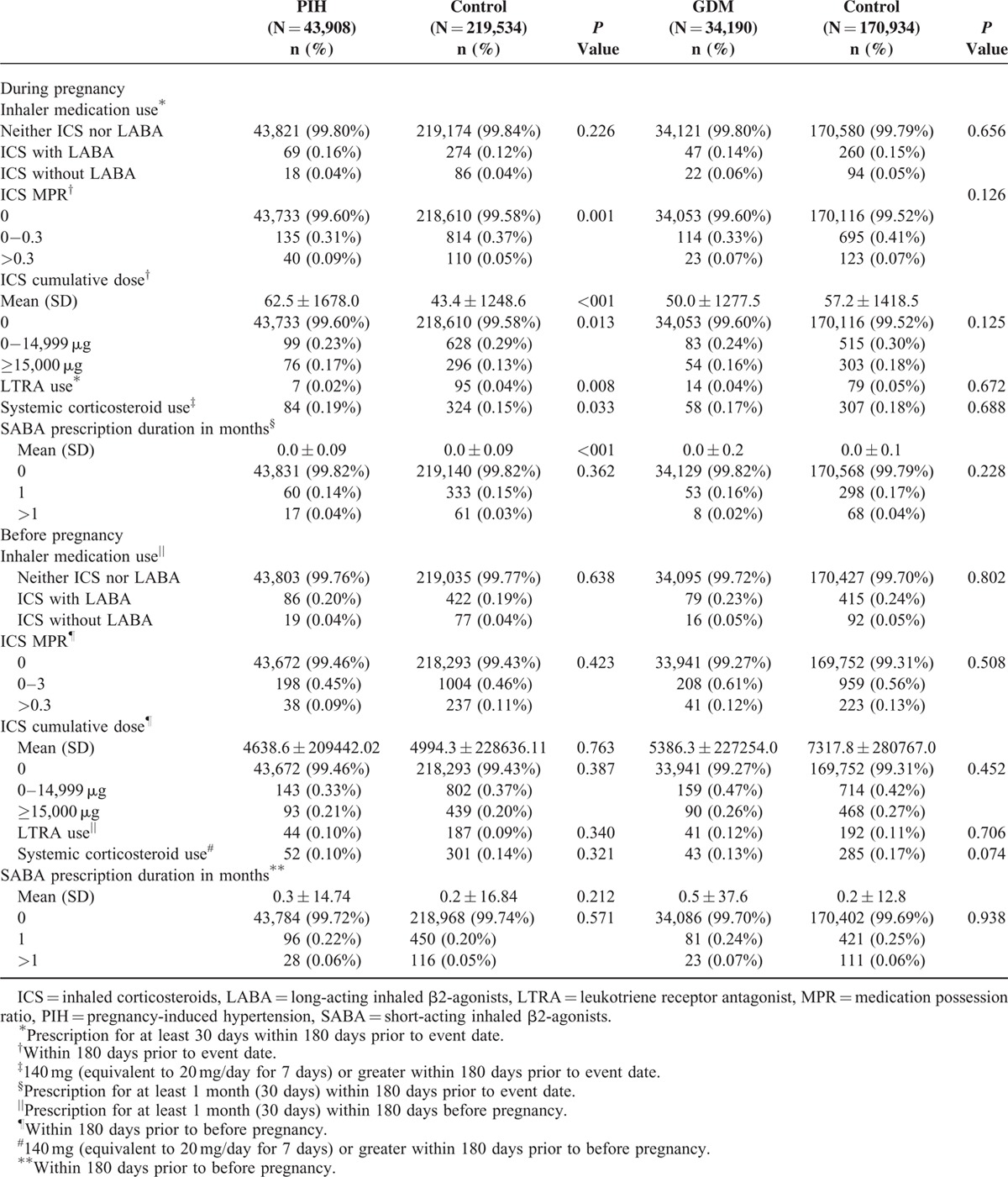
TABLE 3.
