Abstract
Analyze efficacy, safety of endoscopic therapy for duodenal duplication cysts (DDC) by comprehensively reviewing case reports.
Tandem, independent, systematic, computerized, literature searches were performed via PubMed using medical subject headings or Keywords “cyst” and “duodenal” and “duplication”; or “cyst”, and “endoscopy” or “endoscopic”, and “therapy” or “decompression”; with reconciliation of generated references by two experts. Case report followed CARE guidelines.
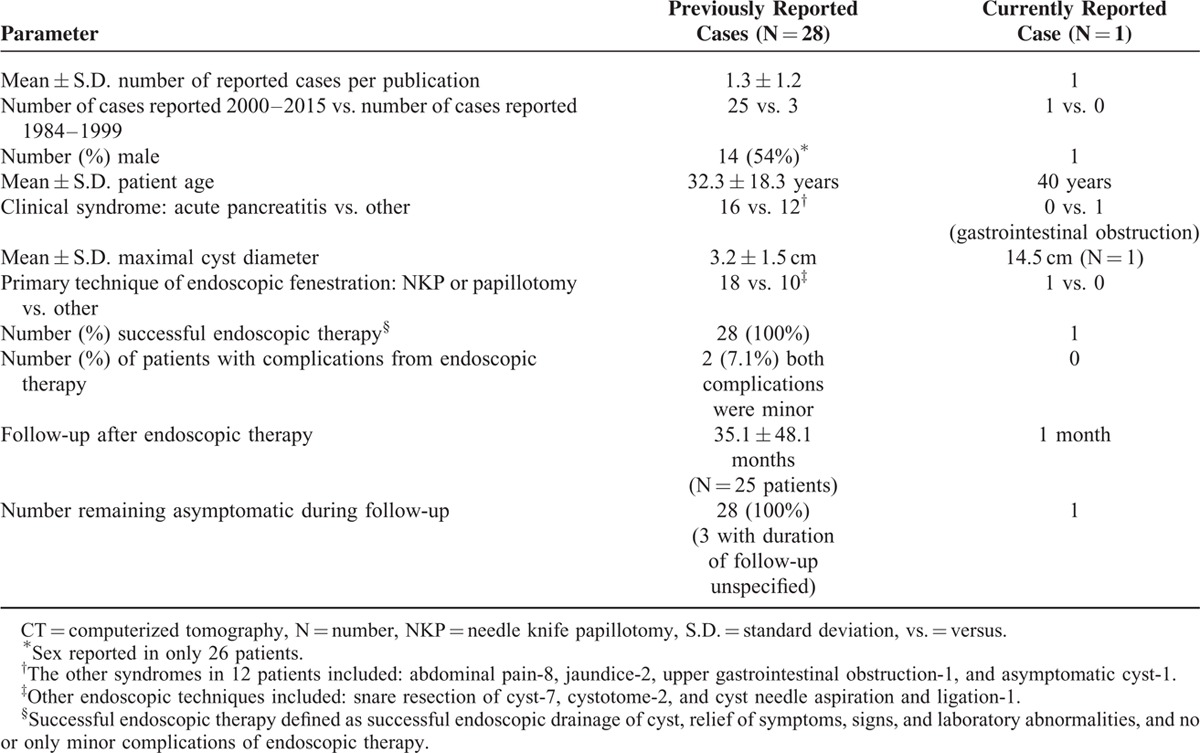
Literature review revealed 28 cases (mean = 1.3 ± 1.2 cases/report). Endoscopic therapy is increasingly reported recently (1984–1999: 3 cases, 2000–2015: 25 cases, P = 0.003, OR = 8.33, 95%-CI: 1.77–44.5). Fourteen (54%) of 26 patients were men (unknown-sex = 2). Mean age = 32.2 ± 18.3 years old. Procedure indications: acute pancreatitis-16, abdominal pain-8, jaundice-2, gastrointestinal (GI) obstruction-1, asymptomatic cyst-1. Mean maximal DDC dimension = 3.20 ± 1.53 cm (range, 1–6.5 cm). Endoscopic techniques included cyst puncture via needle knife papillotomy (NKP)/papillotome-18, snare resection of cyst-7, cystotome-2, and cyst needle aspiration/ligation-1. Endoscopic therapy was successful in all cases. Among 24 initially symptomatic patients, all remained asymptomatic post-therapy without relapses (mean follow-up = 36.5 ± 48.6 months, 3 others reported asymptomatic at follow-up of unknown duration; 1 initially asymptomatic patient remained asymptomatic 3 years post-therapy). Two complications occurred: mild intraprocedural duodenal bleeding related to NKP and treated locally endoscopically.
A patient is reported who presented with vomiting, 15-kg-weight-loss, and profound dehydration for 1 month from extrinsic compression of duodenum by 14 × 6 cm DDC, underwent successful endosonographic cyst decompression with large fenestration of cyst and endoscopic aspiration of 1 L of fluid from cyst with rapid relief of symptoms. At endoscopy the DDC was intubated and visualized and random endoscopic mucosal biopsies were obtained to help exclude malignant or dysplastic DDC.
Study limitations include retrospective literature review, potential reporting bias, limited patient number, variable follow-up.
In conclusion, endoscopic therapy for DDC was efficacious in all 29 reported patients including current case, including patients presenting acutely with acute pancreatitis, or GI obstruction. Complications were rare and minor, suggesting that endoscopic therapy may be a useful alternative to surgery for nonmalignant DDC when performed by expert endoscopists.
INTRODUCTION
Symptomatic duodenal duplication cysts (DDC) were traditionally treated surgically, but have been increasingly treated endoscopically to avoid considerable morbidity from surgery.1 This work comprehensively reviews this subject with review of 28 previous cases, and shows this technique is likely efficacious, relatively safe, and durable. A 29th patient is also reported who presented with severe recurrent vomiting for 1 month from duodenal compression by a huge DDC that was successfully treated by DDC decompression under endosonographic guidance with symptomatic resolution. During decompression at endosonography, the DDC was intubated and biopsied to exclude malignancy/dysplasia. This work suggests that endoscopic treatment is a viable and potentially preferable alternative to surgery for most DCC, provided endoscopic expertise is available and malignancy is excluded.
METHODS
Computerized literature searches independently performed in tandem via PubMed using the medical subject headings (MeSH) or key words of “cyst” and “duodenal” and “duplication”; or “cyst” and “endoscopy” or “endoscopic” or “therapy” and “decompression”; with reconciliation of cited references. One article written in French,2 and one article written in Spanish3 were professionally translated. Case report followed CARE guidelines. This review received exemption/approval by William Beaumont Hospital IRB on August 14, 2015.
CASE REPORT
See Appendix 1.
