Abstract
Laparoscopic surgery has been widely accepted as a feasible and safe treatment modality in many cancers of the gastrointestinal tract. However, most guidelines on gallbladder cancer (GBC) regard laparoscopic surgery as a contraindication, even for early GBC. This study aims to evaluate and compare recent surgical outcomes of laparoscopic and open surgery for T1(a,b) GBC and to determine the optimal surgical strategy for T1 GBC.
The study enrolled 197 patients with histopathologically proven T1 GBC and no history of other cancers who underwent surgery from 2000 to 2014 at 3 major tertiary referral hospitals with specialized biliary-pancreas pathologists and optimal pathologic handling protocols. Median follow-up was 56 months. The effects of depth of invasion and type of surgery on disease-specific survival and recurrence patterns were investigated.
Of the 197 patients, 116 (58.9%) underwent simple cholecystectomy, including 31 (15.7%) who underwent open cholecystectomy and 85 (43.1%) laparoscopic cholecystectomy. The remaining 81 (41.1%) patients underwent extended cholecystectomy. Five-year disease-specific survival rates were similar in patients who underwent simple and extended cholecystectomy (96.7% vs 100%, P = 0.483), as well as being similar in patients in the simple cholecystectomy group who underwent open and laparoscopic cholecystectomy (100% vs 97.6%, P = 0.543). Type of surgery had no effect on recurrence patterns.
Laparoscopic cholecystectomy for T1 gallbladder cancer can provide similar survival outcomes compared to open surgery. Considering less blood loss and shorter hospital stay with better cosmetic outcome, laparoscopic cholecystectomy can be justified as a standard treatment for T1b as well as T1a gallbladder cancer when done by well-experienced surgeons based on exact pathologic diagnosis.
INTRODUCTION
Gallbladder cancer (GBC) is relatively rare in Western populations, and its reported prognosis is poorer than in some of the nations in which GBC is more prevalent, such as Chile, Korea, and Japan.1,2 Curative surgical resection is the treatment of choice, as there are currently no effective systemic treatments. Thus, selection of candidates for surgery and determination of type of surgery are crucial in enhancing survival outcomes in patients with GBC. Survival outcomes have been shown to depend on surgical strategy, pathologic stage, comorbidities, and experience of the surgical unit.3
In contrast to other organs of the gastrointestinal tract, the gallbladder lacks submucosa and partially serosa with a relatively thin proper muscle layer. This can allow tumor cells to easily invade or metastasize to other organs, which results in reduced survival outcomes. Thus, early detection and optimal treatment are key factors determining survival outcomes in patients with GBC. Improvements in radiologic imaging; increased screening of high-risk patients, including those with GB stones and polyps; and the universal use of laparoscopic cholecystectomy have increased the proportion of GBCs detected early. Indeed, many GBCs are detected incidentally during laparoscopic cholecystectomy for the removal of GB stones and/or polyps.4–6 However, some guidelines, including those of the National Comprehensive Cancer Network (NCCN) and the \Japanese Society of Hepato-Biliary-Pancreatic Surgery, do not recommend laparoscopic surgery even for patients with early GBC.7–9
Previous studies reported poor outcomes after laparoscopic surgery on GBC, including port site metastasis and poorer survival.10,11 However, the development of new instruments and advanced surgical techniques has resulted in laparoscopic surgery becoming widely accepted as a feasible and safe treatment modality for many GI tract cancers. Laparoscopic and open surgery have shown similar oncologic outcomes in the management of colon, stomach, and even pancreas cancer.12–14
Surprisingly, consensus guidelines on GBC are based mostly on data reported before 2000. More recent studies on patients with early GBC, however, have reported markedly improved treatment outcomes after laparoscopic than after open surgery.15,16 This study was therefore designed to evaluate and compare recent surgical outcomes of laparoscopic and open surgery for early GBC with pathologically proven T1 and to determine the optimal surgical strategy based on clinical data of patients who underwent surgery for early GBC cohort in the 21st century.
MATERIAL AND METHODS
This study included patients with pathologically proven GBC who underwent surgery for early GBC from 2000 to 2014 at 3 major tertiary referral hospitals in Korea; each of these centers had specialized biliary-pancreas pathologists and an optimal pathologic handling protocol, such as total or near total mapping of GB specimens, especially for diagnosis of early GBC to avoid under-evaluation of pathologic T stage. Clinicopathologic and radiological data were collected using standard clinical and pathologic diagnosis reports determined by a meeting of investigators before initiation of this study.
