Supplemental Digital Content is available in the text
Abstract
Minimally invasive surgery (MIS) of colorectal cancer (CRC) was first introduced over 20 years ago and recently has gained increasing acceptance and usage beyond clinical trials. However, data on dissemination of the method across countries and on long-term outcomes are still sparse.
In the context of a European collaborative study, a total of 112,023 CRC cases from 3 population-based (N = 109,695) and 4 institute-based clinical cancer registries (N = 2328) were studied and compared on the utilization of MIS versus open surgery. Cox regression models were applied to study associations between surgery type and survival of patients from the population-based registries. The study considered adjustment for potential confounders.
The percentage of CRC patients undergoing MIS differed substantially between centers and generally increased over time. MIS was significantly less often used in stage II to IV colon cancer compared with stage I in most centers. MIS tended to be less often used in older (70+) than in younger colon cancer patients. MIS tended to be more often used in women than in men with rectal cancer. MIS was associated with significantly reduced mortality among colon cancer patients in the Netherlands (hazard ratio [HR] 0.66, 95% confidence interval [CI] (0.63–0.69), Sweden (HR 0.68, 95% CI 0.60–0.76), and Norway (HR 0.73, 95% CI 0.67–0.79). Likewise, MIS was associated with reduced mortality of rectal cancer patients in the Netherlands (HR 0.74, 95% CI 0.68–0.80) and Sweden (HR 0.77, 95% CI 0.66–0.90).
Utilization of MIS in CRC resection is increasing, but large variation between European countries and clinical centers prevails. Our results support association of MIS with substantially enhanced survival among colon cancer patients. Further studies controlling for selection bias and residual confounding are needed to establish role of MIS in survival of patients.
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in men and the second in women worldwide, with an estimated 1.4 million cases and about 700,000 deaths occurring in 2012.1,2 In recent years, laparoscopic surgery has gained increasing acceptance and usage for CRC treatment beyond clinical trials since the first report of this minimally invasive surgery (MIS) in 1991.3 Based on the results from both randomized clinical trials (RCTs)4 and observational studies,5 utilization of MIS for resection of colon cancer is as effective as open resectional surgery (ORS) with no negative effect on the overall and disease-free survival rate of patients.6 Furthermore, there is evidence that laparoscopic surgery in CRC patients is associated with lower mortality, lower complication rates, and a shorter median length of hospital stay.7 Recent studies indicate benefits of this procedure also among older patients,8 advanced stage, and incurable patients.9
However, there are also some controversies regarding the short-term and long-term benefits of MIS in comparison with ORS.10,11 Application of MIS in treatment of CRC might be limited by several factors, including surgeons’ experience, and also clinical conditions of patients such as prohibitive abdominal adhesions and acute bowel obstruction.12 Furthermore, considerable controversies surrounded the application of MIS for resection of transverse colon cancer and for proctectomy in rectal cancer patients.11 There are also some patient-specific factors such as high BMI, older age, and disease-specific factors such as T4 cancers that often lead to conversion of MIS to open surgery in treatment of CRC patients.13 Conversion of MIS to open surgery has been reported to occur in more than 20% of colon cancer and more than 40% in rectal cancer treatment.4 Allaix et al14 reported that conversion of MIS per se is though not associated with worse early postoperative outcomes or adverse long-term survival of patients. However, adverse results after converted MIS have also been reported.15,16
This study is a part of the EurocanPlatform project, which is a consortium of major European cancer centers aimed at enhanced translation of progress in oncological research into clinical practice.17 The aim of this study is to compare the implementation of laparoscopic surgery in therapeutic resection of CRC and its impact on outcomes of patients in real clinical practice among European counties. We also aimed to describe the patterns and recent trends in utilization of MIS, with particular attention to age and stage-specific overall survival among patients in various European countries and centers involved in the EurocanPlatform consortium.