RESULTS
Epidemiology and Anatomy
DDC are extremely rare congenital anomalies, with a prevalence of less than 1 per 100,000 live births.4 DDC are the least common small bowel duplication cysts, preceded by ileal and jejunal duplication cysts.5 In a comprehensive meta-analysis, 47 DDC were reported from 1999 to 2009.4 About 60% of cases are diagnosed during infancy and childhood, and 40% are diagnosed in adulthood.4–8
By definition, a DDC must adhere to the duodenum, contain a smooth muscle layer in its walls, and be lined by duodenal epithelium.5,6,9–11 DDC usually share the blood supply with the rest of the duodenum.6 DDC are usually filled with clear fluid, but can contain bile, pancreatic fluid, or gallstones if they connect to the pancreaticobiliary ducts.12–15 DDC can occur in any duodenal segment, but most commonly arise in the second or third parts of the duodenum. They usually arise on the mesenteric side.5,13
DDC can communicate directly with the true lumen via the duodenum, or via the pancreaticobiliary ducts, or have no communication.4–6,9,16 About 30% of DDC communicate with the pancreaticobiliary ducts.4 DDC are also classified according to shape as cystic or elongated/tubular.13 DDC are most commonly cystic.5,12
Clinical Course
DDC are usually asymptomatic and incidentally discovered during radiologic imaging or esophagogastroduodenoscopy (EGD).6,7 They can remain clinically silent for many years.6 Symptoms, when present, most commonly include abdominal pain, followed by nausea in 80%, and vomiting in 40%.4 The most common complication is acute pancreatitis.4,12 Other complications include: jaundice, biliary obstruction, cyst infection, intussusception, cholestasis, and hepatitis.4,14–20 DDC may contain ectopic pancreatic or gastric tissue that can cause duodenal ulcers, gastrointestinal (GI) bleeding, or rarely duodenal perforation.6,11,21,22 DDC are usually benign, but are occasionally malignant.7,23–25
Diagnosis
DDC are strongly suggested by characteristic radiologic imaging findings, but imaging findings are occasionally misleading.12,18 Twin major goals of radiologic imaging are to determine that the lesion is fluid-filled and to locate the cyst attachment site.13 Contrast radiographs reveal a submucosal or extrinsic mass or filling defect.4 Abdominal computerized tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI) are commonly used to detect DDC. DDC appear as discrete, fluid-filled structures attached to the duodenal wall.4,13 CT is useful to depict cyst location and dimensions, potential communications, and other anomalies.26 On CT scan, the wall of duplication shows contrast enhancement, but the cyst content has a density of 0–20 Hounsfield units without contrast enhancement.13 On US, DDC are highly suspected when an outer hypoechoic muscular layer and an inner echogenic mucosal layer is detected, a phenomenon called the “double-wall” or “muscular rim” sign.5,13,26,27 Cysts may contain keratin and desquamated cells, which can appear solid on US.17 MRI typically shows low attenuation signals in T1- and T2-weighted images.13 A Meckel's technetium scan is used to exclude ectopic gastric tissue within DDC.13 Magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP) can also be used diagnostically, and to exclude cyst communication with the pancreaticobiliary ducts.4,16,28,29 If the choledochus drains into the DDC, only the pancreatic duct fills with contrast during ERCP.16 A meta-analysis revealed false negative rates for DDC of 23% with US and 9% with CT or MRI/MRCP.4 Three-dimensional virtual cholangioscopy has been used to exclude communication between DDC and pancreaticobiliary ducts.7,30
Definitive diagnosis requires EGD with endosonographic confirmation, or surgery with pathologic analysis of resected tissue.22,29,31–34 At EGD, DDC appear as a duodenal bulge with normal overlying mucosa, or as a variably long diverticulum. DDC usually have a smooth, regular, mucosal appearance. Other cystic masses in the differential diagnosis include: choledochal cyst, cystic dystrophy of duodenal wall, pancreatic pseudocyst, duodenal polyp, cystic pancreatic tumors, lymphatic malformation, mesenteric cyst, omental cyst, and gastrointestinal stromal tumor.13,35–37 Endoscopic ultrasonography (EUS) can confirm the presence of muscular layer continuity between the duodenum and its duplication.9,13,38 Fine needle aspiration can be performed during EUS if the diagnosis is uncertain, but aspiration occasionally results in cyst infection.17,39–42
Therapy
Asymptomatic cysts are usually managed expectantly, but some authorities recommend intervention based upon potential complications, including malignant transformation,5 especially in patients who are unlikely to follow-up.4,43 Prospective studies of the natural history of DDC are, however, unavailable. Symptomatic DDC generally mandate endoscopic or surgical therapy. Moreno et al,44 however, reported one case of medical management of a symptomatic DDC with prokinetic therapy, and recommended annual follow-up with EGD or abdominal US, if a symptomatic cyst is managed medically. Some authorities recommend complete surgical resection of symptomatic DDC,7,19,45,46 whereas others recommend therapeutic endoscopy to establish free drainage.6,46–48 Surgical extirpation entails higher morbidity, and appreciable mortality as compared with endoscopic therapy, but eliminates the risk of malignant cyst degeneration.6,7 Pancreaticoduodenectomy is sometimes necessary intraoperatively if the DDC is too close to the pancreaticobiliary ducts.7 Less invasive, surgical techniques, including partial cyst resection, internal derivation,19 and marsupialization,4 have become obsolete.
Systematic Literature Review of Therapeutic EGD
Systematic literature review revealed 28 reported cases of endoscopic therapy (Table 1 ,3,8,45–63 number of case reports per publication = 1.3 ± 1.2). Endoscopic therapy is increasingly reported recently: 3 cases reported from 1984 to 1999, versus 25 cases reported from 2000 to 2015 (P = 0.003, OR = 8.33, 95%-CI: 1.77–44.5, Fisher exact test). Among 26 patients in whom the sex was reported, 14 (54%) were men. The mean patient age = 32.2 ± 18.3 years old. Procedure indications included: acute pancreatitis-16 (first episode-10, recurrent-6), abdominal pain-8, jaundice-2, upper GI obstruction-1, and asymptomatic cyst-1 (Table 1 ). Two patients had two simultaneous clinical syndromes: including one patient presenting with acute pancreatitis and chronic GI blood loss (patient-448), and one patient presenting with obstructive jaundice and RUQ abdominal pain (patient-2561). Only 1 patient had chronic GI blood loss attributed to DDC (patient-448). The rarity of this presentation is likely because DDC are uncommonly inflammatory, invasive, or malignant. Patients typically presented with symptoms, signs, and laboratory abnormalities suggestive of the presenting syndrome (e.g., acute pancreatitis), but without clinical features suggesting that the underlying cause was DDC. DDC was, however, generally diagnosed by radiologic imaging, including EUS, CT, ERCP, or MRCP. The mean maximal diameter of the reported DDC = 3.20 ± 1.53 cm.
TABLE 1.
Clinical Presentation and Endoscopic Therapy for Duodenal Duplication Cysts: Comprehensive Review of 28 Cases Reported in the Literature with Patient Outcomes
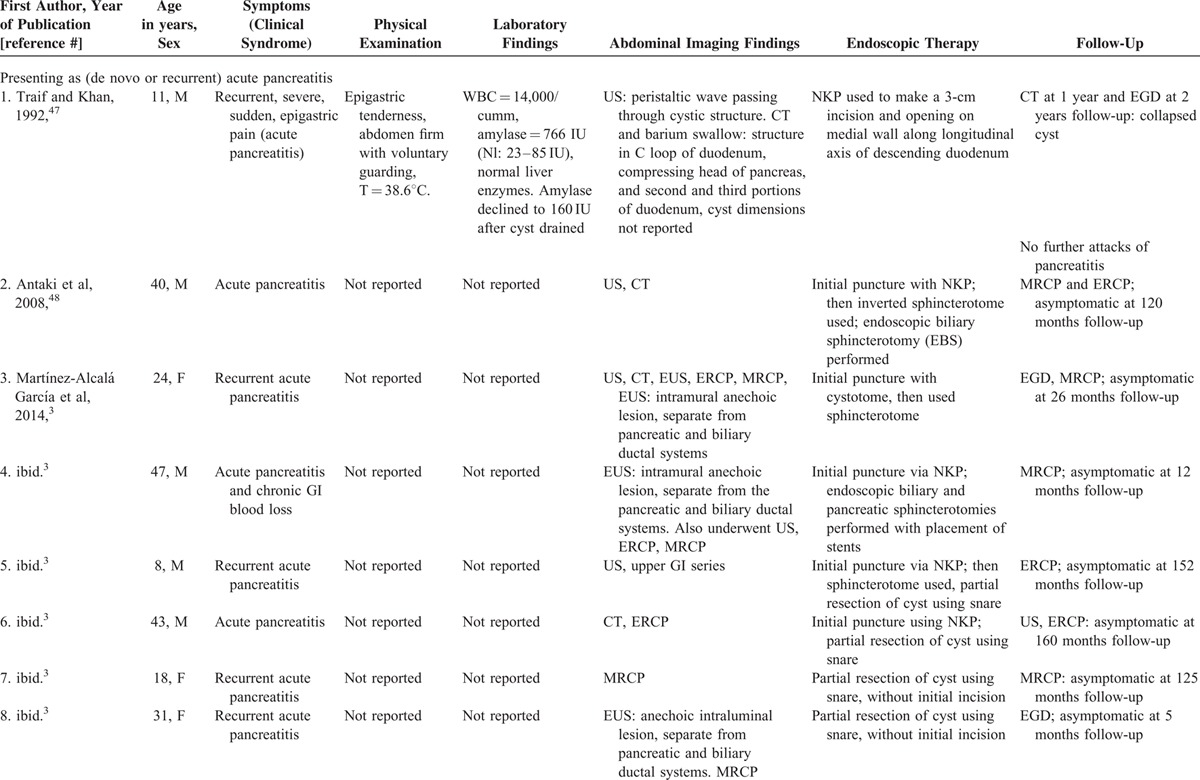
TABLE 1 (Continued).