The early GBC was defined as GBC with pathologically proven T1. The pathologic T1 was subdivided into T1a and T1b, which were defined as tumor invades lamina propria and muscular layer, respectively. The extent of nodal disease was transformed into categorical variables indicating N0 and N1. The pathologic T and N data were defined by AJCC 7th edition. Simple cholecystectomy (SC) was defined as cholecystectomy alone; extended cholecystectomy (EC) also included liver wedge resection, or segments 4b and 5 segmentectomy, and dissection of regional lymph nodes around the pericystic and hepatoduodenal ligaments. Patients who underwent sequential liver resection with lymph node dissection after initial SC were considered as having undergone EC.
If there was no evidence of liver invasion, involvement of the extrahepatic bile duct, or lymph node and distant metastasis in preoperative imaging, laparoscopic cholecystectomy was performed by surgeon's preference. After confirmation of final pathologic staging, further treatments including liver resection and lymph node dissection was determined according to staging and patients’ willingness for operation. In the case of suspected over T2 lesion, extended cholecystectomy was initially planned according to patients’ condition.
Among patients who underwent laparoscopic cholecystectomy, differences in the number of trocars and detailed methods of each center were not considered.
This retrospective study confirmed to the ethical guidelines of the Declaration of Helsinki. The investigational review board or ethics committee at each institution approved the study (SNUH: 1508-081-695, SMC: 201602-120-002, NCC2016-0049).
Continuous data are expressed as means ± SDs. Categorical variables were compared using the Pearson χ2 test, and continuous variables using Student's t test. All parameters with a P value < 0.05 by univariate analysis were included in the multivariate model. Survival and recurrence information were reviewed and confirmed at each hospital at the end of 2014. Overall survival was calculated using the Kaplan–Meier method and compared by the log-rank test. To correct for differences in covariates that could affect choosing method of early gallbladder cancer surgery, and to reduce selection bias inherent to retrospective observational studies, a one-to-one matching analysis was performed between laparoscopic and open surgery. To estimate the propensity score, a function was built by logistic regression model for the method of surgery on the bias of patient's clinical factors. The propensity score was calculated with preoperative factors included age, sex, American Society of Anesthesiologist physical status classification system (ASA score), and pathologic T stage. The c-statistic (0.748) was calculated by the receiver operating characteristic curve.
All statistical analyses were performed using SPSS ver. 21 (SPSS, Chicago, IL).
RESULTS
Demographic Findings
Of the 204 patients initially identified, 7 had a synchronous or metachronous double primary malignancy other than GBC, including 2 each with primary liver cancer and urinary bladder cancer and 1 each with colon cancer bile duct cancer and lung cancer; these 7 patients were excluded from the analysis. This study therefore included 197 patients (Table 1), 85 males and 112 females, with a mean age at diagnosis of 63.4±10.6 years old. 52 (26.4%) had GB stones. Three patients had incidentally detected GBC after operation on symptomatic GB stone. Median follow-up period was 56 months.
TABLE 1.
Demographic and Pathologic Findings
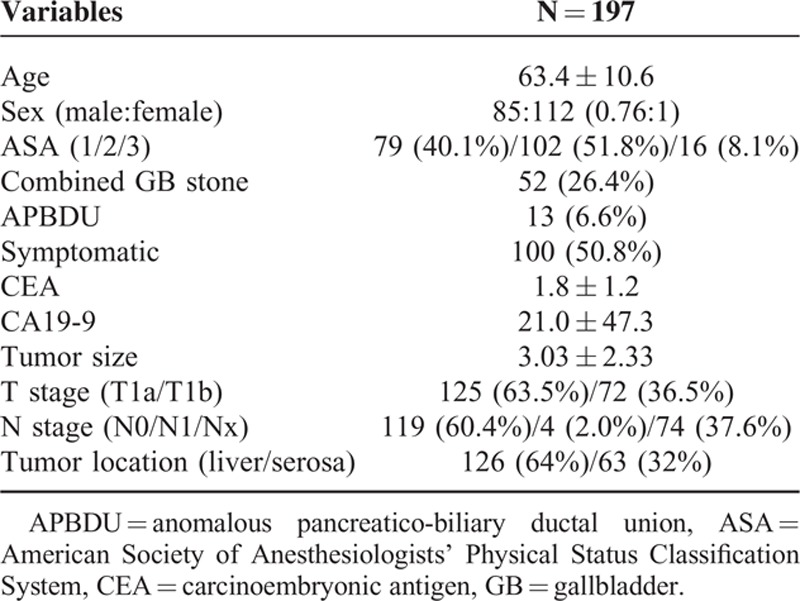
Types of Operation
Of the 197 patients, 94 (47.7%) underwent laparoscopic cholecystectomy, and 103 (52.3%) underwent open surgery including 30 (15.2%) open cholecystectomy (OC) and 73 (37.1%) extended cholecystectomy (Table 2).
