Abstract
Birt–Hogg–Dubé (BHD) is a rare autosomal dominant inherited syndrome that is characterized by the presence of fibrofolliculomas and/or trichodiscomas, pulmonary cysts, spontaneous pneumothorax, and renal tumors. Here, the 2 patients we reported with renal cell carcinomas and dermatological features were suspected to be suffering from BHD syndrome. Blood samples of these patients were sent for whole exon sequencing performed by Sanger sequencing. Eight mutations, including 5 mutations, which were mapped in noncoding region, 1 synonymous mutation, and 2 missense mutations, were detected in the FLCN gene in both patients. The 2 missense mutations, predicted to be disease-causing mutation or affecting protein function by MutationTaster and SIFT, confirmed the diagnosis. In addition, the 2 patients were also affected with papillary thyroid cancer. Total thyroidectomy and prophylactic bilateral central lymph node dissection were performed for them and the BHD-2 also received lateral lymph node dissection. Pathology reports showed that the patients had lymph node metastasis in spite of small size of thyroid lesions.
The 2 missense mutations, not reported previously, expand the mutation spectrum of FLCN gene associated with BHD syndrome. For the thyroid cancer patients with BHD syndrome, total thyroidectomy and prophylactic bilateral central lymph node dissection may be suitable and the neck ultrasound may benefit BHD patients and their family members.
INTRODUCTION
Birt–Hogg–Dubé (BHD) syndrome is a rare autosomal dominant inherited disorder, described firstly in 1977.1 Its clinical characteristics include cutaneous lesions (such as fibrofolliculomas, trichodiscomas), lung cysts, and spontaneous pneumothorax. In 1993, the first case of renal cell carcinoma (RCC) associated with BHD syndrome was reported.2 Subsequently, RCC was also described as an important clinical manifestation of BHD syndrome.3 This syndrome is caused by the germline mutations in FLCN gene, which is located on chromosome 17p11.2.4FLCN gene includes 14 exons and encodes folliculin protein consisting of 579 amino acids. Although this protein was expressed in most tissues, its function has not been elucidated. The FLCN gene expression was found in normal renal tissues and the loss of FLCN expression was found in all histological subtypes of RCC, suggesting that FLCN gene may be a tumor suppressor gene for RCC.5 In addition, the association between BHD syndrome and tumors of other organ systems was also repeatedly described, such as colorectal polyps/cancer, lung cancer, breast cancer, parotid oncocytomas, and so on.6
In this study, we report 2 BHD syndrome patients with RCC and papillary thyroid cancer (PTC).
MATERIALS AND METHODS
Two patients with head and neck skin lesions were diagnosed with PTC in our hospital in 2011 and 2014, respectively. Total thyroidectomy and bilateral central lymph node dissection were performed in patient 1 who had a history of clear cell RCC. Patient 2, who had hypertension well controlled with perindopril, underwent 2 thyroid operations. Lobectomy of the right lobe, isthmectomy, and lateral neck dissection were performed in the first surgery. Two years later, lobectomy of the left lobe and central compartment neck dissection were performed because of recurrence in the left lobe. Meantime, left renal lesion (size 1.8 × 1.8 cm) of this patient was found in the routine examination before the second operation. Then, partial nephrectomy on left was performed. No lung cysts were found in these 2 patients, and no history of spontaneous pneumothorax was found in both patients and their familial members.
As both renal carcinomas and dermatological features were suggestive of BHD syndrome, blood samples of these 2 patients obtained from the Tissue Bank in Tianjin Medical University Cancer Institute and Hospital were sent for sequencing analysis. Sequencing of the whole exons in FLCN gene was performed by the Sanger sequencing. Seventeen pairs of primers were designed for polymerase chain reaction (PCR) on the basis of the sequence of FLNC gene (NM_144997.5). The sequencing results were blasted to the NCBI sequence. This study was approved by Tianjin Medical University Cancer Institute and Hotspital's Ethics Committee.