Clinical Presentation and Endoscopic Therapy for Duodenal Duplication Cysts: Comprehensive Review of 28 Cases Reported in the Literature with Patient Outcomes
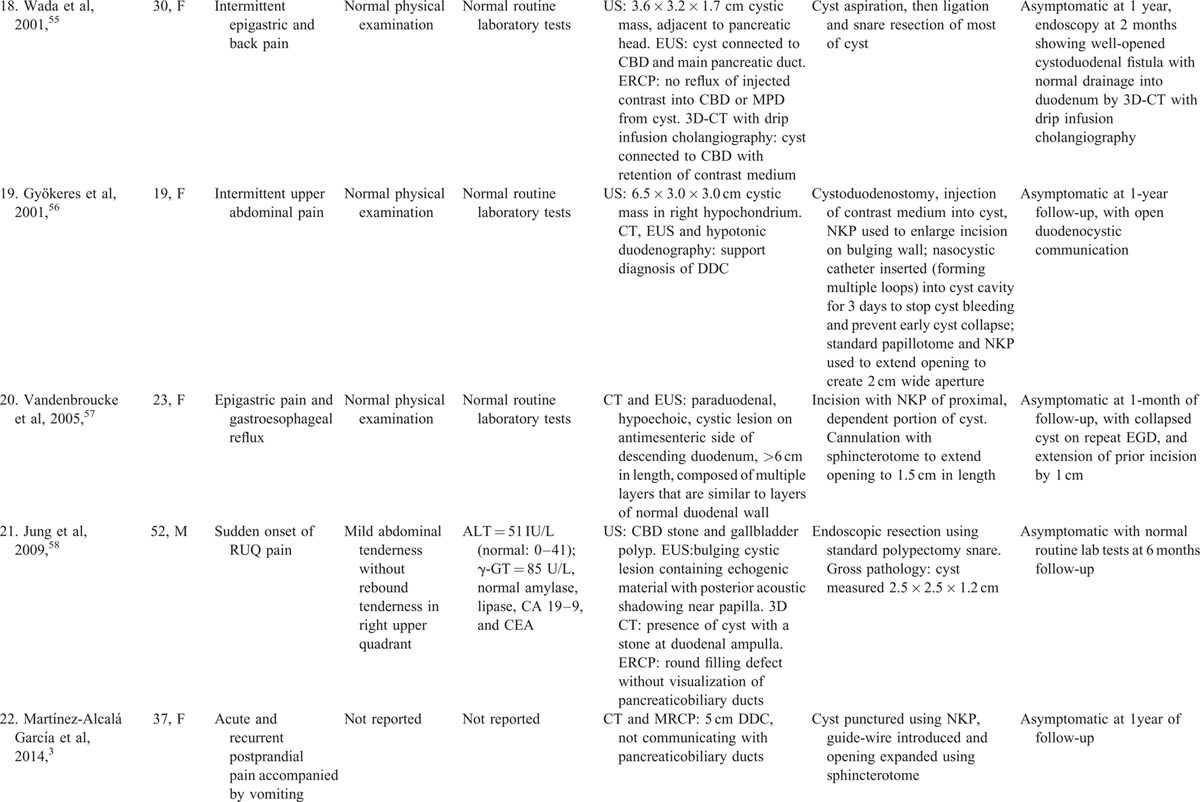
TABLE 1 (Continued).
Clinical Presentation and Endoscopic Therapy for Duodenal Duplication Cysts: Comprehensive Review of 28 Cases Reported in the Literature with Patient Outcomes

TABLE 1 (Continued).
Clinical Presentation and Endoscopic Therapy for Duodenal Duplication Cysts: Comprehensive Review of 28 Cases Reported in the Literature with Patient Outcomes
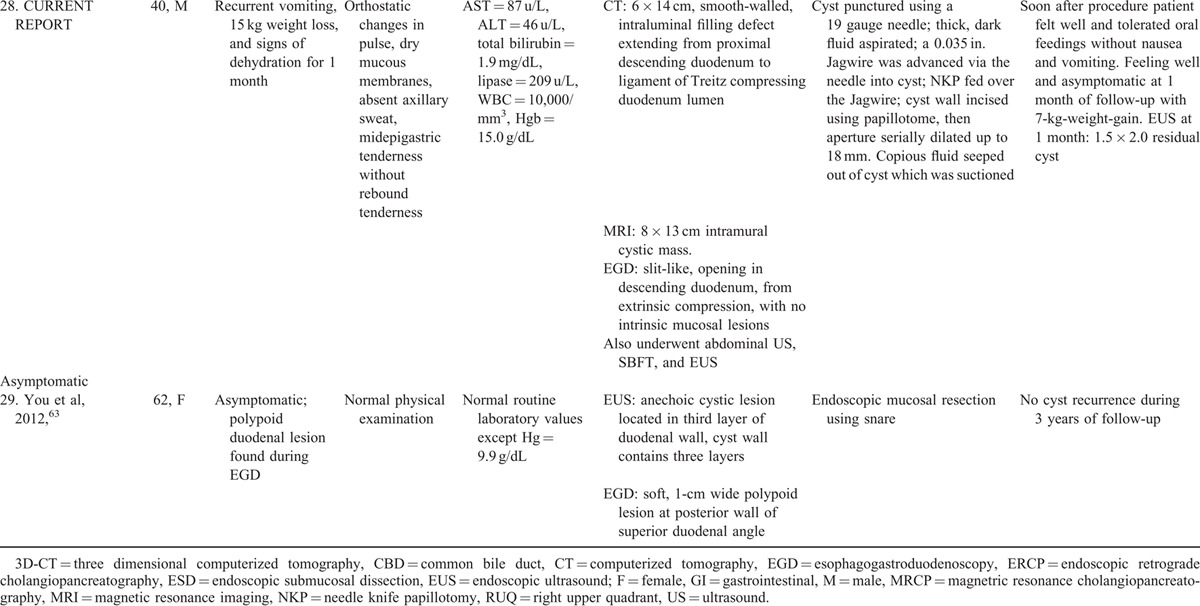
Systematic literature review demonstrates that endoscopic treatment is safe and effective, avoiding the morbidity associated with laparotomy57. Endosonographic therapeutic techniques included cyst puncture via needle knife papillotomy (NKP) or papilotome-18, snare resection of cyst-7, cystotome-2, and cyst needle aspiration and ligation-1. Endosonographic therapy was successful in all 28 prior cases (Table 1 ). Only 2 complications (7.1%) were reported. Both complications were relatively mild: GI bleeding, related to NKP, that were easily managed without any permanent clinical sequelae by dilute subcutaneous epinephrine injection and placement of a cyst catheter for drainage that was removed after 48 hour (patient-1754), or by leaving a cyst drain for 3 days for tamponade and monitoring the bleeding (patient-1956). All patients remained asymptomatic during variable follow-up. Among 25 patients in whom the length of follow-up was reported, all were asymptomatic during mean follow-up of 35.1 ± 48.1 months. Three other patients were reported as asymptomatic at follow-up, but follow-up duration was not reported (patients 23,24,2759–61). One patient who had presented with an asymptomatic DDC remained asymptomatic for 3 years after endoscopic therapy (patient 2963). These data suggest that endoscopic therapy may be durable without symptomatic recurrences. Table 3 summarizes the key findings of this comprehensive review of endosonographic therapy for DDC.
TABLE 1 (Continued).
Clinical Presentation and Endoscopic Therapy for Duodenal Duplication Cysts: Comprehensive Review of 28 Cases Reported in the Literature with Patient Outcomes

Advantages of endoscopic therapy include no visible abdominal scars, decreased post-operative pain, and shorter hospitalization.45 Surgery is necessary if a symptomatic cyst cannot be approached endoscopically.4 Before therapeutic endosonography, the patient should be advised of a potential need for traditional surgery if therapeutic endoscopy fails.45
Comprehensive review of 93 gastric duplication cysts reported in the literature revealed 7 cases treated endoscopically. In all these cases the endsocopic therapy was successful without complications (Table 2 A).9,64–69 Comprehensive review of 80 esophageal duplication cysts reported in the literature revealed 7 cases treated endoscopically. In all these 7 cases the endoscopic therapy was successful (Table 2Table 2 B).70–75 Two minor endoscopic complications occurred: minor esophageal mucosal laceration without GI bleeding in 1, and minor infection at the cyst incision site successfully treated by irrigation and cyst opening extension at repeat EGD in 1. The number of gastric or esophageal duplication cysts treated endoscopically is relatively small. However, these literature reviews on gastric or esophageal duplication cysts further support the efficacy and safety of therapeutic endoscopy for upper gastrointestinal duplication cysts.
