Abstract
Chronic hepatitis C (CHC) disproportionately affects racial minorities in the United States (US). Although prior studies have reported lower treatment rates in Blacks than in Caucasians, the rates of other minorities remain understudied. We aimed to examine antiviral treatment rates by race and to evaluate the effect of other demographic, medical, and psychiatric factors on treatment rates. We performed a population-based study of adult CHC patients identified via ICD-9CM query from OptumInsight's Data Mart from January 2009 to December 2013. Antiviral treatment was defined by pharmaceutical claims for interferon and/or pegylated-interferon. A total of 73,665 insured patients were included: 51,282 Caucasians, 10,493 Blacks, 8679 Hispanics, and 3211 Asians. Caucasians had the highest treatment rate (10.7%) followed by Blacks (8.8%), Hispanics (8.8%), and Asians (7.9%, P < .001). Hispanics had the highest cirrhosis rates compared with Caucasians, Blacks, and Asians (20.7% vs 18.3%, 17.1%, and 14.3%, respectively). Caucasians were the most likely to have a psychiatric comorbidity (20.1%) and Blacks the most likely to have a medical comorbidity (44%). Asians were the least likely to have a psychiatric (6.4%) or medical comorbidity (26.9%). On multivariate analysis, racial minority was a significant predictor of nontreatment with odds ratios of 0.82 [confidence interval (CI): 0.74–0.90] for Blacks, 0.87 (CI: 0.78–0.96) for Hispanics, and 0.73 (CI: 0.62–0.86) for Asians versus Caucasians. Racial minorities had lower treatment rates than Caucasians. Despite fewer medical and psychiatric comorbidities and higher incomes and educational levels, Asians had the lowest treatment rates. Hispanics also had lower treatment rates than Caucasians despite having higher rates of cirrhosis. Future studies should aim to identify underlying racial-related barriers to hepatitis C virus treatment besides socioeconomic status and medical or psychiatric comorbidities.
INTRODUCTION
Chronic hepatitis C (CHC) affects 170 million individuals worldwide and approximately 3.2 million individuals in the United States.1,2 In the US, although the majority of CHC-infected are Caucasians, racial minorities are disproportionately affected. According to the National Health and Nutrition Examination Survey, the prevalence of CHC in Hispanics and Blacks doubles that of Caucasians (2.1% in Hispanics and 3.2% in Blacks vs 1.5% in Caucasians).2 In Asians, community-based studies estimate the prevalence to range from 3% to 6% mirroring prevalence from birth countries.3–7 Race also influences the disease course of CHC with higher rates of cirrhosis in Hispanics that has been attributed to an earlier age of infection and higher rates of nonalcoholic fatty liver disease (NAFLD).8 In contrast, Blacks have a much lower rate of cirrhosis but have higher mortality rates and complications after developing cirrhosis.9
Race also influences nearly all aspects of CHC management including treatment outcomes and access to care and prior studies have suggested that it is poorer for racial minorities.10 For much of the 21st century, the standard of care for CHC was dual therapy with pegylated-interferon (PEG-IFN) and ribavirin (RBV). Compared with Caucasians, Blacks have lower sustained virologic response (SVR) rates to dual therapy, due in part to unfavorable single nucleotide polymorphisms near the IL-28B gene.11,12 There are also differences in treatment response in Hispanics and Asians.13–15 Fortunately, the addition of second-generation protease inhibitors (PIs) and PEG-IFN free regimens have minimized these differences with SVR rates over 90% for patients infected with hepatitis C virus (HCV) genotype 1, regardless of race.16–20
As we develop more efficacious antiviral treatments, it becomes more important to study treatment rates and barriers to treatment initiation. In a large Veteran's affair (VA) cohort, Cheung et al21 reported similar treatment rates in Hispanics than in Caucasians despite higher eligibility rates in the Hispanic cohort. Rousseau et al22 also reported that Blacks were less likely than Caucasians to have appropriate testing, follow-up, and treatment after diagnosis with CHC.
However, reasons for low rates of treatments among CHC-infected minorities and especially in Hispanic and Asian populations are not well studied. Therefore, we conducted a retrospective population-based study of 73,665 patients with CHC from a large national insurance claims database. Our aims were to examine treatment rates of CHC by race and to determine the influence of other demographic characteristics as well as medical and psychiatric comorbidities on treatment rates.
METHODS
Study Population and Data Source
We collected patient information from Optum's Clinformatics Data Mart, a commercially available database of administrative claims submitted for payment by providers and pharmacies. This database includes demographics and medical, lab work, and prescription claims of patients from all 50 states.
Patients were selected on the basis of a diagnosis of hepatitis C infection made by the International Classification of Disease, Ninth Revision (ICD-9CM) coding (070.44, 070.54, 070.70, 070.71, 070.41, and 070.51) from January 2009 to December 2013. The index date was the first date of IFN-prescription for those treated or the earliest date of a medical claim if the patient did not receive treatment. Patients were excluded if their race was not specified or if age was <18 years at index date. Ethical review by an institutional review board was not necessary, as the database contained only deidentified patient information.
Sociodemographic Characteristics
The following socioeconomic data were collected: year of birth, race and ethnicity (Caucasian, Black, Hispanic, or Asian), gender, geographic state of insurance policy, household income, education level, and insurance type (Medicaid or private). US geographic regions (West, Midwest, South, or Northeast) were defined on the basis of designations by the United States Census Bureau.23 Race was self-reported or derived on the basis of patient name.
