Abstract
Patients with oral cavity squamous cell carcinoma (OSCC) undergoing surgery are recommended to receive adjuvant radiation therapy with or without chemotherapy if there are unfavorable prognostic factors. A positive resection margin (PRM) and extra-capsular extension (ECE) of lymph nodes are well-known major prognostic factors. However, there is no agreement on whether oral cavity cancer patients should receive postoperative chemo-radiotherapy (CCRT) if they present with other risk factors or a combination of 2 or more risk factors. In this study, we investigated this issue and provide suggestions for adjuvant treatments.
From January 2002 to December 2013, 567 OSCC patients who had undergone radical surgery were retrospectively reviewed. The 5-year loco-regional control (LRC), distant metastasis-free (DMF), disease-free survival (DFS), and overall survival (OS) were analyzed.
In univariate analysis, pathological T classification, positive node, tumor depth, ECE, lymphatic or vascular or perineural invasion and histology grade are significant prognostic factors for LRC, DMF, DFS, or OS. By multivariate analysis, pathological T4 (pT4), positive node, positive surgical margin are prognostic factors for LRC. pT4, positive node and lymphatic invasion predicted for higher rate of distant metastasis. pT4, positive node, and poor differentiation tumor were prognostic factors for DFS. pT4, positive nodes, and ECE were prognostic factors for OS. These factors were used to define risk groups. We proposed PRM and ECE as major risk factors and pT4, positive nodes, close margin (≤ 5 mm, > 1 mm), tumor depth ≥ 1 cm, lymphatic invasion, vascular invasion, perineural invasion, and poor differentiation as minor risk factors. By subgroups analysis, 192 patients with at least 2 minor prognostic factors and no other major risk factors, postoperative radiotherapy (RT), or CCRT yielded significantly better 5-year LRC, DFS, and OS compared to surgery only group. For 179 patients with at least 3 minor prognostic factors and/or at least 1 major risk factor, patients receiving postoperative CCRT showed significantly better 5-year LRC, DFS, and OS compared with post-OP RT or surgery alone.
Patients with 2 minor risk factors should receive postoperative RT. For patients with PRM, ECE, or >2 minor risk factors, postoperative CCRT is recommended.
INTRODUCTION
Patients with locally advanced oral cavity squamous cell carcinoma (OSCC) undergoing surgery are at high risk of recurrent disease. Adjuvant radiation therapy with or without chemotherapy is recommended for patients with unfavorable prognostic factors.1–6 In 2 large prospective randomized trials conducted in Europe (the European Organization Research and Treatment of Cancer [EORTC])2 and in the United States (the Radiation Therapy Oncology Group [RTOG]),3 postoperative (post-OP) concurrent chemo-radiotherapy (CCRT) with a high dose of cisplatin improved outcomes in high-risk patients with resected head and neck cancer. However, there was a marked difference in the selection criteria between these 2 trials. In the RTOG trial, patients with histologic evidence of invasion into 2 or more regional lymph nodes, extracapsular extension (ECE) of nodal disease and microscopically involved mucosal margins of resection were included. In the EORTC trial, wider inclusion criteria were adopted. Patients with T3 or T4 or N2-N3 or T1-2N0-1 with ECS, positive resection margin (PRM), perineural invasion, vascular tumor embolism or oral cavity, or oropharyngeal cancer with Level IV or V lymph node (LN) metastasis were all included. In both trials, acute toxicities were all significantly increased with post-OP CCRT compared with RT alone.2,3 Therefore, the decision whether to routinely offer post-OP CCRT for patients who fulfilled either the RTOG or EORTC criteria has proven difficult in a clinical setting.
In a subsequent comparative analysis using data pooled from both trials on selection criteria, clinical and pathologic risk factors and treatment outcomes (ECE) and/or PRM were the only risk factors for which the impact of CCRT was significant in both trials. Therefore, PRM and ECE of the lymph node are considered major prognostic factors in the decision to recommend post-OP CCRT for patients. However, there is still no consensus on whether oral cavity cancer patients should receive post-OP CCRT if there are other risk factors (such as T3/T4, N1, N2/N3, perineural invasion or vascular tumor embolism) or clusters of 2 or more risk factors. To clarify the effects of post-OP RT or CCRT in oral cavity patients, this retrospective study was carried out on a consecutive cohort of patients to identify high-risk patients in need of post-OP RT or CCRT.
MATERIALS AND METHODS
Patients and Disease Characteristics
From January 2002 to September 2013, 567 consecutive patients with nonmetastatic OSCC who had received radical surgery were enrolled. Patients aged < 18 or > 80 years old, those with end-stage renal disease, Child-Pugh C liver cirrhosis, or malignancy diagnosed within the preceding 5 years were excluded. This study was approved by the ethics Institutional Review Board (IRB) at our institution. A wavier of informed consent was obtained from the IRB (IRB No. 104–2223B). All patients underwent comprehensive workups, including head and neck examination, complete blood count, routine blood chemistry, bone scan, and abdominal sonography. A computed tomographic or magnetic resonance imaging scan of the head and neck and a chest radiograph were also taken. Primary tumors and involved lymph nodes were staged according to the 2010 staging classification of the American Joint Committee on Cancer.7 Data were retrieved from the Research Database of our hospital (Chang Gung Research Database).
