Abstract
The objective of this study was to assess whether preoperative breast magnetic resonance imaging (MRI) combined with conventional breast imaging techniques decreases the rates of margin involvement and reexcision.
Data on patients who underwent surgery for primary operable breast cancer were obtained from the Changhua Christian Hospital (CCH) breast cancer database. The rate of surgical margin involvement and the rate of reoperation were compared between patients who underwent conventional breast imaging modalities (Group A: mammography and sonography) and those who received breast MRI in addition to conventional imaging (Group B: mammography, sonography, and MRI).
A total of 1468 patients were enrolled in this study. Among the 733 patients in Group A, 377 (51.4%) received breast-conserving surgery (BCS) and 356 (48.6%) received mastectomy. Among the 735 patients in Group B, 348 (47.3%) received BCS and 387 (52.7%) received mastectomy. There were no significant differences in operative method between patients who received conventional imaging alone and those that received MRI and conventional imaging (P = 0.13). The rate of detection of pathological multifocal/multicentric breast cancer was markedly higher in patients who received preoperative MRI than in those who underwent conventional imaging alone (14.3% vs 8.6%, P < 0.01). The overall rate of surgical margin involvement was significantly lower in patients who received MRI (5.0%) than in those who received conventional imaging alone (9.0%) (P < 0.01). However, a significant reduction in rate of surgical margin positivity was only observed in patients who received BCS (Group A, 14.6%; Group B, 6.6%, P < 0.01). The overall BCS reoperation rates were 11.7% in the conventional imaging group and 3.2% in the combined MRI group (P < 0.01). There were no significant differences in rate of residual cancer in specimens obtained during reoperation between the 2 preoperative imaging groups (Group A, 50%; Group B, 81.8%, P = 0.09). In multivariate analysis, multifocal/multicentric breast cancer (odds ratio = 2.38, P = 0.02) and without MRI use (odds ratio = 2.35, P < 0.01) were the major predisposing factors to margin involvement in patients received BCS.
Preoperative breast MRI combined with conventional breast imaging results in a lower rate of surgical margin involvement and reoperations in patients who receive BCS.
INTRODUCTION
Breast cancer treatment involves multidisciplinary and multimodality management, and surgery remains the mainstay of treatment for early stage breast cancer.1,2 The goal of surgical resection is to remove the tumor with adequate safe margins.3,4 A number of studies have shown that positive margins are associated with higher rates of local recurrence after BCS (lumpectomy with adjuvant radiotherapy),5–7 thereby mandating reoperation.8,9 A number of ways to optimize margin clearance have been proposed, including intraoperative surgical techniques, such as radioguided surgery and ultrasound-guided resection, intraoperative pathology assessment techniques, and preoperative imaging.10
Accurate preoperative assessment of tumor location and extension is important for adequate surgical planning.11 MRI is increasingly being used in the preoperative evaluation of breast cancer12–15 and has been reported to have value in estimating tumor size,11,16 high sensitivity for detecting ipsilateral and contralateral occult breast lesions,13–15,17 and therefore could be complementary to conventional breast imaging modalities such as mammography and sonography.
Some studies have demonstrated that the high sensitivity of MRI for detecting cancer aids in the selection of patients for BCS18–20 and increases the likelihood of obtaining negative margins during the first lumpectomy attempt,21,22 reduce reexcision rates,22–24 decreasing the rate of ipsilateral tumor recurrence,25 and the development of new breast cancer on the contralateral side.25 However, other studies have shown that breast MRI is not associated with improved margin status12,24,26–28 or a reduction in reoperation,26–30 but instead is associated with treatment delay12 and an increased rate of mastectomy.12,25,27–30
In this case–control comparative analysis, we evaluated whether combining breast MRI with conventional imaging techniques results in lower rates of margin involvement and reexcision. We compared the surgical methods and margin status between patients who underwent conventional preoperative imaging and those who received conventional imaging combined with MRI group. We also evaluated whether preoperative breast MRI can predict residual cancer requiring further surgery.
MATERIALS AND METHODS
All patients enrolled at this study had primary operable breast cancer, underwent preoperative mammography and sonography with or without MRI examination during January 2009 to December 2013, and received breast cancer surgery at the Changhua Christian Hospital (CCH), a tertiary medical center in central Taiwan. Patients whose primary tumor was removed before definite cancer operation, those who received neoadjuvant chemotherapy, and patients with incomplete data were excluded. The clinical and pathologic data were collected through chart review of medical, surgical, and pathologic records by a well-trained study nurse (SLC), and the accuracy was confirmed by the principle investigator (HWL). This study was approved by the Institutional Review Board (IRB) of the CCH (IRB number #140404).
In this case-controlled analysis, we retrospectively collected 2 groups of patients for evaluating the effect and value of adding preoperative breast MRI to conventional breast images (mammography and sonography). The proportion of patients at CCH received breast MRI increased dramatically since January of 2011 when our hospital started to cover the expense of breast MRI. To prevent the selection bias of patients who did not receive breast MRI, we decided to choose the control group of patients diagnosed and treated during 2009 to 2010. Because during January 2009 to December 2010, breast MRI was rarely performed, and this could prevent the possible selection bias. As after January 2011, when the breast MRI expanse covered program was started, patients selected for conventional breast images only or combined with MRI images might be biased according to physicians or patients’ preference.
A total of 1468 patients fulfilled the inclusion criteria and were enrolled in this study. Patients were stratified into 2 preoperative imaging groups. Group A (n = 733) comprised patients who underwent conventional preoperative imaging (mammography and sonography) and Group B (n = 735) comprised patients who received MRI combined with conventional imaging. Figure 1 shows the flow chart of patients in the present study.
FIGURE 1.
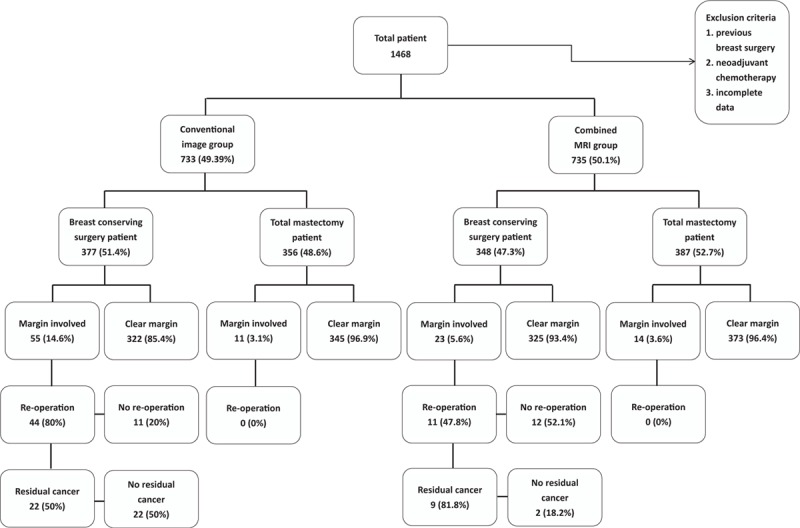
Flow chart of patients in the case-controlled comparative analysis.
