Supplemental Digital Content is available in the text
Abstract
Patients on long-term dialysis are at high risk for tuberculosis (TB). Although latent tuberculosis infection (LTBI) is good target for TB eradication, interferon-gamma release assay-defined LTBI has a high proportion of negative conversion and lacks active TB correlation among patients on dialysis.
Patients on long-term dialysis were screened in multiple centers in Taiwan. QuantiFERON-TB Gold In-tube (QFT-GIT) was used to define LTBI and was performed thrice at 6-month intervals. The primary outcome was active TB diagnosed after LTBI screening. The incidence and predictive value of QFT-GIT were analyzed.
The 940 dialysis patients enrolled had an average age of 59.3 years. The initial QFT-GIT results were positive in 193, including 49.6% with persistent positive results on second check. In an average follow-up period of 3 years, 7 patients had TB. Three (319.1 per 100,000 person-yrs) and 4 (141.8 per 100,000 person-yrs) of them were prevalent and incident TB cases, respectively. Persistent positive QFT-GIT for 2 and 3 times correlated with increased hazard ratio for TB (14.44 and 20.29, respectively) compared with a single positive result (hazard ratio 10.38). Among those with 3 positive QFT-GIT results, TB development rate was 4.5% and incidence rate was 1352.3 per 100,000 person-years. In contrast, none of the incident TB occurred in those with initial positive and then negative conversion of QFT-GIT.
In an area of intermediate TB incidence, dialysis patients have high TB risk. LTBI status is a good predictor of TB development, especially for those with more than 1 positive result. After excluding prevalent TB cases, serial follow-up of LTBI may narrow the target population to reduce treatment costs.
INTRODUCTION
Tuberculosis (TB) remains one of the most important infectious diseases worldwide. According to the World Health Organization estimates, there were 9 million new TB cases and 1.5 million related deaths in 2013.1 For future TB control, preventing transmission by early treatment and reducing reactivation by treatment of latent TB infection (LTBI) are 2 of the most important strategies.2–4 Among high-risk population, patients receiving dialysis have 7.8 to 25 times higher potential for TB development than general population due to the reduced cellular immunity.5–7 However, because extrapulmonary manifestations TB are common, diagnosis of TB is usually delayed in patients with dialysis.8,9 Therefore, detection and treatment for LTBI are important in this specific group.2,4
Positive results of interferon-gamma release assays (IGRAs) and the exclusion of active TB are the current diagnostic bases of LTBI,10–13 which is a precursor of TB reactivation. However, recent studies show a high negative reversion rate of quantiFERON Gold In-tube (QFT-GIT), a kind of IGRA, in cohorts of healthcare workers (33% after 18 wks),14 close TB contacts (35% after 6 mos),15 and patients receiving long-term dialysis (46% after 6 mos).16 This phenomenon questions the clinical significance of a single positive IGRA result. Increasing the IGRA threshold or doing serial follow-up to improve specificity have both been suggested,16,17 but there is no comparison by the risk of TB development.
Moreover, the TB reactivation rate among patients on dialysis is heterogeneously reported, possibly due to different areas, and is not conductive for policy formation. Some small studies show a rate of 8% to 12% in LTBI dialysis patients within 2 to 3 years of follow-up,18,19 whereas the Tuberculosis Network European Trials study in European areas recently reported no TB development in the chronic renal failure group within a median of 1.8-year surveillance.20 A large-scale prospective study is required for the real-world experience of TB development among patients on dialysis living in an intermediate TB incidence country.4
This cohort study aimed to survey TB development in patients receiving dialysis and analyzed its correlation with different status of IGRA-defined LTBI status.
METHODS
Patient Enrollment
This cohort study was conducted at the National Taiwan University Hospital and its 3 branches, and a local hemodialysis clinic. The study was approved by the institutional review board of National Taiwan University Hospital. Adult dialysis patients (age ≥20 yrs) under long-term (>3 mos) dialysis were prospectively identified and recruited between April 2011 and October 2015. Those with human immunodeficiency virus infection, liver cirrhosis Child-Pugh class C,21 active cancer, autoimmune disease receiving chemotherapy, or prior documented TB were excluded. Written informed consent was obtained from all of the enrolled participants.