TABLE 1 (Continued).
Clinical Presentation and Endoscopic Therapy for Duodenal Duplication Cysts: Comprehensive Review of 28 Cases Reported in the Literature with Patient Outcomes
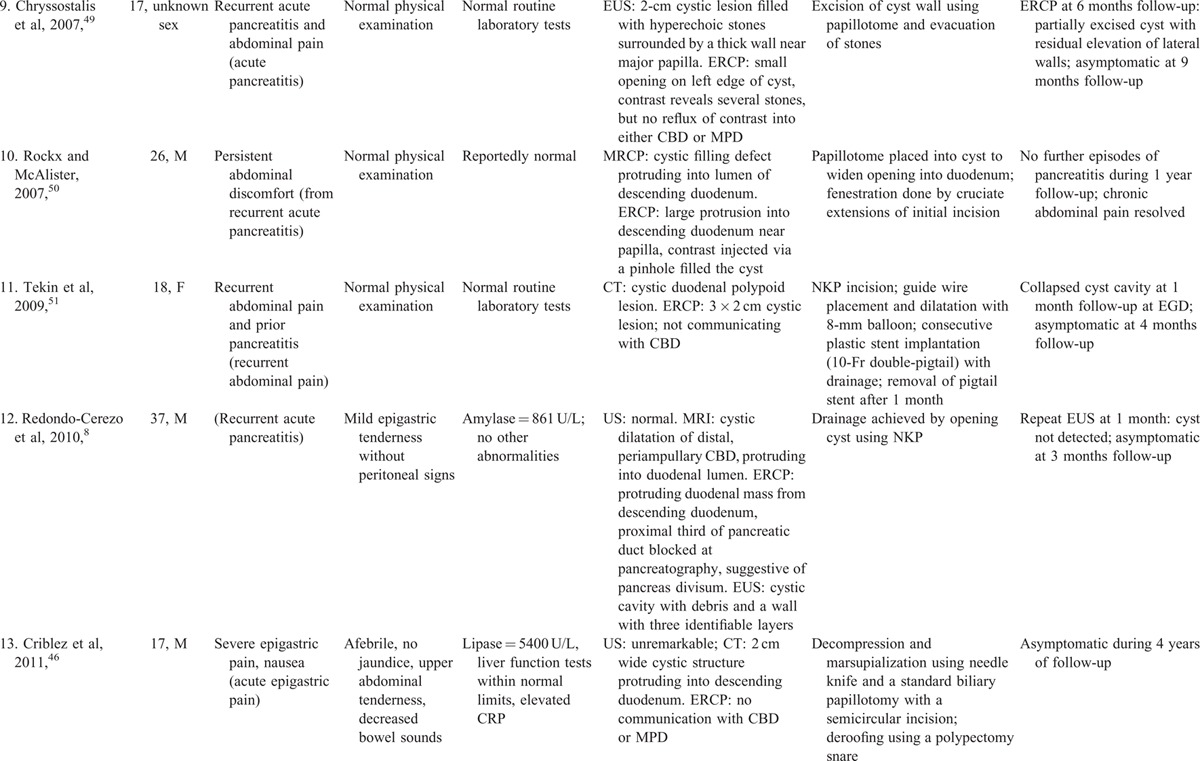
TABLE 2.
Clinical Presentation, Endoscopic Therapy, and Patient Outcomes for Gastric or Esophageal Duplication Cysts: Comprehensive Review of the Literature
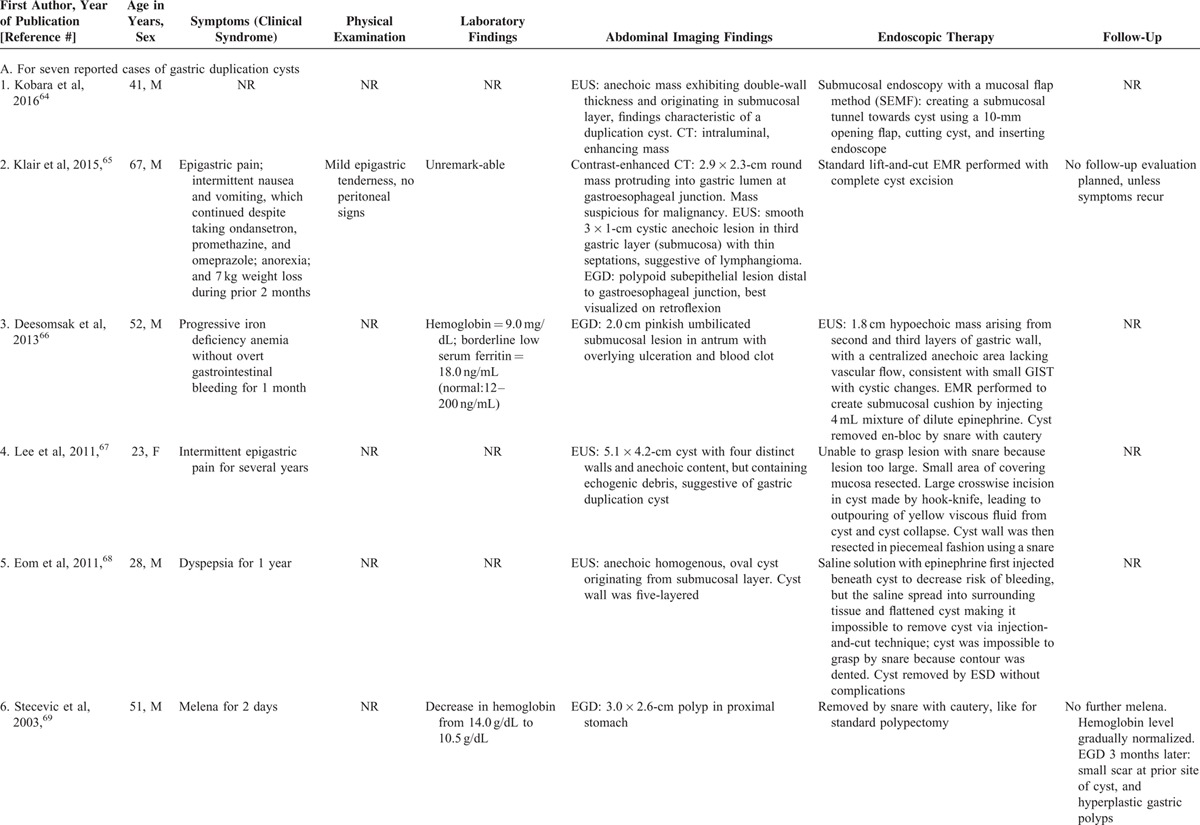
TABLE 2 (Continued).
Clinical Presentation, Endoscopic Therapy, and Patient Outcomes for Gastric or Esophageal Duplication Cysts: Comprehensive Review of the Literature

TABLE 2 (Continued).
Clinical Presentation, Endoscopic Therapy, and Patient Outcomes for Gastric or Esophageal Duplication Cysts: Comprehensive Review of the Literature
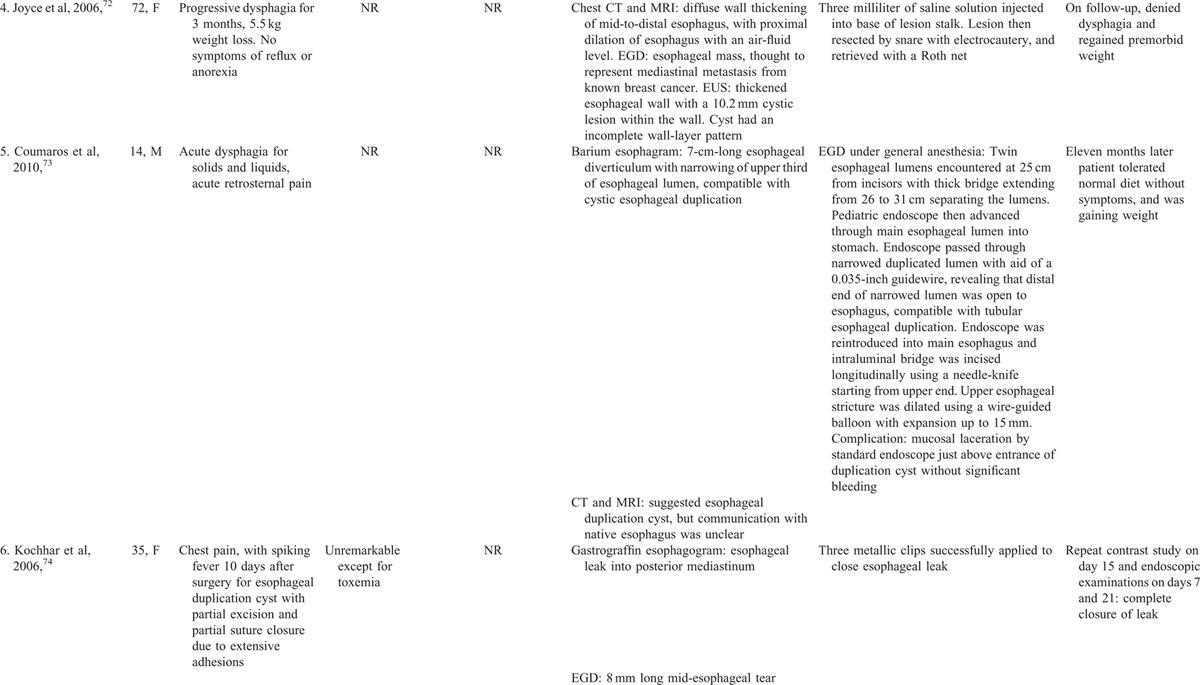
TABLE 2 (Continued).