TABLE 2.
Types of Operation According to T1a and T1b
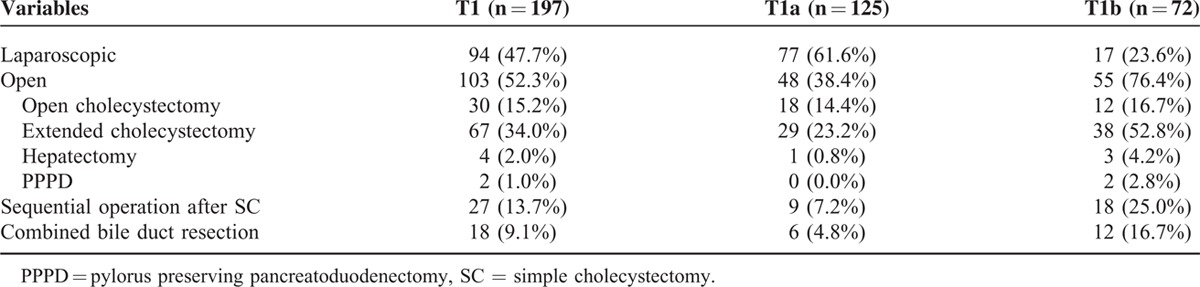
In T1a stage, 77 (61.6%) underwent laparoscopic surgery and 48 (38.4%) underwent open surgery including 29 (23.2%) extended cholecystectomy. In T1b stage, laparoscopic surgery was performed in 17(23.6%) patients and open surgery in 55 (76.4%). Of the 124 patients who underwent simple cholecystectomy, 95 (76.6%) had tumors pathologically classified as T1a and 29 (23.4%) as T1b (P < 0.001).
Eighteen patients (9.1%) underwent combined bile duct resection, 27 (13.7%) underwent a second operation after simple cholecystectomy, and 4 underwent extended right hemihepatectomy. All of these patients were regarded as undergoing extended cholecystectomy.
Four patients underwent R1 resection. All were positive for cancer cells in the cystic duct resection margin. They refused reoperation due to old age and/or the presence of a comorbidity.
According to type of surgery, there was no statistical difference of complication after operation, only 1 case (1.1%) of fluid collection in laparoscopic surgery and 4 patients (3.9%) in open surgery (P = 0.371).
Pathologic Findings
Mean tumor size was 3.0 ± 2.3 cm. Of the 197 patients, 125 (63.5%) were classified as T1a and 72 (36.5%) as T1b (Table 1). In addition, 123 patients (62.3%) underwent lymph node dissection or biopsy. Lymph node metastasis was found in 4 patients classified as T1b, 3.3% of patients who underwent LN biopsy and 5.6% of all patients classified as T1b. Lymph node metastasis was not detected in any patient classified as T1a.
Assessment of tumor location showed that 126 (64.0%) tumors were on the liver side and 63 (32.0%) on the serosal side. The most frequent gross morphology of tumor was papillary type (68.5%), followed by nodular (9.1%) and flat (4.6%) types.
Survival Rate and Recurrence
The 5-year overall rate (5YSR) among all patients was 98.2% in patients with T1a and 96.4% in patients with T1b tumors (P = 0.235) (Figure 1A). The 5YSR did not differ significantly in patients who underwent simple and extended cholecystectomy (96.7% vs 98.3%, P = 0.446). In patients who underwent simple cholecystectomy, 5YSR were similar in those who underwent open and laparoscopic cholecystectomy (100% vs 94.9%, P = 0.982). Simple and extended cholecystectomy showed similar 5YSR in patients with tumors classified as T1a (97.4% vs 100%, P = 0.698) and T1b (95.7% vs 100%, P = 0.846).
FIGURE 1.