LTBI Diagnosis
Peripheral blood was collected from participants at baseline and at 6 and 12 months later. The LTBI status was determined using the QFT-GIT assay (Cellestis, Australia) according to the manufacturer's instructions.22 A 3-tube kit of QFT-GIT was used, and QFT-GIT response was calculated by subtracting interferon (IFN)-γ level in the reaction supernatants of the negative control tube from that in the TB-antigen tube. The results were interpreted as positive, negative, or indeterminate.23,24
Data Collection
Demographic and clinical data included age, sex, underlying comorbidities, blood hemoglobin, and serum albumin levels obtained from the medical records system. Respiratory and constitutional symptoms, smoking status, and TB exposure by history taking and physical examination were reviewed. All data were recorded in a standardized case report form by research assistants. Cough longer than 3 weeks was defined as chronic cough. Current smoker was defined as those who smoked >100 cigarettes, with the last time of smoking within 1 month before the study.25 A radiologist and a pulmonologist reviewed the radiographic findings. Any discrepancy was settled by discussion and consensus. The radiographic findings were labeled as positive if there were lung lesions compatible with prior TB,26 or active lung lesion, or cannot be excluded for TB.27
The primary outcome was active TB development, documented by culture positive for Mycobacterium tuberculosis or typical pathology of M tuberculosis infection.28,29 Patients with active TB within 3 months since the present screen were defined as prevalent cases and those after 3 months were incident cases. Active TB occurrence was confirmed by reviewing the hospital medical records and by the national TB registration. Patients who died not by TB or those who were lost to follow-up were censored.
Statistical Analysis
Intergroup differences were analyzed using the Student t test or Mann–Whitney U test, when appropriate, for numerical variables, and chi-square test for categorical variables. Cox proportional-hazards regression analysis with forward factor selection method was used to identify factors associated with prevalent or incident TB cases. The assumption of the Cox proportional-hazards model was tested using the Schoenfeld residual. Factors applied in the model were QFT-GIT status, age, sex, dialysis mode and duration, current smoking, history of diabetes mellitus or cancer, TB exposure, hemoglobin and albumin levels, presence of respiratory and constitutional symptoms, and radiographic findings of prior TB or active infection lesion. The QFT-GIT status included first QFT-GIT, first QFT-GIT with a cut-off value >1.0 IU/mL, first to second QFT-GIT both (+), and first to third QFT-GIT all (+). Each one was put in the model at 1 time of analysis. A 2-sided P < 0.05 was considered significant. All analyses were performed using the SPSS (Version 19.0, Chicago, IL).
RESULTS
Characteristics of the Enrolled Dialysis Patients
At the initial screening, 940 dialysis patients (53% males) were enrolled. Their average age was 59.3 years (standard deviation [SD] 13.6) and dialysis duration was 5.5 (5.3) years. Among them, 82% received hemodialysis, whereas the remaining patients received peritoneal dialysis. Moreover, 7% had a history of TB contact. In terms of radiographic findings, 42 (4%) had prior TB or active lesion, whereas TB could not be excluded in 3 (0.3%). The respiratory and constitutional symptoms were noted in 199 (21%) including chronic cough in 179, dyspnea in 11, fever in 8, and poor appetite in 1.
Results of QFT-GIT Follow-up
Initial QFT-GIT results were positive in 193 (20.5%) patients, negative in 713 (75.9%), and indeterminate in 34 (3.6%) (Figure 1). Among them, 654 received a second QFT-GIT check and 528 received a third. Populations receiving QFT-GIT at different times had comparable demographic data except for dialysis mode (Table E1, Supplemental File). On follow-up, the number of patients using peritoneal dialysis decreased significantly. On the second QFT-GIT (Figure 1), 70 (49.6%) still had positive results, whereas 68 (47.9%) became negative. For the initial negative result, 6.1% had a positive reversion on the second determination. Among those with positive results on the first and second QFT-GIT determination, 74.6% still had positive results on the third determination.
FIGURE 1.
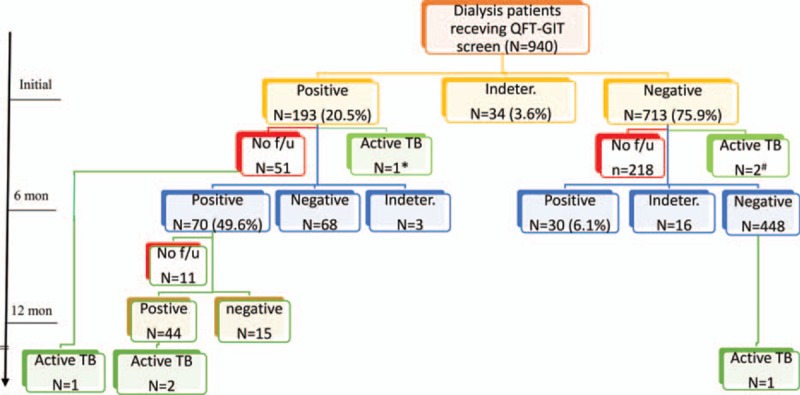
Flow chart of case follow-up by quantiFERON-TB Gold In-tube (QFT-GIT). The results of QFT-GIT were not all shown. If the first check was determinate, the result of the second check was shown. The third check was displayed only when the first 2 checks were both positive. (∗) Lost to follow-up; (+) 1 was lost to follow-up.