Clinical Presentation, Endoscopic Therapy, and Patient Outcomes for Gastric or Esophageal Duplication Cysts: Comprehensive Review of the Literature

DISCUSSION
The currently reported patient was unusual in that the DDC was 14 cm long, more than twice as long as the previously reported longest DDC of 6.5 cm, and that the DDC caused GI obstruction by compressing the duodenal lumen, as demonstrated by abdominal CT and EGD. Only 1 previously reported patient treated endoscopically had presented with GI obstruction (patient-27: gastric outlet obstruction62). However, two children treated surgically had presented with gastric outlet obstruction from compression by pyloroduodenal duplication cysts.76,77
This current case illustrates that even an extremely long DDC can be adequately drained by therapeutic endosonography, without recurrence at least during 1 month of follow-up, and that adequate DDC decompression can reverse nausea, vomiting, and weight loss from the DDC. The key requirement is adequate DDC drainage and decompression to relieve extrinsic compression of the duodenal lumen causing the severe vomiting. The currently reported patient also had acute pancreatitis, likely from pancreatic ductular hypertension from the DDC. The key findings in the currently reported DDC are compared with those in the previously reported DDCs in Table 3.
TABLE 3.
Key Findings in Endoscopic Therapy of Duodenal Duplication Cysts: 28 Previously Reported Cases vs. Currently Reported Case
Keratinized cysts are lined by keratinized squamous mucosa and tend to have thicker and more viscous fluid within individual cysts than non-keratinized cysts. DDC are generally non-keratinized because they are lined by columnar epithelium and tend to have thinner and less viscous fluid within the cyst. The currently reported cyst was non-keratinized histologically and the cyst fluid was thin and, therefore, relatively easy to aspirate.
The current case report adds to the literature by reporting endoscopic intubation and visualization of the DDC with random mucosal endoscopic biopsies. We speculate that this endoscopic maneuver might prove helpful to exclude potential malignancy or dysplasia in a DDC, which would preclude endoscopic therapy.
Limitations of this review include incorporation of retrospectively reported case reports, potential reporting bias in that successful therapeutic endoscopy might have been preferentially reported, variable follow-up, and review of only 29 reported cases of DDC. Review strengths include performance of a systematic literature review; use of tandem, independent, investigators for the literature search with subsequent reconciliation of references to minimize omissions or biases; relatively long mean follow-up of 35.1 months in 25 cases; and illustration of endosonographic therapy to successfully reverse severely symptomatic duodenal compression from one extremely long DDC.
APPENDIX 1: CASE REPORT
A 40-year-old man with prior mild gastroesophageal reflux and no prior gastrointestinal (GI) surgery presented with moderate epigastric pain, and recurrent nausea and vomiting of partly digested food 1 hour post-cibum, associated with 15-kg-weight-loss during the prior month. Physical examination revealed a blood pressure of 106/71 mmHg, pulse of 70 beats/min with orthostasis, dry mucous membranes, poor skin turgor, and absent axillary sweat. Abdominal examination revealed mild epigastric tenderness, without rebound or guarding, and normoactive bowel sounds. Rectal examination revealed no fecal occult blood. Laboratory analysis revealed 10,000 leukocytes/mm3, and hematocrit of 44.9. The serum level of sodium = 131 mmol/L, potassium = 3.6 mmol/L, chloride = 73 mmol/L, bicarbonate = 39 mmol/L, blood urea nitrogen = 80 mg/dL, and creatinine = 2.4 mg/dL. The alkaline phosphatase = 140 U/L, AST = 87 U/L, ALT = 46 U/L, total bilirubin = 1.9 mg/dL, and lipase = 209 U/L. International normalized ratio and platelet count were within normal limits.
The patient was aggressively administered IV fluids with electrolytes for volume resuscitation and electrolyte repletion, with prompt improvement in electrolyte abnormalities. Abdominal CT administered with oral, but not IV, contrast, due to prerenal azotemia, revealed a 6 × 14 cm, smooth-walled, intraluminal filling defect extending from proximal descending duodenum to nearly the ligament of Treitz which compressed the duodenum lumen, but some contrast traversed through the compressed segment to distal small bowel, findings consistent with DDC. CT revealed no pancreatic or peripancreatic inflammation. Abdominal US confirmed a large, simple, retroperitoneal cyst was present. The patient was administered total parenteral nutrition because of intolerance of oral feedings and was administered chlorpromazine for intractable hiccups.
EGD revealed a slit-like, opening in descending duodenum, from extrinsic compression, with no intrinsic mucosal lesions. Histopathologic analysis of biopsies from the slit-like opening revealed mild chronic inflammation without dysplasia. Upper gastrointestinal series with small bowel follow-through revealed a smoothly contoured deformity along the third portion of the duodenum caused by a central mass displacing adjacent bowel loops (Figure 1A). Most of the contrast remained in the stomach, but some contrast traversed through the small bowel with a normal transit time. Abdominal magnetic resonance imaging (MRI), without IV gadolinium contrast, revealed an 8 × 13 cm intramural cystic mass arising along wall of the descending and transverse duodenum that demonstrated T1 and T2 hyperintense signals, and an irregularly thickened posterior cyst wall, findings consistent with a DDC containing complex fluid, such as hemorrhagic or proteinaceous material (Figure 1B). The pancreaticobiliary ducts were not dilated. A Meckel's technetium scan revealed no scintigraphic evidence of ectopic gastric mucosa within the cyst.
FIGURE 1.