METHODS
Participating Centers and Study Populations
As part of activities of Work Package 11 (WP11, clinical epidemiology and outcome research) of the EurocanPlatform project data were obtained from 3 population-based and 4 institute-based clinical cancer registries from 6 European countries. The population-based registries include the national database of the Norwegian Cancer Registry (NCR), national data of the Swedish Colorectal Cancer Registry (SCRCR), and the database from the Netherlands National Cancer Registry (NNCR), which included information on comorbidities from the Eindhoven Cancer Registry (ECR). The institute-based registries include the National Center for Tumor Diseases-Heidelberg (NCT-HD) in Germany, the Institute Jules Bordet (IJB) in Brussels (Belgium), the Netherlands Cancer Institute (NKI) in Amsterdam, and the Portuguese Institute of Oncology in Porto (IPO-Porto). Data on basic patient and tumor characteristics, and also type of surgery (MIS, ORS, and converted) and use of neoadjuvant therapy were requested from all partner registries for consecutive years with available data.
In this study, only first invasive CRC patients with no evidence of metastasis at diagnosis were included. All statistical analyses were performed using the “intention-to-treat” approach, where CRC cases were included according to the originally assigned surgery group, MIS, or ORS, regardless of conversions of MIS to ORS. The reason why intention-to-treat analysis was applied was to avoid the attrition, crossover, and similar sources of bias that are related to noncompletion of the outcome data in a subset of individuals originally assigned to a particular surgical method. All the analyses were stratified by center and tumor location (colon and rectum). Cases with unspecified colon/rectal tumor location or cases that underwent emergent CRC surgery were excluded from all analyses. Period survival estimates were obtained through left truncation of the follow-up of contributing cohorts in addition to the usual right censoring at the end of the study period.
Statistical Methods
We used descriptive statistics to present the distribution of basic patient and tumor characteristics across centers. Trends of MIS utilization during the period 2007 to 2014 (the broadest time period for which data were available from at least 2 centers) were compared by using age-standardized frequencies. The association between surgery type and sex, age group (≤59, 60–69, 70–79, 80+), and tumor stage (I, II, III, IV), after taking missing values into account, were investigated using multiple logistic regression models.
Associations between overall survival and surgery type were assessed for the 3 population-based cancer registries in the Netherlands, Sweden, and Norway. Institute-based centers were not included given the limited number of cases available for stratified analyses. Overall survival was defined as time from surgery to death from any cause or date of last contact. Kaplan–Meier estimates for 1, 3, and 5-year survival after MIS or ORS were compared after stratifying by tumor stage groups (I–III: nonmetastatic, and IV: metastatic). Hazard ratios (HRs) of MIS utilization versus ORS were obtained by Cox regression models adjusted for sex, age group, tumor stage, neoadjuvant therapy (yes/no), count of examined lymph nodes after surgery (<12, ≥12), and colon tumor location (right colon including cecum, ascending colon, and transverse colon/left colon including splenic flexure, descending colon, and sigmoid).
All statistical analyses were performed using SAS software version 9.3. A significance level of 0.05 without multiple comparison corrections was used for statistical tests.
Role of the Funding Source
The sponsor had no role in data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data and had final responsibility to submit for publication. This study was carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki), and was approved by the ethics committee of the Medical Faculty of Heidelberg University in Germany.
RESULTS
Participating Centers and Patient Characteristics
Table 1 summarizes the list of participating centers and the respective numbers of CRC cases with specified surgery type. The population-based registries from the Netherlands, Sweden, and Norway had the largest numbers of cases (NNCR: 58,927; SCRCR: 35,690; NCR: 15,078). Periods of available surgery data varied between centers, with ranges from 2000 to 2014 (IJB), to 2011 to 2012 (IPO-Porto). The majority of CRC patients underwent open surgery, but there was large variation of complete MIS between centers, which ranged from 7% in SCRCR (Sweden) and IPO-Porto (Portugal) to 48% in IJB (Belgium). The proportion of converted MIS to ORS ranged from 1% in NCT-HD (Germany) to 8% in NNCR (Netherlands).
TABLE 1.