Outcome on Follow-up
In an average follow-up period of 3 years (average [SD] 1096.4 [482.6] d), 679 (72.2%) participants continued to be followed up, 156 (16.6%) died, 95 (10.1%) were lost follow-up, and the remaining 10 received kidney transplant. There were 7 patients who had been diagnosed with active TB (Figure 1; Table 1). Three were prevalent TB cases and their average interval between screening and diagnosis was 36.7 days. The prevalence rate was 319.1 (95% confidence interval [CI] 65.8–932.7) per 100,000 person-years. One of the prevalent TB cases had pulmonary TB and positive QFT-GIT status, whereas the other 2 had extrapulmonary or disseminated TB, but negative QFT-GIT (Table 1).
TABLE 1.
Data of Patients With Active Tuberculosis
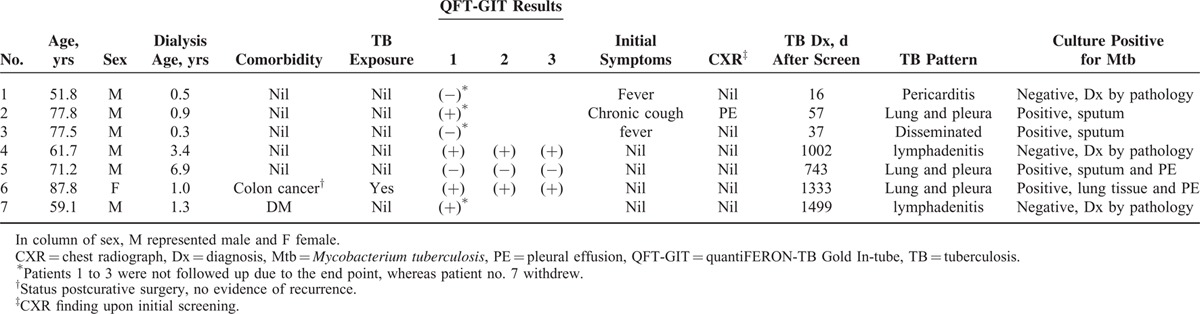
The 4 incident TB cases had an average of 1144.3 days between screening and diagnosis. Two had pulmonary and pleural TB with positive culture, and the remaining 2 had lymphadenitis. Among them, 3 had initial positive QFT-GIT. On the follow-up, 1 withdrew and the reaming 2 had persistent QFT-GIT positivity. The rate of TB development was 1.6%, 2.9%, and 4.5% for those with single, 2, and 3 times of positive QFT-GIT, respectively, and the rate was higher among those continuing follow-up (Figure 2). The TB incidence rate was 141.8 (95% CI 38.6–363.2) per 100,000 person-years for all, and increased to 478.8 (98.7–1399.2), 861.4 (104.3–3111.8), and 1352.3 (164.0–4885.0) per 100,000 person-years for those with first, first and second, and first to third positive QFT-GIT, respectively.
FIGURE 2.
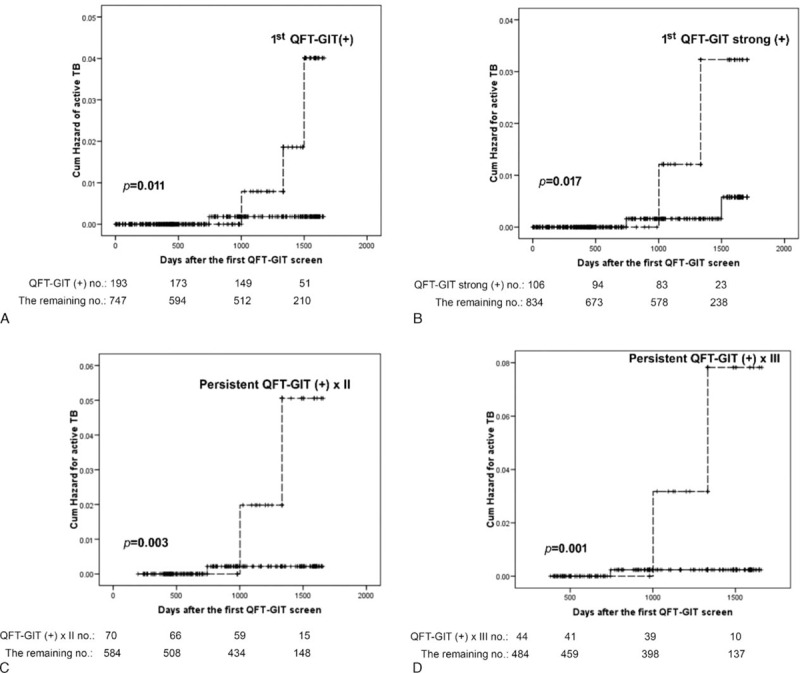
Kaplan—Meier curves of incident tuberculosis by different status of quantiFERON-TB Gold In-tube (QFT-GIT) results. The dashed line showed the indicated group, whereas the solid line represented the remaining group. Strong positive response of QFT-GIT was response >1 IU/mL.
