Abstract
ABCG2 is one of at least three human ATP binding cassette (ABC) transporters which can facilitate the export from cells of a wide range of chemically unrelated drug molecules. This capacity for multidrug transport is not only a confounding factor in chemotherapy, but is also one of the more perplexing phenomena in transporter biochemistry. Since its discovery in the last decade of the 20th century much has been revealed about ABCG2’s localization, physiological function and its broad substrate range. There have also been many investigations of its structure and molecular mechanism. In this mini review article we take a Rumsfeldian approach to ABCG2 and essentially ask what we do know about this transporter, and what we will need to know about this transporter if we wish to use modulation of ABCG2 activity as a therapeutic approach.
Keywords: ABCG2, ABC transporter, ATPase, multidrug pump, pharmacology
Multidrug transporters in biology
Multidrug resistance (MDR) can be defined as the ability of cells or organisms to resist the cytotoxic effects of a diverse range of chemical structures, often with different intracellular targets. MDR is observed across the spectrum of infectious disease therapy, as well as being documented in cancer chemotherapy. In many cases, the acquisition of a MDR phenotype is a poor prognostic indicator. For example, the emergence of drug resistant pathogenic bacteria and the subsequent lack of effective therapies is one of the global challenges for the 21st century. MDR may manifest through many mechanisms, often operating with what one presumes to be a degree of synergy, including chemical modification of the drug molecule rendering it ineffective (or less effective) and the alteration of gene expression to enable cellular metabolism to proceed through other pathways. The mechanism of interest here is the export of the drug from the cell, thus preventing it from achieving clinically effective concentrations. MDR by drug export is catalysed by at least 5 (and almost certainly more) families of membrane proteins with examples well documented in antimicrobial, antiparasitic and anticancer chemotherapy [1].
In humans, MDR is most widely associated with the failure of cancer chemotherapy. A number of cancers have been shown to have poorer prognosis if there is either a pre-existing expression of an MDR pump, or if expression of an MDR pump develops as a result of chemotherapy (see section below). Several human ATP binding cassette (ABC) proteins have been well described to be capable of MDR, although doubtless there will be more from the 48 ABC proteins that comprise the family in humans. Three of these, ABCB1 (P-glycoprotein), ABCC1 (multidrug resistance protein 1) and ABCG2 (breast cancer resistance protein) have been the subject of many decades of investigation, and there are already numerous reviews on many facets of their structure, function, regulation and role in disease [2,3]. The aim here is not to provide an exhaustive overview of one of this triumvirate (ABCG2) but to present a personal view about what we know, and what we need to know about this transporter if we are to develop rational interventions in chemotherapy.
ABCG2 localization and physiological function: an incomplete picture
Although first isolated from multidrug resistant cancer cells, ABCG2’s expression and distribution pattern in normal tissues implies that it must fulfil important physiological roles, such as protecting the organism as a first line of defence against environmental toxins. Schinkel's original study on ABCG2-null mice confirmed this role, demonstrating that these animals are more susceptible to diet-induced protoporphyria and phototoxicity, caused by accumulation of pheophorbide A [4], a chlorophyll degradation product and a confirmed ABCG2 transport substrate [5].
Extensive studies have continued to affirm that in humans and rodents, the localization of ABCG2 can play a vital role in limiting absorption (in the small intestine), mediating distribution (e.g. in the blood–brain and blood–placental barriers) and facilitating elimination and excretion (in the liver and kidney) of drugs or xenobiotics that are ABCG2 transport substrates. This specific ABCG2 distribution profile among the different types of tissues is closely related to the physiological role it assumes in the body. For instance, in the blood–brain barrier, ABCG2 is expressed highest on the luminal side of brain endothelial cells. Here, it serves as a crucial barrier to drug access, significantly limiting the penetration of drugs or xenobiotics into the brain [6,7], potentially in a synergistic manner with ABCB1 [8], with implications for brain tumour therapy and imaging [9].
In addition, ABCG2 is expressed in the apical membrane of epithelial cells in the gastrointestinal (GI) tract, with highest expression in the duodenum and a gradual decrease along the GI tract to the rectum. Here, ABCG2 plays an important role in limiting the absorption of orally administered anticancer drugs and ingested toxins [10,11]. In the liver canalicular membranes, where ABCG2 is constitutively expressed, a role in toxin and metabolite excretion is apparent [11,12]. In the human kidney ABCG2 is expressed on the apical membrane of proximal tubular cells, however with a lower expression level than that in the liver and the small intestine, and is responsible for urate export. Indeed, ABCG2 activity as a urate transporter is known to be abrogated in gout [13].