Treatments
The surgical procedures included primary tumor excision with adequate margins plus unilateral, bilateral, selective or radical neck dissection when needed. The surgical defects were repaired with primary closure or reconstructed immediately by plastic surgeons using free or local flaps. Patients receiving biopsy or excisional biopsy only were excluded. Postoperative RT was suggested for patients with stage III or IV disease and was usually started within 6 weeks after the operation. The majority of pathological stage III and IV patients (219 of 289 patients, 75.8%) received post-OP RT. The initial RT treatment field was to irradiate the entire tumor bed and regional lymphatics with adequate margins with 6MV X-ray beams via 2-dimensional, 3-dimensional or intensity-modulated radiation therapy (IMRT). The prescribed dose was 1.8 to 2 Gy per fraction per day, given 5 days per week. Most patients received RT with a 60 to 66 Gy dose. Post-OP CCRT was suggested for patients with ECE or PRM and was given with a cisplatin-based regimen (cisplatin 30 mg/m2 weekly) or a biweekly cisplatin/tegafur/leucovorin regimen.8,9 Of 110 patients with ECE or PRM, 81 patients (73.6%) received post-OP RT and 56 patients (50.9%) received post-OP CCRT.
End Points and Follow-up Procedures
Study end points were loco-regional control (LRC), distant metastasis-free rate (DMF), disease-free survival (DFS), and overall survival (OS). All patients were followed until death or December 31st, 2013. Physical examinations were carried out monthly during the first 6 months, every 2 months during the second 6 months, every 3 months during the second year, and every 6 months thereafter. A CT scan or MRI of the head and neck was performed 2 to 3 months after RT and then annually for 5 years, or as clinically indicated after surgery. Hemograms and blood chemistry were obtained with image follow-ups. Bone scans and abdominal sonography were arranged when clinically indicated. The survival time and time intervals to recurrence or distant metastasis (DM) were calculated from the date of surgery. The OS time was defined as the time between surgery and death. The DFS was defined as the patient continuing to live with no evidence of recurrent disease during the follow-up period.
STATISTICAL METHODS
Survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate analyses were used to identify the risk factors for LRC, DMF, DFS, and OS. The Cox regression model was used in the multivariate analyses and to estimate hazard ratios (HRs). The level of significance was set at P < 0.05. SPSS ver.17.0 statistical software was used for data processing (IBM Corp., Armonk, NY).
RESULTS
Patient Characteristics
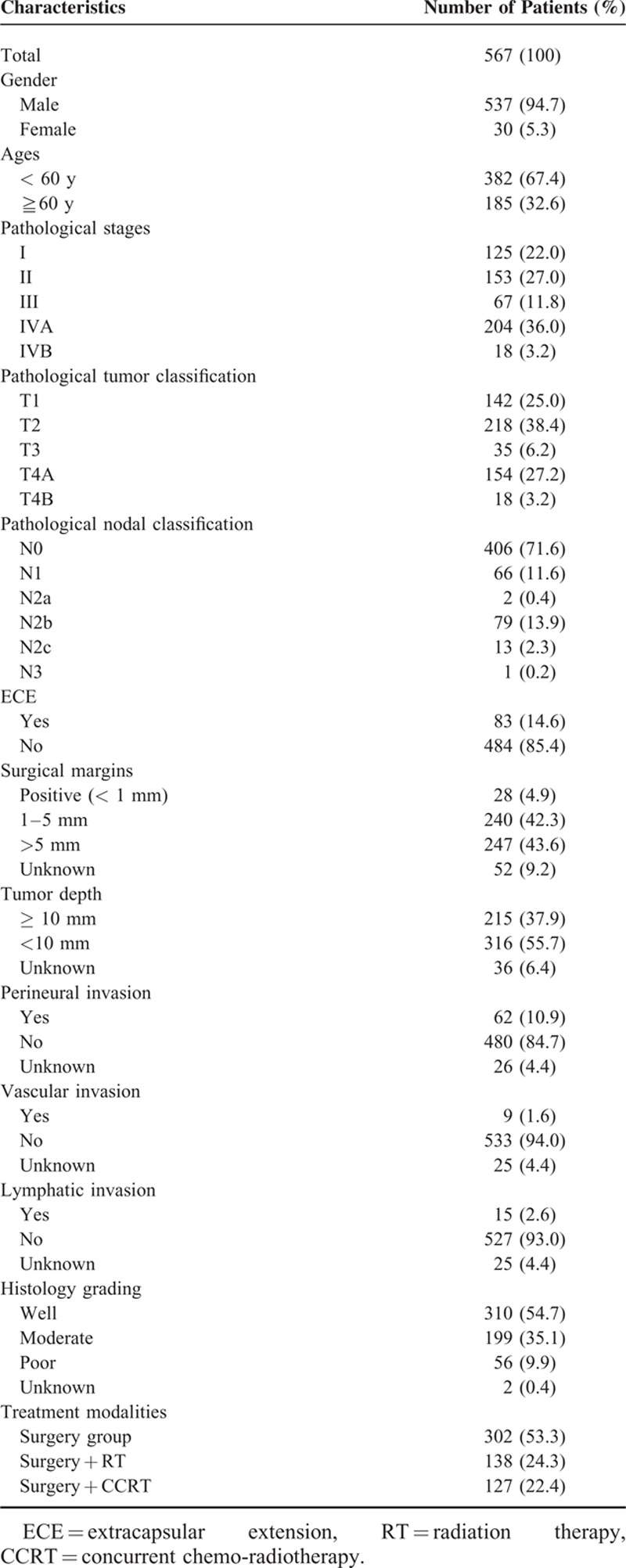
Table 1 lists the clinicopathological characteristics of all patients. There were 537 males and 30 females. The median age of the patients was 54 years old (range: 28–79 years). Of the 567 patients, 138 (24.3%) received adjuvant radiotherapy and 127 (22.4%) received adjuvant CCRT. The AJCC (7th edition) pathologic stage distribution includes I (22.0%), II (27.0%), III (11.8%), IVA (36.0%), and IVB (3.2%), which is summarized in Table 1. The median follow-up time of all patients was 3.5 years (range: 0.1–12.5 years). The median follow-up time of the surviving patients was 3.7 years (range: 0.1–12.5 years). At the time of the last follow-up, 366 (65%) patients were alive and 201 (35%) patients had died. The 5-year OS rate for stage I, II, III, IVA, and IVB patients was 79.8, 70.8, 63.3, 48.2, and 19.7%, respectively (P < 0.001). The 5-year DFS rate for stage I, II, III, IVA, and IVB patients was 65.8, 63.7, 59.0, 39.1, and 30.2%, respectively (P < 0.001). The 5-year LRC rate for stage I, II, III, IVA, and IVB patients was 77.6, 77.6, 76, 67.8, and 42.5%, respectively (P = 0.013). The 5-year DMF rate for stage I, II, III, IVA, and IVB patients was 99.2, 98.6, 96.4, 89.3, and 86.6%, respectively (P < 0.001).
TABLE 1.
Clinicopathological Characteristics of the 567 Patients With Oral Cavity Squamous Cell Carcinoma
Prognostic Factors
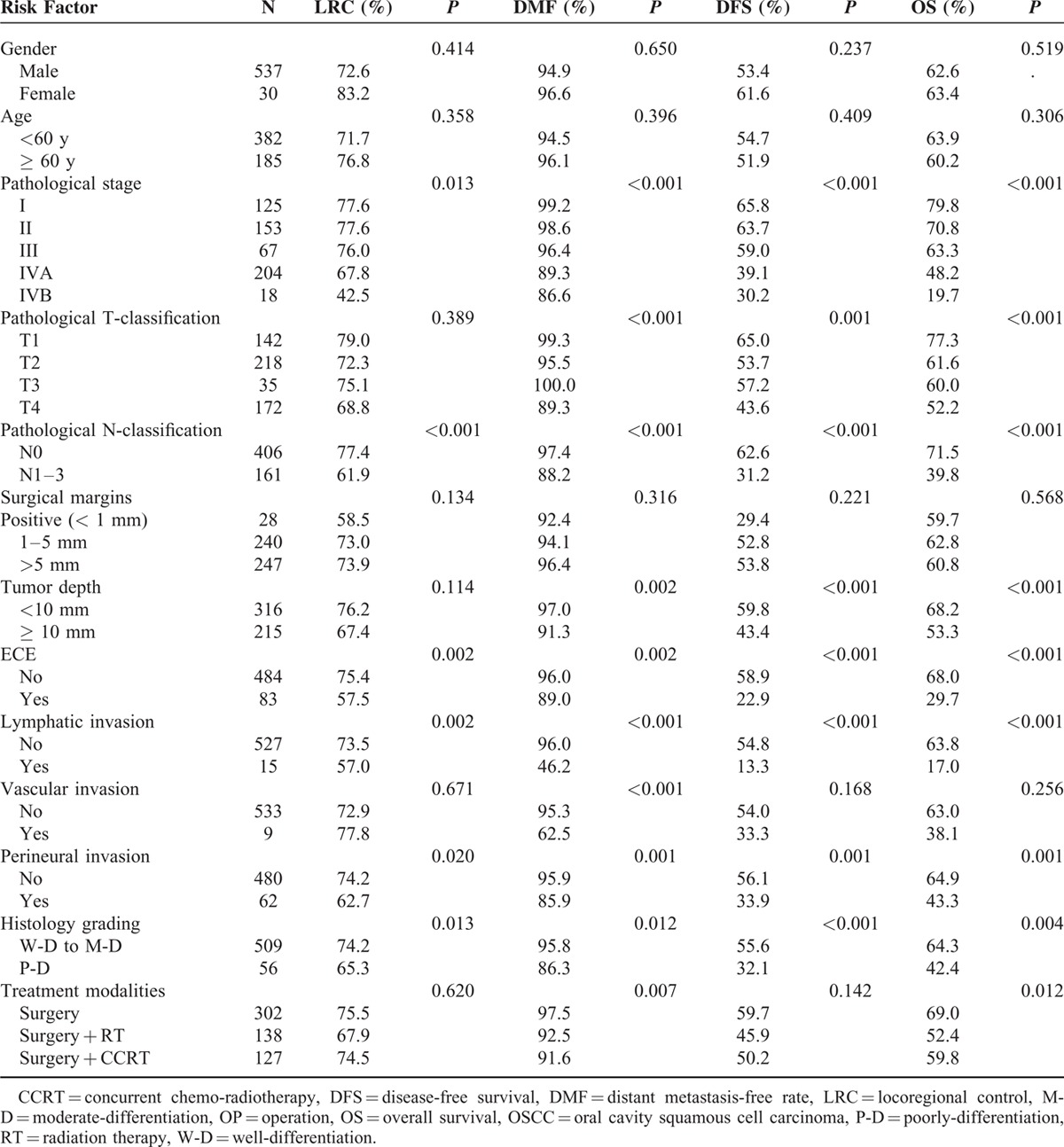
Table 2 lists the prognostic factors identified by the univariate analyses. According to the univariate analyses, the pathological stage (pStage), pathological N classification (pN), ECE, lymphatic invasion, perineural invasion, and histology grading were identified as prognostic factors for 5-year LRC. For distant metastasis, pStage, pathological T classification (pT), pN, tumor depth, ECE, lymphatic invasion, vascular invasion, perineural invasion, histology grading, and treatment modalities were significant prognostic factors. pStage, pT, pN, tumor depth, ECE, lymphatic invasion, perineural invasion, and histology grading were significant prognostic factors for 5-year DFS and OS. Moreover, treatment modality was also a prognostic factor for 5-year OS.