Factors Associated With Prevalent TB
Compared with those who did not develop TB (Table 2), those with prevalent TB had more current smoking, shorter dialysis period, and more radiographic lesions of prior TB or active lesion, TB cannot be excluded, and also the presence of respiratory or constitutional symptoms. Multivariate analysis was not performed due to the small number of prevalent TB for model set-up.
TABLE 2.
Characteristics of Patients on Long-term Dialysis, by Tuberculosis (TB) Development
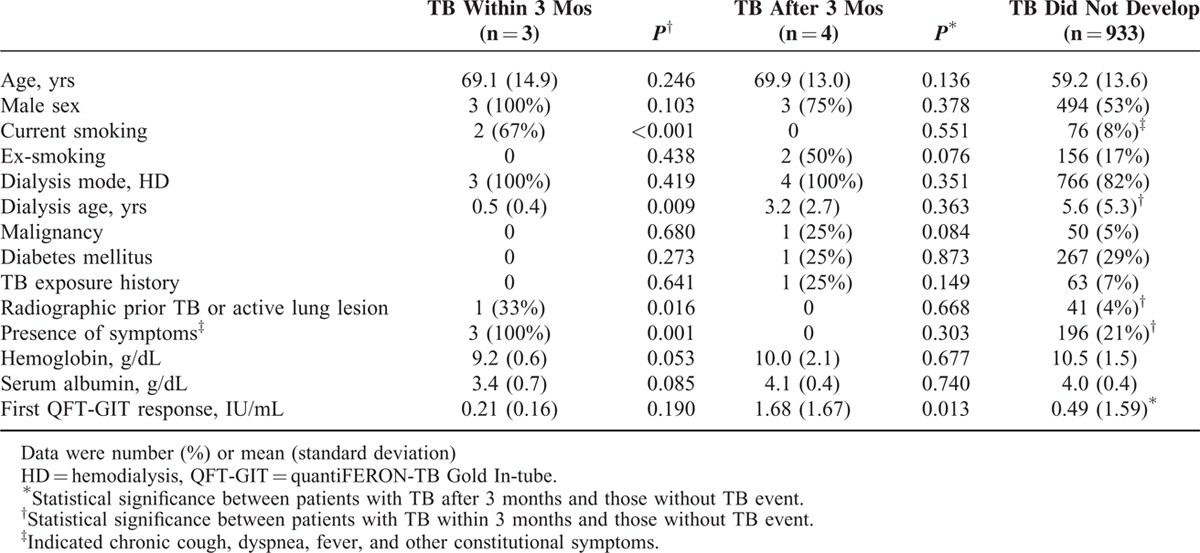
In univariate Cox proportional-hazards analysis (Table E2, Supplemental File), current smoking (hazard ratio [HR] 22.11, 95% CI 2.01–243.88), dialysis age ≤0.5 years (HR 7.54, 95% CI 1.05–53.91), active chest radiograph findings (HR 154.22, 95% CI 13.98–1700.78), and presence of fever (HR 265.90, 95% CI 24.03–2942.47) were significantly associated with prevalent TB. However, QFT-GIT, whether spot or serial results, had no significant association.
If any 2 or 3 of the 4 associated factors were present, sensitivity and specificity for prevalent cases were 100% and 99.6%, respectively (Table E3, Supplement File). Initial assessment for active TB case finding before QFT-GIT was considered before LTBI screening to do both arms of intervention in the high-risk group.
Factors Associated With Incident TB
For incident TB, QFT-GIT response was the only significant difference between patients with incident TB and those without (Table 2; Figure 2). A strong initial QFT-GIT response (≥1.0 IU/mL) significantly correlated with incident TB, but the TB incidence rate was better predicted using persistent QFT-GIT positivity. In Cox proportional-hazards regression using forward conditional factors selection (Table 3), only positive initial QFT-GIT was an independent predictor for incident TB (HR 10.38, 95% CI 1.08–99.91). The Schoenfeld residual showed no violation of the model assumption.
TABLE 3.