Among normal human tissues, ABCG2 is expressed highest on the apical membrane of the placental syncytiotrophoblasts. Here, ABCG2 expels drugs or xenobiotics from the foetal compartment back to the maternal circulation, limiting foetal exposure of the toxic substances and playing a major role in protecting foetus against maternally derived toxins [14]. ABCG2 is also expressed in the mammary gland being induced strongly during the lactation phase in mice, cows, and humans. It has been shown that ABCG2 actively transports not only beneficial vitamins (e.g. riboflavin) but also, seemingly paradoxically, toxic drugs and xenobiotics (e.g. topotecan, cimetidine and 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine) into breast-milk [15,16].
How do we develop a greater understanding of the tissue-specific roles of ABCG2? This question remains important to answer, particularly considering the impact of ABCG2 function on multiple drug pharmacokinetics and the potential for ABCG2 polymorphisms to have differential effects on transporter function (see below).
ABCG2 transport substrate diversity and pharmacology: all mapped out?
As discussed above, the exact physiological roles of ABCG2 remain somewhat enigmatic. The distribution of the transporter within tissues that have a predominantly secretory or barrier function leads to ABCG2 being recognised for a role in controlling the disposition and tissue exposure of endobiotics and xenobiotics, confirmed in numerous in vitro and in vivo studies. These include antibiotics, sterols, immune-suppressants (including anti-HIV drugs), fluorescent dyes (e.g. Hoechst 33342), photosensitizers (pheophorbide A and protoporphyrin IX). The increased expression of ABCG2 has been linked to MDR in cancer (see below) and there have also been numerous studies describing ABCG2 mediated transport of chemotherapeutic drugs including mitoxantrone, methotrexate, topotecans, flavopiridol and tyrosine kinase inhibitors (imatinib, gefitinib and nilotinib) [17].
At present, over 200 transport substrates of ABCG2 have been identified, with some attempts made to analyse structure–activity relationships (SAR). One such study demonstrated the impact of polarity on transport in a series of novel camptothecin analogues, which were actively extruded at a greater rate dependent on their higher polarity [18]. Additionally, small chemical libraries of ABCG2 inhibitors have been investigated to determine important functional groups. For example, inhibitors of casein kinase II were repurposed into a series of ABCG2 inhibitors by an overall increase in hydrophobicity and aromaticity [19]. This argument for polarity being associated with transport, and hydrophobicity with inhibition is interesting when taken alongside the most thorough attempt to determine the typical features of ABCG2 interacting compounds, presented in 2015 by Anna Seelig's group [20,21]. In two papers they employed functional ATPase activity assays, physicochemical data and molecular modelling to attempt to distinguish between the chemistries of ABCB1 and ABCG2 transport substrates. The authors argued that ABCG2 transports chemistries with higher hydrophilicity than ABCB1. However, in common with ABCB1, it does so subsequent to the partitioning of compounds from the aqueous to the lipid phase. A loose set of rules incorporating hydrophobicity, amphipathicity and ionization state was derived from analysis of a test series of chemicals to predict ABCG2’s likely interaction with or inhibition by other compounds [21]. Given the hundreds of compounds within the repertoire of ABCG2’s chemical interactome it would be interesting to see how these rules evolve with the acquisition of further data.
What do we know about how and where these drugs bind? The short answer: surprisingly little! Initial studies aimed to understand ABCG2 pharmacology, and included equilibrium and kinetic radioligand binding assays to study the interaction of radiolabelled daunomycin with ABCG2 expressed in insect (Sf9) cell membranes [22]. This study was performed with an R482G mutant version of ABCG2 due to its broader substrate range (see below). The data demonstrated an affinity (Kd) of ABCG2 for daunomycin of approximately 100 nM, which is still the only quantitative demonstration of an affinity of ABCG2 for a transport substrate. Relative potencies for other drugs (mitoxantrone, Hoechst 33342, doxorubicin and prazosin) to displace daunomycin binding were obtained and led to a picture of multiple drug binding sites showing a complex network of allosteric communications [22]. Changes in transport substrate affinity were demonstrated in subsequent studies upon the binding, rather than the hydrolysis of ATP [23], but other direct, quantitative data for ABCG2:transport substrate interaction remain scarce.
In the absence of such data a great deal of time and effort has been put into in vitro site-directed mutagenesis studies of ABCG2. Arginine 482 to glycine/threonine (TM3; Figure 1) is a classic example of a mutation which impacts substrate binding and/or transport resulting in the ability to transport daunorubicin, rhodamine 123 and lyso-tracker green. The mutant protein is also able to transport most wild type ABCG2 substrates, with the exception of methotrexate [24,25]. Mutations at residues T402(A/R), P485(A), P392(A), M549(A) also appear to be implicated in binding and transport of mitoxantrone, Hoechst 33342 and BODIPY-prazosin [5,26,27].