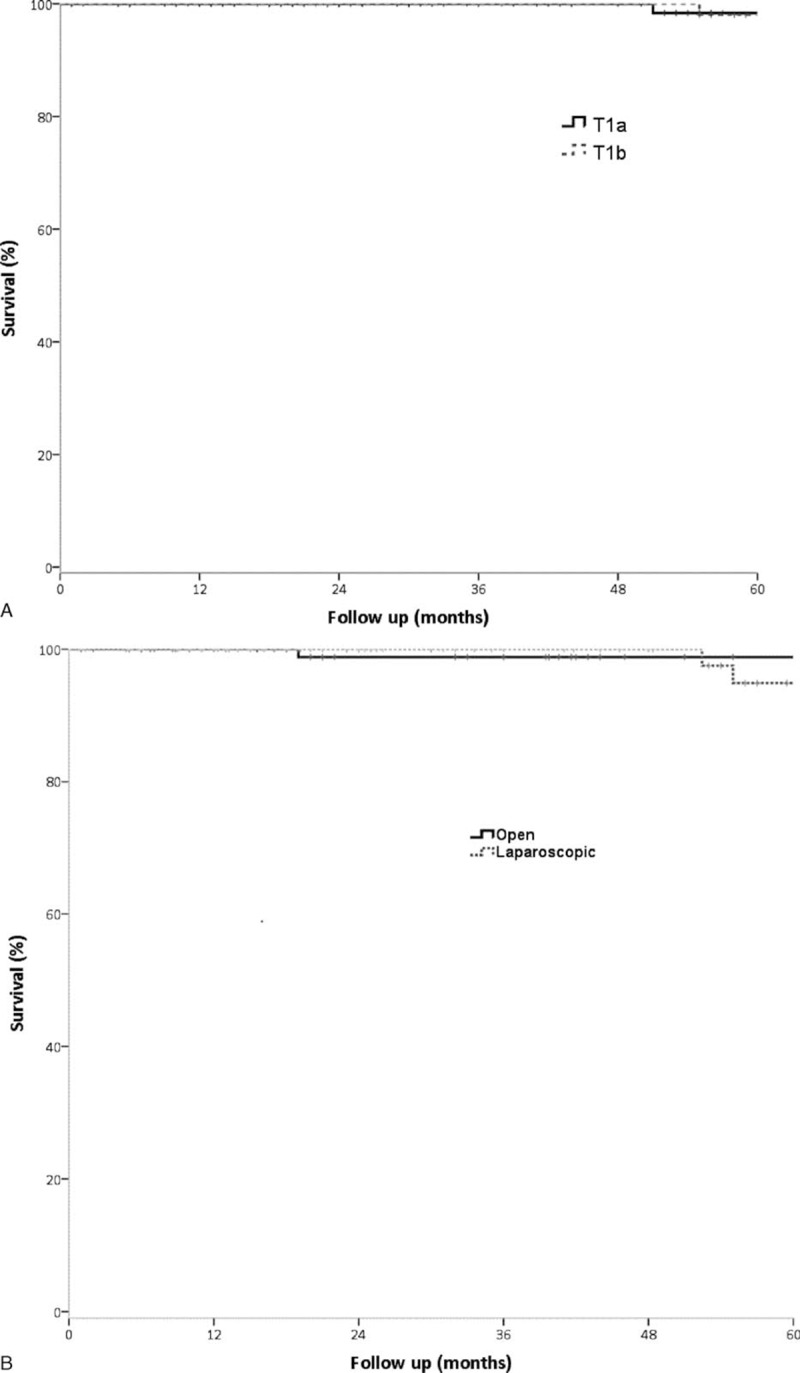
Five-year overall survival in patients with early gallbladder cancer as a function of depth of tumor invasion (A) and type of surgery (B).
Compared 5YSR between laparoscopic surgery (LC) and open surgery (open cholecystectomy, extended cholecystectomy), (LC (94.9%) vs open surgery (98.8%), P = 0.582) (Figure 1B).
The 5 YSR did not differ in those who underwent initially planned EC and sequential EC after SC (95.8% vs 100%, P = 0.325). 5YSR were also similar in patients with tumors on the liver and serosal sides (97.0% vs 100%, P = 0.923), and subgroup analysis of patients with T1a and T1b also showed that tumor location had no effect on survival.
Propensity Score (PS) Matched Analysis
The comparisons among types of surgery after propensity score matching are shown in Table 3. The results showed that perioperative finding such as operation time, estimated blood loss, and hospital stays is shorter or smaller in the laparoscopic group, which results were similar to data before PS matching.
TABLE 3.