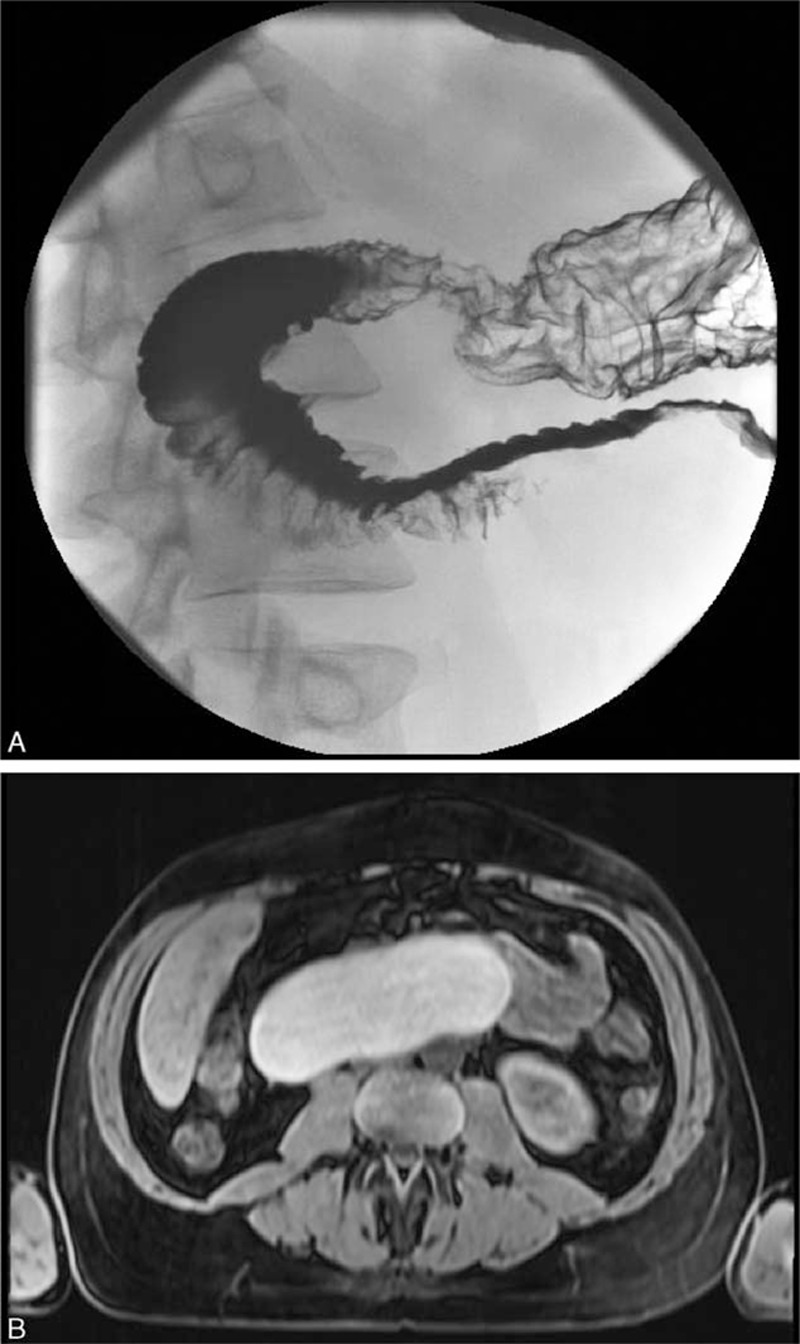
A, Upper gastrointestinal series with small bowel follow-through in a 40-year-old man presenting with severe nausea and vomiting post cibum associated with 15 kg weight loss during the prior month reveals a smoothly contoured deformity along the third portion of the duodenum caused by a central mass displacing and compressing this part of the duodenum. The duodenum is mostly but not completely obstructed by the central mass. B, Abdominal magnetic resonance imaging (MRI) without IV gadolinium contrast reveals an 8 × 13 cm intramural cystic mass arising along wall of the descending and transverse duodenum that demonstrated T1 and T2 hyperintense signals, and an irregularly thickened posterior cyst wall, findings consistent with a duodenal duplication cyst containing complex fluid, such as hemorrhagic or proteinaceous material.
For endoscopic therapy, the patient underwent endotracheal intubation and a linear echoendoscope was advanced into descending duodenum with some difficulty due to the mostly obstructed duodenal lumen from extrinsic compression. Limited EUS revealed an oval, intraluminal (subepithelial), anechoic cystic lesion, that endosonographically had a muscular wall that arose from the muscular wall of the native duodenum, sonographic findings consistent with DDC (Figure 2A). The cyst arose from muscularis propria of descending duodenum just distal to the papilla, extended at least 6 cm into the third portion of duodenum, and contained significant debris within it. Doppler ultrasound excluded pericystic vessels. The cyst was punctured using a 19 gauge needle; thick, dark fluid was aspirated; a 0.035 in. Jagwire was advanced via the needle into the cyst; the needle was withdrawn; a 7 French triple lumen needle-knife papillotome (Wilson Cook Medical, Winston-Salem, USA) was fed over the Jagwire; the cyst wall was incised using the papillotome (Figure 2B), the papillotome was exchanged with a pyloric balloon dilator, and the aperture was serially dilated up to 18 mm using increasingly larger balloons (Figure 2C), creating a large aperture (Figure 2D). Copious dark, thin, fluid seeped out of the cyst and was suctioned. The echoendoscope was withdrawn and a pediatric colonoscope was intubated and advanced into the cyst, and 800 cc of dark, brown fluid was aspirated via the colonoscope (Figure 2E, after cyst aspiration). After confirming that no lesions were endoscopically visible within the DDC, random biopsies were taken of DDC mucosa, which demonstrated no dysplasia or tumor within the DDC by subsequent histological analysis. Insertion of a double pig tail catheter within the cyst was considered to prevent fenestration closure, but was not performed due to a difficult cyst position for this cannulation. No bleeding occurred during the procedure.
FIGURE 2.
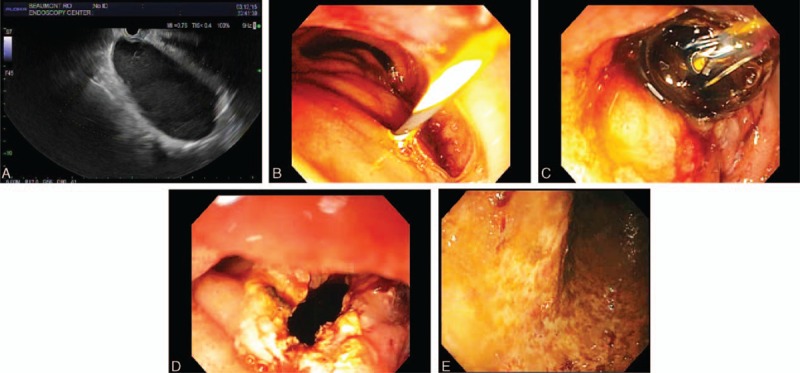
Progressive stages of endosonographic therapy to decompress a highly symptomatic and very long duodenal duplication cyst. A, Endoscopic ultrasound (EUS) revealed an oval, intraluminal (subepithelial), anechoic lesion, that endosonographically originated from within the submucosa (layer 3), consistent with duodenal duplication cyst. The cyst arose from muscularis propria of descending duodenum just distal to the papilla, extended deeply into the third portion of duodenum, and contained significant debris within it. B, The duodenal cyst was punctured using a 19 gauge needle; thick, dark fluid was aspirated; a 0.035 in. Jagwire was advanced via the needle into the cyst; the needle was withdrawn; a needle-knife papillotome was fed over the Jagwire; and the cyst wall was incised using the papillotome. C, The papillotome was exchanged with a pyloric balloon dilator, and the aperture was serially dilated up to 18 mm using increasingly larger balloons. D, EGD reveals a wide aperture after progressive balloon dilatation. E, The echoendoscope was withdrawn and a pediatric colonoscope was intubated and advanced into the cyst, and 800 cc of dark, brown fluid was aspirated via the colonoscope This endoscopic photograph shows the mucosa within the cyst has no evident lesions after cyst aspiration.
The patient was administered IV ciprofloxacin for 3 days after the procedure. The patient was discharged 3 days after the procedure feeling well, and tolerating oral feedings without nausea and vomiting. One month later the patient was asymptomatic, was tolerating oral feedings without nausea and vomiting, and had regained 7 kg in weight. Repeat EGD revealed minimal extrinsic compression of duodenum, and a wide aperture to the cyst. EUS revealed a residual 1.5 × 2.0 cm cyst. The orifice of the prior cyst-duodenostomy could not be identified using either straight-viewing or side-viewing (ERCP) endoscopes. A needle was not passed to aspirate the residual cyst under endosonographic guidance to avoid traversing pancreatic parenchyma.
Footnotes
Abbreviations: CI = confidence interval, CT = computerized tomography, DDC = duodenal duplication cyst, EGD = esophagogastroduodenoscopy, ERCP = endoscopic retrograde cholangiopancreatography, EUS = endoscopic ultrasonography, GI = gastrointestinal, IV = intravenous, MRCP = magnetic resonance cholangiopancreatography, MRI = magnetic resonance imaging, NKP = needle knife papillotomy, OR = odds ratio, RUQ = right upper quadrant, US = ultrasound.
MG and MSC have contributed equally to the article.
IRB Approval: Exempted/approved by William Beaumont Hospital IRB on August 14, 2015.