Cox Proportional-hazards Model for Tuberculosis Risk Among Dialysis Patients, by Different quantiFERON-TB Gold In-tube (QFT-GIT) Results
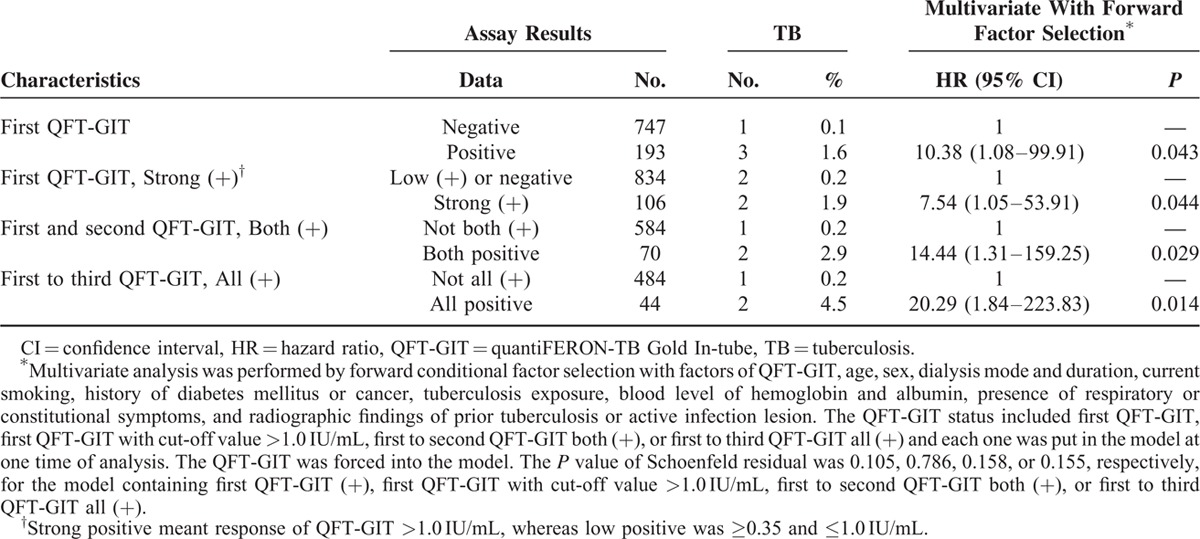
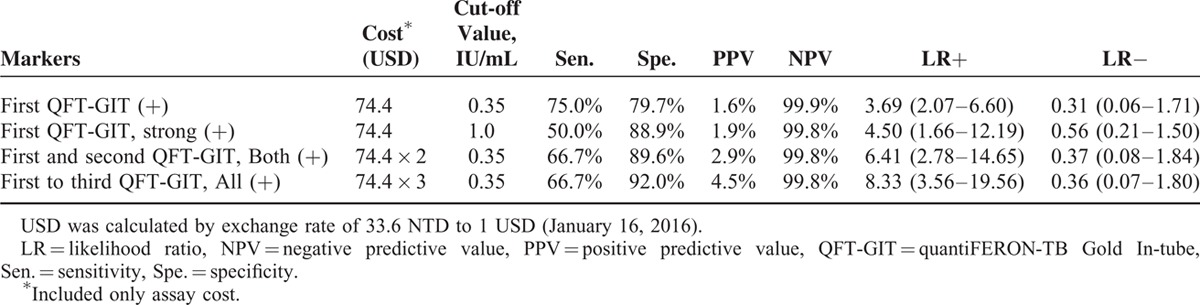
Although strong positivity (≥1.0 IU/mL) of the initial QFT-GIT was used in the model, HR was not obviously elevated (HR 7.54, 95% CI 1.05–53.91). Those with initial positive but then negative conversion report had no correlation with TB development. In contrast, persistent QFT-GIT positivity for 2 and 3 times correlated with increased HR (14.44 and 20.29, respectively) and specificity (89.6% and 92%, respectively) (Tables 3 and 4). The positive likelihood ratio (LR+) from 1 to 2 to 3 positive QFT-GIT results increased from 3.69 to 6.41 to 8.33, respectively. Negative LR was stable at around 0.31 to 0.37 (Table 4).
TABLE 4.