RESULTS
The clinical features of these 2 patients, including age, sex, histology, and diagnosis, are summarized in Table 1. Both of the patients were diagnosed with bilateral and multifocal papillary thyroid carcinoma (PTC). Lymph nodes metastases were found in their central compartment region. Metastases were also found in the lateral region in patient 2. Clear cell RCC was diagnosed in patient 1. No further detailed pathologic information could be obtained, as the renal surgery of patient 1was not performed in our hospital. Renal pathologic examination of patient 2 showed left papillary RCC, Fuhrman II stage, invasion of renal capsule, perirenal fat capsule (−), and immunohistochemistry results were CA9 (+), Vimentin (+), CD117 (−), CK7 (+), P504S (+).
TABLE 1.
Detailed Patients’ Data

Table 2 displays 8 mutations detected in the FLCN gene of the 2 patients. The c.-487G>C mutation was homozygous and others were heterozygous. Three mutations were mapped in the coding region of FLCN, including 1 synonymous mutation (c.1095C>G) and 2 missense mutations (c.1481A>G detected in patient 2, resulting a substitution of asparagine for serine at codon 494; and c.1645C>G detected in patient 1, resulting in a substitution of leucine to valine at codon 549) (Figure 1). Four mutations (c.−487G>C, c.−302G>C, c.2132G>A, and c.2297T>C) in the noncoding region were identified in both patients. Another noncoding region mutation, c.−84G>A, was detected in patient 2 only.
TABLE 2.
Germline Mutations in the 2 BHD Syndrome Patients
FIGURE 1.
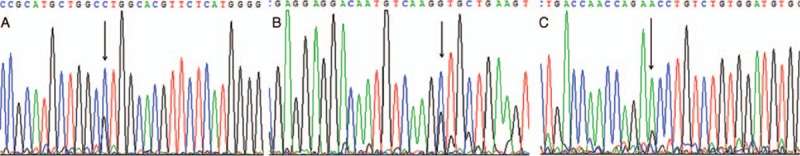
Three mutations detected in the coding region of FLCN. A, A synonymous mutation c.1095C>G was detected in exon 10 in Patient 1. B, The FLCN c.1645C>G, p.L549V was a missense mutation identified in exon 14 in Patient 1. C, The missense mutation c.1481A>G, p.N494S was mapped in exon 13 of FLCN in Patient 2.
The missense mutations (c.1481A>G and c.1645C>G) in FLCN gene were not reported previously in dbSNP or 1000 Genome Project and were also not archived in the ExAC database. We annotated the mutations with MutationTaster, SIFT, and Polyphen2, to predict the impact of the mutations on protein function. They were predicted to be disease-causing mutation or affect protein function by MutationTaster and SIFT. The Polyphen2 predicted the mutation c.1645C>G (L549 V) to be possibly damaging with a score of 0.911 (sensitivity: 0.81; specificity: 0.94) and the mutation c.1481A>G (N494S) to be benign with a score of 0.158 (sensitivity: 0.92; specificity: 0.87).
DISCUSSION AND CONCLUSIONS
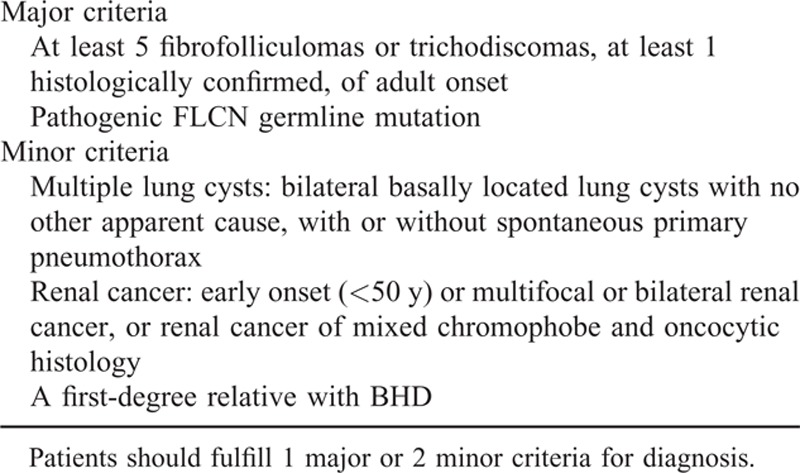
BHD syndrome is characterized by the development of fibrofolliculomas, kidney cancer, lung pneumatocysts, and spontaneous pneumothorax, but not all patients present with these traditional features.7 Patients might present with renal cancer8 or pneumothorax,9 both of which most often occur sporadically. In 2002, defects of FLCN gene were identified in families with BHD syndrome, which made the detection of the FLCN gene for the diagnosis of BHD syndrome possible.4 According to the diagnostic criteria proposed by European Birt–Hogg–Dubé Consortium (Table 3),10 a patient with pulmonary cysts or spontaneous pneumothorax only or RCC only could be diagnosed as suffering from BHD syndrome if he was found to carry a pathogenic germline mutation in the FLCN gene. In this study, BHD syndrome was suspected in the 2 patients with renal carcinomas and dermatological features. The mutations identified in FLCN gene confirmed the diagnosis.