Overview on Participating Centers in the Present Analysis
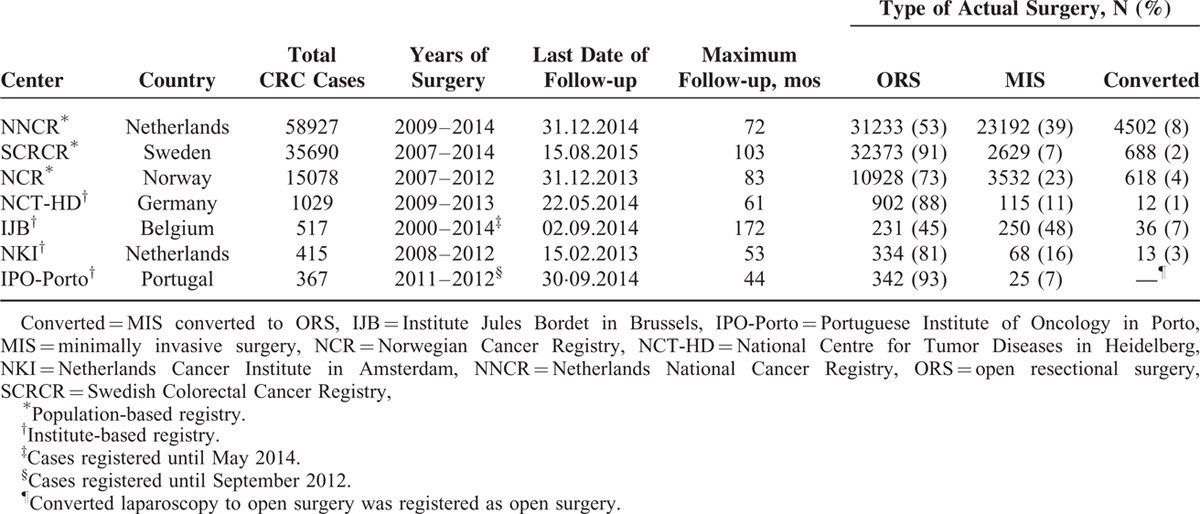
Baseline characteristics of patients, by showing low and mostly negligible proportions of missing data, are displayed in Table 2. Cases with unspecified tumor location colon/rectal (653 cases) or with emergent CRC surgery (5059 cases available in SCRCR) were excluded from further analyses. Colon cancer patients included approximately equal numbers of men and women, except in NCT-HD and IPO-Porto (56% and 59% men, respectively). By contrast, the proportion of men was substantially higher among rectal cancer patients in all centers except in IJB (49%). The age distribution substantially varied across the centers. Moreover, on average, patients from population-based registries were older than patients from institute-based registries (70–72 vs 62–67 years for colon cancer; 67–68 vs 61–66 years for rectal cancer).
TABLE 2.
Baseline Characteristics of Patients With Colorectal Cancer Undergoing Surgical Resection in Each Center

Tumor stages II and III were the most frequent stages of colon cancer in all centers, except in IJB, where stages III and IV were more frequent. In rectal cancer, stage I was the most frequent stage in all centers, except in SCRCR and NCT-HD, where stage III was more frequent (34% and 52%, respectively). For ECR (NNCR) patients, no substantial difference in the frequency of comorbidity types between MIS and ORS groups was observed, except for a slightly higher proportion of diabetes and secondary tumors in the ORS group (Table S1 Supplemental Content).
By inspecting the trend of MIS utilization in 2007 to 2014 (Figure 1), we found that the proportion of colon or rectal cancer patients undergoing MIS steadily increased nationally over time in Netherlands (NNCR, reaching >60% in 2014), Sweden (SCRCR, close to 30% in 2014), and Norway (NCR, around 35% in 2012). Moreover, large differences were observed between centers, particularly in rectal cancer cases, where proportions ranged from 6% in IPO-Porto to 92% in IJB in 2012.
FIGURE 1.