TABLE 2.
Univariate Analysis of Prognostic Factors for 5-Year LRC, DMF, DFS, and OS in 567 OSCC Patients
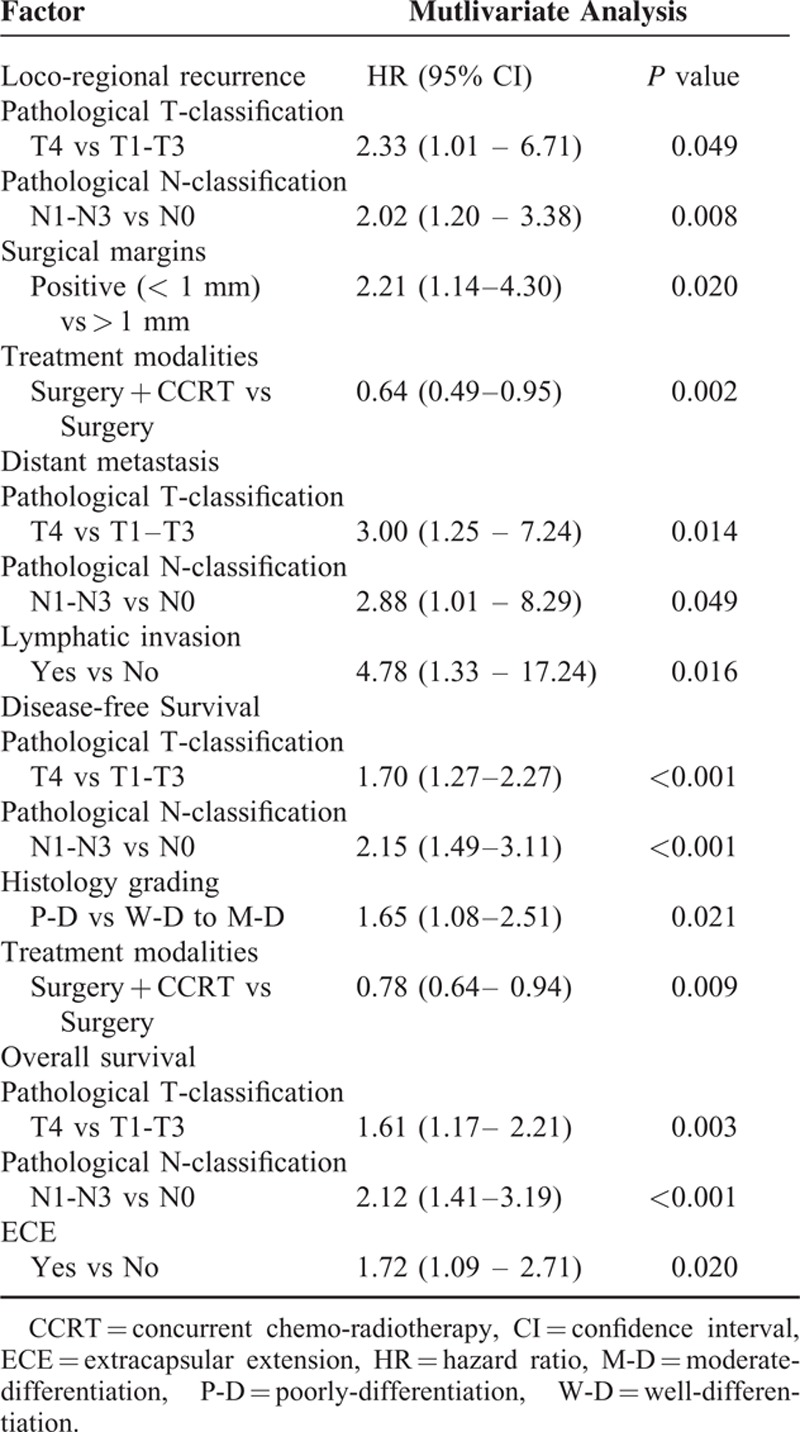
The multivariate analysis of 5-year control and survival rates is shown in Table 3. pN was an independent prognostic factor for 5-year LRC, DMF, DFS, and OS. pT was an independent adverse factor for 5-year LRC, DMF, DFS and OS. Histology grading was an independent factor for 5-year DFS. PRM was a predictor of a high rate of loco-regional recurrence. ECE were predictors for a poor OS. In terms of treatment modality, surgery + CCRT produced a higher 5-year LRC and OS compared with surgery alone.