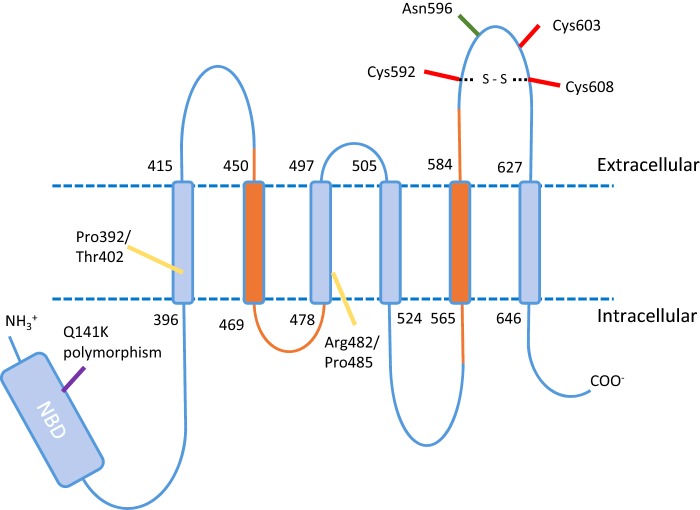
Figure 1. Topology and functionally important residues of ABCG2.
A monomer of ABCG2 consists of a 655 amino acid protein with a 250 amino acid intracellular N-terminal NBD, an uncharacterized linker region followed by six TMDs and associated intra and extracellular loops. Orange colours indicate where the experimental and predicted topology differ. A number of residues have been identified as significant including glycosylation site (green), stability affecting sites (red) and putative drug binding sites (yellow) in addition to the gout associated Q141K polymorphism are shown.
ABCG2 has over 80 single nucleotide polymorphisms (SNPs) residing within its gene coding region. The effect of ABCG2 polymorphisms on clinical pharmacology is a crucial area of current research; given the polyspecificity of the transporter it is inevitable that some polymorphisms will impact on the pharmacokinetics of particular drugs. Perhaps the most well documented of these is the rs2231142 polymorphism, Q141K. This polymorphism (which is associated with increase prevalence of gout [13]) has also been found to increase the bioavailability of topotecan, also more than doubling the exposure to rosuvastatin, commonly used in the treatment of hypercholesterolaemia [28,29]. That a single polymorphism can lead to raised levels of drugs or metabolites by either inhibiting excretion (i.e. renal elimination of urate [13]) or inhibiting absorption (intestinal uptake of rosuvastatin [29]) is intriguing and presents a challenge in understanding drug pharmacokinetics. Clearly, the impact of other SNPs on ABCG2 expression, drug transport and selectivity will be an increasingly important area of research.
ABCG2 structure and oligomerization: building up the picture is taking time
Protein structure is vital for a full understanding of function and could provide an excellent basis for the design of drugs. For the ABC multidrug pumps which are targets for pharmacological intervention this will require high resolution crystal structures in several conformations, including with bound transport substrate. In the absence of this for ABCG2, we have had to rely on the evidence for the transporter's membrane topology, its oligomeric state and available low resolution structural data, as the framework upon which to pin our understanding of mechanism.
Eukaryotic ABC transporters consist of at least one cytoplasmic nucleotide binding domain (NBD) and one transmembrane domain (TMD; Figure 1). The fundamental functional unit of ABC transporters requires the interaction of 2 NBDs to provide composite ATP binding sites, with contributions from both NBDs [30]. Eukaryotic ABC transporters meet this requirement either as ‘full transporters’, typically consisting of two homologous halves comprising a TMD-NBD-TMD-NBD arrangement, or as ‘half-transporters’ (comprising a single TMD-NBD) that form homo-dimers, hetero-dimers or higher order oligomers. The ABCG subfamily falls into the half-transporter category and have their TMD-NBD arrangement reversed. Subsequently, human ABCG2 is a reverse half transporter [31]. Six transmembrane helices are identified by consensus topology prediction algorithms and confirmed by epitope tagging, although there are differences in the predicted, compared with experimental, topology in terms of the location of TM2 and TM5 [32], which may be both shifted C-terminally with respect to the computer-based predictions (Figure 1). Whether this reflects the insertion of tags having an effect on the structure is unclear in the absence of further supporting evidence [2]. Without a high resolution structure, homology modelling has been used to assist in the interpretation of experimental data, although this has been rightly indicated as a hazardous venture due to the low sequence identity (often less than 20% [20]) and the 120 amino acid NBD-TMD linker region which is unique to the ABCG family of transporters and so cannot be modelled reliably from other ABC transporter structures (Figure 1). Models based upon the bacterial NBD MalK (i.e. to model the ABCG2 NBD), bacterial half-transporters Sav1866 and MsbA and the full length mouse ABCB1 have been described and their use to date has been limited to interpreting low resolution electron microscopy (EM) data [33,34]. Improved structural data of ABCG2 in the inward facing, presumably nucleotide-free state have been obtained recently [35], but this is far short of secondary structural resolution; perhaps the incredible advances that have made in data collection and analysis in cryo-EM in recent years (see [36] for a review) will enable us to reach near atomic resolution for ABCG2 in the not too distant future.