Medical and Psychiatric Comorbidities
Rates of medical and psychiatric comorbidities were also assessed by ICD-9CM code. These included autoimmune hepatitis, alcoholic liver disease, NAFLD, mixed cryologlobulinemia, hepatocellular carcinoma (HCC), liver, renal, heart, and/or lung transplantation, cardiac arrhythmias, coronary artery disease, congestive heart failure, chronic obstructive lung disease, human immunodeficiency virus (HIV), cerebrovascular disease, type 2 diabetes, chronic kidney disease, cancer, peripheral vascular disease, seizure disorder, bipolar or major depressive disorder, schizophrenia, substance abuse, and cirrhosis. Cirrhosis was defined by ICD-9CM codes (571.2, 571.5, and 571.6) or by ICD-9CM diagnosis of portal hypertension (including portal hypertensive gastropathy and hepatopulmonary syndrome), esophageal varices, ascites, spontaneous bacterial peritonitis, end-stage liver disease, or hepatic encephalopathy. Where applicable, ICD-9CM codes were selected on the basis of those from the Charlson-Deyo Comorbidity Index.24
HCV Treatment Prescription and Treatment Persistency
Anti-HCV prescription rates were calculated from pharmaceutical claims for any IFN or PEG-IFN containing regimen. Prescriptions of RBV and first-generation PIs (telaprevir and boceprevir) were also collected. Patients receiving RBV, telaprevir, and/or boceprevir without IFN or PEG-IFN were not counted toward as having received antiviral treatment.
Treatment persistency, defined as the act of continuing the treatment for a prescribed duration, was calculated by length of IFN or PEG-IFN therapy from the first day covered by an IFN or PEG-IFN prescription until the last day covered by the final prescription.25 A grace period of 30 days was allotted with time gaps between prescription coverage days. Only the first treatment course was considered for patients who received multiple treatment courses during the study period. As HCV genotype data were not available for the majority of patients, we analyzed treatment persistency up to 24 weeks, which is the shorter duration of treatment for the easier-to-treat HCV genotypes 2/3 for dual therapy.
Data Analysis
Baseline demographic and medical characteristics were reported as mean and standard deviation for continuous variables and percentages for categorical variables. The χ2 test and Mann–Whitney U test were used to compare categorical and nonparametric continuous variables, respectively. To assess factors that may influence anti-HCV treatment practices, univariate and multivariate regression analyses were performed. A 2-tailed P value of ≤0.05 was considered statistically significant. The Kaplan–Meier method was used to illustrate treatment persistence. Comparison of separate time-to-event analyses was done using the log-rank test. All analyses were performed using STATA version 13.0 (STATA Corporation, College Station, TX).
RESULTS
Demographics and Treatment Rates
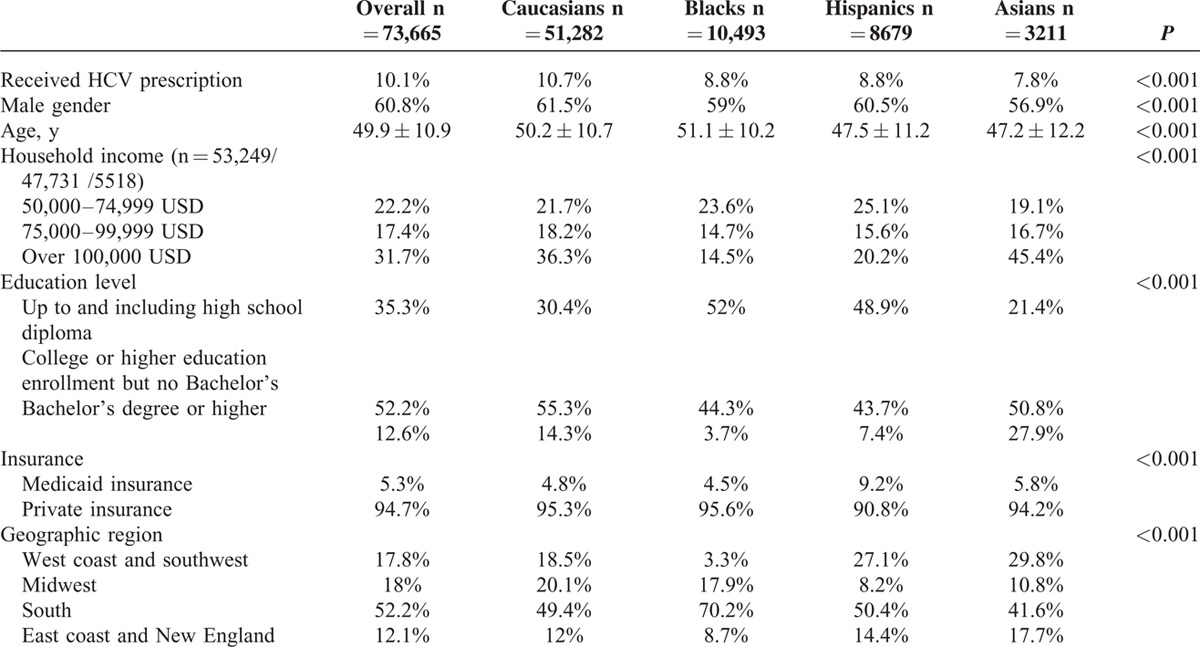
A total of 77,731 patients with CHC were queried with demographic, medical, and prescription data for review. Patients younger than 18 years (n = 740), with unknown race (n = 3193), and without any medical history or pharmaceutical data (n = 133) were excluded. Our final cohort consisted of 51,282 Caucasians, 10,493 Blacks, 8679 Hispanics, and 3211 Asians who were included in the primary analysis (Table 1). The majority of patients were male (60.8%) with a mean age of 49.9 years. Hispanics and Asians were younger than Caucasians and Blacks (mean ages: 47.5 for Hispanics, 47.2 for Asians, 50.2 for Caucasians and 51.1 yrs for Blacks, P < 0.001). The proportion of patients with an educational level at least at a bachelor's degree was highest in Asians (27.9%) followed by Caucasians (14.3%), Hispanics (7.4%), and Blacks (3.7%, P < 0.001). Similarly, proportions of household incomes over 100,000 USD were higher in Asians (45%) and Caucasians (36%) and lower in Hispanics (20%) and Blacks (15%, P < 0.001). Geographically, the majority of our patients were from Southern states (51.7%), and 17.8% were from the West coast and Southwest, 18% were from the Midwest, and 12.1% were from the East coast. Hispanics were more likely to have Medicaid insurance (9.2%) than other racial groups (4.8% for Caucasians, 4.5% for Blacks, 5.8% for Asians, P < 0.001).