The type of operation (BCS, mastectomy or mastectomy with breast reconstruction), and the rate of surgical margin involvement were compared between the 2 groups of patients. To further evaluate the effect of preoperative breast MRI on the rate of margin positivity, index surgeons, defined as surgeons who had performed more than 100 breast cancer operations, were selected and analyzed. We also analyzed and compared the rate of reoperation among patients with positive margins and the rate of residual cancer detection after reoperation between the 2 image study groups.
To prevent bias from confounding factors, a propensity-score matching31 was also perform to select 2 groups of patients for further analysis of factors related to margin involvement in primary operable breast cancer patients.
Definition of Surgical Margins
Tumor margins were assessed microscopically by surgical pathologists. All margins were inked before sectioning. Each specimen was serially sectioned at 3- to 5-mm intervals and then stained with hematoxylin and eosin. Pathologic analysis included the assessment of proximity to or the involvement of each margin for invasive carcinoma or carcinoma in situ. When available, the pathology report was examined for the actual margin. Surgical margin involvement in the present study was defined as the presence of cancer cells at the surgical margin or <1 mm from the peripheral margin. Specimen with surgical margins less than 1 mm from the superficial (away from skin flap) or deep (away from pectoralis major muscle) layer of the fascia, where the fibroglandular boundary of the skin and chest wall was located, were not regarded as margin involvement.
IMAGE STUDY
Mammography
The mammograms were performed by using 1 of 3 digital mammography systems, a Hologic Selenia Dimension full-field digital mammography system (Hologic, Danbury, CT), Siemens Mammomat Inspiration (Siemens AG, Healthcare Sector, Erlangen, Germany) and the GE Senographe Essential digital mammography system (General Electric Medical Systems, Milwaukee, WI). All women received the standard mediolateral oblique (MLO) and craniocaudal (CC) views. Three radiologists who specialize in breast imaging independently interpreted the mammograms using a 5 MP premium diagnostic grayscale display system (Coronis 5MP Mammo, Barco, Kortrijk, Belgium) on a Picture Archiving and Communication System without using the aid of clinical information, physical examination, or sonography results.
Sonography
The ultrasound sonography procedures were performed with the patient in the supine position. A high-resolution 5 to 12 MHz linear array transducer, including color Doppler ultrasonography (Voluson 530D and 730D, Kretz Technik, Zipf, Austria), was used for imaging acquisition. Recorded images were reported according to the Breast Image Reporting and Data System (BI-RADS).32 The measurement of tumor size took the echo-poor center of the lesion and the echogenic halo into account. Experienced, board-certified breast physicians performed the whole sonographic examinations.
MRI
The MRI protocol is described in our previous study.11 Briefly, a Siemens (Verio) 3.0 T magnet MR imaging was used. All patients were imaged with both breasts placed into a dedicated 16-channel breast coil in the prone position. Both breasts were examined with a 60-second interval between each dynamic phase image in the transverse plane. A commercially available MRI computer aid diagnosis (CAD) system (DynaCAD Version 2.1 for Breast MRI; Invivo, Gainesville, FL) was used to help analyzing MR images. Experienced, board-certified radiologist specializing in breast imaging (HKW) performed the whole breast MRI readings.
Statistical Analyses
Data are expressed as mean ± standard deviation (SD) for continuous variables. Differences in means of continuous measurements were tested by the Student t test. The Chi-square test was used for categorical comparisons of data. Univariate and multivariate analysis were used to find factors affecting margin involvement and to reduce the possible bias due to confounding variables. Propensity score matching analysis was performed with the package MatchIt in software R (version 3.2.2) to prevent bias from confounding factors. All tests were 2-tailed. A P-value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed with the statistical package SPSS (IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp., Chicago) and accuracy of statistic results was confirmed by statistic expert (YJL).
RESULTS
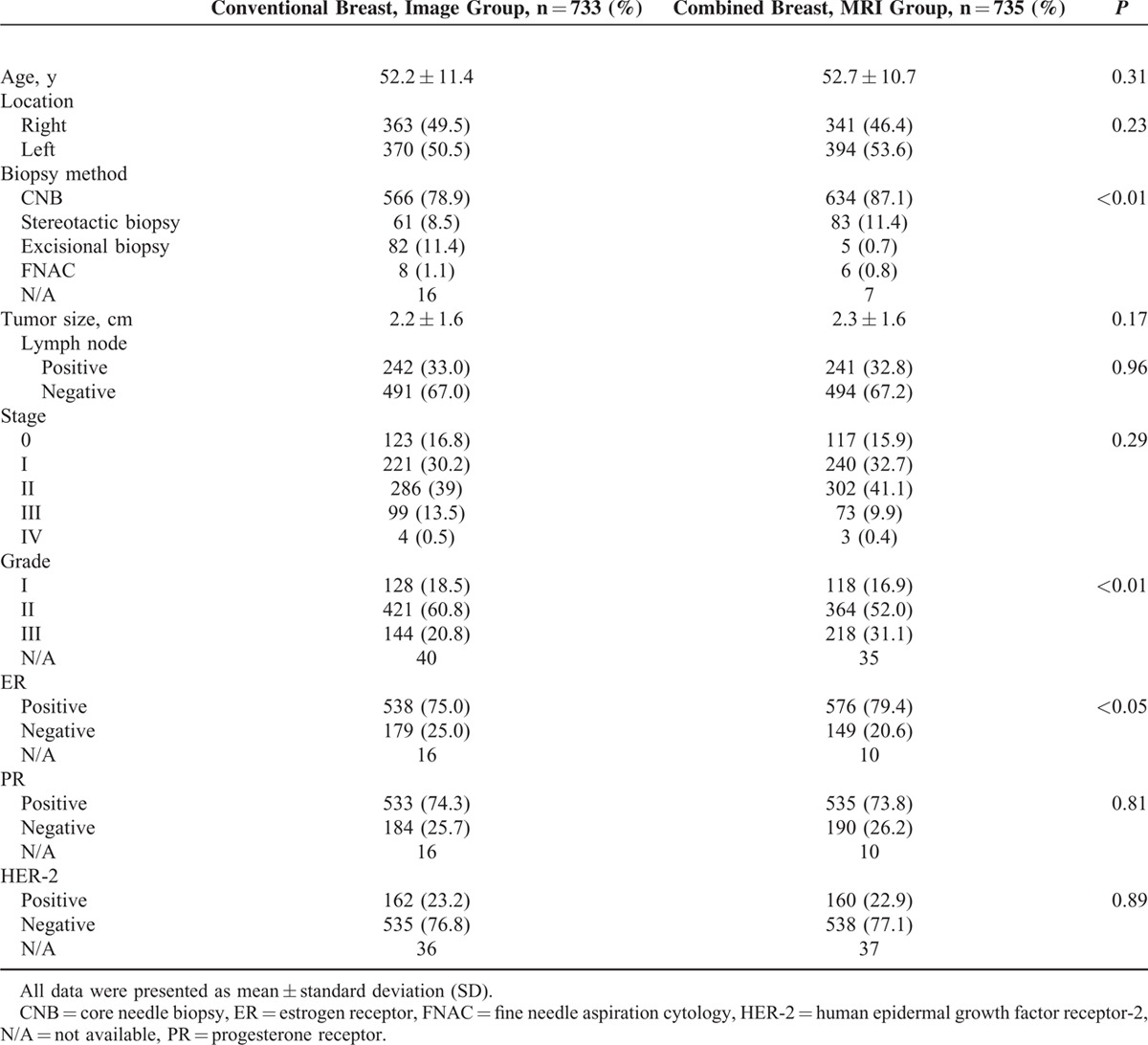
There were no significant differences between patients who received conventional preoperative imaging alone (Group A, n = 733) and those who underwent preoperative MRI in addition to conventional imaging (Group B, n = 735) in age (52.2 ± 11.4 vs 52.7 ± 10.7, P = 0.31), tumor laterality, tumor size (2.2 ± 1.6 cm vs 2.3 ± 1.6 cm, P = 0.17), positive lymph node rate (33.0% vs 32.8%, P = 0.96), cancer stage, progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) expression (Table 1).