Based on knowledge of other ABC transporters it was probable that ABCG2 would require at least dimerization to form a functional unit. However, evidence for higher order oligomers has come thick and fast. The demonstration of tetramer formation has included native gel electrophoresis and EM [37,38] and other EM studies identified a stable homo-octameric association [33]. More recently, the oligomeric state has been studied in intact membranes in live and fixed cells; both fluorescence correlation spectroscopy and stepwise photobleaching enabled the observation of a predominantly tetrameric organization of ABCG2 in the presence and absence of transport substrates [39]. Understanding the structural basis of oligomerization remains problematic; the entire TM5–TM6 region appears to be involved with some evidence that this helical pair is sufficient for oligomerization to occur [40]. Within this region the single glycosylation site (N596; [41]) and an intramolecular disulfide bond between C592 and C608 [42] are implicated in protein trafficking and stability. A further cysteine residue in the TM5–TM6 extracellular loop (C603) is involved in inter-molecular disulfide bond formation although mutation of this residue does not impede oligomerization nor transport activity [43,44].
When will this structural knowledge enable us to inhibit any unwanted actions of ABCG2? Tellingly, more than a decade on from the initial discovery of ABCG2-specific inhibitors, the mechanism of fumitremorgin C and Ko143 remains obscure [45]. It remains to be seen whether a better understanding of the structural basis for drug interaction, or a better understanding of oligomerization is required to provide a route towards therapeutic inhibition of ABCG2.
ABCG2 in cancer: did we cry wolf?
ABCG2 was originally cloned from breast tumour cell lines and placental tissue in 1998 [46–48] and its isolation from a multidrug resistant breast cancer (Mcf7-derived) cell line quickly led to its assignation as the ‘breast cancer resistance protein’. But how justifiable has that moniker proven to be? Indeed, how much of a prognostic indicator is ABCG2 expression in cancer? A trawl of the literature reveals relatively few cancer types where ABCG2 expression (either protein or mRNA) is an independent prognostic indicator of poor clinical outcome. For haematological malignancies there is considerable evidence that ABCG2 is associated with poor outcome in acute myeloid leukaemia [49,50], even after bone marrow transplantation [51], and diffuse large B-cell lymphoma [52]. In some of these studies, co-expression with other MDR pumps (notably ABCB1) was associated with even higher hazard ratios [53,54]. For acute lymphoblastic leukaemia (ALL) the evidence is rather less convincing [55], and in paediatric ALL multiple studies report no association between ABCG2 expression and clinical outcome [56,57]. In solid tumours the picture is very mixed. For many tumour types there are frequently only 1 or 2 studies available precluding any conclusions from being drawn. Even for breast cancer the number of studies remains low with conflicting data presented [58,59]. For lung and oesophageal cancer, which have dismal 5-year survival rates (less than 20%), there is a consensus developing that ABCG2 is a prognostic indicator. In both small cell and non-small cell lung cancer (NSCLC) multiple studies now identify ABCG2 expression as being correlated with lower overall survival, shorter progression-free survival and the response to therapy [52,60–64]. Intriguingly, this applies when the therapy is platinum based, although ABCG2 is not identified as a transporter of Pt-containing drugs.
The identification of ABCG2 as a cancer stem cell marker [65] has led to many papers attempting to identify cancer stem cells co-expressing the marker CD133 and ABCG2, although in only a limited number of cases is co-expression of CD133 and ABCG2 of prognostic significance [61]. Attention is also turning in clinical studies to whether particular SNPs of ABCG2 are predictive of response to treatment, or chemotherapy induced toxicity. Again, haematological malignancies lead the way in these studies although recent data on NSCLC and ovarian cancer indicate that a more fine-grained analysis of ABCG2 haplotypes will uncover clinically relevant findings [66].