TABLE 3.
Multivariate Analysis of Prognostic Factors for 5-Year LRC, DFS, and OS in 567 OSCC Patients
Risk Stratification Groups
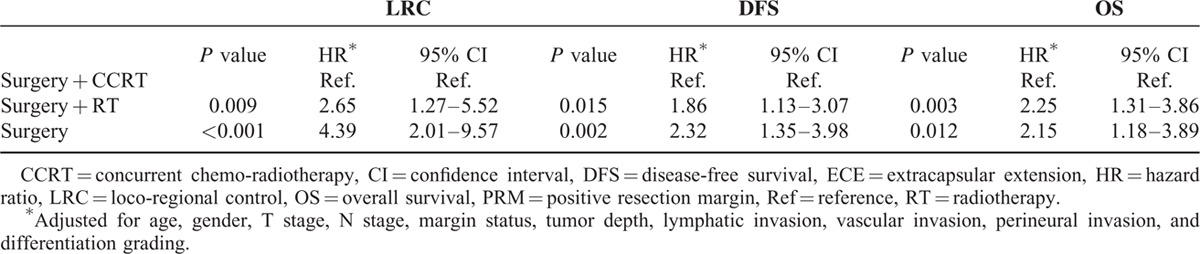
To fully understand the effects of treatment modality on patients with different prognostic factors, subgroup analyses were performed. Based on the findings of this study and several important randomized trials, we proposed that PRM and ECE, 2 well-known risk factors, would be considered major risk factors. The remaining risk factors (including pathological T4, positive node, close margin 1–5 mm, tumor depth ≥1 cm, lymphatic invasion, vascular invasion, perineural invasion, and poor differentiation) were defined as minor risk factors. As shown in Figure 1, 192 patients with at least 2 minor prognostic factors and without any major risk factors, post-OP RT or CCRT yielded significant better 5-year LRC (surgery + CCRT: 76.7%, surgery + RT:75.4% vs surgery 66.3%, P = 0.044), DFS (surgery + CCRT: 58.6%, surgery + RT:55.9% vs surgery 41.7%, P = 0.006) and OS (surgery + CCRT: 69.8%, surgery + RT:62.1% vs surgery 48.6%, P = 0.030) compared to the surgery only group. After adjusting for age, gender, pT, pN, margin status, tumor depth, lymphatic invasion, vascular invasion, perineural invasion, and differentiation grading, post-OP RT or CCRT still significantly improved the outcomes (LRC, DFS and OS) compared with surgery alone (Table 4). For 179 patients with at least 3 minor prognostic factors and/or at least one of the major risk factors, those receiving post-OP CCRT showed a significantly better 5-year LRC (surgery + CCRT: 70.1% vs surgery + RT:48.7%, surgery: 46.0%, P = 0.007), and there was a trend toward improved DFS (surgery + CCRT: 39.8% vs surgery + RT:24.8%, surgery: 29.7%, P = 0.111) and OS (surgery + CCRT: 53.3% vs surgery + RT:31.3%, surgery: 44.2%, P = 0.146) compared with post-OP RT or surgery alone (Figure 2). After adjusting for age, gender, pT, pN, margin status, tumor depth, lymphatic invasion, vascular invasion, perineural invasion, and differentiation grading, post-OP CCRT significantly improved all outcomes (LRC, DFS, and OS) compared with post-OP RT or surgery alone (Table 5).
FIGURE 1.
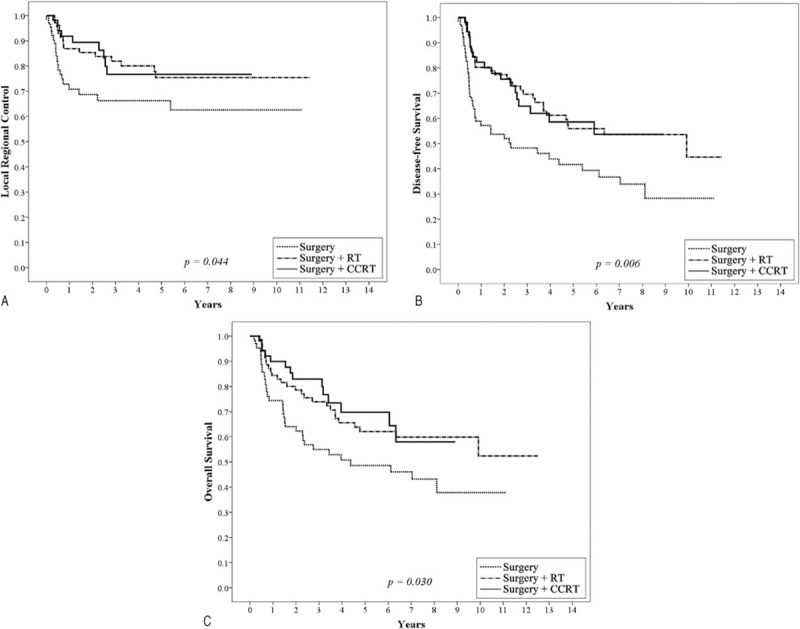
Kaplan–Meier analysis of the 5-year local regional control (A), disease-free survival (B), and overall survival rate (C) for 192 patients with at least 2 minor prognostic factors and without any major risk factors stratified by surgery, surgery plus postoperative radiotherapy (RT), or postoperative chemoradiotherapy (CCRT). Major risk factors include positive resection margin and extracapsular extension of nodal disease. Minor risk factors include pathological T4, positive node, close margin 1 to 5 mm, tumor depth ≥1 cm, lymphatic invasion, vascular invasion, perineural invasion, and poor differentiation. CCRT = chemo-radiotherapy.