TABLE 3.
Diagnosis Criteria for BHD Syndrome Proposed by the European Birt–Hogg–Dubé Consortium
Only 2 missense mutations (c.1481A>G and c.1645C>G) were detected in FLCN gene of the 2 patients in this study. The germline mutation c.1481A>G (N494S) in exon 13 was detected in patient 2. According to Toro et al,11 the asparagine 494 amino acid of folliculin is highly conserved in vertebrate species (chimpanzee, monkey, horse, mouse, rat, dog, cow, chicken, frog) suggesting functional significance. The N494S mutation was present in this patient with fibrofolliculoma, papillary RCC, PTC, and hypertension, suggesting that it is likely a disease-causing mutation. In patient 1 with clear cell RCC and PTC, the c.1645C>G (L549V) mutation was identified. The 2 mutations were predicted to be disease-causing mutation or affect protein function by MutationTaster and SIFT.
Some studies reported that the risk of pneumothorax in patients with BHD syndrome was 50 times greater than in the normal population7 and up to 80% of BHD syndrome patients had lung cysts.7,11–14 According to the study by Toro et al,12 mutations in exon 11 were more frequent in patients with a history of pneumothorax and mutations in exon 9 and 12 were, respectively, associated with the size and number of lung cysts. In this study, the 2 patients did not have the history of pneumothorax and multiple lung cysts, which may be explained by the fact that the missense mutations we reported were detected in exon 13 and 14. To date, however, the association between genotype and phenotype has not been established.
In 1999, Toro et al3 described that RCC was an important clinical manifestation of BHD syndrome, confirmed by the further studies.4,11,13,15,16 One study found that patients with BHD syndrome were about 6.9 times as likely to develop renal tumors as those not affected with BHD syndrome.13 According to the reported researches,11,13 the most common histological type of RCC in BHD syndrome patients was oncocytic-chromophobe hybrid carcinoma (50%), followed by purely chromophobe carcinomas (34%), purely oncocytic carcinomas (5%), clear cell carcinomas (3%), and papillary carcinomas (2%). Generally, the RCC patients with BHD syndrome had an earlier age (between 20 and 55 years). The BHD-associated renal tumors were most often multiple and bilateral and associated with microscopic lesions in the surrounding renal parenchyma.13,17,18 In this study, the 2 patients were diagnosed with RCC at the age of 49 and 45 years, respectively. The onset age of the 2 patients was consistent with the age characteristic of the BHD syndrome. Even though the histologic types (clear cell carcinoma and papillary carcinoma) were not the common form of BHD syndrome, they could also occur.19,20
A population-based study showed that patients with a history of thyroid cancer had an elevated risk of subsequent renal cancer, a 2.0-fold increase in women and a 4.5-fold increase in men, and in patients with renal cancer, there was a 1.5-fold and a 3.0-fold increase in the prevalence of subsequent thyroid cancer in women and men, respectively.21 This was consistent with the results of other studies.22–25 The co-occurrence of thyroid cancer and RCC could be associated with risk factors such as environmental, genetic predisposition, and therapy-related. The bidirectional associations between thyroid cancer and RCC were more likely explained by germline mutations, which could lead to the occurrence of them at the role of the environment.26