Predictive Power of Different QFT-GIT Assay Results for Incident Tuberculosis in the Dialysis Population
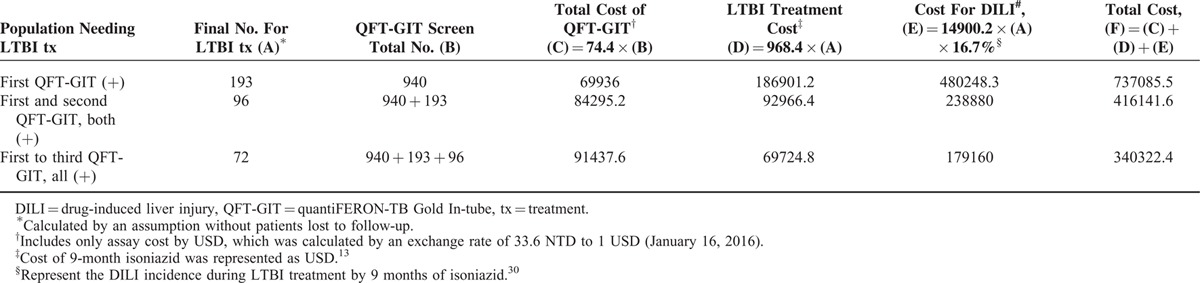
In the present study, initial QFT-GIT positivity was persistent in around 49.6% and 37.0% on the second and third determinations, respectively. The population of persistent QFT-GIT was used to define the targeted LTBI treatment population. In the present study, if the initial QFT-GIT (+) participants all received a second QFT-GIT, 96 of 193 patients with initial positive QFT-GIT needed LTBI treatment (Table 5). If isoniazid LTBI treatment was not given in the 97 patients with QFT-GIT results of first (+) and second (−), the estimated cost reduction would be 320,944 USD because treatment fee and the cost of drug-induced liver injury were saved.13,30 Similarly, if only patients with persistent positive results in all 3 QFT-GIT determinations were included, 396,763 USD could be saved.
TABLE 5.
Possible Cost Consumption in Using Different QFT-GIT Results for Implementing Treatment of Latent Tuberculosis Infection
DISCUSSION
The present study surveyed TB risk among patients on dialysis and studied the usefulness of serial follow-up of LTBI status by QFT-GIT. For all participants, prevalent TB was notified in 319.1 per 100,000 persons and QFT-GIT had no predictive role. In contrast, clinical information of current smoking, short dialysis duration, active radiographic lesion, and presence of fever were associated with prevalent TB. On follow-up, the incidence of TB was 478.8, 861.4, and 1352.3 per 100,000 person-years in patients with 1, 2, and 3 persistent QFT-GIT positive determinations. Positive QFT-GIT independently correlated with incident TB development, with HR of 10.38, 14.44, and 20.29 by the single, twice, and thrice QFT-GIT positivity, respectively.
The dialysis population is enlarging31 and is suggested as a high-priority group for LTBI treatment,4 given their increased TB risk.32 The possible explanations for the high risk of TB acquisition and progression to active TB in renal failure includes increased immune cell apoptosis, lymphocyte depletion, and polymorphonuclear leukocytes dysfunction due to blood oxidative stress and uremic toxins.33–36 In the present study, dialysis patients with initial QFT-GIT positivity had an HR of 10.38 compared with those without LTBI. The TB incidence among dialysis patients with LTBI was 478.8 per 100,000 person-years, which was higher than that in the general comparable age population (105 per 100,000 person-yrs), but less than that in the cohort of household contacts in Taiwan (3440 cases per 100,000 person-yrs).37
When the threshold of first QFT-GIT was elevated, the HR for incident TB was not obviously increased and might not be a good surrogate for serial testing, although a previous study showed that strong titer of the initial QFT-GIT could predict persistent positivity.16 For those with initial positive QFT-GIT, but sequential negative conversion, no TB reactivation was reported. These could be assumed as transiently positive or false-positive. On the contrary, persistent positive QFT-GIT correlated with increasing HR for TB incidence, up to 20.29 for those with 3 positive determinations for 1 year. The high specificity of persistent QFT-GIT can be applied to narrow the target treatment population.13,30 Thus, costs can be saved from fewer patients who require LTBI treatment and decreased drug-induced adverse events. But evaluating the cost-effectiveness warrants more investigations in the future.
When a TB screening strategy is applied to the dialysis population, prevalent and LTBI cases are both the concerns in an intermediate TB-incidence area. If QFT-GIT is applied to all, and clinical information and chest radiograph for those with positive QFT-GIT are taken together (Figure E1, Supplement File), costs for chest radiograph and history review in negative QFT-GIT (75.9%) can be saved, but 67% of prevalent cases may be missed or have delayed diagnosis.13 However, whether chest radiograph and review of history should be done before or after the QFT-GIT test remains an open question. Future studies on cost-effectiveness are needed. Because prevalent TB remains 3 times higher in the present study than that in the 60-year-old general population in study country,7,29 reviewing history and symptoms, which is relatively easy to implement, are suggested before or together with clinical QFT-GIT determination in an area with intermediate TB incidence.
This study has several limitations. First, the case number of active TB is small and many potential factors may become statistically insignificant. The CIs of HRs of significant factors are wide so data should be interpreted carefully. Second, dialysis patients were continuously enrolled, so the follow-up duration was not the same. The TB incidence may vary during the follow-up period.29 Third, although dialysis patients were consecutively enrolled, the enrollment and follow-up were based on patients’ cooperation. About 27% of the data are censored and the results may be biased. Lastly, generalizability is limited in dialysis patients and may not be applied to other populations.