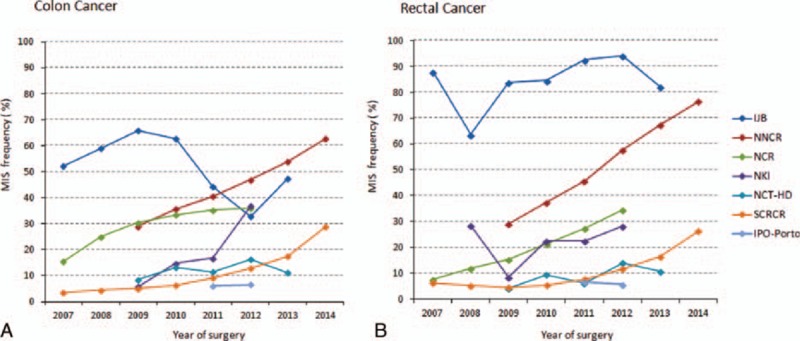
Age-standardized trend of utilization of MIS in patients with colon (A) and rectal cancer (B) between 2007 and 2014. The by far largest database (NNCR) was used as standard population for age standardization. IJB = Institute Jules Bordet in Brussels, IPO-Porto = Portuguese Institute of Oncology in Porto, NCR = Norwegian Cancer Registry, NCT-HD = National Centre for Tumor Diseases in Heidelberg, NKI = Netherlands Cancer Institute, NNCR = Netherlands National Cancer Registry, SCRCR = Swedish Colorectal Cancer Registry.
Use of MIS and ORS According to Patients’ Characteristics
A strongly divergent pattern of MIS use by tumor location was seen across centers (Table 3; and Table S2 Supplemental Content, for frequency of cases). MIS was used significantly less often in rectal than in colon cancer patients at SCRCR (odds ratio [OR] 0.88, 95% confidence interval [CI] 0.81–0.95), NCR (OR 0.56, 95% CI 0.51–0.62), and in NCT-HD (OR 0.51, 95% CI 0.33–0.79). By contrast, MIS was used significantly more often in rectal than in colon cancer patients at NNCR (OR 1.10, 95% CI 1.06–1.15) and in IJB (OR 1.59, 95% CI 1.06–2.39).
TABLE 3.
Odds Ratio of Utilization of MIS to ORS for Colon and Rectal Cancer, by Baseline Patient Characteritics

Colon Cancer
Minimally invasive surgery was significantly less often used in women than in men at NNCR (OR 0.86, 95% CI 0.83–0.89) and SCRCR (OR 0.82, 95% CI 0.75–0.89). A similar pattern was observed at IJB and IPO-Porto, whereas women tended to undergo more often MIS at NCR, NCT-HD, and NKI. There was a clear tendency toward less frequent use of MIS with increasing age of the patient or higher tumor stage in all centers (Table 3). In addition, MIS was significantly less used in obese patients (body mass index [BMI] ≥30 kg/m2) (OR 0.80, 95% CI 0.70–0.92) in SCRCR (available only in SCRCR, data not shown).
Rectal Cancer
Utilization of MIS for rectal cancer was significantly higher in women than in men in SCRCR (OR 1.33, 95% CI 1.17–1.51), with a similar tendency in NCR, NCT-HD, and NKI. Older patients (80+ years) in NNCR underwent significantly less often MIS than younger patients. By contrast, MIS use increased by increasing age of the patients in SCRCR and NCR. Moreover, MIS was significantly less often used in patients with high tumor stages in NNCR, SCRCR, and IJB, and significantly more often used in patients with stage IV cancer in the NCR (Table 3). In addition, MIS was significantly less often used in obese patients (BMI ≥30 kg/m2) (OR 0.70, 95% CI 0.60–0.89) in SCRCR (data not shown).
Overall Survival After MIS and ORS
Survival analyses were restricted to patients from the population-based registries. After excluding patients with missing follow-up (443 cases), a total of 103,540 cases (NNCR: colon/rectal = 44,367/14,505; SCRCR: colon/rectal = 20,185/10,425; NCR: colon/rectal = 10,684/3374) were eligible for survival analysis. Median follow-up time was 35, 54 and 46 months for patients in NNCR, SCRCR, and NCR, respectively.