TABLE 1.
Clinical Features of Patients of Current Case-Controlled Analysis
Among the 733 patients in Group A, 377 (51.4%) received BCS and 356 (48.6%) received mastectomy. In Group B, 348 (47.3%) received BCS and 387 (52.7%) received mastectomy. There were no differences in surgical methods employed between the 2 groups of patients (P = 0.13, Table 2). However, the percentage of patients who underwent sentinel lymph node surgery and the percentage of patients who received breast reconstruction surgery were higher in Group B than in Group A (P < 0.01). The rate of detection of pathological multifocal/multicentric breast cancer was markedly higher in patients who received preoperative MRI than in those who underwent conventional imaging alone (14.3% vs 8.6%, P < 0.01, Table 2).
TABLE 2.
Operation Methods and Clinical Outcome According to Different Group of Image Survey
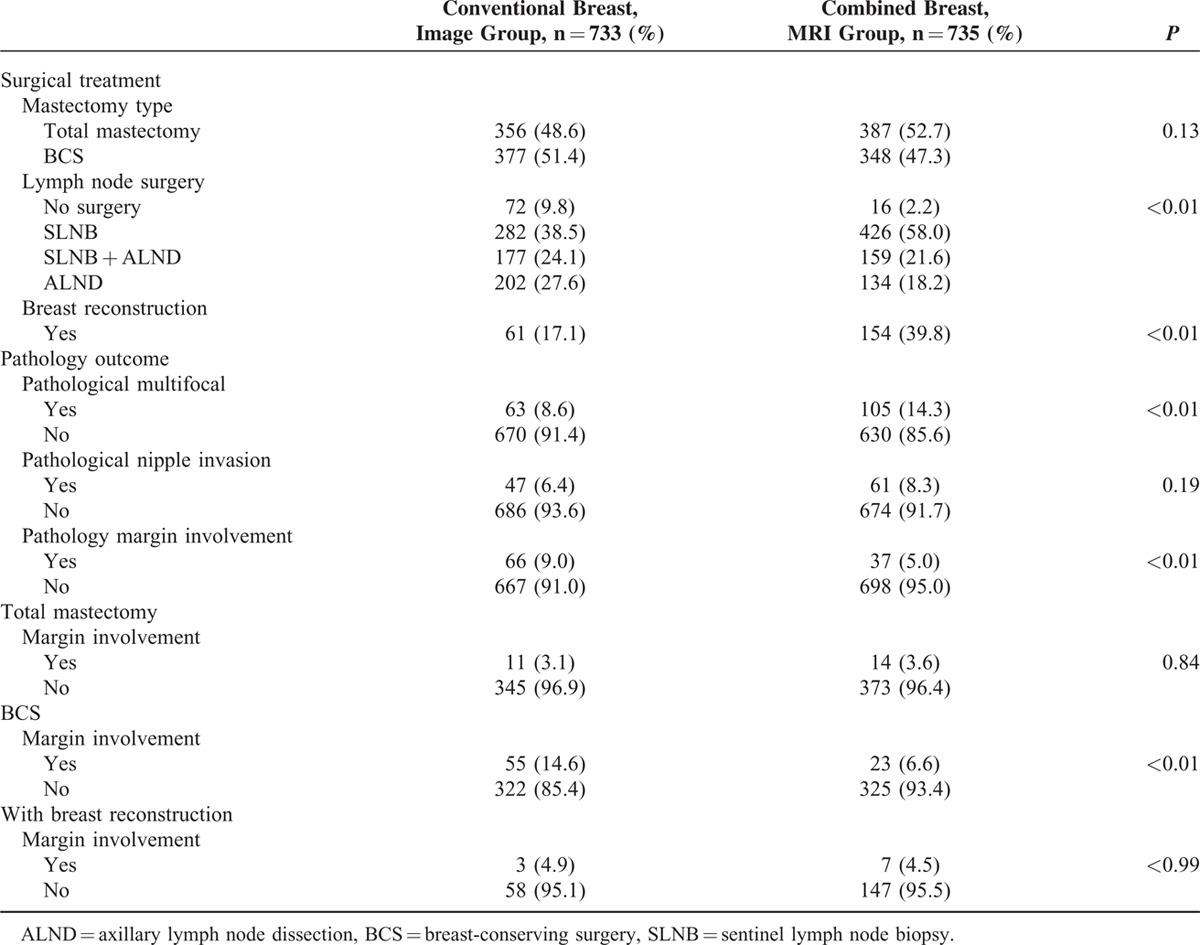
The overall rate of surgical margin involvement was significantly higher among patients who received conventional preoperative imaging alone (9%) than among those who combined with preoperative MRI (5%) (P < 0.01). There were no significant differences in rate of margin positivity between patients in Group A and those in Group B who received total mastectomy (3.1% vs 3.6%, P = 0.84) or breast reconstruction (4.9% vs 4.5%, P > 0.99). However, the rate of positive margin involvement was significantly lower in patients who received BCS and preoperative MRI than in those who received conventional imaging alone (6.6% vs 14.6%, P < 0.01, Table 2).