TABLE 1.
Baseline Demographics by Race and Ethnicity
Overall, 7430 of 73,665 (10.1%) adult patients with CHC were prescribed antiviral treatment during the study period. A slightly higher number of patients were treated in the pre-PI period from January 2009 to May 2011 (3988 of 7430, 53.6%) than in the post-PI period from June 2011 to December 2013 (3442 of 7430, 46.4%) (Figure 1). Caucasians had the highest treatment rates (10.7%) followed by Blacks and Hispanics (8.8%). Asians had the lowest treatment rates (7.9%, P < 0.001). When stratified by gender and race, a significantly lower proportion of Asian females (6.9%) were prescribed antiviral treatment than any other gender and racial group (Figure 2).
FIGURE 1.
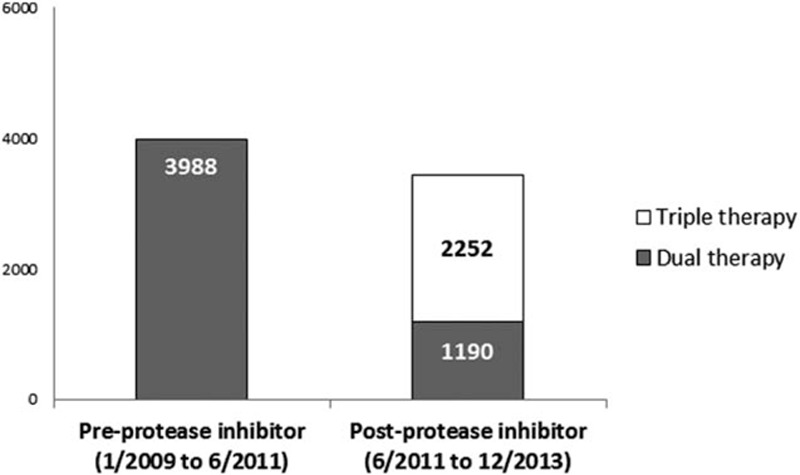
Number of antiviral prescriptions from January 2009 to June2011 (before introduction of protease inhibitors) and June 2011 to December 2013 (after the introduction of protease inhibitors). N = 3988 in the pre-protease inhibitor period and n = 3442 (2252 prescriptions of triple therapy and 1190 of dual therapy) in the post-protease inhibitor period.
FIGURE 2.
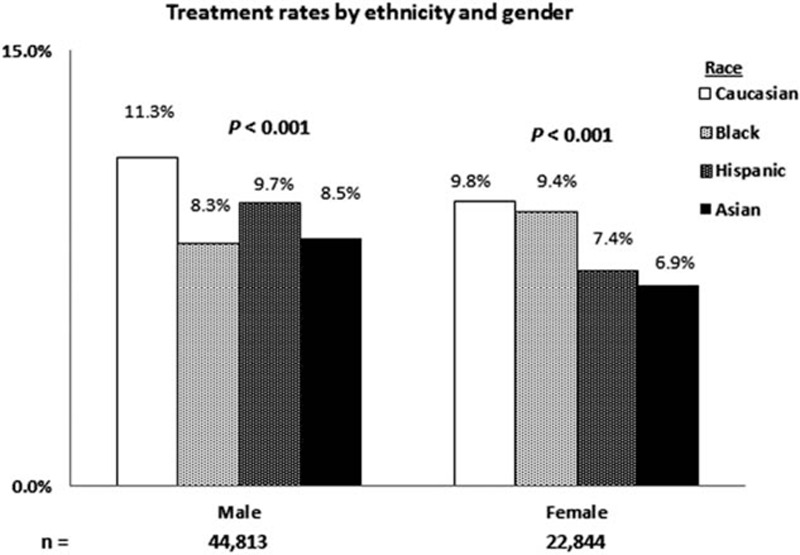
Treatment rates across the study period as stratified by ethnicity and gender. In both genders, Caucasians had the highest treatment rates and Asians had the lowest (P = 0.001). Caucasian males had the highest treatment rates (11.3%), whereas Asian females had the lowest treatment rates (6.9%).