Colon Cancer
In all centers and in both metastatic and nonmetastatic groups, colon cancer patients consistently showed longer overall survival after MIS (eg, 5-yr survival in nonmetastatic patients in NNCR was 73.7% (95% CI 72.4%–74.9%) compared with ORS (60.3%; 95% CI 59.3%–61.3%) (Table 4).
TABLE 4.
Overall Unadjusted 1, 3, and 5-Year Survival Estimates for Colon and Rectal Cancer Patients According to Type of Surgery and Tumor Stage Group (I–III: nonmetastatic, IV: metastatic) in Each Population-based Center

The HRs after adjustment for prognostic factors indicated significant lower mortality for colon cancer patients who underwent MIS compared with those who underwent ORS in NNCR (HR 0.66, 95% CI 0.63–0.69); SCRCR (HR 0.68, 95% CI 0.60–0.76); and NCR (HR 0.73, 95% CI 0.67–0.79). The significant survival advantage of MIS was observed in all subgroups in NNCR and in most subgroups in SCRCR and NCR (Table 5). Comparable results were observed from hazard ratios between complete (nonconverted) MIS and ORS (Table S3 Supplemental Content). Adjusted survival curves are illustrated in Figure 2(A–C).
TABLE 5.
Association of Surgery Type (MIS and ORS) With Overall Survival in Each Center, After Adjustment for Confounding Factors, and Stratified by Tumor Location and Baseline Characteristics of Patients

FIGURE 2.

Survival of patients undergoing minimally invasive surgery (MIS) or open resectional surgery (ORS) estimated from Cox regression models with adjustment for sex, age group, tumor stage, neoadjuvant therapy, examined lymph nodes, colon tumor location (for colon cancer group only) in Netherlands (NNCR), Sweden (SCRCR), and Norway (NCR). Emergent surgeries excluded in SCRCR data (n = 5074; 14%). NCR = Norwegian Cancer Registry, NNCR = Netherlands National Cancer Registry, SCRCR = Swedish Colorectal Cancer Registry.
Rectal Cancer
In all centers, overall survival of nonmetastatic rectal cancer patients was higher in the MIS group (eg, 5-year survival in NNCR was 73.9% (95% CI 71.7%–76.0%) than in the ORS group (66.8%; 95% CI 65.1%–68.5%). The limited number of metastatic patients precluded an evaluation of the effect of surgical resection type on survival (Table 4). The HRs indicated significant lower mortality in rectal cancer patients who underwent MIS compared with those who underwent ORS in NNCR (HR 0.74. 95% CI 0.68–0.80) and SCRCR (HR 0.77, 95% CI 0.66–0.90). The significant survival advantage of MIS was observed in most subgroups in NNCR and SCRCR, and in only 2 subgroups (age group 60–69, and tumor stage I) in NCR (Table 5). Comparable results were observed from HRs between complete (nonconverted) MIS and ORS, except that age group <60 in NCR showed lower survival for patients with completed MIS (Table S3 Supplemental Content). Figure 2(D–F) illustrates the adjusted survival curves.
DISCUSSION
In this large multicenter study, we investigated the utilization of MIS and ORS in newly diagnosed CRC patients among 7 European major clinical centers and population-based cancer registries. MIS was more likely applied in younger patients and in early-stage tumors; this finding is in agreement with a recent population-based study in England.18 MIS in colon cancer patients was associated with higher overall short-term and long-term survival compared with ORS in all 3 population-based clinical registries (NCR, SCRCR, and NNCR), also after adjustment for demographic and clinical factors. MIS in rectal cancer patients was significantly associated with increased survival in NNCR and SCRCR. The study has, however, not been able to adjust for the potential bias in selection of patients to the surgery groups that may have affected survival.