Perspective
So what now for ABCG2 research? In our own field (structure and function) we would hope that the bounty of functional data soon has more robust structural models within which these data can be interpreted. That will help us to develop a more complete understanding of how drugs are bound and transported by this MDR pump. It will also help us to interpret the action of small molecule inhibitors of ABCG2, which may emerge to be important in clinical practice. In this regard, we need more studies to examine ABCG2 expression in solid tumours to consolidate the data already in the literature and provide a more definitive picture of this pump's role in cancer biology.
Abbreviations
- ABC
ATP binding cassette
- ALL
acute lymphoblastic leukaemia
- EM
electron microscopy
- MDR
multidrug resistance
- NBD
nucleotide binding domain
- NSCLC
non-small cell lung cancer
- SNP
single nucleotide polymorphism
- TMD
transmembrane domain
Footnotes
Membrane Proteins From A to Z: Held at University of Leeds, U.K., 16–17 December 2015
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentships [grant number BB/J014508/1 (to A.J.H. and M.H.C.)]; and the University of Nottingham Developing Solutions Scholarship (to S.S.).
References
- 1.Wong K., Ma J., Rothnie A., Biggin P.C., Kerr I.D. Towards understanding promiscuity in multidrug efflux pumps. Trends Biochem. Sci. 2014;39:8–16. doi: 10.1016/j.tibs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Mao Q., Unadkat J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport–an update. AAPS J. 2015;17:65–82. doi: 10.1208/s12248-014-9668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darby R.A., Callaghan R., McMahon R.M. P-glycoprotein inhibition; the past, the present and the future. Curr. Drug Metab. 2011;12:722–731. doi: 10.2174/138920011798357006. [DOI] [PubMed] [Google Scholar]
- 4.Jonker J.W., Buitelaar M., Wagenaar E., Van Der Valk M.A., Scheffer G.L., Scheper R.J., Plosch T., Kuipers F., Elferink R.P., Rosing H., et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haider A.J., Cox M.H., Jones N., Goode A.J., Bridge K.S., Wong K., Briggs D., Kerr I.D. Identification of residues in ABCG2 affecting protein trafficking and drug transport, using co-evolutionary analysis of ABCG sequences. Biosci. Rep. 2015;35:e00241. doi: 10.1042/BSR20150150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronica E., Gorter J.A., Redeker S., van Vliet E.A., Ramkema M., Scheffer G.L., Scheper R.J., van der Valk P., Leenstra S., Baayen J.C., et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46:849–857. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W., Mojsilovic-Petrovic J., Andrade M.F., Zhang H., Ball M., Stanimirovic D.B. Expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–2087. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S., Sane R., Ohlfest J.R., Elmquist W.F. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J. Pharmacol. Exp. Ther. 2011;336:223–233. doi: 10.1124/jpet.110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer M., Karch R., Zeitlinger M., Stanek J., Philippe C., Wadsak W., Mitterhauser M., Jager W., Haslacher H., Muller M., Langer O. Interaction of 11C-tariquidar and 11C-elacridar with P-glycoprotein and breast cancer resistance protein at the human blood–brain barrier. J. Nucl. Med. 2013;54:1181–1187. doi: 10.2967/jnumed.112.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutmann H., Hruz P., Zimmermann C., Beglinger C., Drewe J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem. Pharmacol. 2005;70:695–699. doi: 10.1016/j.bcp.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Kruijtzer C.M., Beijnen J.H., Rosing H., ten Bokkel Huinink W.W., Schot M., Jewell R.C., Paul E.M., Schellens J.H. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J. Clin. Oncol. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 12.Maliepaard M., Scheffer G.L., Faneyte I.F., van Gastelen M.A., Pijnenborg A.C., Schinkel A.H., van De Vijver M.J., Scheper R.J., Schellens J.H. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 13.Woodward O.M., Kottgen A., Coresh J., Boerwinkle E., Guggino W.B., Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm. Res. 2008;25:1244–1255. doi: 10.1007/s11095-008-9537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonker J.W., Merino G., Musters S., van Herwaarden A.E., Bolscher E., Wagenaar E., Mesman E., Dale T.C., Schinkel A.H. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 16.van Herwaarden A.E., Wagenaar E., Merino G., Jonker J.W., Rosing H., Beijnen J.H., Schinkel A.H. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell. Biol. 2007;27:1247–1253. doi: 10.1128/MCB.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basseville A., Hall M.D., Chau C.H., Robey R.W., Gottesman M., Figg W.D., Bates S.E. The ABCG2 multidrug transporter. In: George A.M., editor. In ABC Transporters–40 Years On. Heidelberg: Springer; 2016. pp. 195–226. [DOI] [Google Scholar]