Concurrent Liver-Related Diseases and Characteristics by Race
HCV genotype data were available for 3361 of 73,665 (4.6%) patients, and of them, approximately 95% had HCV genotype 1 infection, 2.8% had genotype 2, and 0.1% had genotype 6 (Table 2). On subanalysis, 819 of 3361 (24.3%) patients received antiviral treatment (data described only). A significant proportion of patients had liver-related comorbidities, including NAFLD (13.6%) and alcoholic liver disease (5.7%). Hispanics were also most likely to have NAFLD (17.3%) and alcoholic liver disease (7.2%).
TABLE 2.
Virologic Characteristics, Dual Infection, Dual Liver Disease, and Extrahepatic Manifestations by Race and Ethnicity
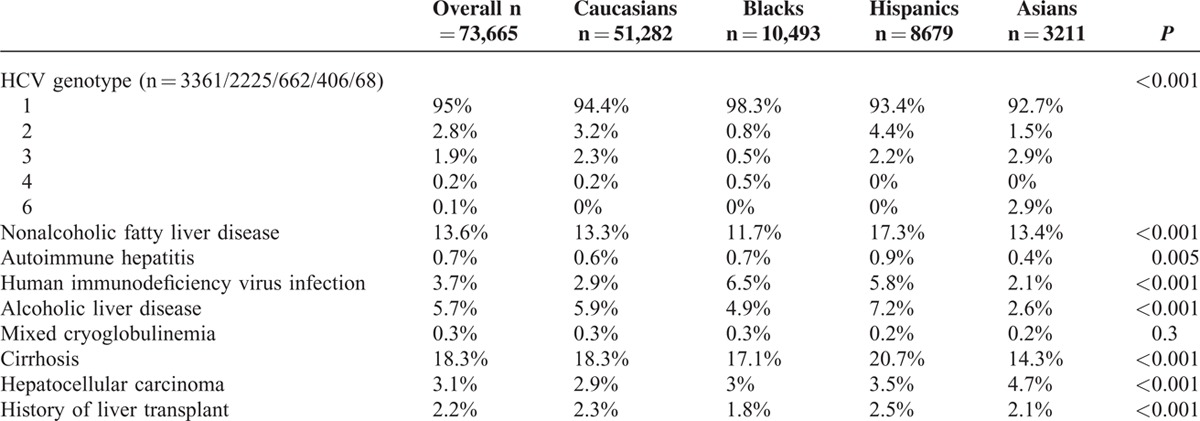
The overall rate of cirrhosis was 18.3% with the highest proportion in Hispanics (20.7%) and the lowest in Asians (14.3%). Cirrhosis rates were intermediate in Caucasians (18.3%) and Blacks (17.1%, P < 0.001). Rates of HCC were highest in Asians (4.7%) compared with Hispanics (2.5%), Caucasians (2.3%), and Blacks (1.8%, P < 0.001).
Medical and Psychiatric Comorbidities by Race
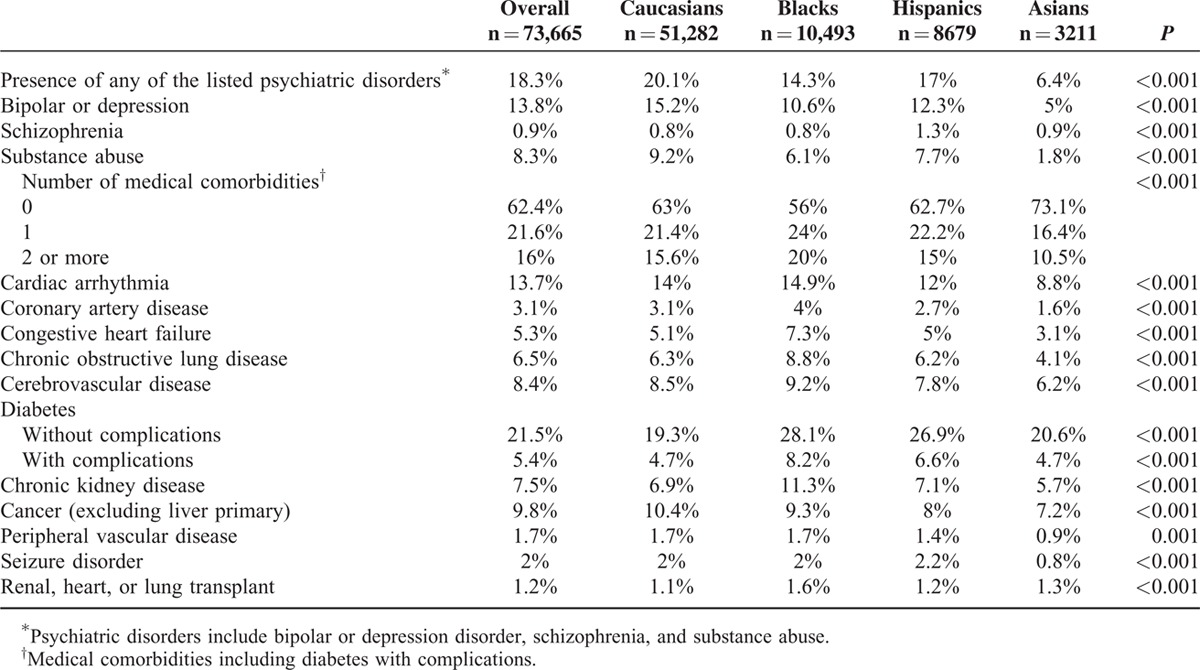
Prevalence of individual medical and psychiatric comorbidities is listed in Table 3 and analyzed by race. Blacks were most likely to have a listed medical comorbidity (44%) followed by Caucasians and Hispanics (∼37%), and Asians were least likely to have a comorbidity (26.9%, P < 0.001). Caucasians were more likely to have a psychiatric disorder (20.1%) than Blacks (14.3%), Hispanics (17%), and Asians (6.4%, p < .001).