Risk of PIH According to ICS Use
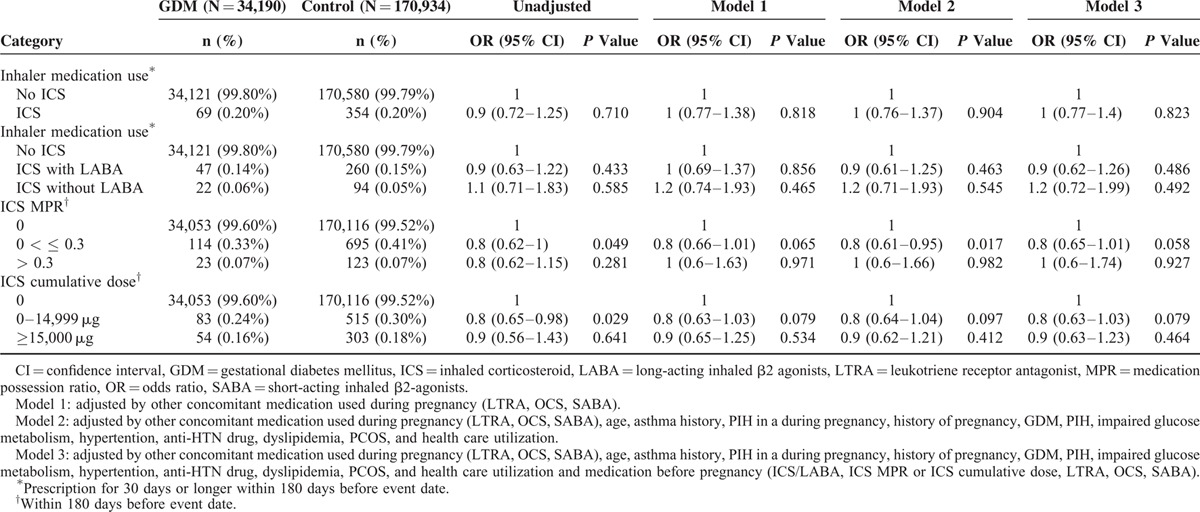
Effects of ICSs on GDM
The frequency of respiratory medications including ICSs was not different in cases and controls as shown in Table 2. The use of ICS within 180 days before the index date was not significantly associated with an increased risk of GDM, and this lack of association remained significant after adjustment for confounding covariates in different multivariable analysis models. ICS use was significantly associated with a lower risk of GDM in certain models (ICS MPR 0–0.3 vs. 0; aOR, 0.80; 95% CI, 0.61–0.95; P = 0.017 after adjustment for other concomitant respiratory medication, age, associated comorbidities, and healthcare utilization (model 2)) (Table 4).
TABLE 4.
Risk of GDM According to ICS Use
DISCUSSION
Our nationwide nested case-control studies showed that ICS use is not associated with the risk of PIH and GDM. However, after adjustment for other concomitant respiratory medication, ICS use significantly was associated with increase in the risk of PIH, although this association was no longer significant in other multivariate models. There was also a statistically significant dose–response relationship between ICS use and development of PIH. ICS use was not associated with an increased risk of GDM.