Thyroid cancer had been found with a greater frequency than expected in the Cowden syndrome, familial adenomatous polyposis (FAP), and multiple endocrine neoplasia type 2A (MEN2A).27–29 Thyroid cancer was also reported in BHD syndrome patients.1,11,15,20,30 The original BHD syndrome patients were found in a family in which 6 of 9 siblings had medullary thyroid carcinoma.1 A recent case report described a BHD patient with a primary clear cell carcinoma of the thyroid, and the c.1062G>C mutation in exon 9 of FLCN was identified both in the patient's blood DNA and thyroid tumor DNA, which seemed to provide molecular evidence for this association.30 However, Warren et al31 did not find any FLCN mRNA expression in normal thyroid. Nowadays, no firm conclusion could be stated about the association between BHD syndrome and thyroid cancer. Notably, in our study, PTCs of the 2 patients were bilateral and multifocal. Although thyroid lesions were small, lymph node metastases occurred, and patient 2 even had lateral lymph node involvement. It is unsure whether thyroid cancer is susceptible to exhibit bilaterality and lymph node metastasis in BHD syndrome because the case number in our study was too small. However, it suggests that total thyroidectomy and central compartment neck dissection may be suitable for thyroid cancer patients with BHD syndrome. According to Kluger et al,15 there was at least 1 member with a thyroid nodule in 90% of the affected BHD families and 65% of the screened individuals displaying this lesion. So, the neck ultrasound is recommended for BHD patients and their family members. And, we are in agreement with that large-scale investigation should be conducted to evaluate the prevalence of thyroid cancer or thyroid nodule in patients with BHD syndrome.
Skin lesions, lung disease, and renal cancer could not appear simultaneously in a patient with BHD syndrome. This may be an explanation that BHD syndrome is often overlooked. For patients whose clinical features are atypical, detection of germline mutation in FLCN gene would help confirm diagnosis. The 2 mutations we reported would expand the mutation spectrum of FLCN gene associated with BHD syndrome. For the thyroid cancer patients with BHD syndrome, total thyroidectomy and prophylactic bilateral central lymph node dissection may be suitable and the neck ultrasound may benefit BHD patients and their family members.
Footnotes
Abbreviations: BHD = Birt–Hogg–Dubé, FAP = familial adenomatous polyposis, MEN2A = multiple endocrine neoplasia type 2A, PTC = papillary thyroid carcinoma, RCC = renal cell carcinoma.
LD and MG have contributed equally to this work.
This work was supported by National Natural Science Foundation of China (No. 81472580 and No. 81272282), the Natural Science Foundation of Tianjin, China (No. 13JCQNJC10200), and the Science and Technology Foundation of Tianjin Public Health Bureau, China (No. 2013KZ087).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977; 113:1674–1677. [PubMed] [Google Scholar]
- 2.Roth JS, Rabinowitz AD, Benson M, et al. Bilateral renal cell carcinoma in the Birt-Hogg-Dube syndrome. J Am Acad Dermatol 1993; 29:1055–1056. [DOI] [PubMed] [Google Scholar]
- 3.Toro JR, Glenn G, Duray P, et al. Birt-Hogg-Dube syndrome: a novel marker of kidney neoplasia. Arch Dermatol 1999; 135:1195–1202. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects,;1; and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2002; 2:157–164. [DOI] [PubMed] [Google Scholar]
- 5.van Steensel MA, Verstraeten VL, Frank J, et al. Novel mutations in the BHD gene and absence of loss of heterozygosity in fibrofolliculomas of Birt-Hogg-Dube patients. J Invest Dermatol 2007; 127:588–593. [DOI] [PubMed] [Google Scholar]
- 6.Welsch MJ, Krunic A, Medenica MM. Birt-Hogg-Dube syndrome. Int J Dermatol 2005; 44:668–673. [DOI] [PubMed] [Google Scholar]
- 7.Kunogi M, Kurihara M, Ikegami TS, et al. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet 2010; 47:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluijt I, de Jong D, Teertstra HJ, et al. Early onset of renal cancer in a family with Birt-Hogg-Dube syndrome. Clin Genet 2009; 75:537–543. [DOI] [PubMed] [Google Scholar]
- 9.Diamond JM, Kotloff RM. Recurrent spontaneous pneumothorax as the presenting sign of the Birt-Hogg-Dube syndrome. Ann Intern Med 2009; 150:289–290. [DOI] [PubMed] [Google Scholar]