- 18.Yoshikawa M., Ikegami Y., Hayasaka S., Ishii K., Ito A., Sano K., Suzuki T., Togawa T., Yoshida H., Soda H., et al. Novel camptothecin analogues that circumvent ABCG2-associated drug resistance in human tumor cells. Int. J. Cancer. 2004;110:921–927. doi: 10.1002/ijc.20216. [DOI] [PubMed] [Google Scholar]
- 19.Gozzi G.J., Bouaziz Z., Winter E., Daflon-Yunes N., Honorat M., Guragossian N., Marminon C., Valdameri G., Bollacke A., Guillon J., et al. Phenolic indeno[1,2-b]indoles as ABCG2-selective potent and non-toxic inhibitors stimulating basal ATPase activity. Drug Des. Dev. Ther. 2015;9:3481–3495. doi: 10.2147/DDDT.S84982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y., Egido E., Li-Blatter X., Muller R., Merino G., Berneche S., Seelig A. Allocrite sensing and binding by the breast cancer resistance protein (ABCG2) and P-glycoprotein (ABCB1) Biochemistry. 2015;54:6195–6206. doi: 10.1021/acs.biochem.5b00649. [DOI] [PubMed] [Google Scholar]
- 21.Egido E., Muller R., Li-Blatter X., Merino G., Seelig A. Predicting activators and inhibitors of the breast cancer resistance protein (ABCG2) and P-glycoprotein (ABCB1) based on mechanistic considerations. Mol. Pharm. 2015;12:4026–4037. doi: 10.1021/acs.molpharmaceut.5b00463. [DOI] [PubMed] [Google Scholar]
- 22.Clark R., Kerr I.D., Callaghan R. Multiple drugbinding sites on the R482G isoform of the ABCG2 transporter. Br. J. Pharmacol. 2006;149:506–515. doi: 10.1038/sj.bjp.0706904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDevitt C.A., Crowley E., Hobbs G., Starr K.J., Kerr I.D., Callaghan R. Is ATP binding responsible for initiating drug translocation by the multidrug transporter ABCG2? FEBS J. 2008;275:4354–4362. doi: 10.1111/j.1742-4658.2008.06578.x. [DOI] [PubMed] [Google Scholar]
- 24.Ejendal K.F., Diop N.K., Schweiger L.C., Hrycyna C.A. The nature of amino acid 482 of human ABCG2 affects substrate transport and ATP hydrolysis but not substrate binding. Protein Sci. 2006;15:1597–1607. doi: 10.1110/ps.051998406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robey R.W., Honjo Y., Morisaki K., Nadjem T.A., Runge S., Risbood M., Poruchynsky M.S., Bates S. E. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br. J. Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni Z., Bikadi Z., Cai X., Rosenberg M.F., Mao Q. Transmembrane helices 1 and 6 of the human breast cancer resistance protein (BCRP/ABCG2): identification of polar residues important for drug transport. Am. J. Physiol., Cell Physiol. 2010;299:C1100–C1109. doi: 10.1152/ajpcell.00160.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni Z., Bikadi Z., Shuster D.L., Zhao C., Rosenberg M.F., Mao Q. Identification of proline residues in or near the transmembrane helices of the human breast cancer resistance protein (BCRP/ABCG2) that are important for transport activity and substrate specificity. Biochemistry. 2011;50:8057–8066. doi: 10.1021/bi200573t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sissung T.M., Baum C.E., Kirkland C.T., Gao R., Gardner E.R., Figg W.D. Pharmacogenetics of membrane transporters: an update on current approaches. Mol. Biotechnol. 2010;44:152–167. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keskitalo J.E., Zolk O., Fromm M.F., Kurkinen K.J., Neuvonen P.J., Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin. Pharmacol. Ther. 2009;86:197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 30.Kerr I.D. Structure and association of ATP binding cassette transporter nucleotide-binding domains. Biochem. Biophys. Acta. 2002;1561:47–64. doi: 10.1016/S0304-4157(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 31.Kerr I.D., Haider A.J., Gelissen I.C. The ABCG family of membrane-associated transporters: you don't have to be big to be mighty. Br. J. Pharmacol. 2011;164:1767–1779. doi: 10.1111/j.1476-5381.2010.