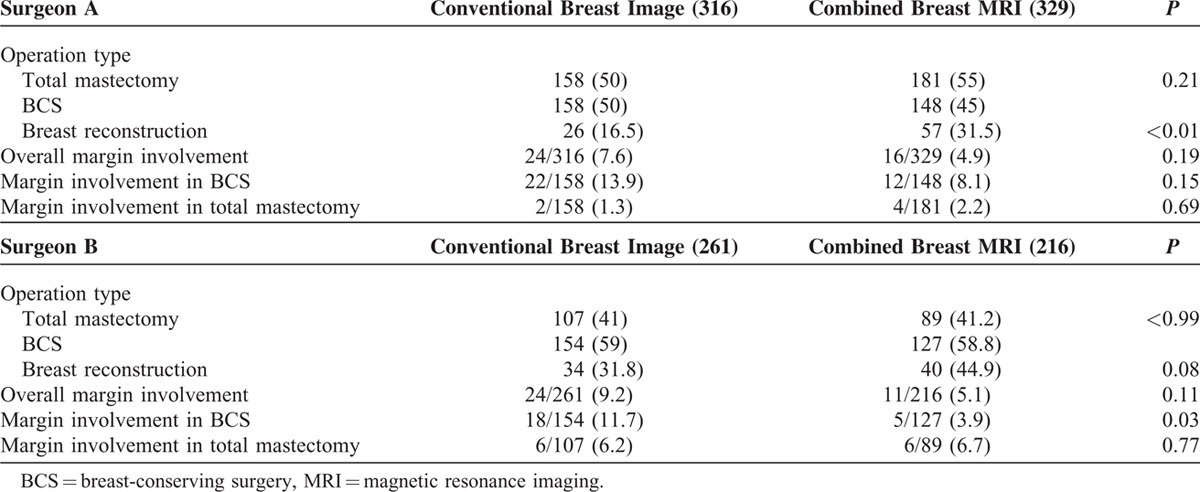
Two index surgeons (A and B), defined as surgeons who have performed more than 100 surgeries for breast cancer, were selected to evaluate whether breast MRI affected the surgeon's choice of operation and whether the imaging method was associated with margin positivity in resected specimens (Table 3). For surgeon A, there was no significant difference in number of BCS procedures performed between the conventional imaging group (158/316) and the MRI group (148/329) (P = 0.21). There were also no significant differences in overall rate of surgical margin involvement between the two groups (7.6% vs 4.9%, P = 0.19) and no significant differences in rates of margin involvement after total mastectomy (1.3% vs 2.2%, P = 0.69), or BCS (13.9% vs 8.1%, P = 0.15) between the 2 groups. For surgeon B, there was no significant difference in number of BCS procedures performed between the conventional imaging group (154/261) and the MRI group (127/216) (P > 0.99). There were also no significant differences in overall rate of surgical margin involvement between the 2 groups (9.2% vs 5.1%, P = 0.11) and no significant differences in rates of margin involvement after total mastectomy (6.2% (Group A) vs 6.7% (Group B), P = 0.77). However, the rate of margin positivity in resected specimens in patients who underwent BCS was significantly lower among patients who received MRI than among patients who received conventional preoperative imaging (3.9% vs 11.7%, P = 0.03, Table 3).
TABLE 3.
Effect of Preoperative Breast MRI on Index Surgeons on Margin Involved Rate
We also analyzed whether there were differences in the rates of reoperation for cases of margin involvement and the rates of residual cancer detection after reoperation between the 2 image study groups (Table 4). Of the 103 (7%) patients with margin involvement, 66 had undergone preoperative conventional image studies and 37 had undergone MRI as well as conventional imaging. The overall rate of reoperations in margin involved conditions was significantly higher among patients in the conventional imaging group (66.7%) than among those in the combined MRI group (29.7%, P < 0.01). None of the patients with margin involvement who received total mastectomy in either imaging group underwent a second surgery. The rate of reoperation for patients with margin involvement who received BCS was significantly higher among those who underwent conventional imaging alone (80%) than among those who received preoperative MRI in addition to conventional imaging (47.8%, P < 0.01). The overall BCS reoperation rates were 11.7% (44/377) in the conventional imaging group and 3.2% (11/348) in the combined MRI group (P < 0.01).
TABLE 4.
Margin Involved BCS Patients With Reexcision and Residual Cancer Found in Reexcised Specimens
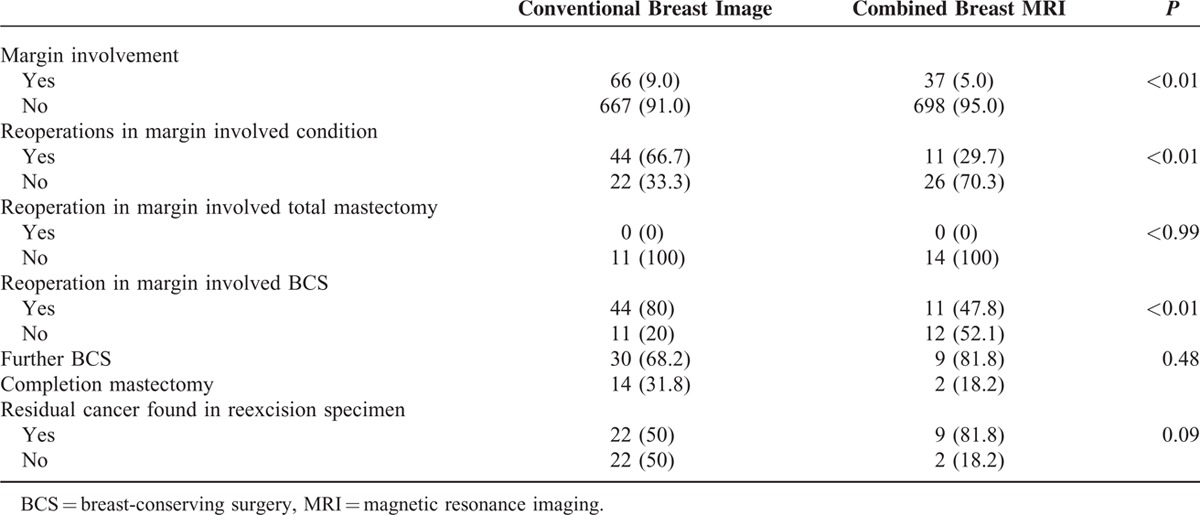
In patients with margin involved and undergone reoperations, further BCS could be performed in 68.2% (30/44) of patients in conventional image group and 81.8% (9/11) in combined with MRI group (P = 0.48, Table 4). There was no difference in rate of residual breast cancer found at reexcised specimens after BCS between the 2 imaging groups (50% vs 81.8%, P = 0.09). The results of rates about margin involvement, reoperations, mastectomy, and local recurrences in our case-controlled comparative study were compared with other literature reported series and summarized in Table 5.
TABLE 5.