- 10.Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dube syndrome: diagnosis and management. Lancet Oncol 2009; 10:1199–1206. [DOI] [PubMed] [Google Scholar]
- 11.Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet 2008; 45:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dube syndrome. Am J Respir Crit Care Med 2007; 175:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zbar B, Alvord WG, Glenn G, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev 2002; 11:393–400. [PubMed] [Google Scholar]
- 14.Hudon V, Sabourin S, Dydensborg AB, et al. Renal tumour suppressor function of the Birt-Hogg-Dube syndrome gene product folliculin. J Med Genet 2010; 47:182–189. [DOI] [PubMed] [Google Scholar]
- 15.Kluger N, Giraud S, Coupier I, et al. Birt-Hogg-Dube syndrome: clinical and genetic studies of 10 French families. Br J Dermatol 2010; 162:527–537. [DOI] [PubMed] [Google Scholar]
- 16.Hartman TR, Nicolas E, Klein-Szanto A, et al. The role of the Birt-Hogg-Dube protein in mTOR activation and renal tumorigenesis. Oncogene 2009; 28:1594–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ornstein DK, Lubensky IA, Venzon D, et al. Prevalence of microscopic tumors in normal appearing renal parenchyma of patients with hereditary papillary renal cancer. J Urol 2000; 163:431–433. [PubMed] [Google Scholar]
- 18.Walther MM, Lubensky IA, Venzon D, et al. Prevalence of microscopic lesions in grossly normal renal parenchyma from patients with von Hippel-Lindau disease, sporadic renal cell carcinoma and no renal disease: clinical implications. J Urol 1995; 154:2010–2014. [PubMed] [Google Scholar]
- 19.Pavlovich CP, Grubb RR, Hurley K, et al. Evaluation and management of renal tumors in the Birt-Hogg-Dube syndrome. J Urol 2005; 173:1482–1486. [DOI] [PubMed] [Google Scholar]
- 20.Fahmy W, Safwat AS, Bissada NK, et al. Multiple/bilateral renal tumors in patients with Birt-Hogg-Dube syndrome. Int Urol Nephrol 2007; 39:995–999. [DOI] [PubMed] [Google Scholar]
- 21.Van Fossen VL, Wilhelm SM, Eaton JL, et al. Association of thyroid, breast and renal cell cancer: a population-based study of the prevalence of second malignancies. Ann Surg Oncol 2013; 20:1341–1347. [DOI] [PubMed] [Google Scholar]
- 22.Lal G, Groff M, Howe JR, et al. Risk of subsequent primary thyroid cancer after another malignancy: latency trends in a population-based study. Ann Surg Oncol 2012; 19:1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandeep TC, Strachan MW, Reynolds RM, et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab 2006; 91:1819–1825. [DOI] [PubMed] [Google Scholar]
- 24.Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer 2005; 117:281–288. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CH, Huang CL, Hsu YH, et al. Co-occurrence of second primary malignancy in patients with thyroid cancer. QJM 2014; 107:643–648. [DOI] [PubMed] [Google Scholar]
- 26.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer 2003; 89:1638–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cetta F, Montalto G, Gori M, et al. Germline mutations of the APC gene in patients with familial adenomatous polyposis-associated thyroid carcinoma: results from a European cooperative study. J Clin Endocrinol Metab 2000; 85:286–292. [DOI] [PubMed] [Google Scholar]
- 28.Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 1997; 16:64–67. [DOI] [PubMed] [Google Scholar]
- 29.Biscolla RP, Ugolini C, Sculli M, et al. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid 2004; 14:946–952. [DOI] [PubMed] [Google Scholar]
- 30.Benusiglio PR, Gad S, Massard C, et al. Case report: expanding the tumour spectrum associated with the Birt-Hogg-Dube cancer susceptibility syndrome. F1000Res 2014; 3:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren MB, Torres-Cabala CA, Turner ML, et al. Expression of Birt-Hogg-Dube gene mRNA in normal and neoplastic human tissues. Mod Pathol 2004; 17:998–1011. [DOI] [PubMed] [Google Scholar]