The overall percentage of CRC patients operated by MIS increased from 2007 to 2014 in most of the centers. This increase was expectedly in line with trends of MIS utilization in other nationwide studies.19 However, the percentages varied substantially between centers, with wider variations for rectal than for colon cancer. Other studies showed similar variations of MIS utilization regionally and across centers (academic and nonacademic), although RCTs support the benefit of MIS in treatment of CRC patients.20,21 Yeo et al21 reported an increase in the utilization of MIS for CRC patients of institutions of the National Comprehensive Cancer Network in the United States between 2006 and 2010, with statistically significant variability across institutions even after accounting for differences in stage and comorbidities. In a study using the National Inpatient Sample data, it was shown that academic centers were adopting MIS technology more quickly than other centers.22 In the current study, the highest rate of MIS utilization was seen in IJB (an institutional center), specially for patients with primary nonmetastatic rectal and left colon tumors. Moderate percentages of MIS use were seen in the national registries of the Netherlands (NNCR) and Norway (NCR). The national registries include a large number of hospitals, some of which have a low volume of patients with CRC, probably affecting the possibility of implementing MIS, although this is not shown in the current study.
In a recent meta-analysis of RCTs on colon cancer, higher 5-year survival after MIS compared with ORS has been reported.23 Furthermore, expert opinions in the literature supporting the acceptability of MIS for treatment of colon cancer have been shown to be correlated closely with published results from large RCTs.24 Results from the COLOR (Colon cancer Laparoscopic or Open Resection) trial (n = 1248) demonstrated that utilization of MIS for resection of colon cancer is clinically acceptable.6 Moreover, a recently published report from MRC CLASICC (The Medical Research Council Conventional versus Laparoscopic-Assisted Surgery In Colorectal Cancer) trial (n = 794) showed no statistically significant difference in long-term disease-free survival of CRC patients between MIS and ORS groups.25 In the current study, a major survival advantage of MIS for resection of colon cancer was also observed, even after adjustment for main prognostic factors. Data from the current study suggest that MIS is an acceptable alternative to open surgery in curative resection of colon cancer in older and advanced-stage patients, which is also supported by a recent report of 10-year outcomes of a prospective clinical trial.26 It is very important to interpret the differences in survival in the current study with caution, because the groups of patients in the MIS and the ORS are most likely not exactly comparable, but the epidemiologic data of the study imply that MIS can be safely introduced in a population.
A comprehensive review on RCTs revealed that laparoscopic surgery for rectal cancer has been increasingly accepted since 2006, but remains still controversial.24 In a recently published report of the COLOR II trial, the overall 3-year survival rates of rectal cancer patients in MIS (86.7%) and ORS group (83.6%) were not significantly different.27 Results from the MRC CLASICC trial also showed nearly identical long-term outcomes of MIS and ORS in rectal cancer.25 In the current study, results for rectal cancer were inconsistent, as we found better survival after MIS compared with ORS in the Netherlands (NCCR) and Sweden (SCRCR), but comparable survival between both groups in Norway (NCR). Utilization of MIS depended on age group and tumor stage. However, whereas less MIS was used with increasing age of the patients in the Netherlands and Sweden, the opposite was observed in Norway. It has to be considered that diverse time periods were included in the analyses, with more recent data from the Netherlands and Sweden. Further studies with more detailed data on clinical and patient-related factors are needed to explain the observed differences.
This study is the first European level study that used routinely available institution and population-based detailed data, reflecting the implementation and outcomes of MIS in routine clinical practice in a diverse patient population. Strengths of the study include the presence of high-quality population-based,28,29 and also institutional clinical cancer registry data. The limitations of this study include the fact that some possibly influential factors, such as patients’ comorbidities, the Eastern Cooperative Oncology Group (ECOG) Performance scales, or the Charlson comorbidity index, were not available in sufficient completeness across the registries to be considered in the analyses. However, these factors do not primarily affect the outcomes of CRC patients who underwent MIS as demonstrated in a large multicenter case-control study.30 Although the survival estimates in this study have been adjusted for majority of influential factors, the selection criterion of CRC patients for each type of surgical resections may widely differ within studied countries and it needs to be investigated in further studies.
Furthermore, owing to the lack of information on obesity of CRC patients in more centers, further assessments of this factor on MIS utilization and clinical outcomes were not possible. Additionally, absence of recurrence data precluded assessment of disease-free survival of CRC patients. Survival of patients from institution-based centers could not be studied because of limited numbers of cases or due to missing active follow-up of operated patients.