TABLE 3.
Medical and Psychiatric Comorbidities by Race and Ethnicity
Multivariate Logistic Regression Model for Treatment Prescription
In a multivariate logistic regression model, significant predictors of nontreatment were female gender [odds ratio (OR) = 0.91, confidence interval (CI): 0.86–0.97, P = 0.003], older age (75 and older vs 18 to 54 yrs, OR = 0.44, CI: 0.26–0.76, P = 0.003), any minority race versus Caucasians (OR = 0.82, CI: 0.74–0.9, P < 0.001 for Blacks; 0.87, CI: 0.78–0.96, P = 0.004 for Hispanics; 0.73; CI: 0.62–0.86, P < 0.001 for Asians), HIV coinfection (OR = 0.77, CI: 0.65–0.92, P = 0.004), and diabetes (OR = 0.8; CI: 0.74–0.86, P < 0.001) (Table 4). On univariate analysis, a history of major psychiatric disorder was a weak predictor of nontreatment and was not a significant predictor on multivariate analysis (OR = 1, CI: 0.97–1.1, P = 0.27). A higher education level (OR = 1.02, CI: 0.97–1.07, P = 0.39) was also not predictive of treatment prescription on univariate analysis and was not included in the multivariate model.
TABLE 4.
Logistic Regression Analysis Predicting Anti-HCV Prescription
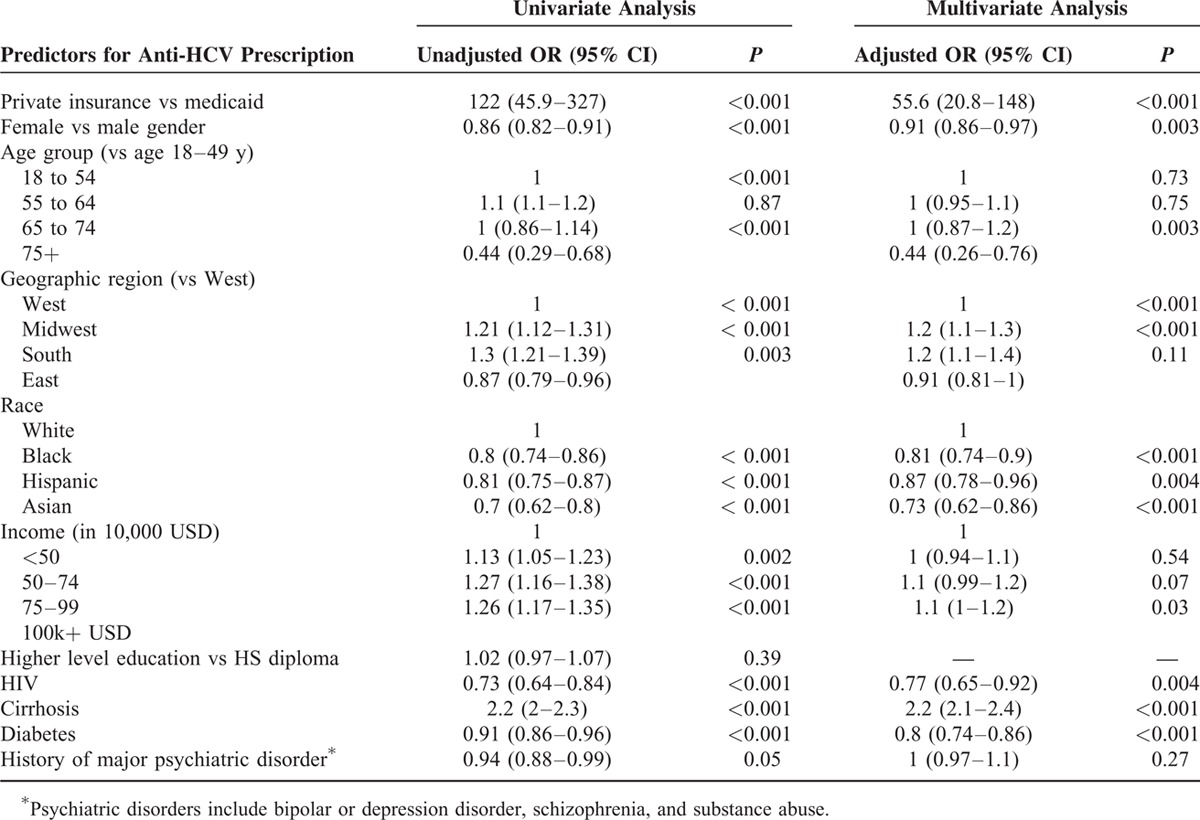
Significant predictors of receiving antiviral treatment were private insurance versus Medicaid (OR = 55.6, CI: 20.8–148, P < 0.001), cirrhosis (OR 2.2, CI: 2.1–2.4, P < 0.001), geographic residence in the Midwest (OR = 1.2, CI: 1.1–1.3, P < 0.001) or South (OR = 1.3, CI: 1.1–1.4, P < 0.001) versus West, and a household income of <50,000 USD vs > 100,000 USD (OR = 1.1, CI: 1–1.2, P = 0.03).