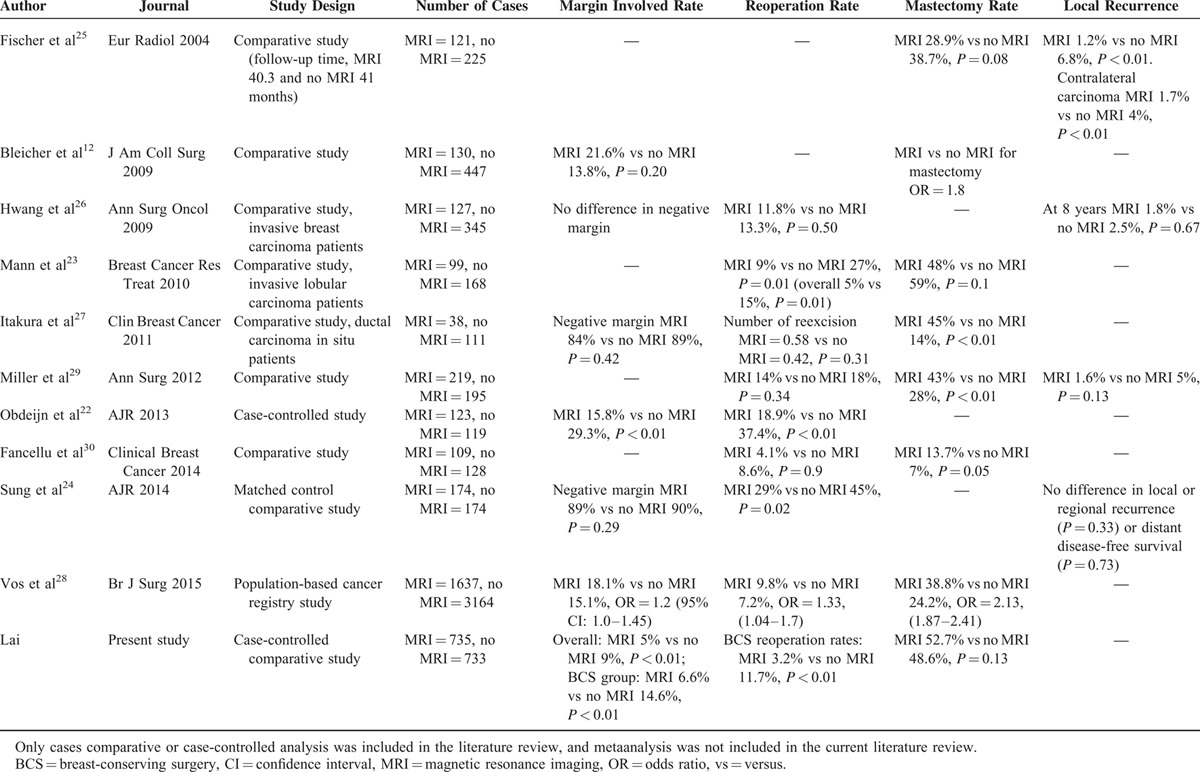
Literature Review of Comparative Studies of Preoperative MRI on Rates of Margin Involvement, Reoperation, Mastectomy, and Local Recurrence
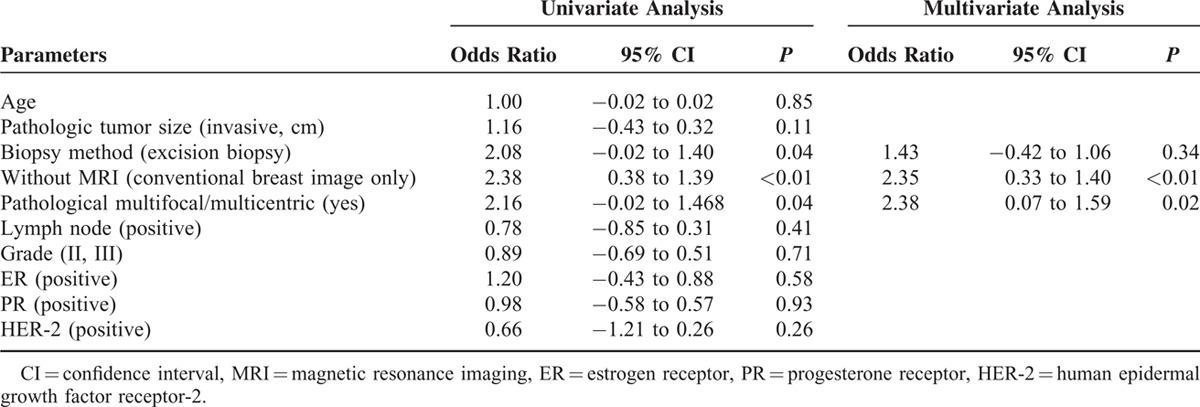
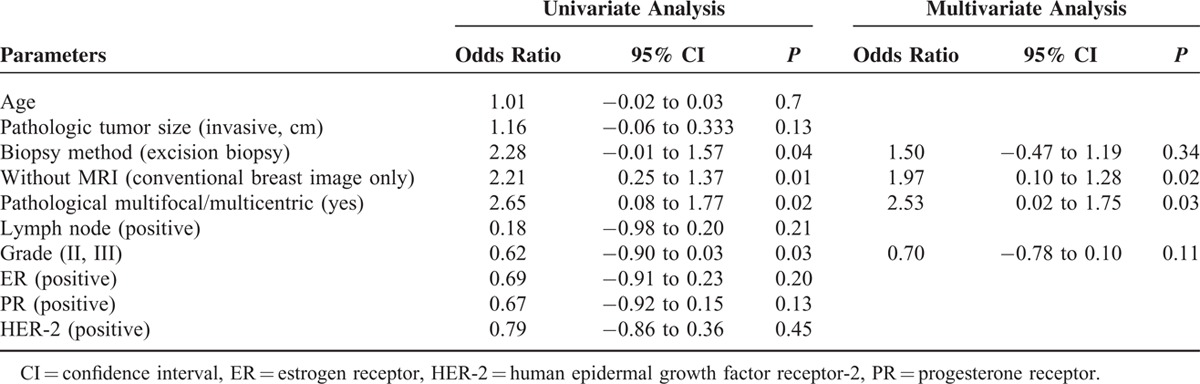
The factors affecting margin involvement in patients received BCS were further analyzed with univariate and multivariate analysis. In Table 6 univariate analysis, excision biopsy (odds ratio = 2.08, P = 0.04), without MRI use (conventional breast image only) (odds ratio = 2.38, P = 0.01), and pathological multifocal/multicentric breast cancer (odds ratio = 2.16, P = 0.04) were risk factors for margin involvement in patients received BCS. In multivariate analysis, multifocal/multicentric breast cancer (odds ratio = 2.38, P = 0.02) and without MRI use (odds ratio = 2.35, P < 0.01) were the major predisposing factors for margin involvement.
TABLE 6.
Risk Factors for Margin Involvement in Patients Received Breast-Conserving Surgery
To prevent bias from confounding factors, a propensity-score matching was also perform to select 2 groups of patients for further analysis of factors related to margin involvement in primary operable breast cancer patients (Table 7). We have 641 patients in conventional image group and combined with breast MRI group. The pathologic margin involvement was 8.3% in conventional image group, and 5% in combined with MRI group (P = 0.03). The margin involvement rate in patients received BCS was 13.5% in conventional image group versus 6.6% in combined with MRI group (P = 0.01). In multivariate analysis (Table 8), multicentric/multifocal breast cancer (odds ratio=2.53, P = 0.03), and without MRI use (odds ratio = 1.97, P = 0.02) remained the 2 major factors related to margin involvement in BCS patients.
TABLE 7.