Patients Characteristics (Unadjusted and Propensity Score [PS] Matched Between Laparoscopic and Open Surgery
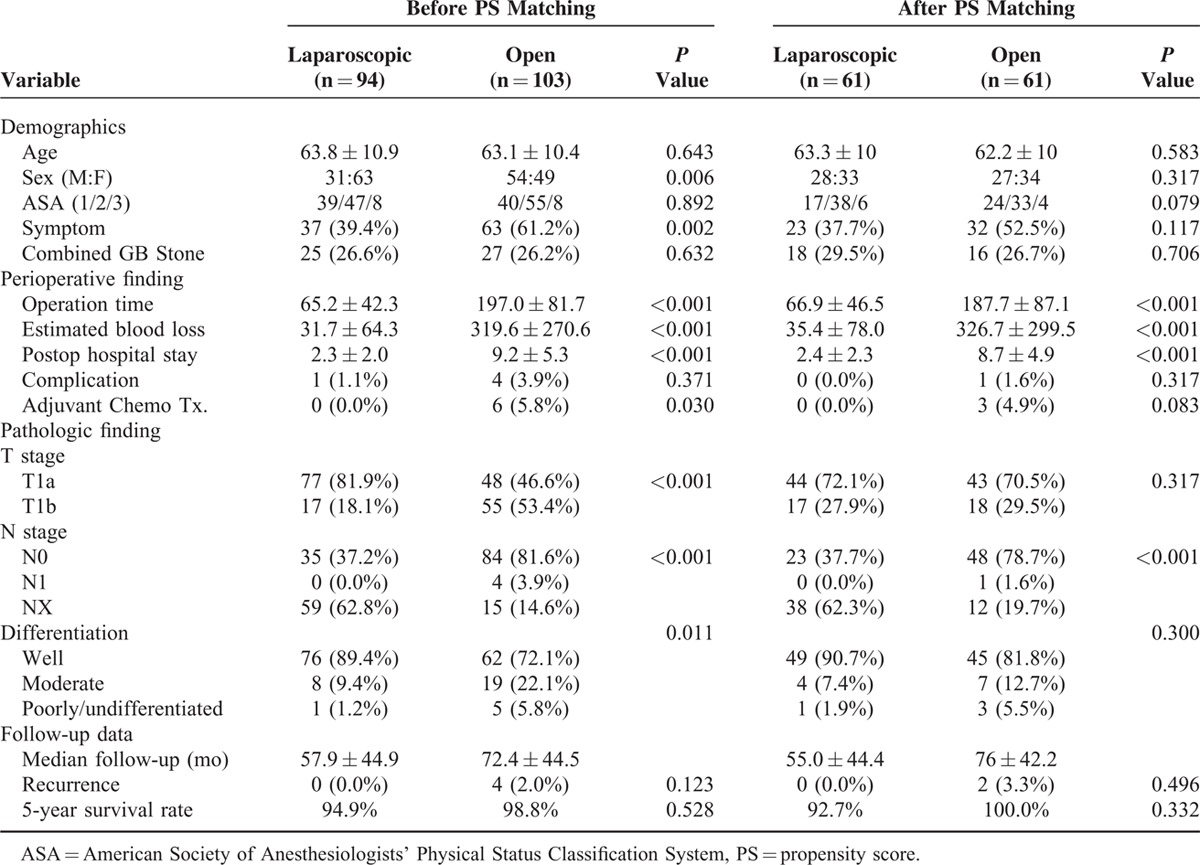
After matching, 5YSR of the laparoscopic surgery (92.7%) was similar to open surgery (100%). Two patients from laparoscopic cholecystectomy died of non-GB cancer recurrence (cardiac diseases). The 5-year disease specific survival was 100% in the laparoscopic cholecystectomy group.
DISCUSSION
The early GBC was usually defined as tumors confined to the mucosa (T1a) or muscular layer (T1b). However, accurate preoperative diagnosis of early GBC is difficult because many patients are asymptomatic. Thus, these patients are frequently not assessed by high-resolution image techniques. In addition, the GB wall itself is anatomically complex, with the lack of submucosa and the Rokitansky–Aschoff sinus making it difficult to accurately predict the depth of tumor invasion.17 The lack of uniform specimen handling and pathologic diagnosis protocols among nations and hospitals has also complicated the diagnosis of early GBCs.18–20 Many early GBCs are therefore detected incidentally, without precise imaging work-up.