Treatment Persistency by Race
At 12 weeks of therapy, the overall persistency rate was 68%. Rates were highest in Asians and Caucasians, and lowest in Blacks (Caucasians = 69.2%, Black = 64%, Hispanic = 66.7%, Asian at 69.2%) (Figure 3). At 24 weeks, nearly two-thirds of patients dropped out of treatment and only 41.3% remained persistent. Drop-out rates at this time point were also highest in Blacks and Hispanics compared with Caucasians and Asians resulting in a persistency rate of only 35.3% in Blacks, 41.4% in Hispanics, 42.2% in Caucasians, and 44.4% in Asians.
FIGURE 3.
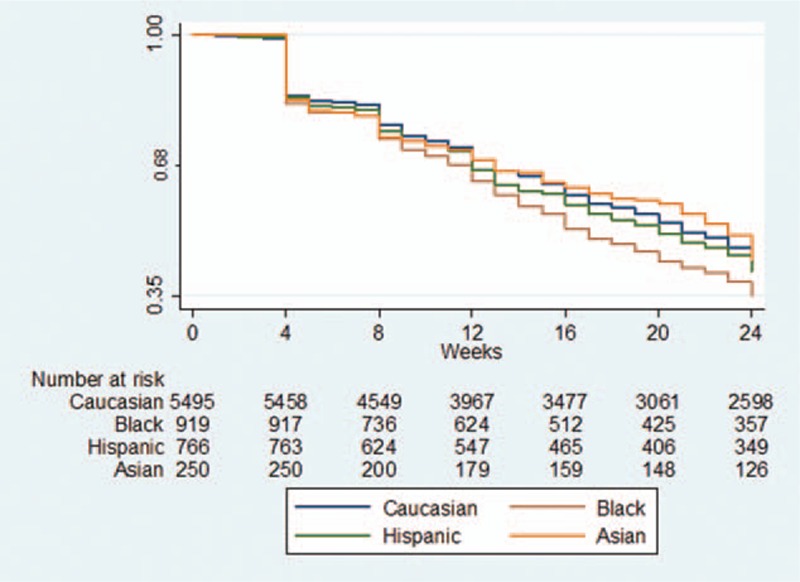
Antiviral treatment persistency rates by race. The blue curve represents Caucasians, green curve Hispanics, brown curve Blacks, and orange curve Asians. At 24 weeks, nearly two-thirds of patients dropped out of treatment and only 41.3% remained persistent.
DISCUSSION
In this large US cohort of insured patients with CHC, we found that only 10.1% (7430 of 73,665) were prescribed antiviral therapy during the 5-year study period and racial minorities were independently associated with not receiving treatment even after adjusting for socioeconomic status and medical and psychiatric comorbidities.
Our treatment prescription rate is consistent with other population-based studies from VA databases and suggests treatment underutilization in the US. Several factors may contribute to lower treatment rates. Kramer et al,26 who reported treatment rates at 11.6%, noted that 40% of patients did not receive appropriate testing including HCV genotype testing. In our study, we had limited access to HCV genotype data. However, the treatment rate for patients with available HCV genotype data was still low at 819 of 3361 (24.4%), though significantly higher than the overall treatment rate of 10.1%. In addition, it is likely that the absence of HCV genotype testing is due to the lack of anti-HCV therapy intention. Our findings are divergent from those of another US population-based study conducted by Shatin et al,27 who reported a higher treatment rate of 30.3% in 3259 patients with CHC. Underlying differences in our methodologies and timing may play a role, as Shatin et al27 examined patient claims from 1997 to 1999, which was a period of transition from IFN monotherapy to dual therapy with RBV. The higher efficacy of dual therapy at that time may have persuaded many providers to treat patients as a result.
Other major barriers to treatment include medical or psychiatric contraindication to HCV therapy and patient-provider reluctance to initiate treatment given adverse effects. Although we were not able to directly measure the effects of these barriers, it is likely that these barriers contributed to the persistently low rate of treatment even as the more efficacious triple therapy (PEG-IFN, RBV, and telaprevir or boceprevir) was introduced in May 2011 (Figure 1). For those who initiated triple therapy, SVR rates in real-life practice have turned out to be much lower than the reported rates from clinical trials at (50% vs 70%).28
We also found significant disparities with lower treatment rates in racial minorities. Compared with Caucasians, the odds of treatment in Blacks, Hispanics, and Asians were 19%, 13%, and 27% lower, respectively, on multivariate analysis. Racial minority status remained an independent predictor of nontreatment even after controlling for socioeconomic factors and medical and psychiatric comorbidities. Butt et al29 also reported lower treatment rates in Blacks and Hispanics than in Caucasians, and Melia et al30 found that Blacks may also have higher treatment ineligibility rates due to comorbidities including uncontrolled diabetes, chronic kidney disease, and cytopenias. In our study, we did find Blacks to have higher rates of medical comorbidities, however, even after adjusting for these factors, suggesting that there is race-related factor affecting treatment rate beyond socioeconomic and comorbidity factors. Blacks also had the highest dropout rates while on HCV therapy, with only 35.3% remaining persistent on therapy at 24 weeks, perhaps owing to higher baseline medical comorbidity rates at presentation.