Our study is somewhat different from previous studies that reported no relationship between asthma drugs, including ICS, and PIH. However, only a few studies investigated the relationship between ICS use and the risk of PIH.1,2,21,22 A prospective cohort study reported that the ICSs-only group did not increase the risk of preeclampsia.1 One randomized trial did not find any significant differences in obstetric outcomes including PIH between inhaled beclomethasone group and theophylline group.22 A larger nested case-control study using administrative databases in Quebec demonstrated that ICSs did not increase the risk of PIH.2 However, these studies have several weak points in supporting the neutral effect of ICSs on PIH. First, each study included only pregnant women with asthma. Because asthma itself increases the risk of PIH,23 the effects of ICSs, which are the principal asthma-controller medications, on PIH could have been weakened. Our nested case-control study using a nationwide database included all pregnant women with or without asthma. Second, several studies had only small numbers of PIH cases.21,22 Our study analyzed more than 40,000 cases with PIH. Third, although previous studies did not find any statistically significant increases in PIH risk among ICS users, the majority showed trends that ICSs increase the risk of PIH. For example, 1 prospective study demonstrated that 10.9% of ICS users versus 7.8% of individuals who did not use ICSs experienced PIH,1 while a previous nested case-control study computed an aOR for PIH of 1.06 with ICS use, with a prevalence of PIH among ICS users of 0.20%, similar to our results.1,2
We also showed that no significant increase in the risk of GDM was observed among pregnant women who were prescribed ICS. These results could be explained by various factors. First, unlike systemic corticosteroid treatment, ICSs may have little impact on serum glucose levels.34 Although several studies reported a positive association between ICS and DM,34–39 other studies found contradicting results.34,36,37,39 Second, a relatively short duration of ICS treatment during pregnancy (at most 10 months) could be associated with fewer effects. In fact, studies showed that total systemic glucocorticoid dose and duration could influence glucocorticoid-induced hyperglycemia.9 However, it remains unclear whether or not ICS dose and duration are significantly associated with the development of hyperglycemia. An analysis evaluating randomized trials conducted over 4 years did not find any significant relationship between DM/hyperglycemia and ICS.36
Our study has several advantages. Most of all, as it was based on a nationwide insurance claims database, the likelihood of selection bias is minimal28 and relatively great number of cases could be used. Also, because we included not only women with asthma but also nonasthmatic pregnant women, the possibility of masking the risk of PIH by asthma itself, as mentioned above, was avoided.23 In addition, a large prospective cohort study showed that severe asthma is significantly associated with GDM in multivariate analysis,40 which suggests that asthma may also be a risk factor for GDM. Therefore, the effect of ICS on GDM may have been also weakened in an analysis only with asthmatic women. Furthermore, various possible confounders including drugs used during and before pregnancy and comorbidities were adjusted in the multivariate analyses for a better examination of the relationships between ICS use and the risk of PIH and GDM.
We should acknowledge limitations. First, the definitions of PIH and GDM were based only on ICD-10 codes. However, thanks to the coverage of national health insurance, almost all pregnant women in Korea undergo PIH and GDM screening during pregnancy. In fact, there was no difference in the number of visits to obstetricians between cases and controls in our studies. Second, third, we could not find the exact biological mechanism by which ICS increase the risk of PIH. Vitamin D deficiency might be a link. It has been reported that glucocorticoid use is associated with low 25-hydroxyvitamin D levels,41 and that low vitamin D levels are associated with the risk of preeclampsia.42
Our data should be applied cautiously to the management of pregnant women with asthma. The use of ICSs may reduce the risk of exacerbation among pregnant women with asthma without an increase in adverse pregnancy outcomes.43 Therefore, 1 guideline stresses that it is safer for pregnant women with asthma to be treated with asthma drugs than to have asthma symptoms and exacerbation.44 We do not think that ICSs should be avoided due to the risk of PIH based on our study. Our study included all pregnant women with or without asthma because asthma itself has been regarded as a risk factor for PIH.23 In addition, we also could not identify the population most prone to the risk. However, our results can be summarized in a statement that although few drugs have definitely proven teratogenic in humans, no drug can be considered completely safe.45
In conclusion, our nationwide nested case-control studies showed that ICS use is not associated with an increased risk of PIH and GDM. However, the possibility of increased risk of PIH by ICS could not be excluded. Further studies are needed.
Footnotes
Abbreviations: DM = diabetes mellitus, DPP-4 = dipeptidyl peptidase-4, GDM = gestational diabetes mellitus, GLP-1 = glucagon-like peptide-1, HIRA = Health Insurance Review and Assessment Service, ICD-10 = International Statistical Classification of Diseases and Related Health Problems, 10th edition, ICSs = inhaled corticosteroids, LABAs = long-acting inhaled β2-agonists, LAMAs = long-acting inhaled muscarinic antagonists, LTRAs = leukotriene receptor antagonists, MPRs = medication possession ratios, NHI = National Health Insurance, PCOS = polycystic ovary syndrome, PIH = pregnancy-induced hypertension, SABAs = short-acting inhaled β2-agonists.
All authors participated in study design, data analysis, and interpretation of the data. All authors were involved in writing and revising the manuscript, and agreed the submission of the paper.