Clinical Features of Patients After Propensity Score Matching
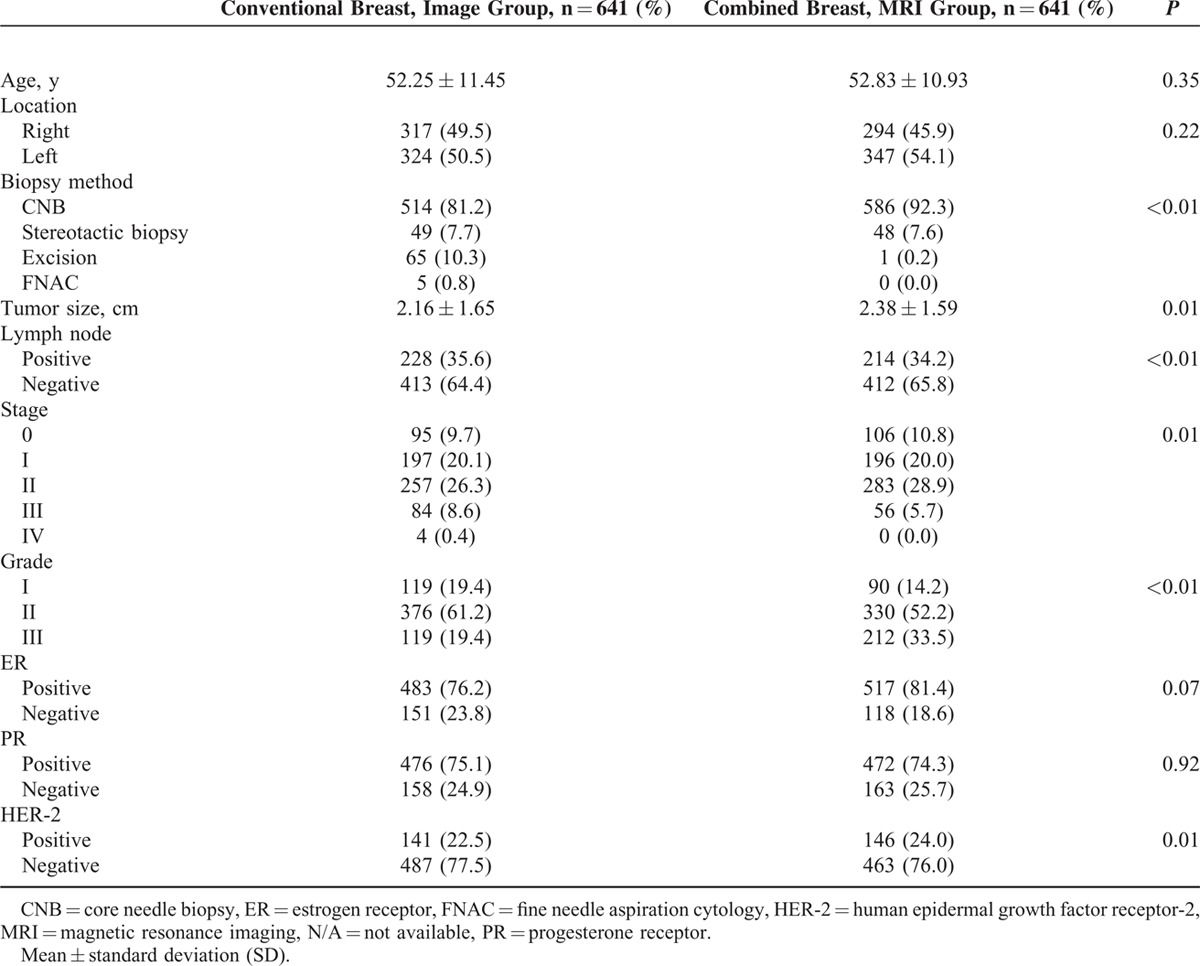
TABLE 8.
Risk Factors for Margin Involvement in Patients Received Breast-Conserving Surgery After Propensity Score Matching
DISCUSSION
Surgical resection with clear histologic margin remained the main task of surgeons either in BCS or mastectomy. Factors that influence local recurrence include patient age, tumor stage, tumor grade, lymphovascular invasion, molecular subtype, and positive surgical margins.6,33–37 Positive surgical margin has been demonstrated to be the most important and preventable factor associated with recurrence of operable breast cancers.6,35,37 Methods that show promise for minimizing the rate of positive margin involvement10 include margin index, nomograms, intraoperative ultrasound-guided resection, wire-guided localization, radioactive seed localization, standardize cavity shaving, frozen section analysis, and MRI.
In a systematic review and meta-analysis, MRI was determined to be the most sensitive preoperative imaging tool for detection of additional or occult disease.15 In our study, the rate of detection of pathological multifocal/multicentric breast cancer was markedly higher in patients who received preoperative MRI than in those who underwent conventional imaging alone (14.3% vs 8.6%, P < 0.01, Table 2). Similar findings have been reported elsewhere.15,38 Adding breast MRI to conventional breast imaging did increase the findings of more multifocal/multicentric breast cancer or disease extent more severe than expected, and therefore might increase the decision to perform a mastectomy. In our case-controlled comparison analysis, the combination of preoperative breast MRI did increase 4.1% mastectomy rate, however, it was statistically nonsignificantly (P = 0.13). In Killelea et al's study,39 they found that “when compared with the women who did not have an MRI, the women with a normal MRI or a benign biopsy actually had an increased lumpectomy rate (66% and 62%). Thus, some women who were considering mastectomy may have chosen lumpectomy based on the MRI results. This may explain why the use of MRI had a relatively modest effect overall on the lumpectomy rate.” Our study also supported this finding that when breast MRI showed unifocal breast cancer, which was consistent with previous mammography and/or sonographic findings, then patients and surgeons were more convinced to receive BCS.
From Table 2, about 7.9% (4.1%/51.4%) of patients would change their surgery from BCS to mastectomy due to the addition of breast MRI. The margin involved rate in patients received BCS decreased from 14.6% (conventional breast image group) to 6.6% (combined with preoperative MRI group) (P < 0.01). About 54.8% (8%/14.6%) of previous margin involved BCS patients was prevented after combining preoperative breast MRI to conventional breast images. Combining with preoperative breast MRI could help us to find a group of patients with higher risk of margin involvement for BCS. The allocation of this high-risk group of patients to mastectomy (with or without breast reconstruction) would greatly decrease margin involved rate for patients receiving BCS. We found that a 54.8% decrease ratio of margin involvement in BCS patients was derived from the change of 7.9% patients, who might not be suitable for BCS. The development of nipple sparing type of mastectomy40 did increase patients’ will to perform mastectomy with reconstruction when preoperative MRI revealed disease extent larger than expected (39.8% breast reconstruction rate in combined MRI group vs 17.1% in conventional image group, P < 0.01, Table 2). From the observation of this case-controlled comparison analysis, we speculated that adding preoperative breast MRI to conventional breast images could help physicians to pick up a group of patients who were not suitable for BCS and allocate them to receive mastectomy could greatly decrease the surgical margin involved rate.