In conclusion, the utilization of MIS for curative resection of CRC patients is increasing among European countries and cancer centers with substantial differences between them. Utilization of MIS in curative resection of colon cancer may enhance overall survival rates even in advanced-stage and older patients. The use of MIS techniques in rectal cancer seems to provide equivalent long-term overall survival to open surgery. Further investigations are needed to ascertain whether there are rectal cancer patients who will benefit from the application of MIS.
Supplementary Material
Acknowledgments
We thank all staff from the contributing registries for their efforts to collect and process detailed clinical cancer information, and for providing data for this study. Thanks to the Swedish Colorectal Cancer Study Group for providing data from the SCRCR. Thanks to Flávio Videira and Tatiana Domingues for their valuable contribution to data collection in IPO-Porto. Thanks to Dr Phillip Knebel from Department of surgery of Heidelberg University Hospital, Heidelberg, Germany, for his contribution in discussion of results.
Footnotes
Abbreviations: adj = adjusted, BMI = body mass index, COLOR = Colon cancer Laparoscopic or Open Resection trial, CRC = colorectal cancer, ECOG = Eastern Cooperative Oncology Group, ECR = Eindhoven Cancer Registry, HR = hazard ratio, IJB = Institute Jules Bordet, IPO-Porto = Portuguese Institute of Oncology in Porto, MIS = minimally invasive surgery, MRC CLASICC = The Medical Research Council Conventional versus Laparoscopic-Assisted Surgery In Colorectal Cancer trial, NCR = Norwegian Cancer Registry, NCT-HD = National Center for Tumor Diseases–Heidelberg, NE = Not enough available data, NNCR = Netherlands National Cancer Registry, OR = odds ratio, ORS = open resectional surgery, RCT = randomized clinical trial, SCRCR = Swedish Colorectal Cancer Registry, WP11 = Work Package 11.
MB, YB, and LJ contributed to this work equally.
PS-K and HB contributed to this work equally.
MB is the study coordinator, acquired data from registries, participated in data harmonization and interpretation of results, and wrote the manuscript. YB and LJ participated in data harmonization, performed statistical analysis and interpretation of results, and wrote the manuscript. AG participated in protocol development and acquisition of data. VL, AS, TBJ, MM, LG, AFG, MJB, TvdV, LRK, AU, and NB provided valuable data from their respective participating registries, reviewed the manuscript and contributed in discussion of results. CMU is former co-leader from the WP11 and participated in protocol development, reviewed the manuscript, and contributed to discussion and interpretation of results. PSK is the co-leader from the WP11, participated in protocol development and interpretation of results. HB is leader from the WP11, developed the study protocol, interpreted results, and contributed to manuscript writing. All authors critically revised the manuscript and approved the final version.