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., Lee E.W., Cai X., Ni Z., Zhou L., Mao Q. Membrane topology of the human breast cancer resistance protein (BCRP/ABCG2) determined by epitope insertion and immunofluorescence. Biochemistry. 2008;47:13778–13787. doi: 10.1021/bi801644v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDevitt C.A., Collins R., Kerr I.D., Callaghan R. Purification and structural analyses of ABCG2. Adv. Drug Deliv. Rev. 2009;61:57–65. doi: 10.1016/j.addr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg M.F., Bikadi Z., Chan J., Liu X., Ni Z., Cai X., Ford R.C., Mao Q. The human breast cancer resistance protein (BCRP/ABCG2) shows conformational changes with mitoxantrone. Structure. 2010;18:482–493. doi: 10.1016/j.str.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg M.F., Bikadi Z., Hazai E., Starborg T., Kelley L., Chayen N.E., Ford R.C., Mao Q. Three-dimensional structure of the human breast cancer resistance protein (BCRP/ABCG2) in an inward-facing conformation. Acta. Crystallogr. D. Biol. Crystallogr. 2015;71:1725–1735. doi: 10.1107/S1399004715010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai X.C., McMullan G., Scheres S.H. How cryo-EM is revolutionizing structural biology. Trends Biochem. Sci. 2015;40:49–57. doi: 10.1016/j.tibs.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Dezi M., Fribourg P.F., Di Cicco A., Arnaud O., Marco S., Falson P., Di Pietro A., Levy D. The multidrug resistance half-transporter ABCG2 is purified as a tetramer upon selective extraction from membranes. Biochim. Biophys. Acta. 2010;1798:2094–2101. doi: 10.1016/j.bbamem.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Xu J., Liu Y., Yang Y., Bates S., Zhang J.T. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J. Biol. Chem. 2004;279:19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 39.Wong K., Briddon S.J., Holliday N.D., Kerr I.D. Plasma membrane dynamics and tetrameric organisation of ABCG2 transporters in mammalian cells revealed by single particle imaging techniques. Biochim. Biophys. Acta. 2015;1863:19–29. doi: 10.1016/j.bbamcr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Mo W., Qi J., Zhang J.T. Different roles of TM5, TM6, and ECL3 in the oligomerization and function of human ABCG2. Biochemistry. 2012;51:3634–3641. doi: 10.1021/bi300301a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa H., Wakabayashi-Nakao K., Tamura A., Toyoda Y., Koshiba S., Ishikawa T. Disruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. FEBS J. 2009;276:7237–7252. doi: 10.1111/j.1742-4658.2009.07423.x. [DOI] [PubMed] [Google Scholar]
- 42.Henriksen U., Fog J.U., Litman T., Gether U. Identification of intra- and intermolecular disulfide bridges in the multidrug resistance transporter ABCG2. J. Biol. Chem. 2005;280:36926–36934. doi: 10.1074/jbc.M502937200. [DOI] [PubMed] [Google Scholar]
- 43.Haider A.J., Briggs D., Self T.J., Chilvers H.L., Holliday N.D., Kerr I.D. Dimerization of ABCG2 analysed by bimolecular fluorescence complementation. PLoS One. 2011;6:e25818. doi: 10.1371/journal.pone.0025818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kage K., Fujita T., Sugimoto Y. Role of Cys-603 in dimer/oligomer formation of the breast cancer resistance protein BCRP/ABCG2. Cancer Sci. 2005;96:866–872. doi: 10.1111/j.1349-7006.2005.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen J.D., van Loevezijn A., Lakhai J.M., van der Valk M., van Tellingen O., Reid G., Schellens J.H., Koomen G.J., Schinkel A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002;1:417–425. doi: 10.4161/cbt.1.4.20. [DOI] [PubMed] [Google Scholar]
- 46.Allikmets R., Schriml L.M., Hutchinson A., Romano-Spica V., Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 47.Doyle L.A., Yang W., Abruzzo L.V., Krogmann T., Gao Y., Rishi A.K., Ross D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyake K., Mickley L., Litman T., Zhan Z., Robey R., Cristensen B., Brangi M., Greenberger L., Dean M., Fojo T., Bates S.E. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 49.Damiani D., Tiribelli M., Calistri E., Geromin A., Chiarvesio A., Michelutti A., Cavallin M., Fanin R. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotype. Haematologica. 2006;91:825–828. [PubMed] [Google Scholar]