TABLE 4.
Hazard Ratios of LRC, DFS, and OS of 192 Patients With at Least Two Minor Risk Factors Without ECE or PRM in Different Treatment Groups
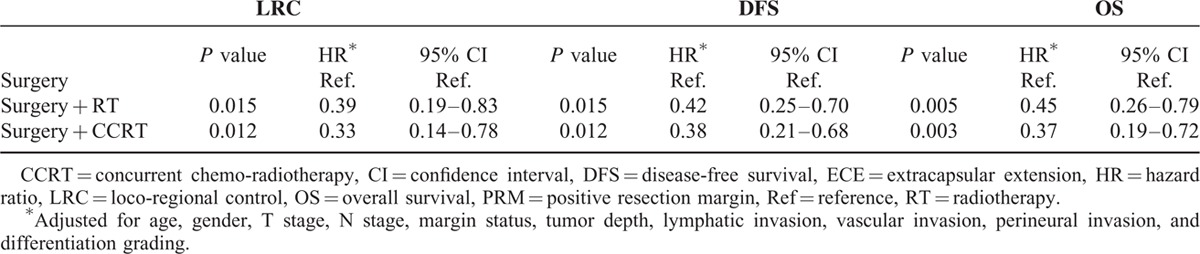
FIGURE 2.
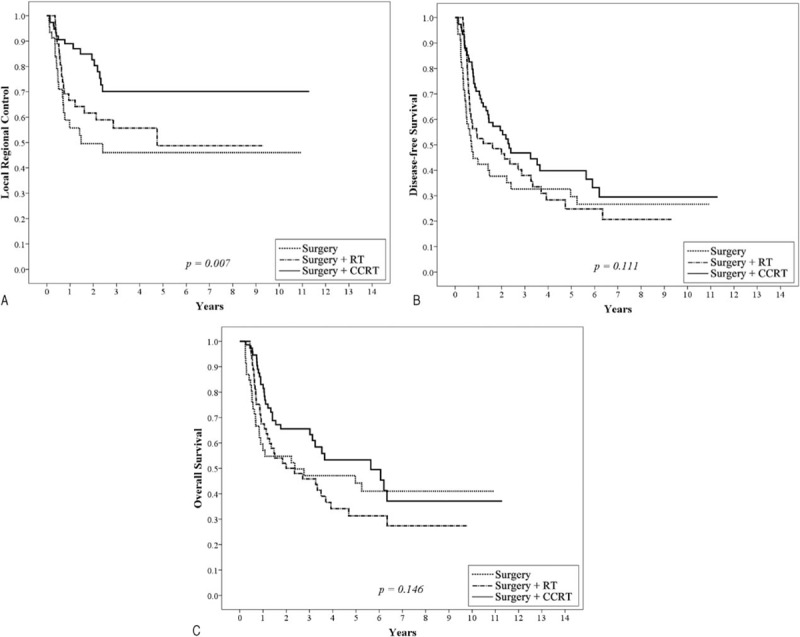
Kaplan–Meier analysis of the 5-year local regional control (A), disease-free survival (B), and overall survival rate (C) for 179 patients with at least 3 minor prognostic factors and/or at least one of the major risk factors stratified by surgery, surgery plus postoperative radiotherapy (RT), or postoperative chemo-radiotherapy (CCRT). Major risk factors include positive resection margin and extracapsular extension of nodal disease. Minor risk factors include pathological T4, positive node, close margin 1 to 5 mm, tumor depth ≥1 cm, lymphatic invasion, vascular invasion, perineural invasion, and poor differentiation. CCRT = chemo-radiotherapy, RT = radiotherapy.
TABLE 5.
Hazard Ratios of LRC, DFS, and OS of 179 Patients With at Least 3 Minor Risk Factors or ECE/PRM in Different Treatment Groups
DISCUSSION
Based on the NCCN 2015 guidelines,10 post-OP CCRT is the preferred treatment for advanced head and neck cancer with the adverse features of ECE or PRM. For patients with other risk factors (pT3 or T4 primary, N2 or N3 nodal disease, nodal disease level IV or V, perineural invasion, vascular embolism or lymphovascular invasion), either post-OP or CCRT is suggested. There is consensus on offering post-OP CCRT to patients with ECE or PRM5; however, the role of post-OP CCRT in patients with risk factors other than ECE or PRM remains poorly understood, especially for patients with 2 or more risk factors. In a prospective randomized trial conducted by Ang et al1 and in several large retrospective trials,6,11 the combination of 2 or more adverse features apart from ECE or PRM predicted poor loco-regional control and survival, even though these patients received post-OP RT. To clarify this issue, this retrospective analysis of a large cohort of consecutive patients with oral cancer was conducted in our hospital.
