Genetic deletion of the hydrogen peroxide producing NADPH oxidase 4 (Nox4), as shown in the present study, leads to endothelial dysfunction and increased atherosclerosis under pathological conditions. Consequently, endothelial activation of Nox4 may represent a promising novel strategy for preventing endothelial dysfunction and atherosclerosis and its severe clinical complications. This also suggests that in contrast to the deleterious effects of oxidative stress certain reactive oxygen species might mediate beneficial effects in the vessel wall.
Keywords: NADPH oxidase 4, Nox4, Endothelial dysfunction, Ldlr−/− mice, Atherosclerosis, Flow-mediated dilation
Abstract
Aims
Endothelial dysfunction is an early step in the development of atherosclerosis. Increased formation of superoxide anions by NADPH oxidase Nox1, 2, and 5 reduces nitric oxide availability and can promote endothelial dysfunction. In contrast, recent evidence supports a vasoprotective role of H2O2 produced by main endothelial isoform Nox4. Therefore, we analysed the impact of genetic deletion of Nox4 on endothelial dysfunction and atherosclerosis in the low-density lipoprotein receptor (Ldlr) knockout model.
Methods and results
Ex vivo analysis of endothelial function by Mulvany myograph showed impaired endothelial function in thoracic aorta of Nox4−/−/Ldlr−/− mice. Further progression of endothelial dysfunction due to high-fat diet increased atherosclerotic plaque burden and galectin-3 staining in Nox4−/−/Ldlr−/− mice compared with Ldlr−/− mice. Under physiological conditions, loss of Nox4 does not influence aortic vascular function. In this setting, loss of Nox4-derived H2O2 production could be partially compensated for by nNOS upregulation. Using an innovative optical coherence tomography approach, we were able to analyse endothelial function by flow-mediated vasodilation in the murine saphenous artery in vivo. This new approach revealed an altered flow-mediated dilation in Nox4−/− mice, indicating a role for Nox4 under physiological conditions in peripheral arteries in vivo.
Conclusions
Nox4 plays an important role in maintaining endothelial function under physiological and pathological conditions. Loss of Nox4-derived H2O2 could be partially compensated for by nNOS upregulation, but severe endothelial dysfunction is not reversible. This leads to increased atherosclerosis under atherosclerotic prone conditions.
Translational perspective.
Genetic deletion of the hydrogen peroxide producing NADPH oxidase 4 (Nox4), as shown in the present study, leads to endothelial dysfunction and increased atherosclerosis under pathological conditions. Consequently, endothelial activation of Nox4 may represent a promising novel strategy for preventing endothelial dysfunction and atherosclerosis and its severe clinical complications. This also suggests that in contrast to the deleterious effects of oxidative stress certain reactive oxygen species might mediate beneficial effects in the vessel wall.
Introduction
Endothelial dysfunction is an early event in atherosclerosis that precedes clinical symptoms and has prognostic value for future cardiovascular events.1–8 It is characterized by an imbalance between vasodilation and vasoconstriction, inhibition and promotion of proliferation and migration of smooth muscle cells, prevention and stimulation of adhesion of monocytes and aggregation of platelets.9 Furthermore, increased formation of reactive oxygen species (ROS) such as superoxide anions can contribute to impaired endothelium-dependent vasodilation by decreased bioavailability of nitric oxide (NO).10,11
NADPH oxidase (Nox) enzyme complexes are predominant sources of ROS in the vessel wall.12,13 Isoforms Nox1, Nox2, Nox4, and Nox5 are expressed in the human vasculature.14 Nox4 is the predominant isoform in endothelial cells15 and mainly produces H2O2, which might explain functional differences to superoxide anion producing Nox isoforms.16–18
The role of different Nox isoforms in cardiovascular physiology and pathophysiology is controversial.11 Increased expression of Nox1 and Nox2 in vascular cells and macrophages has been reported as contributing to atherosclerosis and vascular diseases.19–21 Patients with genetic Nox2 deficiency show an enhanced endothelium-dependent relaxation.22 Nox5 can contribute to the oxidative stress in human coronary artery disease as well.23 Increasing evidence supports mainly beneficial effects of Nox4 in the cardiovascular system, overexpression of Nox4 reduced angiotensin II-induced increase in blood pressure.24 Mechanistically, H2O2 increases endothelial NO release by AKT-dependent (Ser473/Thr308) phosphorylation.25–28 In addition, H2O2 acts as an endothelium-derived hyperpolarizing factor (EDHF) in mice and man leading to vasorelaxation.29–31 Global Nox4 knockout mice revealed contractile impairment, cardiac hypertrophy, and intestinal fibrosis after chronic overload.32 In tamoxifen-inducible Nox4 knockout mice, angiotensin II-mediated aortic inflammation, media hypertrophy, and endothelial dysfunction were enhanced.33 On the other hand, Nox4−/− mice were protected from oxidative stress, blood–brain barrier leakage, and neuronal apoptosis in a stroke model.34 Cardiac hypertrophy, fibrosis, and apoptosis after pressure overload were reduced in a model of cardiac-specific deletion of Nox4.35 These data suggest different cell- and dose-dependent effects of Nox4 in cardiovascular diseases.36
To investigate the diverse role of Nox4, we analysed the effects of genetic deletion of Nox4 in the development of endothelial dysfunction and atherosclerosis in mice in vivo.