C-HL and JK are co-first authors who equally contributed to this study.
This study was supported by the National Evidence-Based Healthcare Collaborating Agency (NA13-004).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Schatz M, Zeiger RS, Harden K, et al. The safety of asthma and allergy medications during pregnancy. J Allergy Clin Immunol 1997; 100:301–306. [DOI] [PubMed] [Google Scholar]
- 2.Martel MJ, Rey E, Beauchesne MF, et al. Use of inhaled corticosteroids during pregnancy and risk of pregnancy induced hypertension: nested case-control study. BMJ 2005; 330:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000; 183:S1–S22. [PubMed] [Google Scholar]
- 4.Weinstein SP, Wilson CM, Pritsker A, et al. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metabolism 1998; 47:3–6. [DOI] [PubMed] [Google Scholar]
- 5.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia 2005; 48:2119–2130. [DOI] [PubMed] [Google Scholar]
- 6.Buren J, Lai YC, Lundgren M, et al. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem Biophys 2008; 474:91–101. [DOI] [PubMed] [Google Scholar]
- 7.Henriksen JE, Alford F, Vaag A, et al. Intracellular skeletal muscle glucose metabolism is differentially altered by dexamethasone treatment of normoglycemic relatives of type 2 diabetic patients. Metabolism 1999; 48:1128–1135. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrand A, Schalin-Jantti C, Lofman M, et al. The effect of (steroid) immunosuppression on skeletal muscle glycogen metabolism in patients after kidney transplantation. Transplantation 1996; 61:889–893. [DOI] [PubMed] [Google Scholar]
- 9.Kwon S, Hermayer KL. Glucocorticoid-induced hyperglycemia. Am J Med Sci 2013; 345:274–277. [DOI] [PubMed] [Google Scholar]
- 10.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract 2009; 15:469–474. [DOI] [PubMed] [Google Scholar]
- 11.Pagano G, Cavallo-Perin P, Cassader M, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest 1983; 72:1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group HSCR, Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 13.Dodd JM, Crowther CA, Antoniou G, et al. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust N Z J Obstet Gynaecol 2007; 47:307–312. [DOI] [PubMed] [Google Scholar]
- 14.Jensen DM, Damm P, Sorensen B, et al. Clinical impact of mild carbohydrate intolerance in pregnancy: a study of 2904 nondiabetic Danish women with risk factors for gestational diabetes mellitus. Am J Obstet Gynecol 2001; 185:413–419. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara A, Weiss NS, Hedderson MM, et al. Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycaemia and hyperbilirubinaemia. Diabetologia 2007; 50:298–306. [DOI] [PubMed] [Google Scholar]
- 16.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007; 30 Suppl 2:S251–260. [DOI] [PubMed] [Google Scholar]
- 17.Rocklin RE. Asthma, asthma medications and their effects on maternal/fetal outcomes during pregnancy. Reprod Toxicol 2011; 32:189–197. [DOI] [PubMed] [Google Scholar]
- 18.Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc 2004; 1:338–344. [DOI] [PubMed] [Google Scholar]
- 19.Todd GR, Acerini CL, Buck JJ, et al. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur Respir J 2002; 19:1207–1209. [DOI] [PubMed] [Google Scholar]
- 20.Holme J, Tomlinson JW, Stockley RA, et al. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J 2008; 32:1047–1052. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi R, Nikfar S, Abdollahi M. Meta-analysis finds use of inhaled corticosteroids during pregnancy safe: a systematic meta-analysis review. Hum Exp Toxicol Aug 2006; 25:447–452. [DOI] [PubMed] [Google Scholar]
- 22.Dombrowski MP, Schatz M, Wise R, et al. Randomized trial of inhaled beclomethasone dipropionate versus theophylline for moderate asthma during pregnancy. Am J Obstet Gynecol Mar 2004; 190:737–744. [DOI] [PubMed] [Google Scholar]
- 23.Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG Oct 2011; 118:1314–1323. [DOI] [PubMed] [Google Scholar]