In this study, the 5-year OS of patients at stage I, II, III, IVA, and IVB were 79.7%, 70.8%, 65.8%, 49.0%, and 17.7%, respectively. These results are comparable to or even slightly better than the survival results listed in the AJCC staging manual.7 Univariate analysis revealed pathological stage as a significant prognostic factor in LRC, DMF, DFS, and OS. For patients with PRM (margin < 1 mm), poor clinical results (5-year LRC—58.5%, DMF—92.4%, DFS—29.4%, and OS —59.7%) were observed. PRM also predicted a lower LRC by multivariate analysis. ECE was also a negative predictor of 5-year LRC, DMF, DFS, and OS. ECE remained statistically significantly in predicting poor 5-year OS by multivariate analysis. The prognostic significance of other clinicopathologic factors, such as pT, pN, tumor depth, surgical margin, lymphatic invasion, vascular invasion, perineural invasion, and poor differentiation of the tumor, was identified from previous retrospective analyses.6,12–18 Our study confirmed that these factors were significant prognostic factors in 5-year LRC, DMF, DFS, and OS, either in univariate or multivariate analyses.
Bernier et al5 conducted a comparative analysis of pooled data from the EORTC 22931 and RTOG 95-01 trials. This analysis confirmed that microscopically involved resection margins and extracapsular spread of the tumor from the neck nodes were the most significant prognostic factors for poor outcomes in resected head and neck cancer. Those patients could have benefited from the addition of concomitant cisplatin to their post-OP radiotherapy. Our results showed that patients with ECE or PRM also had poor clinical outcomes. Therefore, we proposed that PRM and ECE were major risk factors and that other risk factors (such as pathological T4, positive node, close margin 1–5 mm, tumor depth ≥1 cm, lymphatic invasion, vascular invasion, perineural invasion, and poor differentiation) would be considered minor risk factors. We showed that 192 patients with at least 2 minor prognostic factors and no major risk factor (ECE or PRM), post-OP RT or CCRT had significantly better 5-year LRC, DFS, and OS (Figure 1A–C and Table 4) compared with the surgery only group. For 179 patients with at least 3 minor prognostic factors and/or at least 1 major risk factor, post-OP CCRT showed significantly better 5-year LRC, and there was a trend toward improved DFS and OS compared with post-OP RT or surgery alone (Figure 2A–C). After correction of the prognostic variables, post-OP CCRT significantly improved all outcomes (LRC, DFS, and OS) compared with post-OP RT or surgery alone (Table 5). In a randomized trial conducted by Ang et al1, a prospective assessment by clusters of surgical-pathologic features (oral cavity site, mucosal margin status, nerve invasion, > 1 positive nodes, > 1 positive nodal groups, largest node > 3 cm, ECE, and treatment delay > 6 weeks) was carried out to differentiate the need for and dosage of post-OP RT. For patients with 1 adverse feature other than ECE, the 5-year OS (66%) and LRC (94%) were excellent after receiving 57.6 Gy of post-OP RT. In contrast, for patients with ECE or at least 2 other adverse factors, the 5-year OS (42%) and LRC (68%) were poorer despite higher radiation doses (63 Gy). Ang et al1 proposed a combination chemotherapy or biological modifiers with RT in these high-risk patients. In a large retrospective analysis of resected oral cavity cancer patients by Liao et al6, 4 significant prognostic factors, including a surgical margin of ≤7 mm, a tumor depth ≤10 mm, being pathological node-positive, and betel quid chewing were used to predict the local control rate. Adjuvant therapy was suggested for patients with 3 or more factors. In addition to ECE and PRM, our study investigated the combination of risk factors from these 2 studies as well as the EORTC randomized trial and suggested updated risk classification groups for oral cavity cancer. For patients with 2 minor risk factors and no major risk factors, RT alone after surgery is sufficient in terms of the increasing toxicities of CCRT comparing with post-OP RT alone.2,3 However, for patients with 3 or more minor risk factors and with or without any major risk factors, post-OP CCRT is needed in order to improve their outcomes.
However, some weaknesses remain in the present risk classification system. For example, patients with stage IVB (T4b or N3) disease had a significant poor prognosis, especially for supra-notch T4b tumors.19,20 In this study, the 5-year OS, DFS, LRC, and DMF rates for stage IVB patients were 19.7, 30.2, 42.5, and 86.6%, respectively, which were much worse than in patients with 3 minor risk factors or at least 1 major risk factor. Therefore, for patients with only 1 risk factor, such as T4b or N3 and carrying no major risk factors, post-OP CCRT should still be considered in clinical practice. Secondly, due to the limited number of patients with N-positive disease (161 of 567), patients were not stratified for N2 or N3 nodal disease or nodal disease in levels IV or V for further analysis. A larger sample size or prospective trials would be needed to clarify whether patients with N2 or N3 nodal disease or nodal disease in levels IV or V should receive adjuvant CCRT. The phase III RTOG 0920 trial compared RT alone vs RT plus cetuximab in patients with intermediate-risk disease (perineural invasion, lymphovascular invasion, single lymph node > 3 cm or ≥2 lymph nodes, close margin, i.e., within 5 mm of a surgical margin, pT3 or pT4a, andT2 oral cavity cancer with > 5-mm depth of invasion). This trial may be able to answer any outstanding questions in the near future.