Methods
All materials and methods are available in Supplementary material online.
Results
Nox4−/−/Ldlr−/− mice develop endothelial dysfunction
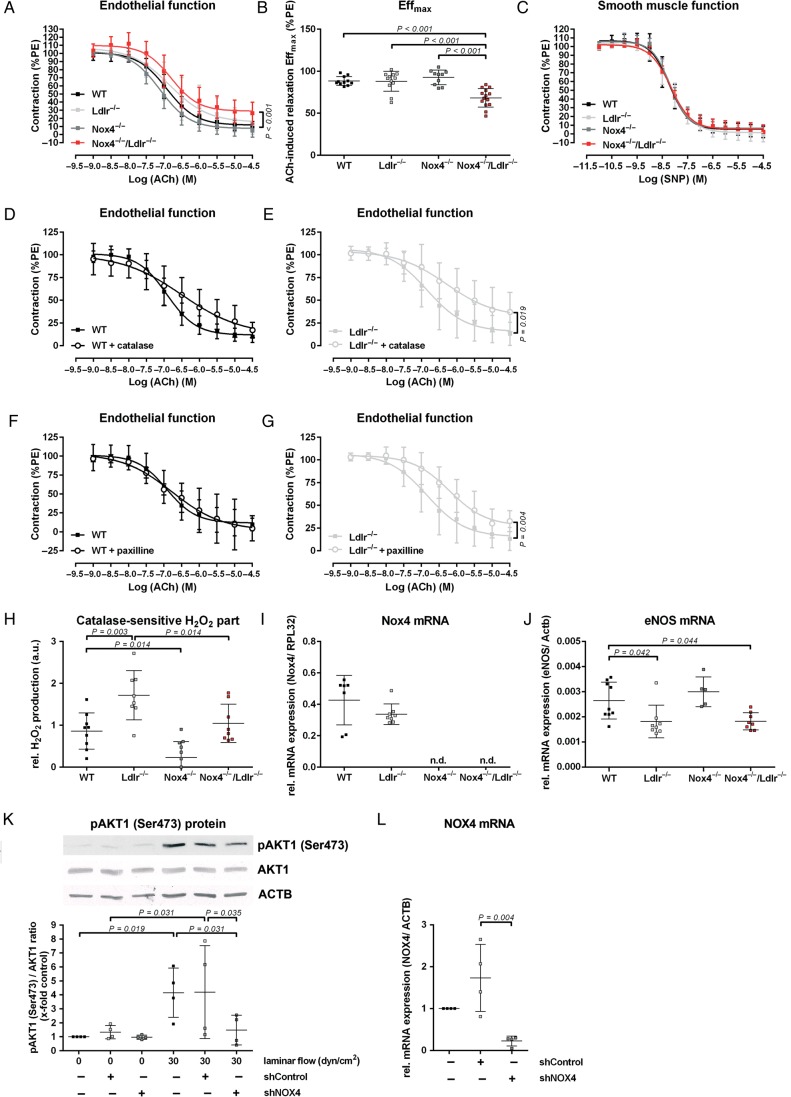
First, we analysed endothelial function in aortas of 10-week-old C57BL/6J (wild type), Ldlr−/−, and Nox4−/− mice. We observed no changes in endothelium-dependent relaxation. In contrast, Nox4−/−/Ldlr−/− double knockout mice of the same age developed endothelial dysfunction (Figure 1A and B). No changes in smooth muscle function were observed between the strains of mice (Figure 1C). In addition, endothelial dysfunction could be induced by application of catalase to aortic segments of Ldlr−/− mice (Figure 1D and E). By blocking the calcium-activated potassium channels with large conductance (BK channels) with paxilline endothelial function of Ldlr−/− mice, but not wild-type mice, was declined to the level of endothelial dysfunction in aortic rings of Nox4−/−/Ldlr−/− mice (Figure 1F and G).