Studies that have investigated whether preoperative MRI results in lower rates of margin involvement, and local recurrence have provided conflicting results (Table 5).12,15,25,28,30,41 Obdeijn et al22 in a case-controlled study showed that the margin positive rate was significantly decreased in MRI group compared with controlled no MRI group (15.8% vs 29.3%, P < 0.01). However, other studies have failed to show that breast MRI is associated with improved margin status.12,24,26–28 Fischer et al25 showed that the ipsilateral tumor recurrence and the development of new breast cancer on the contralateral side were decreased in cased who received preoperative breast MRI evaluation. Other studies did not show adding preoperative MRI to conventional breast imaging would statistically decrease local recurrence.24,26,29 In our present study, without MRI use (conventional breast image only) was associated with increased risk (Odds ratio = 2.35, P < 0.01, Table 6) of margin involvement in patients received BCS in multivariate analysis. To reduce the bias from possible confounding factors, the propensity score matching were performed and we repeat the analysis (Tables 7 and 8). In multivariate analysis, multicentric/multifocal breast cancer (odds ratio = 2.53, CI = 0.02–1.75, P = 0.03), and without MRI use (odds ratio = 1.97, CI = 0.10–1.28, P = 0.02) remained the 2 major factors related to margin involvement in BCS patients. An increased rate of mastectomy were observed in some studies,12,25,27–30 however, other studies showed MRI was not associated with increased mastectomy rate.23,25 In contrast to the “negative impact” results,12,27,28 which found that MRI was associated with an increased mastectomy rate but was not associated with improved margin status, we found that preoperative MRI was associated with a lower rate of margin involvement without an apparent increase in mastectomy rate.
No consensus exists among surgeons as to what constitutes a safe surgical margin (1–2, >5, or >1 cm).42 There is increasing evidence that a negative margin should be defined in samples with no tumor on the inked margin.3,43 However, in this study, we defined a positive margin as one in which tumor cells were seen within 1 mm from peripheral inked margins because many studies have shown that margins <1 mm are associated with high risk of residual disease.9,44,45
Patients with positive margins are usually advised to receive further surgery to prevent local recurrence.7,8,44 However, up to 50% of specimens taken during reoperation do not show evidence of residual breast cancer.8,9,45 Some studies showed that preoperative breast MRI could reduce reexcision rates,22–24 but other studies did not revealed a reduction in reoperations26–30 (Table 5). In our study, we found that the use of preoperative breast MRI was associated with a significantly lower rate of reoperation than conventional imaging among patients with positive margins after BCS (47.8% vs 80%, P < 0.01). This decreased of reoperation in combined with MRI group might be that the surgeons would suppose that the residual cancer would be less as preoperative MRI did not show other multifocal or multicentric lesions for patients selected for BCS. Thus the overall rates of reoperation among patients who underwent BCS were 11.7% (44/377) in the conventional imaging group and 3.2% (11/348) in the MRI group (P < 0.01). Although we found that combining MRI with mammography and sonography was associated with a reduction in the number of reoperations, there was no significant difference in the rate of residual cancer detection in the reexcised specimens between patients who received conventional imaging only and those who received MRI preoperatively (50% vs 81.8%, P = 0.09).
In present study, we tried to understand the impact of adding breast MRI to conventional breast images on the effect of individual breast surgeons. In the past, most studies reported the impact of breast MRI was derived from a mixture of different experience of surgeons and disease severity of patients. This could mask the real effect of adding preoperative breast MRI upon physicians and their performance on patients’ outcome. Fortunately, we had 2 index surgeons, defined as having more than 100 breast cancer operations in each of 2 imaging survey periods, for evaluation. During the study period in our hospital, more than 10 surgeons were found for the operations done in the 1468 patients. Index surgeon was chose to prevent the bias of inadequate surgical techniques or inexperience about breast imaging tools (either conventional breast images or combined with breast MRI) on the outcome of margin involvement. From these 2 index breast surgeons, we could see the impact of breast MRI on the practice or medical behavior on the breast cancer patients (Table 3). We found that for experienced breast surgeons, adding breast MRI did not increase mastectomy rate significantly, and a decrease of surgical margin involved rate in BCS patients were observed.
Limitations in this study include its retrospective nature and possible selection bias. Designing a case-controlled comparison analysis, we try to have 2 groups of patients with comparable characteristics, like patients’ age, tumor size, lymph node status, and stages. However, as most of the retrospective cases collective analysis, we could not have all the characteristics (or variables) comparable or equal. The related lower estrogen receptor (ER) expression (75% vs 79%, P < 0.05), and higher histologic grade in conventional image group than combined with breast MRI group might be also selection bias related (Table 1). Furthermore, patients diagnosed and treated during 2009 to 2010, would have slightly higher proportion of patients diagnosed with excision biopsy or less percentage of patients received sentinel lymph node biopsy than patients diagnosed after January 2011. To prevent bias derived from confounding factors, we had performed the propensity score matching to reduce possible selection bias in this retrospective study. The lack of long-term follow-up results in present study could not answer whether preoperative MRI would decrease ipsilateral tumor recurrence or prolong disease-free survival. Nonetheless, our results clearly demonstrate that preoperative MRI combined with mammography and sonography results in a lower rate of positive surgical margins and reoperations than conventional preoperative imaging.
In conclusion, we found that preoperative breast MRI combined with conventional breast imaging would detect more multifocal/multicentric breast cancer, which was the major predisposing factor for margin involvement. The combination of breast MRI resulted in a lower rate of surgical margin involvement in patients who underwent BCS but not in patients who underwent mastectomy. Breast MRI was also associated with a higher rate of breast reconstruction in patients who underwent mastectomy and a lower rate of reoperation in patients with margin involvement who underwent BCS. MR images obtained preoperatively, however, were not sufficient for predicting residual cancer after excision.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, BCS = breast-conserving surgery, BI-RADS = Breast Image Reporting and Data System, CAD = computer aid diagnosis, CC = craniocaudal, CCH = Changhua Christian Hospital, ER = estrogen receptor, HER-2 = human epidermal growth factor receptor-2, IRB = Institutional Review Board, MLO = mediolateral oblique, MRI = magnetic resonance imaging, PR = progesterone receptor, SD = standard deviation, VIBE = volumetric interpolated breath-hold examination.
Synopsis: In our case–control study, we found that preoperative breast magnetic resonance imaging (MRI) combined with conventional breast imaging detected more multifocal/multicentric breast cancer, and resulted in a lower rate of surgical margin involvement in patients who underwent breast-conserving surgery (BCS). Breast MRI was also associated with a higher rate of breast reconstruction in patients who underwent mastectomy and a lower rate of reoperation in patients with margin involvement who underwent BCS. MR images obtained preoperatively, however, were not sufficient for predicting residual cancer after excision.