This paper does not discuss any confidential pharmaceutical industry data reviewed by Dr. Cappell as a consultant for the United States Food & Drug Administration (FDA) Advisory Committee on Gastrointestinal Drugs. Dr. Cappell is a speaker for Movantik, a drug jointly manufactured by AstraZeneca and Daiichi Sankyo. This work does not discuss any drug or medical device manufactured or marketed by AstraZeneca or Daiichi Sankyo.The authors have no conflict of interest to disclose
REFERENCES
- 1.Stringer MD, Spitz L, Abel R, et al. Management of alimentary tract duplication in children. Br J Surg 1995; 82:74–78. [DOI] [PubMed] [Google Scholar]
- 2.Destruys L, Guinard-Samuel V, Peycelon M, et al. Duodenal duplication cyst causing acute obstructive pancreatitis in a young girl with Crohn disease [Article in French]. Arch Pediatr 2014; 21:532–534. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Alcalá García F, Peréz Pozo JM, Martínez-Alcalá García A, et al. Duodenal duplication cyst and its endoscopic resolution [Article in Spanish]. Gastroenterol Hepatol 2014; 37:274–275. [DOI] [PubMed] [Google Scholar]
- 4.Chen JJ, Lee HC, Yeung CY, et al. Meta-analysis: the clinical features of the duodenal duplication cyst. J Pediatr Surg 2010; 45:1598–1606. [DOI] [PubMed] [Google Scholar]
- 5.Liu R, Adler DG. Duplication cysts: diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound 2014; 3:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo YC, Joo KR, Kim DH, et al. Duodenal duplicated cyst manifested by acute pancreatitis and obstructive jaundice in an elderly man. J Korean Med Sci 2004; 19:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeliger B, Piardi T, Marzano E, et al. Duodenal duplication cyst: a potentially malignant disease. Ann Surg Oncol 2012; 19:3753–3754. [DOI] [PubMed] [Google Scholar]
- 8.Redondo-Cerezo E, Pleguezuelo-Díaz J, de Hierro ML, et al. Duodenal duplication cyst and pancreas divisum causing acute pancreatitis in an adult male. World J Gastrointest Endosc 2010; 2:318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolfolk GM, McClave SA, Jones WF, et al. Use of endoscopic ultrasound to guide the diagnosis and endoscopic management of a large gastric duplication cyst. Gastrointest Endosc 1998; 47:76–79. [DOI] [PubMed] [Google Scholar]
- 10.Faigel DO, Burke A, Ginsberg GG, et al. The role of endoscopic ultrasound in the evaluation and management of foregut duplications. Gastrointest Endosc 1997; 45:99–103. [DOI] [PubMed] [Google Scholar]
- 11.Gross RE, Holcomb GW, Jr, Farber S. Duplications of the alimentary tract. Pediatrics 1952; 9:448–468. [PubMed] [Google Scholar]
- 12.Sefa T, Mikail C, Anil S, et al. Duodenal duplication cyst extending into the posterior mediastinum. Int J Surg Case Rep 2015; 10:252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laskowska K, Gałązka P, Daniluk-Matraś I, et al. Use of diagnostic imaging in the evaluation of gastrointestinal tract duplications. Pol J Radiol 2014; 79:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lad RJ, Fitzgerald P, Jacobson K. An unusual cause of recurrent pancreatitis: duodenal duplication cyst. Can J Gastroenterol 2000; 14:341–345. [DOI] [PubMed] [Google Scholar]
- 15.Ko SY, Ko SH, Ha S, et al. A case of a duodenal duplication cyst presenting as melena. World J Gastroenterol 2013; 19:6490–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geller A, Wang KK, DiMagno EP. Diagnosis of foregut duplication cysts by endoscopic ultrasonography. Gastroenterology 1995; 109:838–842. [DOI] [PubMed] [Google Scholar]
- 17.Chong VY, Loveday BP, Weilert F, et al. Delayed infection in duodenal duplication cyst after endoscopic ultrasound. ANZ J Surg 2014; Nov 12. [Epub ahead of print]. PMID: 25388220. [DOI] [PubMed] [Google Scholar]
- 18.Al-Harake A, Bassal A, Ramadan M, et al. Duodenal duplication cyst in a 52-year-old man: A challenging diagnosis and management. Int J Surg Case Rep 2013; 4:296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrot T, Anastasescu R, Pankevych T, et al. Duodenal duplications. Clinical characteristics, embryological hypotheses, histological findings, treatment. Eur J Pediatr Surg 2006; 16:18–23. [DOI] [PubMed] [Google Scholar]
- 20.Arbell D, Lebenthal A, Blashar A, et al. Duplication cyst of the duodenum as an unusual cause of massive gastrointestinal bleeding in an infant. J Pediatr Surg 2002; 37:E8. [DOI] [PubMed] [Google Scholar]
- 21.Rubin RB, Saltzman JR, Zawacki JK, et al. Duodenal duplication cyst with massive gastrointestinal bleeding. J Clin Gastroenterol 1995; 21:72–74. [DOI] [PubMed] [Google Scholar]
- 22.Rai BK, Zaman S, Mirza B, et al. Duodenal duplication cyst having ectopic gastric and pancreatic tissues. APSP J Case Rep 2012; 3:15. [PMC free article] [PubMed] [Google Scholar]
- 23.Falk GL, Young CJ, Parer J. Adenocarcinoma arising in a duodenal duplication cyst: a case report. Aust N Z J Surg 1991; 61:551–553. [DOI] [PubMed] [Google Scholar]
- 24.Hata H, Hiraoka N, Ojima H, et al. Carcinoid tumor arising in a duplication cyst of the duodenum. Pathol Int 2006; 56:272–278. [DOI] [PubMed] [Google Scholar]
- 25.Inoue M, Nishimura O, Andachi H, et al. Early cancer of duodenal duplication. A case report. Gastroenterol Jpn 1979; 14:233–237. [DOI] [PubMed] [Google Scholar]
- 26.Lee NK, Kim S, Jeon TY, et al. Complications of congenital and developmental abnormalities of the gastrointestinal tract in adolescents and adults: evaluation with multimodality imaging. Radiographics 2010; 30:1489–1507. [DOI] [PubMed] [Google Scholar]
- 27.Segal SR, Sherman NH, Rosenberg HK, et al. Ultrasonographic features of gastrointestinal duplications. J Ultrasound Med 1994; 13:863–870. [DOI] [PubMed] [Google Scholar]
- 28.Lam WW, Le SD, Chan KL, et al. Transverse testicular ectopia detected by MR imaging and MR venography. Pediatr Radiol 2002; 32:126–129. [DOI] [PubMed] [Google Scholar]
- 29.Carbognin G, Guarise A, Biasiutti C, et al. Duodenal duplication cyst identified with MRCP. Eur Radiol 2000; 10:1277–1279. [DOI] [PubMed] [Google Scholar]
- 30.Simone M, Mutter D, Rubino F, et al. Three-dimensional virtual cholangioscopy: a reliable tool for the diagnosis of common bile duct stones. Ann Surg 2004; 240:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guarise A, Faccioli N, Ferrari M, et al. Duodenal duplication cyst causing severe pancreatitis: imaging findings and pathological correlation. World J Gastroenterol 2006; 12:1630–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanna PC, Gawand V, Nawale AJ, et al. Complete large bowel duplication with paraduodenal cyst: prenatal sonographic features. Prenat Diagn 2004; 24:312–314. [DOI] [PubMed] [Google Scholar]
- 33.Narlawar RS, Rao JR, Karmarkar SJ, et al. Sonographic findings in a duodenal duplication cyst. J Clin Ultrasound 2002; 30:566–568. [DOI] [PubMed] [Google Scholar]
- 34.Rotondo A, Scialpi M, Pellegrino G, et al. Duodenal duplication cyst: MR imaging appearance. Eur Radiol 1999; 9:890–893. [DOI] [PubMed] [Google Scholar]
- 35.Stoupis C, Ros PR, Abbitt PL, et al. Bubbles in the belly: imaging of cystic mesenteric or omental masses. Radiographics 1994; 14:729–737. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Jakhmola CK, Chauhan SS, et al. Atypical presentation of gastrointestinal stromal tumor masquerading as a large duodenal cyst: a case report. Int J Surg Case Rep 2015; 9:123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You HS, Park SB, Kim JH, et al. A case of duodenal duplication cyst manifested by duodenal polyp. Clin Endosc 2012; 45:425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhutani MS, Hoffman BJ, Reed C. Endosonographic diagnosis of an esophageal duplication cyst. Endoscopy 1996; 28:396–397. [DOI] [PubMed] [Google Scholar]