Figure 1.
Nox4−/−/Ldlr−/− mice develop endothelial dysfunction. (A) Concentration–response curve for acetylcholine in aortic segments of 10-week-old wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 10) precontracted with phenylephrine. (B) Maximal effect of 30 µmol/L ACh on aortic segments of wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 10). (C) Concentration–response curves for sodium nitroprusside in aortic segments of wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 7). (D and E) Concentration–response curve for acetylcholine in aortic segments of 10-week-old wild-type and Ldlr−/− mice with catalase (n ≥ 9). (F and G) Concentration–response curve for acetylcholine in aortic segments of 10-week-old wild-type and Ldlr−/− mice with paxilline (n ≥ 9). (H) Amplex red assay for hydrogen peroxide generation of Aorta thoracalis segments of wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/−. H2O2 formation was measured as the catalase-sensitive part of the signal (a.u.: arbitrary units) (n ≥ 8). (I and J) Real-time polymerase chain reaction of murine aorta from wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 5). (K) Western blot analysis of pAKT1 (Ser473) in human umbilical vein endothelial cells transduced with shControl or shNOX4 for 48 h. Cells were either kept under static conditions or exposed to laminar shear stress (30 dyn/cm2) for 5 min (n=4. Statistics: one-way analysis of variance Duncan's method). (L) Real-time polymerase chain reaction of NOX4 in human umbilical vein endothelial cells. Cells were transduced for 72 h with shControl or shNOX4 (n = 4).
Hydrogen peroxide release of aortas revealed significantly higher levels in Ldlr−/− mice. Aortas of Nox4−/−/Ldlr−/− mice released significantly lower levels of hydrogen peroxide compared with the Ldlr−/− mice (Figure 1H). Interestingly, both Ldlr−/− and Nox4−/−/Ldlr−/− mice expressed significant lower eNOS mRNA in the aorta compared with wild-type mice. This suggests that hydrogen peroxide might partially compensate NO as a vasodilator in Ldlr−/− mice (Figure 1I and J). In vitro experiments in human umbilical vein endothelial cells showed activation of pAKT1 (Ser473) by high laminar flow of 30 dyn/cm2. Downregulation of NOX4 by shRNA diminished pAKT1 (Ser473) phosphorylation (Figure 1K and L). We assume that hydrogen peroxide derived from NOX4 acts via pAKT1 (Ser473) phosphorylation on eNOS activation. We observed severely increased levels of LDL cholesterol, total cholesterol, and triglyceride in the serum of Ldlr−/− and Nox4−/−/Ldlr−/− mice (see Supplementary material online). Thus, hydrogen peroxide seems to be particularly essential under pathological conditions.
Nox4−/−/Ldlr−/− mice on high-fat diet develop increased atherosclerosis
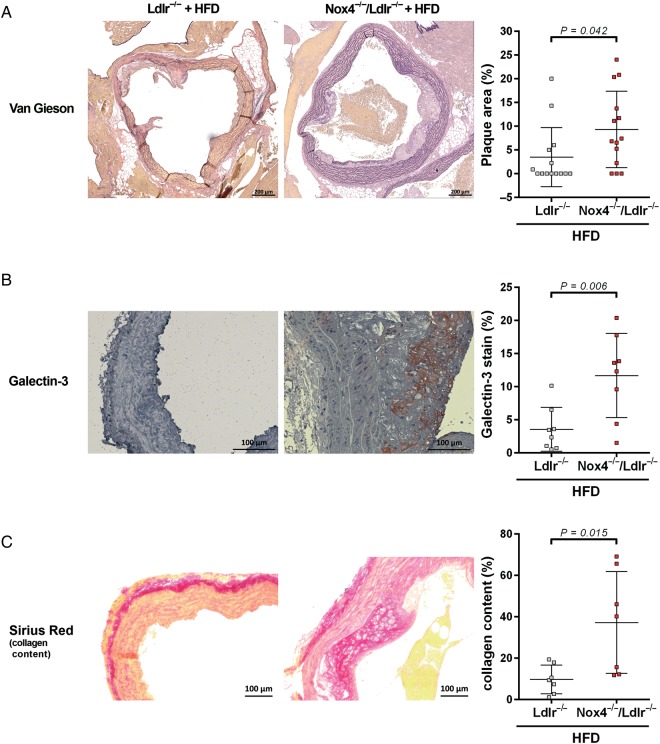
To determine the outcome of the severe endothelial dysfunction, we investigated Nox4−/−/Ldlr−/− mice after 20 weeks on a high-fat diet. Body weight development, energy intake, blood glucose, serum lipid parameters, weight of white adipose tissue, kidney, heart, and liver did not differ between Ldlr−/− and Nox4−/−/Ldlr−/− mice (see Supplementary material online, Figure S3 and Tables S2, S3). Plaque burden in the aortic arch of Nox4−/−/Ldlr−/− mice was significantly higher, compared with Ldlr−/− mice, as indicated by Elastica van Gieson staining (Figure 2A). Similarly, Galectin-3 staining was significantly higher in aortic arch sections of Nox4−/−/Ldlr−/− mice, compared with Ldlr−/− mice, further supporting a more severe atherosclerotic phenotype in the double knockout mice (Figure 2B). Collagen content in the media analysed by Sirius Red staining was significantly increased in Nox4−/−/Ldlr−/− mice (Figure 2C). Under the severe conditions of LDL receptor knockout, the loss of Nox4 led to a higher progression of atherosclerosis.