This study was sponsored by research funding provided by the Changhua Christian Hospital (103-CCH-IRP-022 and 104-CCH-ICO-006).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015; 26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology-Breast Cancer. 2014; http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf Accessed November 6, 2015. [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347:1233–1241. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347:1227–1232. [DOI] [PubMed] [Google Scholar]
- 5.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 2002; 184:383–393. [DOI] [PubMed] [Google Scholar]
- 6.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010; 46:3219–3232. [DOI] [PubMed] [Google Scholar]
- 7.Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys 1999; 44:1005–1015. [DOI] [PubMed] [Google Scholar]
- 8.Biglia N, Ponzone R, Bounous VE, et al. Role of re-excision for positive and close resection margins in patients treated with breast-conserving surgery. Breast 2014; 23:870–875. [DOI] [PubMed] [Google Scholar]
- 9.Cellini C, Hollenbeck ST, Christos P, et al. Factors associated with residual breast cancer after re-excision for close or positive margins. Ann Surg Oncol 2004; 11:915–920. [DOI] [PubMed] [Google Scholar]
- 10.Angarita FA, Nadler A, Zerhouni S, et al. Perioperative measures to optimize margin clearance in breast conserving surgery. Surg Oncol 2014; 23:81–91. [DOI] [PubMed] [Google Scholar]
- 11.Lai HW, Chen DR, Wu YC, et al. Comparison of the diagnostic accuracy of magnetic resonance imaging with sonography in the prediction of breast cancer tumor size: a concordance analysis with histopathologically determined tumor size. Ann Surg Oncol 2015; 22:3816–3823. [DOI] [PubMed] [Google Scholar]
- 12.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg 2009; 209:180–187.quiz 185–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberman L, Morris EA, Dershaw DD, et al. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. Am J Roentgenol 2003; 180:901–910. [DOI] [PubMed] [Google Scholar]
- 14.Esserman L, Hylton N, Yassa L, et al. Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging. J Clin Oncol 1999; 17:110–119. [DOI] [PubMed] [Google Scholar]
- 15.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008; 26:3248–3258. [DOI] [PubMed] [Google Scholar]
- 16.Gruber IV, Rueckert M, Kagan KO, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer 2013; 13:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orel SG, Schnall MD, Newman RW, et al. MR imaging-guided localization and biopsy of breast lesions: initial experience. Radiology 1994; 193:97–102. [DOI] [PubMed] [Google Scholar]
- 18.Hollingsworth AB, Stough RG, O’Dell CA, et al. Breast magnetic resonance imaging for preoperative locoregional staging. Am J Surg 2008; 196:389–397. [DOI] [PubMed] [Google Scholar]
- 19.Lalonde L, David J, Trop I. Magnetic resonance imaging of the breast: current indications. Can Assoc Radiol J 2005; 56:301–308. [PubMed] [Google Scholar]
- 20.Grobmyer SR, Mortellaro VE, Marshall J, et al. Is there a role for routine use of MRI in selection of patients for breast-conserving cancer therapy? J Am Coll Surg 2008; 206:1045–1050.discussion 1042–1050. [DOI] [PubMed] [Google Scholar]
- 21.Bassett LW, Dhaliwal SG, Eradat J, et al. National trends and practices in breast MRI. Am J Roentgenol 2008; 191:332–339. [DOI] [PubMed] [Google Scholar]
- 22.Obdeijn IM, Tilanus-Linthorst MM, Spronk S, et al. Preoperative breast MRI can reduce the rate of tumor-positive resection margins and reoperations in patients undergoing breast-conserving surgery. Am J Roentgenol 2013; 200:304–310. [DOI] [PubMed] [Google Scholar]
- 23.Mann RM, Loo CE, Wobbes T, et al. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat 2010; 119:415–422. [DOI] [PubMed] [Google Scholar]
- 24.Sung JS, Li J, Da Costa G, et al. Preoperative breast MRI for early-stage breast cancer: effect on surgical and long-term outcomes. Am J Roentgenol 2014; 202:1376–1382. [DOI] [PubMed] [Google Scholar]
- 25.Fischer U, Zachariae O, Baum F, et al. The influence of preoperative MRI of the breasts on recurrence rate in patients with breast cancer. Eur Radiol 2004; 14:1725–1731. [DOI] [PubMed] [Google Scholar]
- 26.Hwang N, Schiller DE, Crystal P, et al. Magnetic resonance imaging in the planning of initial lumpectomy for invasive breast carcinoma: its effect on ipsilateral breast tumor recurrence after breast-conservation therapy. Ann Surg Oncol 2009; 16:3000–3009. [DOI] [PubMed] [Google Scholar]
- 27.Itakura K, Lessing J, Sakata T, et al. The impact of preoperative magnetic resonance imaging on surgical treatment and outcomes for ductal carcinoma in situ. Clin Breast Cancer 2011; 11:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vos EL, Voogd AC, Verhoef C, et al. Benefits of preoperative MRI in breast cancer surgery studied in a large population-based cancer registry. Br J Surg 2015; 102:1649–1657. [DOI] [PubMed] [Google Scholar]
- 29.Miller BT, Abbott AM, Tuttle TM. The influence of preoperative MRI on breast cancer treatment. Ann Surg Oncol 2012; 19:536–540. [DOI] [PubMed] [Google Scholar]
- 30.Fancellu A, Soro D, Castiglia P, et al. Usefulness of magnetic resonance in patients with invasive cancer eligible for breast conservation: a comparative study. Clin Breast Cancer 2014; 14:114–121. [DOI] [PubMed] [Google Scholar]
- 31.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol 1999; 150:327–333. [DOI] [PubMed] [Google Scholar]
- 32.American College of Radiology. Breast Imaging Reporting and Data System Atlas (BI-RADS Atlas). 4th ed.Reston, VA, USA: American College of Radiology; 2003. [Google Scholar]
- 33.Millar EK, Graham PH, O’Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol 2009; 27:4701–4708. [DOI] [PubMed] [Google Scholar]
- 34.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 35.Mechera R, Viehl CT, Oertli D. Factors predicting in-breast tumor recurrence after breast-conserving surgery. Breast Cancer Res Treat 2009; 116:171–177. [DOI] [PubMed] [Google Scholar]
- 36.Bernardi S, Bertozzi S, Londero AP, et al. Influence of surgical margins on the outcome of breast cancer patients: a retrospective analysis. World J Surg 2014; 38:2279–2287. [DOI] [PubMed] [Google Scholar]
- 37.Bhatti AB, Khan A, Muzaffar N, et al. Safe negative margin width in breast conservative therapy: results from a population with a high percentage of negative prognostic factors. World J Surg 2014; 38:2863–2870. [DOI] [PubMed] [Google Scholar]
- 38.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg 2013; 257:249–255. [DOI] [PubMed] [Google Scholar]
- 39.Killelea BK, Grube BJ, Rishi M, et al. Is the use of preoperative breast MRI predictive of mastectomy? World J Surg Oncol 2013; 11:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petit JY, Veronesi U, Luini A, et al. When mastectomy becomes inevitable: the nipple-sparing approach. Breast 2005; 14:527–531. [DOI] [PubMed] [Google Scholar]
- 41.Solin LJ, Orel SG, Hwang WT, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol 2008; 26:386–391. [DOI] [PubMed] [Google Scholar]
- 42.Azu M, Abrahamse P, Katz SJ, et al. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol 2010; 17:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol 2014; 21:704–716. [DOI] [PubMed] [Google Scholar]
- 44.Vicini FA, Goldstein NS, Pass H, et al. Use of pathologic factors to assist in establishing adequacy of excision before radiotherapy in patients treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 2004; 60:86–94. [DOI] [PubMed] [Google Scholar]
- 45.Kurniawan ED, Wong MH, Windle I, et al. Predictors of surgical margin status in breast-conserving surgery within a breast screening program. Ann Surg Oncol 2008; 15:2542–2549. [DOI] [PubMed] [Google Scholar]