Unlike other organs of the gastrointestinal tract, the GB has a thin mucosal layer without submucosa. Moreover, the presence of a naturally invaginated mucosa into muscle layer (Rokitansky–Aschoff sinus) may complicate pathologic staging, especially in early GBC.21 Neoplastic lesions of the GB often show subtle differences, making it important to determine the associations of different epithelial lesions with the findings of elaborate pathologic examinations based on optimal sampling protocols. In the absence of GB specimen mapping or thorough examination, the depth of invasion of many GBC specimens might be underevaluated and the tumors can be understaged.18,19,22 This resulted in very low survival rates, even for patients with T1 GBC, especially based on pathologically unstandardized national cancer registry data from the USA and Germany.23,24
Due to the complexities of the diagnosis of early GBC, the reported survival rate of patients with early GBC has been reported to range from 40% to 100%.25,26 To reduce the likelihood of pathologic understaging, we evaluated patients treated at 3 major hospitals, which routinely sample tissue specimens at 5- to7-mm intervals, thus optimizing the likelihood of a correct pathologic diagnosis of early GBC.
The development of better instrumentation and technical advances have resulted in the more widespread use of laparoscopic surgery to treat most cancers of GI tract, including colon and stomach cancers. Laparoscopic surgery has therefore become a standard treatment in patients with these early stage tumors, showing survival outcomes similar to those of open surgery.12,27
Despite promising reports on laparoscopic cholecystectomy for early GB cancer,16,23,26,28–30 most current guidelines do not recommend laparoscopic surgery for GB cancer, even early stage disease. These contraindications were based on studies showing increased risks of port site recurrence and peritoneal dissemination.8,9,31–34 Unlike these initial findings, however, recent studies have shown similar survival outcomes for laparoscopic and open surgery, especially for early tumors. Most recent reports have shown survival rates of >95% for patients with T1a tumors who underwent laparoscopic cholecystectomy.29,30,35 Experience has shown that, when performing laparoscopic procedure, care should be taken to avoid perforation and to use a specimen retrieval bag to get a same oncologic outcome with open surgery. However, the optimal management for T1b GBC remains unclear due to the lack of reports on laparoscopic surgery.3,16,36–39
NCCN 2015 guidelines and Japanese 2015 guidelines recommend hepatic resection with lymphadenectomy for T1b gallbladder cancer.8,9 A recent meta-analysis found that radical resection provided a survival benefit of 3.43 years compared with simple cholecystectomy alone.40 However, the studies included in this meta-analysis reported survival rates ranging from 0% to 100%. Most reports with poor survival outcomes (5 year survival rates <50%) following laparoscopy were published before 2000, involved <10 patients, and assessed overall survival, which included non-GBC-related deaths. Inasmuch as patients with severe comorbidities tend to undergo simple cholecystectomy, especially minimally invasive surgery, survival outcomes must be interpreted carefully to avoid selection bias.25,41–43
This study found that laparoscopic and open surgery had similar oncological outcomes (survival and recurrence rates) in patients with T1b as well as T1a GBC. Considering the functional and cosmetic advantages of laparoscopic surgery, there is no reason not to recommend laparoscopic surgery in the management of early GBC. In the case of incidentally found GB cancer, further resection including liver and lymph node is not needed if the final pathologic diagnosis is T1 according to our results.
Recently, a more advanced laparoscopic surgical approach including extended cholecystectomy has been shown to be feasible, with outcomes comparable to those of open surgery, in patients with T2 GBC at selected referral centers.30,44,45 However, the advanced laparoscopic procedure could not be generalized in all hospitals. Surgical outcomes may differ between high- and low-volume centers.3,46 Thus, the criteria for referral of patients with suspected GBC are crucial to improve survival and treatment outcomes.
In conclusion, laparoscopic cholecystectomy for T1 gallbladder cancer can provide similar survival outcomes compared to open surgery. Considering less blood loss and shorter hospital stay with better cosmetic outcome, laparoscopic cholecystectomy can be justified as a standard treatment for T1b as well as T1a gallbladder cancer when done by well-experienced surgeons based on exact pathologic diagnosis.
Footnotes
Abbreviations: GB = gallbladder, GBC = gallbladder cancer, LC = laparoscopic cholecystectomy, PS = propensity score.