Figure 2.
Nox4−/−/Ldlr−/− mice develop increased atherosclerosis on high-fat diet. (A) Atherosclerotic plaque burden: Plaque area relative to total vessel area of aortic arch sections was shown with Elastica Van Gieson staining from Ldlr−/− and Nox4−/−/Ldlr−/− mice after 20 weeks on high-fat diet (n = 14). (B) Galectin-3 staining of aortic arch sections of Ldlr−/− and Nox4−/−/Ldlr−/− mice after 20 weeks on high-fat diet (n ≥ 8). (C) Collagen content: Sirius Red-positive staining of plaque and media of aortic arch sections from Ldlr−/− and Nox4−/−/Ldlr−/− mice after 20 weeks on high-fat diet (n = 7).
Compensation of hydrogen peroxide release in older Nox4−/− mice
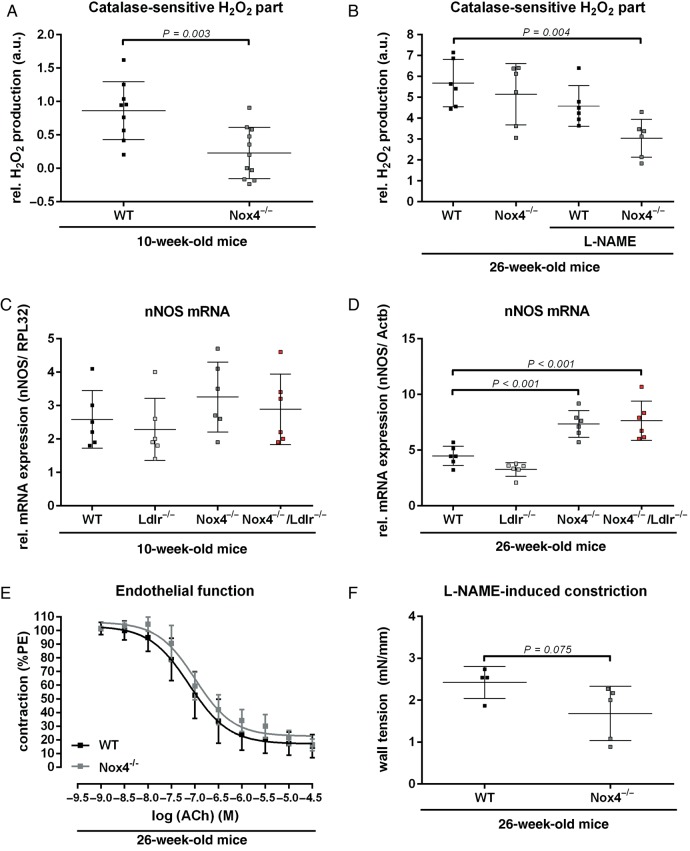
The importance of hydrogen peroxide is also supported by the fact that decreased aortic H2O2 levels in young Nox4−/− mice were restored to levels of wild-type mice at 26 weeks of age (Figure 3A and B). This effect could be blocked when incubating aortic segments with NOS blocker L-NAME (Figure 3B), indicating a role of nNOS as potential source of H2O2. Similar to the compensation of hydrogen peroxide in the 26-week-old Nox4−/− mice, nNOS mRNA expression was significantly increased in aortas of 26-week-old but not 10-week-old Nox4−/− mice (Figure 3C and D). Aortic endothelial function of 26-week-old Nox4−/− was not altered (Figure 3E). However, NO availability seemed to be decreased in aortic segments of Nox4−/− mice. These mice showed a trend to decreased L-NAME-induced endothelium-dependent constriction (Figure 3F).