In conclusion, TB risk in the dialysis population is high, and QFT-GIT-positive LTBI indicates high risk for TB even with a 49.6% negative conversion. Persistently positive QFT-GIT correlates with increased HR and specificity for TB development. The possibility of prevalent TB is suggested to be reviewed before or together with LTBI screening. After prevalent TB cases are excluded, serial follow-up of LTBI may narrow the target population and reduce the costs of LTBI preventive therapy.
Supplementary Material
Acknowledgments
The authors thank the staff of the Eighth Core Lab of the Department of Medical Research of National Taiwan University Hospital for their technical support, and also the Department of Medical Research.
Footnotes
Abbreviations: CXR = chest radiograph, DILI = drug-induced liver injury, Dx = diagnosis, HD = hemodialysis, HR = hazard ratio, IGRA = interferon-gamma release assays, LR = likelihood ratio, LTBI = latent tuberculosis infection, Mtb = Mycobacterium tuberculosis , NPV = negative predictive value, PE = pleural effusion, PPV = positive predictive value, QFT-GIT = QuantiFERON-TB Gold In-tube, SD = standard deviation, Sen = sensitivity, Spe = specificity, TB = tuberculosis.
Parts of the study results were presented as a poster discussion in the 46th Union World Conference on Lung Health, 2015 and 2016 ATS meeting.
Author contributions: Dr C-CS conceptualized the study. Drs J-YW, C-CS, Y-FW, C-LH, C-YL, H-HL, V-CW, and F-JY participated in the sample and clinical data collection. Drs C-CS, Professor H-HL, J-SC, L-NL, and Professor C-JY were involved in the data analysis and manuscript writing.
Funding: This study was funded by grants from the Research Center for Biotechnology and Medicine Policy in Taiwan, the Center of Disease Control, Ministry of Health and Welfare, Taiwan (MOHW105-CDC-C-114-000103, 4-3), and the Ministry of Science and Technology, Taiwan (103-2325-B-002 -005; and 103-2314-B-002-152-MY2).
Conflict of interest statement: Dr C-CS and Professor J-YW received travel funds from QIAGEN to attend TB experts meeting in Bali, 2015.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
All of the authors declare no financial, professional, or other personal interests of any nature or kind in any related product, service, and/or company.
Supplemental Digital Content is available for this article.
REFERENCES
- 1.Global Tuberculosis Report 2014. World Health Organization, Geneva, Switzerland; 2014. [Google Scholar]
- 2.Rose DN. Benefits of screening for latent Mycobacterium tuberculosis infection. Arch Intern Med 2000; 160:1513–1521. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015; 46:1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidelines on the Management of Latent Tuberculosis Infection. World Health Organization, Geneva, 2015. [PubMed] [Google Scholar]
- 5.Smirnoff M, Patt C, Seckler B, et al. Tuberculin and anergy skin testing of patients receiving long-term hemodialysis. Chest 1998; 113:25–27. [DOI] [PubMed] [Google Scholar]
- 6.Lundin AP, Adler AJ, Berlyne GM, et al. Tuberculosis in patients undergoing maintenance hemodialysis. Am J Med 1979; 67:597–602. [DOI] [PubMed] [Google Scholar]
- 7.Li SY, Chen TJ, Chung KW, et al. Mycobacterium tuberculosis infection of end-stage renal disease patients in Taiwan: a nationwide longitudinal study. Clin Microbiol Infect 2011; 17:1646–1652. [DOI] [PubMed] [Google Scholar]
- 8.Venkata RK, Kumar S, Krishna RP, et al. Tuberculosis in chronic kidney disease. Clin Nephrol 2007; 67:217–220. [DOI] [PubMed] [Google Scholar]
- 9.Fang HC, Lee PT, Chen CL, et al. Tuberculosis in patients with end-stage renal disease. Int J Tuberc Lung Dis 2004; 8:92–97. [PubMed] [Google Scholar]
- 10.Simsek H, Alpar S, Ucar N, et al. Comparison of tuberculin skin testing and T-SPOT.TB for diagnosis of latent and active tuberculosis. Jpn J Infect Dis 2010; 63:99–102. [PubMed] [Google Scholar]
- 11.Brock I, Weldingh K, Lillebaek T, et al. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 2004; 170:65–69. [DOI] [PubMed] [Google Scholar]
- 12.Diel R, Loddenkemper R, Meywald-Walter K, et al. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med 2008; 177:1164–1170. [DOI] [PubMed] [Google Scholar]
- 13.Kowada A. Cost effectiveness of the interferon-gamma release assay for tuberculosis screening of hemodialysis patients. Nephrol Dial Transplant 2013; 28:682–688. [DOI] [PubMed] [Google Scholar]