In contrast, Asians, despite a more favorable socioeconomic and medical/psychiatric profile and highest HCC rates, had the lowest treatment rates. Asian American females in particular had the lowest treatment rates of any racial/gender group with treatment rates nearly 40% lower than Caucasian males and 30% lower than Caucasian females (Figure 2). Prior studies of not only CHC populations but also more broadly of Asian populations with chronic diseases have reported that Asians were less likely than other races to see a physician for chronic illnesses.31,32 Poor linkage to care for CHC in Asians may mirror that for chronic hepatitis B, a much more appreciated disease in this population.
Finally, Hispanics had lower treatment rates than Caucasians but similar to Blacks. Socioeconomic factors may play a role, as Hispanics had lower household incomes and the highest proportion of Medicaid insurance (9.2%). Cheung et al21 noted in a large VA population that despite higher treatment eligibility rates in Hispanics than Caucasians, treatment rates were comparable and hypothesized that socioeconomic status and higher concurrent rates of alcohol abuse may have played a role. Similarly, we found Hispanics to have the highest rates of alcoholic liver disease compared with other racial groups. In addition, Hispanics also had the highest rates of cirrhosis (a strong independent predictor of HCV treatment), perhaps owing to a higher prevalence of NAFLD and alcoholic liver disease, but again despite of this, Hispanics had a lower observed HCV treatment rate.
Our study is limited by its retrospective design and had weaknesses inherent to its use of insurance claims data with lack of detailed clinical and laboratory information. We also did not have comprehensive access to laboratory data and were unable to ascertain HCV genotype, HCV RNA levels, or liver tests such as alanine transferase or aspartate transferase enzyme levels in many of our patients. As we did not have HCV RNA levels for most patients, we were unable to exclude the proportion of patients without active HCV viremia, a population who would not need anti-HCV therapy, leading to potential underestimation of treatment. However, considering that approximately 80% of individuals with exposure to HCV infection (anti-HCV antibody positive) will remain viremic,33 our treatment rates would only increase from 10.1% to 12.5%: an absolute increase of 2.4%. There is also some level of ascertainment bias with psychiatric and medical comorbidity rates; as with any study based on insurance claims, we relied on providers’ accuracy in recording and reporting ICD-9CM codes. The receipt of a prescription claim also does not necessarily mean that the patient consumed the medication; however, approximately 85% of patients had more than 1 prescription for IFN or PEG-IFN suggesting that most patients who were prescribed therapy did consume some quantity of it. Our results may be better interpreted in terms of odds and risk estimates instead of absolute values for this reason. Nevertheless, the above biases were likely to lead to an overestimation of true treatment rate and treatment persistency.
In summary, HCV treatment is underutilized even in a large population of insured patients with a treatment rate of only 10.1%. We found significant racial disparities with minorities being associated with lower treatment rates, and race remained an independent predictor even after adjusting for socioeconomic status, other demographic factors, and medical/psychiatric comorbidities. Specifically Hispanics, despite having fewer psychiatric comorbidities and higher rates of cirrhosis than Caucasians, had lower treatment rates. Asians also had lower treatment rates despite higher income and educational levels and lower psychiatric and medical comorbidities. Black patients also had the lower treatment rate and the lowest treatment persistency rate for those few patients who were started on therapy. Future studies should aim to identify underlying racial and ethnic barriers to antiviral treatment besides socioeconomic status and medical/psychiatric comorbidities and further educational, clinical, and research efforts are needed to optimize CHC treatment rates. Such barriers are even more relevant with the introduction of efficacious and well-tolerated antiviral acting agents that should eliminate most barriers related to comorbidities, thus making the remaining socioeconomic and racial factors even more prominent.
Footnotes
Abbreviations: CHC = Chronic hepatitis C, HCC = Hepatocellular carcinoma, ICD-9CM = International Classification of Diseases Ninth Revision, Clinical Modification, IFN = interferon, PEG-IFN = pegylated interferon, PI = protease inhibitor, RBV = ribavirin, SVR = sustained virologic response, VA = Veteran's affairs.
PV planned and conducted the study, collected and interpreted data, drafted the manuscript, provided statistical expertise, and has approved the submitted final draft. JH collected and interpreted data and has approved the submitted final draft. LBJr collected and interpreted data and has approved the submitted final draft. NHN planned and interpreted the data and has approved the submitted final draft. MHN planned and conducted the study, collected and interpreted data, provided statistical expertise, drafted the manuscript, and has approved the submitted final draft.
This study was supported by a grant from Gilead Sciences Inc. The study sponsor provided funding for the study and reviewed and approved the final version of this manuscript. Gilead Sciences had no role in the design and conduct of the study, analysis, interpretation, or preparation of the article.
Louis Brooks Jr. is a current employee of Optum Inc.
MHN is a member of Advisory board honorarium and received research support from Janssen Pharmaceuticals, Gilead Sciences, Bristol Myer Squibbs, and Roche Laboratories.
P.V., J.H., and N.H.N. have no conflicts of interests to disclose.
L.B.Jr. is a current employee of Optum Inc.
M.H.N. receives advisory board honorarium and research support from Janssen Pharmaceuticals, Gilead Sciences, Bristol Myer Squibbs, and Roche Laboratories.