Figure 3.
Compensation of hydrogen peroxide release during aging in Nox4−/− mice. (A and B) Amplex red assay for hydrogen peroxide generation of Aorta thoracalis segments of 10-week-old wild-type and Nox4−/− mice (n ≥ 9) or 26-week-old mice with or without L-NAME pre-incubation (n ≥ 6). H2O2 formation was measured as the catalase-sensitive part of the signal. (C and D) Real-time polymerase chain reaction of murine aorta from 10- and 26-week-old wild-type, Ldlr−/−, Nox4−/−, and Nox4−/−/Ldlr−/− mice (n ≥ 6). (E) Concentration–response curves of acetylcholine in aortic segments of 26-week-old wild-type and Nox4−/− mice (n ≥ 12) precontracted with phenylephrine. (F) L-NG-Nitroarginine methyl ester induced constriction of aortic segments of 26-week-old wild-type and Nox4−/− mice (n ≥ 4).
Saphenous artery of Nox4−/− mice show impaired flow-mediated dilation in vivo
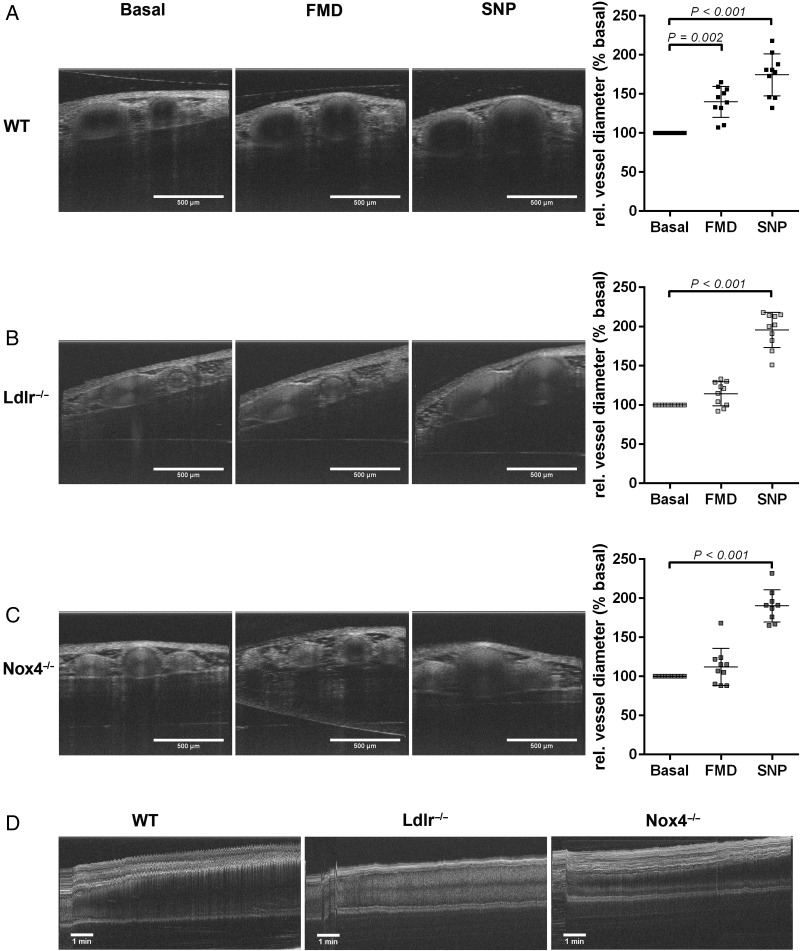
Finally, we analysed endothelial function of Arteria saphena of 26-week-old wild-type, Nox4−/−, and Ldlr−/− mice in vivo. We developed a method of determining flow-mediated dilation by optical coherence tomography (OCT). Cross-sectional tomographic images (25 B-scans per second) at resting conditions, after vessel clamp release and after sodium nitroprusside application were analysed (Figure 4A–C). In addition, a continuous OCT recording was performed over a 10-min period after vessel clamp release to analyse flow-mediated vasodilation (Figure 4D). Analysis showed significant increase in vessel diameter due to flow-mediated dilation in wild-type mice, while no significant increase in vessel diameter after clamp release could be detected in either Nox4−/− or Ldlr−/− mice (Figure 4C). Sodium nitroprusside caused similar increases of vessel diameter in mice strains.
Figure 4.