- 14.Ringshausen FC, Nienhaus A, Schablon A, et al. Predictors of persistently positive Mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect Dis 2010; 10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adetifa IM, Ota MO, Jeffries DJ, et al. Interferon-gamma ELISPOT as a biomarker of treatment efficacy in latent tuberculosis infection: a clinical trial. Am J Respir Crit Care Med 2013; 187:439–445. [DOI] [PubMed] [Google Scholar]
- 16.Shu CC, Wu VC, Yang FJ, et al. Dynamic changes in positive interferon-gamma release assay in a dialysis population: An observational cohort study. J Infect 2013; 67:529–535. [DOI] [PubMed] [Google Scholar]
- 17.Bartalesi F, Goletti D, Spinicci M, et al. Serial QuantiFERON TB-gold in-tube testing during LTBI therapy in candidates for TNFi treatment. J Infect 2013; 66:346–356. [DOI] [PubMed] [Google Scholar]
- 18.Christopoulos AI, Diamantopoulos AA, Dimopoulos PA, et al. Risk factors for tuberculosis in dialysis patients: a prospective multi-center clinical trial. BMC Nephrol 2009; 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SS, Chou KJ, Su IJ, et al. High prevalence of latent tuberculosis infection in patients in end-stage renal disease on hemodialysis: comparison of QuantiFERON-TB GOLD, ELISPOT, and tuberculin skin test. Infection 2009; 37:96–102. [DOI] [PubMed] [Google Scholar]
- 20.Sester M, van Leth F, Bruchfeld J, et al. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med 2014; 190:1168–1176. [DOI] [PubMed] [Google Scholar]
- 21.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 22.Lalvani A, Pathan AA, McShane H, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med 2001; 163:824–828. [DOI] [PubMed] [Google Scholar]
- 23.Dyrhol-Riise AM, Gran G, Wentzel-Larsen T, et al. Diagnosis and follow-up of treatment of latent tuberculosis; the utility of the QuantiFERON-TB Gold In-tube assay in outpatients from a tuberculosis low-endemic country. BMC Infect Dis 2010; 10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banach DB, Harris TG. Indeterminate QuantiFERON(R)-TB Gold results in a public health clinic setting. Int J Tuberc Lung Dis 2011; 15:1623–1630. [DOI] [PubMed] [Google Scholar]
- 25.Lin HH, Ezzati M, Chang HY, et al. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med 2009; 180:475–480. [DOI] [PubMed] [Google Scholar]
- 26.Jasmer RM, Snyder DC, Chin DP, et al. Twelve months of isoniazid compared with four months of isoniazid and rifampin for persons with radiographic evidence of previous tuberculosis: an outcome and cost-effectiveness analysis. Am J Respir Crit Care Med 2000; 162:1648–1652. [DOI] [PubMed] [Google Scholar]
- 27.Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008; 191:834–844. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Controlling tuberculosis in the United States. Am J Respir Crit Care Med 2005; 172:1169–1227. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control MoHaW, R.O.C. (Taiwan). Taiwan Tuberculosis Control Report 2013. Centers for Disease Control, Ministry of Health and Welfare, R.O.C. (Taiwan); 2014. [Google Scholar]
- 30.Vikrant S, Agarwal SK, Gupta S, et al. Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl Infect Dis 2005; 7:99–108. [DOI] [PubMed] [Google Scholar]
- 31.Yang WC, Hwang SJ. Taiwan Society of N. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transplant 2008; 23:3977–3982. [DOI] [PubMed] [Google Scholar]
- 32.Horsburgh CR., Jr Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 2004; 350:2060–2067. [DOI] [PubMed] [Google Scholar]
- 33.D’Intini V, Bordoni V, Fortunato A, et al. Longitudinal study of apoptosis in chronic uremic patients. Semin Dial 2003; 16:467–473. [DOI] [PubMed] [Google Scholar]
- 34.de Cal M, Cruz DN, Corradi V, et al. HLA-DR expression and apoptosis: a cross-sectional controlled study in hemodialysis and peritoneal dialysis patients. Blood Purif 2008; 26:249–254. [DOI] [PubMed] [Google Scholar]
- 35.Peraldi MN, Berrou J, Metivier F, et al. Natural killer cell dysfunction in uremia: the role of oxidative stress and the effects of dialysis. Blood Purif 2013; (35 Suppl 2):14–19. [DOI] [PubMed] [Google Scholar]
- 36.Xiang FF, Zhu JM, Cao XS, et al. Lymphocyte depletion and subset alteration correlate to renal function in chronic kidney disease patients. Ren Fail 2016; 38:7–14. [DOI] [PubMed] [Google Scholar]
- 37.Wang JY, Shu CC, Lee CH, et al. Interferon-gamma release assay and rifampicin therapy for household contacts of tuberculosis. J Infect 2012; 64:291–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