REFERENCES
- 1.1998; Di Bisceglie AM, Hepatitis C. Lancet. 351:351–355. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714. [DOI] [PubMed] [Google Scholar]
- 3.Dev A, Sundararajan V, Sievert W. Ethnic and cultural determinants influence risk assessment for hepatitis C acquisition. J Gastroenterol Hepatol 2004; 19:792–798. [DOI] [PubMed] [Google Scholar]
- 4.Ho EY, Ha NB, Ahmed A, et al. Prospective study of risk factors for hepatitis C virus acquisition by Caucasian, Hispanic, and Asian American patients. J Viral Hepat 2012; 19:e105–e111. [DOI] [PubMed] [Google Scholar]
- 5.Kin KC, Lin B, Chaung KT, et al. Less-established risk factors are common in Asian Americans with hepatitis C virus: a case-controlled study. Dig Dis Sci 2013; 58:3342–3347. [DOI] [PubMed] [Google Scholar]
- 6.Kin KC, Lin B, Ha NB, et al. High proportion of hepatitis C virus in community Asian American patients with non-liver-related complaints. J Clin Gastroenterol 2013; 47:367–371. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther 2013; 37:921–936. [DOI] [PubMed] [Google Scholar]
- 8.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Kramer J, Duan Z, et al. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol 2014; 109:1427–1435. [DOI] [PubMed] [Google Scholar]
- 10.Ditah I, Al Bawardy B, Gonzalez HC, et al. Lack of health insurance limits the benefits of hepatitis C virus screening: insights from the National Health and Nutrition Examination Hepatitis C follow-up study. Am J Gastroenterol 2015; 110:1126–1133. [DOI] [PubMed] [Google Scholar]
- 11.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461:399–401. [DOI] [PubMed] [Google Scholar]
- 12.Muir AJ, Bornstein JD, Killenberg PG. Atlantic Coast Hepatitis Treatment G. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 2004; 350:2265–2271. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen NH, VuTien P, Garcia RT, et al. Response to pegylated interferon and ribavirin in Asian American patients with chronic hepatitis C genotypes 1 vs 2/3 vs 6. J Viral Hepat 2010; 17:691–697. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Torres M, Slim J, Bhatti L, et al. Peginterferon alfa-2a plus ribavirin for HIV-HCV genotype 1 coinfected patients: a randomized international trial. HIV Clin Trials 2012; 13:142–152. [DOI] [PubMed] [Google Scholar]
- 15.Vutien P, Nguyen NH, Trinh HN, et al. Similar treatment response to peginterferon and ribavirin in Asian and Caucasian patients with chronic hepatitis C. Am J Gastroenterol 2010; 105:1110–1115. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman ML, James AM, Long AG, et al. Treatment of chronic HCV with sofosbuvir and simeprevir in patients with cirrhosis and contraindications to interferon and/or ribavirin. Am J Gastroenterol 2015; 110:1179–1185. [DOI] [PubMed] [Google Scholar]
- 17.Wyles DL, Rodriguez-Torres M, Lawitz E, et al. All-oral combination of ledipasvir, vedroprevir, tegobuvir, and ribavirin in treatment-naive patients with genotype 1 HCV infection. Hepatology 2014; 60:56–64. [DOI] [PubMed] [Google Scholar]
- 18.Everson GT, Sims KD, Rodriguez-Torres M, et al. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology 2014; 146:420–429. [DOI] [PubMed] [Google Scholar]
- 19.Reddy KR, Bourliere M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 2015; 62:79–86. [DOI] [PubMed] [Google Scholar]
- 20.Reddy KR, Zeuzem S, Zoulim F, et al. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis 2015; 15:27–35. [DOI] [PubMed] [Google Scholar]
- 21.Cheung RC, Currie S, Shen H, et al. Chronic hepatitis C in Latinos: natural history, treatment eligibility, acceptance, and outcomes. Am J Gastroenterol 2005; 100:2186–2193. [DOI] [PubMed] [Google Scholar]
- 22.Rousseau CM, Ioannou GN, Todd-Stenberg JA, et al. Racial differences in the evaluation and treatment of hepatitis C among veterans: a retrospective cohort study. Am J Public Health 2008; 98:846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regions and Divisions. Organization: United States Census Bureau. Available at: http://www.census.gov/econ/census/help/geography/regions_and_divisions.html Accessed April 1, 2016. [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619. [DOI] [PubMed] [Google Scholar]
- 25.Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006; 15:565–574.discussion 575-567. [DOI] [PubMed] [Google Scholar]
- 26.Kramer JR, Kanwal F, Richardson P, et al. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol 2012; 56:320–325. [DOI] [PubMed] [Google Scholar]
- 27.Shatin D, Schech SD, Patel K, et al. Population-based hepatitis C surveillance and treatment in a national managed care organization. Am J Manag Care 2004; 10:250–256. [PubMed] [Google Scholar]
- 28.Vo KP, Vutien P, Akiyama MJ, et al. Poor sustained virological response in a multicenter real-life cohort of chronic hepatitis C patients treated with pegylated interferon and ribavirin plus telaprevir or boceprevir. Dig Dis Sci 2015; 60:1045–1051. [DOI] [PubMed] [Google Scholar]
- 29.Butt AA, Justice AC, Skanderson M, et al. Rate and predictors of treatment prescription for hepatitis C. Gut 2007; 56:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melia MT, Muir AJ, McCone J, et al. Racial differences in hepatitis C treatment eligibility. Hepatology 2011; 54:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tohme RA, Xing J, Liao Y, et al. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009-2010. Am J Public Health 2013; 103:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Chen CC, Nie X, et al. Racial and socioeconomic disparities in access to primary care among people with chronic conditions. J Am Board Fam Med 2014; 27:189–198. [DOI] [PubMed] [Google Scholar]
- 33.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol 2014; 61:S58–68. [DOI] [PubMed] [Google Scholar]