- 24.Braman SS. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest Jan 2006; 129 (1 Suppl):138S–146S. [DOI] [PubMed] [Google Scholar]
- 25.Brightling CE. Chronic cough due to nonasthmatic eosinophilic bronchitis: ACCP evidence-based clinical practice guidelines. Chest Jan 2006; 129 (1 Suppl):116S–121S. [DOI] [PubMed] [Google Scholar]
- 26.Refuerzo JS, Garg A, Rech B, et al. Continuous glucose monitoring in diabetic women following antenatal corticosteroid therapy: a pilot study. Am J Perinatol May 2012; 29:335–338. [DOI] [PubMed] [Google Scholar]
- 27.Gurbuz A, Karateke A, Ozturk G, et al. Is 1-hour glucose screening test reliable after a short-term administration of antenatal betamethasone? Am J Perinatol Oct 2004; 21:415–420. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH, Kim K, Hyun MK, et al. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax Dec 2013; 68:1105–1113. [DOI] [PubMed] [Google Scholar]
- 29.Park YT, Yoon JS, Speedie SM, et al. Health insurance claim review using information technologies. Healthc Inform Res Sep 2012; 18:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CH, Hyun MK, Jang EJ, et al. Inhaled corticosteroid use and risks of lung cancer and laryngeal cancer. Respir Med Aug 2013; 107:1222–1233. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Lee E, Lee T, et al. Economic burden of acute coronary syndrome in South Korea: a national survey. BMC Cardiovasc Disord 2013; 13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Kim J, Kim K, et al. Healthcare use and prescription patterns associated with adult asthma in Korea: analysis of the NHI claims database. Allergy Nov 2013; 68:1435–1442. [DOI] [PubMed] [Google Scholar]
- 33.Boulet LP, Becker A, Berube D, et al. Canadian Asthma Consensus Report, 1999. Canadian Asthma Consensus Group. CMAJ 1999; 161 (11 Suppl):S1–S61. [PMC free article] [PubMed] [Google Scholar]
- 34.Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med Sep 2002; 17:717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med Nov 2010; 123:1001–1006. [DOI] [PubMed] [Google Scholar]
- 36.O’Byrne PM, Rennard S, Gerstein H, et al. Risk of new onset diabetes mellitus in patients with asthma or COPD taking inhaled corticosteroids. Respir Med Nov 2012; 106:1487–1493. [DOI] [PubMed] [Google Scholar]
- 37.Dendukuri N, Blais L, LeLorier J. Inhaled corticosteroids and the risk of diabetes among the elderly. Br J Clin Pharmacol Jul 2002; 54:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slatore CG, Bryson CL, Au DH. The association of inhaled corticosteroid use with serum glucose concentration in a large cohort. Am J Med May 2009; 122:472–478. [DOI] [PubMed] [Google Scholar]
- 39.Faul JL, Wilson SR, Chu JW, et al. The effect of an inhaled corticosteroid on glucose control in type 2 diabetes. Clin Med Res 2009; 7:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dombrowski MP, Schatz M, Wise R, et al. Asthma during pregnancy. Obstet Gynecol 2004; 103:5–12. [DOI] [PubMed] [Google Scholar]
- 41.Skversky AL, Kumar J, Abramowitz MK, et al. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: results from the National Health and Nutrition Examination Survey (NHANES): 2001-2006. J Clin Endocrinol Metab 2011; 96:3838–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabesh M, Salehi-Abargouei A, Tabesh M, et al. Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis. J Clin Endocrinol Metab 2013; 98:3165–3173. [DOI] [PubMed] [Google Scholar]
- 43.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 2006; 61:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dombrowski MP, Schatz M, Bulletins-Obstetrics ACoP. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 90, February 2008: asthma in pregnancy. Obstet Gynecol 2008; 111 (2 Pt 1):457–464. [DOI] [PubMed] [Google Scholar]
- 45.Vlastarakos PV, Manolopoulos L, Ferekidis E, et al. Treating common problems of the nose and throat in pregnancy: what is safe? Eur Arch Otorhinolaryngol 2008; 265:499–508. [DOI] [PubMed] [Google Scholar]