Nox4−/− mice show altered flow-mediated dilation in vivo. (A) Representative optical coherence tomography images of Arteria saphena and evaluation of images of 26-week-old (A) wild-type, (B) Ldlr−/−, and (C) Nox4−/− mice under control conditions (basal), flow-mediated dilation and after application of sodium nitroprusside. (D) Representative optical coherence tomography scan of the Arteria saphena of 26-week-old wild-type, Ldlr−/−, and Nox4−/− mice showing the process of flow-mediated dilation during 10 min after occlusion (n = 10).
Discussion
In the present study, we were able to show that loss of H2O2-releasing Nox4 in a genetic background of hypercholesterolaemia leads to severe endothelial dysfunction. The reduced vasodilation capacity in the Nox4−/−/Ldlr−/− mice was identical to the declined maximal acetylcholine-induced relaxation of aortic segments of Ldlr−/− mice which were pre-incubated with hydrogen peroxide-degrading catalase or BKCa channel inhibitor paxilline. This implies a role of H2O2 as a vasodilator under pathological conditions. In agreement with this assumption, we did not find changes in vascular function in 10-week-old Nox4−/− mice. It was recently shown that increased H2O2 release by overexpression of Nox4 enhances vasodilation in mice.24 Aortas of Ldlr−/− mice released more H2O2 than wild-type mice. Compared with Ldlr−/−, Nox4−/−/Ldlr−/− double knockout mice showed significantly lower levels of H2O2. This indicates a specific role for Nox4-released H2O2 in Ldlr−/− mice. In support of this, we found a lower eNOS mRNA expression in Ldlr−/− mice as well as Nox4−/−/Ldlr−/− mice, which could explain the importance of H2O2 for vasorelaxation in these mice. H2O2 might act as an EDHF in mice and man.29–31 Endothelium-derived hyperpolarizing factors can open calcium-activated potassium channels leading to a hyperpolarized membrane of the vascular smooth muscle cell and subsequent vasorelaxation.37 Under pathological conditions of atherosclerosis and hypertension H2O2 can be involved in compensation of vasorelaxation in large vessels.27 In mice with DOCA-salt hypertension and uncoupled eNOS, endothelium-derived H2O2 compensated to maintain vasodilation.38 We observed an altered endothelial function in Ldlr−/− mice after application of BKCa channel inhibitor paxilline. The reduction was comparable with the endothelial dysfunction found in Nox4−/−/Ldlr−/− mice. This suggests that H2O2 in Ldlr−/− mice leads via opening of potassium channels to hyperpolarization-mediated dilation. Thereby, H2O2 compensates the loss of other vasodilator mechanisms. Furthermore, unlike superoxide, H2O2 does not further decrease bioavailability of NO. H2O2 is also described as increasing expression and activation of AKT/eNOS pathway.25,26 Our observation, that downregulation of NOX4 leads to less phosphorylation of pAKT1 (Ser473) under laminar shear stress, suggests a protective role of Nox4 generated H2O2 via this pathway.
We analysed the consequences of the endothelial dysfunction in Nox4−/−/Ldlr−/− mice by feeding a high-fat diet for 20 weeks. Plaque burden was significantly higher in the Nox4−/−/Ldlr−/− double knockout compared with Ldlr−/− mice. Inflammation marker Galectin-3 and collagen content was also increased in Nox4−/−/Ldlr−/− compared with Ldlr−/− mice. Therefore, we are the first to document a potential protective role of Nox4 in the background of hypercholesterolaemia.