The study was funded by the FP7 program of the European Commission as part of a EUROCANPlatform project (project number: 260791).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991; 1:144–150. [PubMed] [Google Scholar]
- 4.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005; 365:1718–1726. [DOI] [PubMed] [Google Scholar]
- 5.Aziz O, Constantinides V, Tekkis PP, et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol 2006; 13:413–424. [DOI] [PubMed] [Google Scholar]
- 6.Group, CCLoORS. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009; 10:44–52. [DOI] [PubMed] [Google Scholar]
- 7.Juo YY, Hyder O, Haider AH, et al. Is minimally invasive colon resection better than traditional approaches?: first comprehensive national examination with propensity score matching. JAMA Surg 2014; 149:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou SA, Antoniou GA, Koch OO, et al. Laparoscopic colorectal surgery confers lower mortality in the elderly: a systematic review and meta-analysis of 66,483 patients. Surg Endosc 2015; 29:322–333. [DOI] [PubMed] [Google Scholar]
- 9.Hida K, Hasegawa S, Kinjo Y, et al. Open versus laparoscopic resection of primary tumor for incurable stage IV colorectal cancer: a large multicenter consecutive patients cohort study. Ann Surg 2012; 255:929–934. [DOI] [PubMed] [Google Scholar]
- 10.Brouquet A, Nordlinger B. Minimally-invasive approach for rectal cancer surgery. Lancet Oncol 2014; 15:680–681. [DOI] [PubMed] [Google Scholar]
- 11.Mathis KL, Nelson H. Controversies in laparoscopy for colon and rectal cancer. Surg Oncol Clin N Am 2014; 23:35–47. [DOI] [PubMed] [Google Scholar]
- 12.Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012; 23:2479–2516. [DOI] [PubMed] [Google Scholar]
- 13.Thorpe H, Jayne DG, Guillou PJ, et al. Patient factors influencing conversion from laparoscopically assisted to open surgery for colorectal cancer. Br J Surg 2008; 95:199–205. [DOI] [PubMed] [Google Scholar]
- 14.Allaix ME, Degiuli M, Arezzo A, et al. Does conversion affect short-term and oncologic outcomes after laparoscopy for colorectal cancer? Surg Endosc 2013; 27:4596–4607. [DOI] [PubMed] [Google Scholar]
- 15.Clancy C, O’Leary DP, Burke JP, et al. A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery. Colorectal Dis 2015; 17:482–490. [DOI] [PubMed] [Google Scholar]
- 16.Moghadamyeghaneh Z, Masoomi H, Mills SD, et al. Outcomes of conversion of laparoscopic colorectal surgery to open surgery. JSLS 2014; 18:e2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McVie G, Ringborg U. EurocanPlatform, an FP7 project of the European Commission-first year commentary. Ecancermed-icalscience 2012; 6:ed13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor EF, Thomas JD, Whitehouse LE, et al. Population-based study of laparoscopic colorectal cancer surgery 2006–2008. Br J Surg 2013; 100:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp JA, Finlayson SR. Nationwide trends in laparoscopic colectomy from 2000 to 2004. Surg Endosc 2008; 22:1181–1187. [DOI] [PubMed] [Google Scholar]
- 20.Boller AM, Nelson H. Colon and rectal cancer: laparoscopic or open? Clin Can Res 2007; 13:6894s–6896s. [DOI] [PubMed] [Google Scholar]
- 21.Yeo H, Niland J, Milne D, et al. Incidence of minimally invasive colorectal cancer surgery at National Comprehensive Cancer Network Centers. J Natl Cancer Inst 2015; 107: dju362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson CN, Chen GJ, Balentine CJ, et al. Minimally invasive surgery is underutilized for colon cancer. Ann Surg Oncol 2011; 18:1412–1418. [DOI] [PubMed] [Google Scholar]
- 23.Di BS, Li Y, Wei KP, et al. Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol 2013; 22:E39–E43. [DOI] [PubMed] [Google Scholar]
- 24.Martel G, Crawford A, Barkun JS, et al. Expert opinion on laparoscopic surgery for colorectal cancer parallels evidence from a cumulative meta-analysis of randomized controlled trials. PloS One 2012; 7:e35292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 2013; 100:75–82. [DOI] [PubMed] [Google Scholar]
- 26.Allaix ME, Giraudo G, Mistrangelo M, et al. Laparoscopic versus open resection for colon cancer: 10-year outcomes of a prospective clinical trial. Surg Endosc 2015; 29:916–924. [DOI] [PubMed] [Google Scholar]
- 27.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 28.Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009; 45:1218–1231. [DOI] [PubMed] [Google Scholar]
- 29.Coebergh JW, Crommelin MA, van der Heijden LH, et al. Trends in cancer survival in the southeastern Netherlands since 1975: the Eindhoven Cancer Registry experience. Health Rep 1993; 5:105–110. [PubMed] [Google Scholar]
- 30.Niitsu H, Hinoi T, Kawaguchi Y, et al. Laparoscopic surgery for colorectal cancer is safe and has survival outcomes similar to those of open surgery in elderly patients with a poor performance status: subanalysis of a large multicenter case-control study in Japan. J Gastroenterol 2015; 51:43–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