J-YJ and JSH contributed equally to this study.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.White K, Kraybill WG, Lopez MJ. Primary carcinoma of the gallbladder: TNM staging and prognosis. J Surg Oncol 1988; 39:251–255. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on day/month/year. [Google Scholar]
- 3.Yip VS, Gomez D, Brown S, et al. Management of incidental and suspicious gallbladder cancer: focus on early referral to a tertiary centre. HPB 2014; 16:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darabos N, Stare R. Gallbladder cancer: laparoscopic and classic cholecystectomy. Surg Endosc 2004; 18:144–147. [DOI] [PubMed] [Google Scholar]
- 5.Cavallaro A, Piccolo G, Di Vita M, et al. Managing the incidentally detected gallbladder cancer: algorithms and controversies. Int J Surg 2014; 12 Suppl 2:S108–S119. [DOI] [PubMed] [Google Scholar]
- 6.Jin K, Lan H, Zhu T, et al. Gallbladder carcinoma incidentally encountered during laparoscopic cholecystectomy: how to deal with it. Clin Transl Oncol 2011; 13:25–33. [DOI] [PubMed] [Google Scholar]
- 7.Kondo S, Takada T, Miyazaki M, et al. Japanese Association of Biliary Surgery; Japanese Society of Hepato-Biliary-Pancreatic Surgery; Japan Society of Clinical Oncology. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg 2008; 15:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki M, Yoshitomi H, Miyakawa S, et al. Clinical practice guidelines for the management of biliary tract cancers 2015. J Hepatobiliary Pancreat Sci 2015; 22:249–273. [DOI] [PubMed] [Google Scholar]
- 9.The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) Hepatobiliary Cancers. (Version 2.2015). © 2015. Available at: www.NCCN.org. [Google Scholar]
- 10.Reddy YP, Sheridan WG. Port-site metastasis following laparoscopic cholecystectomy: a review of the literature and a case report. Eur J Surg Oncol 2000; 26:95–98. [DOI] [PubMed] [Google Scholar]
- 11.Wullstein C, Woeste G, Barkhausen S, et al. Do complications related to laparoscopic cholecystectomy influence the prognosis of gallbladder cancer? Surg Endosc 2002; 16:828–832. [DOI] [PubMed] [Google Scholar]
- 12.Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomized controlled trial. Lancet Oncol 2014; 15:767–774. [DOI] [PubMed] [Google Scholar]
- 13.Chen XZ, Wen L, Rui YY, et al. Long-term survival outcomes of laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta-analysis. Medicine 2015; 94:e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Küper MA, Eisner F, Königsrainer A, et al. Laparoscopic surgery for benign and malign diseases of the digestive system: indications, limitations, and evidence. World J Gastroenterol 2014; 20:4883–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Aretxabala XA, Roa IS, Mora JP, et al. Laparoscopic cholecystectomy: its effect on the prognosis of patients with gallbladder cancer. World J Surg 2004; 28:544–547. [DOI] [PubMed] [Google Scholar]
- 16.Lee SE, Jang JY, Kim SW, et al. Korean Pancreas Surgery Club. Surgical strategy for T1 gallbladder cancer: a nationwide multicenter survey in South Korea. Ann Surg Oncol 2014; 21:3654–3660. [DOI] [PubMed] [Google Scholar]
- 17.Jang JY, Kim SW, Lee SE, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg 2009; 250:943–949. [DOI] [PubMed] [Google Scholar]
- 18.Adsay V, Saka B, Basturk O, et al. Criteria for pathologic sampling of gallbladder specimens. Am J Clin Pathol 2013; 140:278–280. [DOI] [PubMed] [Google Scholar]
- 19.Renshaw AA, Gould EW. Submitting the entire gallbladder in cases of dysplasia is not justified. Am J Clin Pathol 2012; 138:374–376. [DOI] [PubMed] [Google Scholar]
- 20.Ito H, Ito K, D’Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011; 254:320–325. [DOI] [PubMed] [Google Scholar]
- 21.Roa JC, Tapia O, Manterola C, et al. Early gallbladder carcinoma has a favorable outcome but Rokitansky–Aschoff sinus involvement is an adverse prognostic factor. Virchows Arch 2013; 463:651–661. [DOI] [PubMed] [Google Scholar]
- 22.Adsay NV, Bagci P, Tajiri T, et al. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol 2012; 29:127–141. [DOI] [PubMed] [Google Scholar]