The role of other Nox isoforms in atherosclerosis is controversial. Some studies using knockouts of subunits of the Nox2 complex showed anti-atherosclerotic effects21,39,40 while others did not.41 The non-selective Nox1/Nox4 inhibitor GKT137831 slightly reduced atherosclerotic plaque area in diabetic ApoE−/− mice.42 Furthermore, Nox1 deletion, but not Nox4, decreased vascular adhesion of leukocytes and expression of inflammation markers,43 indicating that the effects of inhibitor GKT137831 might mainly result from blocking Nox1. Nox4 expression was mainly upregulated in the atheroma stage of plaques, but downregulated in more advanced stages of atherosclerosis.44 For the NADPH oxidase inhibitor apocynin protective effects for the vascular function are described as well.45 Apocynin is described to inhibit Nox activity by blocking p47phox phosphorylation.14 Thereby, apocynin has a lower effect on the Nox4/p22phox complex than on Nox2 activity.18,46 Furthermore, apocynin has been suggested to be not a specific inhibitor of vascular Nox but rather an antioxidant.47 Therefore, the protective effects of apocynin observed in the study by Liang et al. might be more due to inhibition of Nox2 rather than Nox4.14
The importance of Nox4-released H2O2 might be emphasized by the fact that loss of Nox4 led to compensation of H2O2 release in 26-week-old Nox4−/− mice. This upregulation to a level found in wild-type mice was partially blocked by NOS inhibitor L-NAME. In agreement with these findings, nNOS mRNA expression was significantly elevated in the older Nox4−/− mice compared with wild-type mice. A role for nNOS in vascular function has been described.48 In eNOS knockdown mice, nNOS inhibition reduced H2O2 production and further reduced vasorelaxation.49
Finally, we analysed flow-mediated dilation in saphenous arteries of 26-week-old wild-type and Nox4−/− mice using OCT. We observed a significantly lower flow-mediated dilation in the Nox4−/− mice compared with wild-type mice. Although ex vivo analysis of vascular function in the aorta of Nox4−/− mice showed no difference in endothelium-dependent relaxation compared with wild-type mice, flow-mediated dilation in saphenous artery in the Nox4−/− mice was significantly reduced. This supports a much higher sensitivity of the in vivo method to objectify alterations in vessel function. While ex vivo vessel ring analyses are based on the effects of pharmacological stimuli, the OCT approach allows us to monitor the effects of endogenous mechanical and chemical stimuli. Furthermore, this novel approach allows measurements of vasodynamics of a blood vessel segment integrated in the complex anatomical and physiological vascular network. Our data show evidence for a role of Nox4-released H2O2 in vascular function of smaller arteries like the saphenous artery and vessels of microcirculation. Paravicini et al. showed a role for Nox-derived H2O2 in flow-induced cerebral vasodilation by performing cranial window preparation in rats.50 Catalase transgenic mice show impaired endothelium-dependent relaxation in mesenteric resistance arteries. These mice also revealed a reduction in blood flow recovery and capillary formation after hindlimb ischaemia and disorganized microvasculature.51 Significantly attenuated blood flow recovery was also described for Nox4−/− mice.33
In conclusion, Nox4 plays an important role under physiological and pathological conditions in maintaining endothelial function. In states of increased proatherosclerotic risk such as hypercholesterolemia, Nox4 can protect against endothelial dysfunction and atherosclerosis.
Authors’ contributions
H.L., C.B.: performed statistical analysis; C.B., S.R.B., A.D., E.K., H.M.: handled funding and supervision; H.L., C.B., M.B.: acquired the data; H.L., C.B., A.D., E.K., H.M.: conceived and designed the research; H.L., C.B., H.M.: drafted the manuscript; H.L., C.B., A.H., P.C., S.R.B., A.D., E.K., H.M.: made critical revision of the manuscript for key intellectual content.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (DFG) (Grant MO 1695/4-1 to H.M.), by funding of the Excellence Initiative by the German Federal and State Governments (Institutional Strategy, measure ‘support the best’ to H.M., E.K., and A.D.), Else Kröner-Fresenius-Stiftung (Grant 2010_A105 to H.M.) and German Federal Ministry of Education and Research (BMBF) (professorship of Vascular Endothelium and Microcirculation to H.M.). Funding to pay the Open Access publication charges for this article was provided by the University Hospital and the Research Committee of the Medical Faculty Carl Gustav Carus of the Technische Universität Dresden.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We are grateful to Ralf P. Brandes and Katrin Schröder for providing us with the Nox4−/− mice and Jennifer Mittag, Sindy Giebe, and Annika Frenzel for excellent technical support.