In conclusion, in addition to ECE and PRM, pT4, positive node, close margin ≤5 mm, pathological tumor depth ≥10 mm, lymphatic invasion, vascular invasion, perineural invasion, and poorly differentiated grading were identified as poor prognostic factors for LRC and survival outcomes for OSCC patients. Patients with 2 minor risk factors should receive postoperative RT. For patients with PRM, ECE or >2 minor risk factors, postoperative CCRT is recommended.
Footnotes
Abbreviations: CCRT = chemo-radiotherapy, DFS = disease-free survival, DMF = distant metastasis-free, ECE = extra-capsular extension, EORTC = the European Organization Research and Treatment of Cancer, HRs = hazard ratios, LN = lymph node, LRC = loco-regional control, OS = overall survival, OSCC = oral cavity squamous cell carcinoma, post-OP = postoperative, PRM = positive resection margin, pT4 = pathological T4, RT = radiotherapy, RTOG = the Radiation Therapy Oncology Group.
It was presented at the ASTRO 2015 Annual Meeting (e-Poster section).
Author contributions: WCC conceived and designed the study, collected, analyzed, and interpreted the data, prepared the draft and gave final approval of the version to be submitted. CHL and CCF collected the data, undertook data analysis and interpretation, and performed the statistical analysis. YHY, PCC, and CPL also performed the statistical analysis and carried out clinical revision of the data.
MFC critically reviewed the intellectual content and gave final approval of the version to be submitted. All authors read and approved the final manuscript.
Funding: this work was supported by research grants from Chang Gung Memorial Hospital (CORPG6D0161–2, CORPG6D0251–2).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 51:571–578. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004; 350:1937–1944. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J, Cooper JS. Chemoradiation after surgery for high-risk head and neck cancer patients: how strong is the evidence? Oncologist 2005; 10:215–224. [DOI] [PubMed] [Google Scholar]
- 5.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005; 27:843–850. [DOI] [PubMed] [Google Scholar]
- 6.Liao CT, Chang JT, Wang HM, et al. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann Surg Oncol 2008; 15:915–922. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Compton CC, et al. , eds. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer, 2009. [Google Scholar]
- 8.Bachaud JM, Cohen-Jonathan E, Alzieu C, et al. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys 1996; 36:999–1004. [DOI] [PubMed] [Google Scholar]
- 9.Wang HM, Wang CS, Chen JS, et al. Cisplatin, tegafur, and leucovorin: a moderately effective and minimally toxic outpatient neoadjuvant chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Cancer 2002; 94:2989–2995. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network (NCCN). NCCN Clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (Accessed on Sep30, 2015). [Google Scholar]
- 11.Liao CT, Huang SF, Chen IH, et al. Risk stratification of patients with oral cavity squamous cell carcinoma and contralateral neck recurrence following radical surgery. Ann Surg Oncol 2009; 16:159–170. [DOI] [PubMed] [Google Scholar]
- 12.Liao CT, Huang SF, Chen IH, et al. Outcome analysis of patients with pN2 oral cavity cancer. Ann Surg Oncol 2010; 17:1118–1126. [DOI] [PubMed] [Google Scholar]
- 13.Liao CT, Lin CY, Fan KH, et al. Identification of a high-risk group among patients with oral cavity squamous cell carcinoma and pT1-2N0 disease. Int J Radiat Oncol Biol Phys 2012; 82:284–290. [DOI] [PubMed] [Google Scholar]
- 14.Parsons JT, Mendenhall WM, Stringer SP, et al. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys 1997; 39:137–148. [DOI] [PubMed] [Google Scholar]
- 15.Lin YT, Chien CY, Lu CT, et al. Triple-positive pathologic findings in oral cavity cancer are related to a dismal prognosis. Laryngoscope 2015; 125:E300–E305.Available from URL: http://www.ncbi.nlm.nih.gov/pubmed/26152458 [Accessed Sep 30, 2015]. [DOI] [PubMed] [Google Scholar]
- 16.Carter RL, Tanner NS, Clifford P, et al. Perineural spread in squamous cell carcinomas of the head and neck: a clinicopathological study. Clin Otolaryngol Allied Sci 1979; 4:271–281. [DOI] [PubMed] [Google Scholar]
- 17.Jardim JF, Francisco AL, Gondak R, et al. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int J Oral Maxillofac Surg 2015; 44:23–28. [DOI] [PubMed] [Google Scholar]
- 18.Anderson CR, Sisson K, Moncrieff M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol 2015; 51:464–469. [DOI] [PubMed] [Google Scholar]
- 19.Liao CT, Ng SH, Chang JT, et al. T4b oral cavity cancer below the mandibular notch is resectable with a favorable outcome. Oral Oncol 2007; 43:570–579. [DOI] [PubMed] [Google Scholar]
- 20.Kao J, Lavaf A, Teng MS, et al. Adjuvant radiotherapy and survival for patients with node-positive head and neck cancer: an analysis by primary site and nodal stage. Int J Radiat Oncol Biol Phys 2008; 71:362–370. [DOI] [PubMed] [Google Scholar]