- 23.Goetze T, Paolucci V. Does laparoscopy worsen the prognosis for incidental gallbladder cancer? Surg Endosc 2006; 20:286–293. [DOI] [PubMed] [Google Scholar]
- 24.Downing SR, Cadogan KA, Ortega G, et al. Early-stage gallbladder cancer in the Surveillance, Epidemiology, and End Results database: effect of extended surgical resection. Arch Surg 2011; 146:734–738. [DOI] [PubMed] [Google Scholar]
- 25.Goetze TO, Paolucci V. Immediate re-resection of T1 incidental gallbladder carcinomas: a survival analysis of the German Registry. Surg Endosc 2008; 22:2462–2465. [DOI] [PubMed] [Google Scholar]
- 26.Kim EK, Lee SK, Kim WW. Does laparoscopic surgery have a role in the treatment of gallbladder cancer? J Hepatobiliary Pancreat Surg 2002; 9:559–563. [DOI] [PubMed] [Google Scholar]
- 27.Lee HJ, Shiraishi N, Kim HH, et al. Standard of practice on laparoscopic gastric cancer surgery in Korea and Japan: experts’ survey. Asian J Endosc Surg 2012; 5:5–11. [DOI] [PubMed] [Google Scholar]
- 28.Chan KM, Yeh TS, Jan YY, et al. Laparoscopic cholecystectomy for early gallbladder carcinoma: long-term outcome in comparison with conventional open cholecystectomy. Surg Endosc 2006; 20:1867–1871. [DOI] [PubMed] [Google Scholar]
- 29.Tian YH, Ji X, Liu B, et al. Surgical treatment of incidental gallbladder cancer discovered during or following laparoscopic cholecystectomy. World J Surg 2015; 39:746–752. [DOI] [PubMed] [Google Scholar]
- 30.Yoon YS, Han HS, Cho JY, et al. Is laparoscopy contraindicated for gallbladder cancer? A 10-year prospective cohort study. J Am Coll Surg 2015; 221:847–853. [DOI] [PubMed] [Google Scholar]
- 31.Aloia TA, Járufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB 2015; 17:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiland ST, Mahvi DM, Niederhuber JE, et al. Should suspected early gallbladder cancer be treated laparoscopically? J Gastrointest Surg 2002; 6:50–56. [DOI] [PubMed] [Google Scholar]
- 33.Sano T, Ajiki T, Hirata K, et al. A recurrent case of an early gallbladder carcinoma after laparoscopic cholecystectomy. Hepatogastroenterology 2004; 51:672–674. [PubMed] [Google Scholar]
- 34.Yoshida T, Matsumoto T, Sasaki A, et al. Laparoscopic cholecystectomy in the treatment of patients with gall bladder cancer. J Am Coll Surg 2000; 191:158–163. [DOI] [PubMed] [Google Scholar]
- 35.You DD, Lee HG, Paik KY, et al. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg 2008; 247:835–838. [DOI] [PubMed] [Google Scholar]
- 36.Goetze TO, Paolucci V. Benefits of reoperation of T2 and more advanced incidental gallbladder carcinoma: analysis of the German registry. Ann Surg 2008; 247:104–108. [DOI] [PubMed] [Google Scholar]
- 37.Costi R, Violi V, Roncoroni L. Gallbladder cancer and radical surgery. Ann Surg 2008; 248:494–496. [DOI] [PubMed] [Google Scholar]
- 38.Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008; 98:485–489. [DOI] [PubMed] [Google Scholar]
- 39.Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 2007; 11:1478–1486. [DOI] [PubMed] [Google Scholar]
- 40.Abramson MA, Pandharipande P, Ruan D, et al. Radical resection for T1b gallbladder cancer: a decision analysis. HPB 2009; 11:656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puhalla H, Wild T, Bareck E, et al. long-term follow-up of surgically treated gallbladder cancer patients. Eur J Surg Oncol 2002; 28:857–863. [DOI] [PubMed] [Google Scholar]
- 42.Cangemi V, Fiori E, Picchi C, et al. Early gallbladder carcinoma: a single-center experience. Tumori 2006; 92:487–490. [DOI] [PubMed] [Google Scholar]
- 43.Wagholikar GD, Behari A, Krishnani N, et al. Early gallbladder cancer. J Am Coll Surg 2002; 194:137–141. [DOI] [PubMed] [Google Scholar]
- 44.Itano O, Oshima G, Minagawa T, et al. Novel strategy for laparoscopic treatment of pT2 gallbladder carcinoma. Surg Endosc 2015; 29:3600–3607. [DOI] [PubMed] [Google Scholar]
- 45.Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic hepatic bisegmentectomy (s4b+s5) and hilar lymphadenectomy for incidental gallbladder cancer. Ann Surg Oncol 2015; 22 Suppl 3:S336–S339. [DOI] [PubMed] [Google Scholar]
- 46.Matthews JB. Planned laparoscopic approach for early-stage gallbladder cancer: the glass is one-third full. Arch Surg 2010; 145:133. [DOI] [PubMed] [Google Scholar]