- 50.van den Heuvel-Eibrink M.M., van der Holt B., Burnett A.K., Knauf W. U., Fey M.F., Verhoef G.E., Vellenga E., Ossenkoppele G.J., Lowenberg B., Sonneveld P. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann. Hematol. 2007;86:329–337. doi: 10.1007/s00277-007-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damiani D., Tiribelli M., Geromin A., Michelutti A., Cavallin M., Sperotto A., Fanin R. ABCG2 overexpression in patients with acute myeloid leukemia: impact on stem cell transplantation outcome. Am.J. Hematol. 2015;90:784–789. doi: 10.1002/ajh.24084. [DOI] [PubMed] [Google Scholar]
- 52.Kim J.E., Singh R.R., Cho-Vega J.H., Drakos E., Davuluri Y., Khokhar F.A., Fayad L., Medeiros L.J., Vega F. Sonic hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod. Pathol. 2009;22:1312–1320. doi: 10.1038/modpathol.2009.98. [DOI] [PubMed] [Google Scholar]
- 53.Bartholomae S., Gruhn B., Debatin K.M., Zimmermann M., Creutzig U., Reinhardt D., Steinbach D. Coexpression of multiple ABC-transporters is strongly associated with treatment response in childhood acute myeloid leukemia. Pediatr. Blood Cancer. 2016;63:242–247. doi: 10.1002/pbc.25785. [DOI] [PubMed] [Google Scholar]
- 54.Benderra Z., Faussat A.M., Sayada L., Perrot J.Y., Chaoui D., Marie J.P., Legrand O. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin. Cancer Res. 2004;10:7896–7902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 55.Suvannasankha A., Minderman H., O'Loughlin K.L., Nakanishi T., Ford L.A., Greco W.R., Wetzler M., Ross D.D., Baer M.R. Breast cancer resistance protein (BCRP/MXR/ABCG2) in adult acute lymphoblastic leukaemia: frequent expression and possible correlation with shorter disease-free survival. Br. J. Haematol. 2004;127:392–398. doi: 10.1111/j.1365-2141.2004.05211.x. [DOI] [PubMed] [Google Scholar]
- 56.Sauerbrey A., Sell W., Steinbach D., Voigt A., Zintl F. Expression of the BCRP gene (ABCG2/MXR/ABCP) in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2002;118:147–150. doi: 10.1046/j.1365-2141.2002.03550.x. [DOI] [PubMed] [Google Scholar]
- 57.Kourti M., Vavatsi N., Gombakis N., Sidi V., Tzimagiorgis G., Papageorgiou T., Koliouskas D., Athanassiadou F. Expression of multidrug resistance 1 (MDR1), multidrug resistance-related protein 1 (MRP1), lung resistance protein (LRP), and breast cancer resistance protein (BCRP) genes and clinical outcome in childhood acute lymphoblastic leukemia. Int. J. Hematol. 2007;86:166–173. doi: 10.1532/IJH97.E0624. [DOI] [PubMed] [Google Scholar]
- 58.Faneyte I.F., Kristel P.M., Maliepaard M., Scheffer G.L., Scheper R.J., Schellens J.H., van de Vijver M.J. Expression of the breast cancer resistance protein in breast cancer. Clin. Cancer Res. 2002;8:1068–1074. [PubMed] [Google Scholar]
- 59.Xiang L., Su P., Xia S., Liu Z., Wang Y., Gao P., Zhou G. ABCG2 is associated with HER-2 expression, lymph node metastasis and clinical stage in breast invasive ductal carcinoma. Diagn. Pathol. 2011;6:90. doi: 10.1186/1746-1596-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hang D., Dong H.C., Ning T., Dong B., Hou D.L., Xu W.G. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis. Esophagus. 2012;25:638–644. doi: 10.1111/j.1442-2050.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- 61.Li F., Zeng H., Ying K. The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med. Oncol. 2011;28:1458–1462. doi: 10.1007/s12032-010-9646-5. [DOI] [PubMed] [Google Scholar]
- 62.Ota S., Ishii G., Goto K., Kubota K., Kim Y.H., Kojika M., Murata Y., Yamazaki M., Nishiwaki Y., Eguchi K., Ochiai A. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64:98–104. doi: 10.1016/j.lungcan.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Tsunoda S., Okumura T., Ito T., Kondo K., Ortiz C., Tanaka E., Watanabe G., Itami A., Sakai Y., Shimada Y. ABCG2 expression is an independent unfavorable prognostic factor in esophageal squamous cell carcinoma. Oncology. 2006;71:251–258. doi: 10.1159/000106787. [DOI] [PubMed] [Google Scholar]
- 64.Yoh K., Ishii G., Yokose T., Minegishi Y., Tsuta K., Goto K., Nishiwaki Y., Kodama T., Suga M., Ochiai A. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin. Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.CCR-0937-3. [DOI] [PubMed] [Google Scholar]
- 65.Hirschmann-Jax C., Foster A.E., Wulf G.G., Nuchtern J.G., Jax T.W., Gobel U., Goodell M.A., Brenner M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian C., Ambrosone C.B., Darcy K.M., Krivak T.C., Armstrong D.K., Bookman M.A., Davis W., Zhao H., Moysich K., Gallion H., DeLoia J.A. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecol. Oncol. 2012;124:575–581. doi: 10.1016/j.ygyno.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]