- 39.Van Dam J, Rice TW, Sivak MV., Jr Endoscopic ultrasonography and endoscopically guided needle aspiration for the diagnosis of upper gastrointestinal tract foregut cysts. Am J Gastroenterol 1992; 87:762–765. [PubMed] [Google Scholar]
- 40.Janssen J, König K, Knop-Hammad V, et al. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc 2004; 59:339–344. [DOI] [PubMed] [Google Scholar]
- 41.Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 2006; 63:622–629. [DOI] [PubMed] [Google Scholar]
- 42.Wildi SM, Hoda RS, Fickling W, et al. Diagnosis of benign cysts of the mediastinum: the role and risks of EUS and FNA. Gastrointest Endosc 2003; 58:362–368. [PubMed] [Google Scholar]
- 43.Gyökeres T, Schwab R, Burai M, et al. Intraluminal duodenal duplication-endoscopic therapy is recommended even without previous pancreatitis and jaundice. Gastrointest Endosc 2008; 68:1244. [DOI] [PubMed] [Google Scholar]
- 44.Moreno P, Lazo MD, Andrade RJ, et al. Unusual duodenal duplication cyst associated with partial gastric diverticulum in a middle-aged woman: are they congenital or acquired? Dig Dis Sci 2002; 47:304–308. [DOI] [PubMed] [Google Scholar]
- 45.Meier AH, Mellinger JD. Endoscopic management of a duodenal duplication cyst. J Pediatr Surg 2012; 47:e33–e35. [DOI] [PubMed] [Google Scholar]
- 46.Criblez D, Mitschele T, Scheiwiller A. A rare cause of acute pancreatitis in an adolescent. Juxtapapillary duodenal duplication cyst as a rare cause of acute pancreatitis in an adolescent. Gastroenterology 2011; 140:783. [DOI] [PubMed] [Google Scholar]
- 47.al Traif I, Khan MH. Endoscopic drainage of a duodenal duplication cyst. Gastrointest Endosc 1992; 38:64–65. [DOI] [PubMed] [Google Scholar]
- 48.Antaki F, Tringali A, Deprez P, et al. A case series of symptomatic intraluminal duodenal duplication cysts: presentation, endoscopic therapy, and long-term outcome (with video). Gastrointest Endosc 2008; 67:163–168. [DOI] [PubMed] [Google Scholar]
- 49.Chryssostalis A, Ribiere O, Prat F. Endoscopic management of a duodenal duplication cyst filled with stones and revealed by recurrent pancreatitis. Clin Gastroenterol Hepatol 2007; 5:e31–e32. [DOI] [PubMed] [Google Scholar]
- 50.Rockx MA, McAlister VC. Endoscopic fenestration of a duodenal duplication cyst to resolve recurrent pancreatitis. JOP 2007; 8:795–798. [PubMed] [Google Scholar]
- 51.Tekin F, Ozutemiz O, Ersoz G, et al. A new endoscopic treatment method for a symptomatic duodenal duplication cyst. Endoscopy 2009; 41 Suppl 2:E32–E33. [DOI] [PubMed] [Google Scholar]
- 52.Arantes V, Nery SR, Starling SV, et al. Duodenal duplication cyst causing acute recurrent pancreatitis, managed curatively by endoscopic marsupialization. Endoscopy 2012; 44(Suppl 2) UCTN:E117–E118. [DOI] [PubMed] [Google Scholar]
- 53.Antaki N, Abboud D, Lemmers A, et al. Acute recurrent pancreatitis secondary to the rare association of a duodenal duplication cyst and a pancreas divisum. Clin Res Hepatol Gastroenterol 2013; 37:e32–e36. [DOI] [PubMed] [Google Scholar]
- 54.Johanson JF, Geenen JE, Hogan WJ, et al. Endoscopic therapy of a duodenal duplication cyst. Gastrointest Endosc 1992; 38:60–64. [DOI] [PubMed] [Google Scholar]
- 55.Wada S, Higashizawa T, Tamada K, et al. Endoscopic partial resection of a duodenal duplication cyst. Endoscopy 2001; 33:808–810. [DOI] [PubMed] [Google Scholar]
- 56.Gyökeres T, Schwab R, Burai M, et al. Duodenal duplication cyst. Endoscopy 2002; 34:503–504. [DOI] [PubMed] [Google Scholar]
- 57.Vandenbroucke F, Dagenais M, Létourneau R, et al. Endoscopic treatment of a duodenal duplication cyst. Endoscopy 2005; 37:601. [DOI] [PubMed] [Google Scholar]
- 58.Jung MK, Park SY, Jeon SW, et al. Duodenal duplication cysts of ampulla of vater containing stone. Gut Liver 2009; 3:356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurien RT, Chowdhury SD, Unnikrishnan LS, et al. Endoscopic treatment of a duodenal duplication cyst. Endoscopy 2014; 46(Suppl 1) UCTN:E583–E584. [DOI] [PubMed] [Google Scholar]
- 60.Johnson EA, Gopal D. Endoscopic management of a symptomatic duodenal duplication cyst. Gastrointest Endosc 2015; 82:172. [DOI] [PubMed] [Google Scholar]
- 61.Sezgin O, Altiparmak E, Yilmaz U, et al. Endoscopic management of a duodenal duplication cyst associated with biliary obstruction in an adult. J Clin Gastroenterol 2001; 32:353–355. [DOI] [PubMed] [Google Scholar]
- 62.Dave P, Romeu J, Clary S, et al. Endoscopic removal of an obstructing duodenal duplication cyst. Endoscopy 1984; 16:75–76. [DOI] [PubMed] [Google Scholar]
- 63.You HS, Park SB, Kim JH, et al. A case of duodenal duplication cyst manifested by duodenal polyp. Clin Endosc 2012; 45:425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobara H, Mori H, Masaki T. Gastric duplication cyst with heterotopic pancreas and ectopic submucosal gland on submucosal endoscopy. Dig Endosc 2016; 28:223. [DOI] [PubMed] [Google Scholar]
- 65.Klair JS, Girotra M, Rego RF. A rare, gastric, multiseptated duplication cyst resembling gastric lymphangioma. Clin Gastroenterol Hepatol 2015; 13:e161–e162. [DOI] [PubMed] [Google Scholar]
- 66.Deesomsak M, Aswakul P, Junyangdikul P, et al. Rare adult gastric duplication cyst mimicking a gastrointestinal stromal tumor. World J Gastroenterol 2013; 19:8445–8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YC, Kim YB, Kim JK, et al. Endoscopic treatment of a large gastric duplication cyst with hook-knife and snare (with video). Gastrointest Endosc 2011; 73:1039–1040. [DOI] [PubMed] [Google Scholar]
- 68.Eom JS, Kim GH, Song GA, et al. Gastric duplication cyst removed by endoscopic submucosal dissection. Korean J Gastroenterol 2011; 58:346–349. [DOI] [PubMed] [Google Scholar]
- 69.Stecevic V, Karim R, Jacobs R. Gastric duplication cyst treated by endoscopic electrosurgical snare resection. Gastrointest Endosc 2003; 57:615–616. [DOI] [PubMed] [Google Scholar]
- 70.Mou Y, Wen D, Liu Q, et al. Endoscopic resection of an esophageal duplication cyst with spraying of anhydrous alcohol. Endoscopy 2015; 47(suppl 1) UCTN:E348–E349. [DOI] [PubMed] [Google Scholar]
- 71.Ivekovic H, Jouret-Mourin A, Deprez PH. Endoscopic fenestration of esophageal duplication cysts. Endoscopy 2012; (suppl 2 UCTN):E404–E405. [DOI] [PubMed] [Google Scholar]
- 72.Joyce AM, Zhang PJ, Kochman ML. Complete endoscopic resection of an esophageal duplication cyst (with video). Gastrointest Endosc 2006; 64:288–289. [DOI] [PubMed] [Google Scholar]
- 73.Coumaros D, Schneider A, Tsesmeli N, et al. Endoscopic management of a tubular esophageal duplication diagnosed in adolescence (with videos). Gastrointest Endosc 2010; 71:827–830. [DOI] [PubMed] [Google Scholar]
- 74.Kochhar R, Saluja H, Singh RS, et al. Endoscopic clip application for postoperative residual esophageal duplication cyst. Endscopy 2006; 38:424–425. [DOI] [PubMed] [Google Scholar]
- 75.Will U, Meyer F, Bosseckert H. Successful endoscopic treatment of an esophageal duplication cyst. Scand J Gastroenterol 2005; 40:995–999. [DOI] [PubMed] [Google Scholar]
- 76.Annigeri VM, Hegde HV, Patil PB, et al. Pyloroduodenal duplication cyst. J Indian Assoc Pediatr Surg 2012; 17:80–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Troebs RB, Wunsch R, Neid M. Pyloroduodenal duplication cysts: treatment of eleven cases. Eur J Pediatr Surg 2013; 23:505–506. [DOI] [PubMed] [Google Scholar]