References
- 1. Authors/Task Force Members Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 2. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwoger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ, Guidelines ESCCfP. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–2926. [DOI] [PubMed] [Google Scholar]
- 3. Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation 2004;110:1926–1932. [DOI] [PubMed] [Google Scholar]
- 4. Heitzer T, Baldus S, von Kodolitsch Y, Rudolph V, Meinertz T. Systemic endothelial dysfunction as an early predictor of adverse outcome in heart failure. Arterioscler Thromb Vasc Biol 2005;25:1174–1179. [DOI] [PubMed] [Google Scholar]
- 5. Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, Hert SD, Ford I, Gonzalez-Juanatey JR, Gorenek B, Heyndrickx GR, Hoeft A, Huber K, Iung B, Kjeldsen KP, Longrois D, Luscher TF, Pierard L, Pocock S, Price S, Roffi M, Sirnes PA, Sousa-Uva M, Voudris V, Funck-Brentano C, Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383–2431. [DOI] [PubMed] [Google Scholar]
- 6. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 7. Task Force Memebers Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL, Guidelines ESCCfP. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 8. Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2014;15:736–746. [DOI] [PubMed] [Google Scholar]
- 9. Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 2006;291:H985–H1002. [DOI] [PubMed] [Google Scholar]
- 10. Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coronary Artery Dis 2014;25:713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morawietz H. Endothelial NADPH oxidases: friends or foes? Basic Res Cardiol 2011;106:521–525. [DOI] [PubMed] [Google Scholar]
- 12. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245–313. [DOI] [PubMed] [Google Scholar]
- 13. Muller G, Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal 2009;11:1711–1731. [DOI] [PubMed] [Google Scholar]
- 14. Drummond GR, Sobey CG. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol Metab 2014;25:452–463. [DOI] [PubMed] [Google Scholar]
- 15. Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med 2010;49:687–706. [DOI] [PubMed] [Google Scholar]
- 16. Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 2011;286:13304–13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 2008;45:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 2007;406:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 2010;56:490–497. [DOI] [PubMed] [Google Scholar]
- 20. Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 2005;112:2677–2685. [DOI] [PubMed] [Google Scholar]
- 21. Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol 2010;298:H24–H32. [DOI] [PubMed] [Google Scholar]
- 22. Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, Loffredo L. Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation 2009;120:1616–1622. [DOI] [PubMed] [Google Scholar]
- 23. Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 2008;52:1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 2011;31:1368–1376. [DOI] [PubMed] [Google Scholar]
- 25. Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 2000;86:347–354. [DOI] [PubMed] [Google Scholar]
- 26. Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 2002;277:6017–6024. [DOI] [PubMed] [Google Scholar]
- 27. Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res 2005;68:26–36. [DOI] [PubMed] [Google Scholar]
- 28. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999;399:601–605. [DOI] [PubMed] [Google Scholar]
- 29. Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 2003;92:e31–e40. [DOI] [PubMed] [Google Scholar]
- 30. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 2000;106:1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. [DOI] [PubMed] [Google Scholar]
- 32. Zhang M, Brewer AC, Schroder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 2010;107:18121–18126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schroder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 2012;110:1217–1225. [DOI] [PubMed] [Google Scholar]
- 34. Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 2010;8:e1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 2010;107:15565–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Touyz RM, Montezano AC. Vascular Nox4: a multifarious NADPH oxidase. Circ Res 2012;110:1159–1161. [DOI] [PubMed] [Google Scholar]
- 37. Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch 2010;459:915–922. [DOI] [PubMed] [Google Scholar]
- 38. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003;111:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 2001;108:1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol 2007;27:2714–2721. [DOI] [PubMed] [Google Scholar]
- 41. Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2000;20:1529–1535. [DOI] [PubMed] [Google Scholar]
- 42. Di Marco E, Gray SP, Chew P, Koulis C, Ziegler A, Szyndralewiez C, Touyz RM, Schmidt HH, Cooper ME, Slattery R, Jandeleit-Dahm KA. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune-inflammatory responses in diabetic Apoe(−/−) mice. Diabetologia 2014;57:633–642. [DOI] [PubMed] [Google Scholar]
- 43. Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JP, Touyz RM, Wingler K, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013;127:1888–1902. [DOI] [PubMed] [Google Scholar]
- 44. Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002;105:1429–1435. [DOI] [PubMed] [Google Scholar]
- 45. Liang CF, Liu JT, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol 2013;33:777–784. [DOI] [PubMed] [Google Scholar]
- 46. Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 1994;11:95–102. [DOI] [PubMed] [Google Scholar]
- 47. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008;51:211–217. [DOI] [PubMed] [Google Scholar]
- 48. Takaki A, Morikawa K, Tsutsui M, Murayama Y, Tekes E, Yamagishi H, Ohashi J, Yada T, Yanagihara N, Shimokawa H. Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J Exp Med 2008;205:2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Capettini LS, Cortes SF, Lemos VS. Relative contribution of eNOS and nNOS to endothelium-dependent vasodilation in the mouse aorta. Eur J Pharmacol 2010;643:260–266. [DOI] [PubMed] [Google Scholar]
- 50. Paravicini TM, Miller AA, Drummond GR, Sobey CG. Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol-3 kinase, NADPH-oxidase, and nitric oxide synthase. J Cereb Blood Flow Metab 2006;26:836–845. [DOI] [PubMed] [Google Scholar]
- 51. Urao N, Sudhahar V, Kim SJ, Chen GF, McKinney RD, Kojda G, Fukai T, Ushio-Fukai M. Critical role of endothelial hydrogen peroxide in post-ischemic neovascularization. PLoS ONE 2013;8:e57618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.