Abstract
Nationally, health care providers wrote 259 million prescriptions for narcotic analgesics in 2012, or roughly one bottle of narcotics per US adult (1). In an effort to combat this ever-growing problem, the Drug Enforcement Administration changed the schedule of hydrocodone combination products from schedule III to schedule II on October 6, 2014. Fourteen Baylor Scott & White pharmacies encompassing a 200-mile radius in Central Texas were queried for prescription information on hydrocodone/acetaminophen, morphine, codeine/acetaminophen, and tramadol before and after the rescheduling to evaluate trends in prescription drug usage. While the rescheduling of hydrocodone combination products resulted in a reduced number of prescriptions and the total quantity dispensed of both the hydrocodone/acetaminophen 5/325 mg (Norco 5/325) and 10/325 mg (Norco 10/325) formulations, this was offset by a dramatic increase in alternative narcotic analgesics such as tramadol, codeine/acetaminophen 30/300 mg (Tylenol #3), and codeine/acetaminophen 60/300 mg (Tylenol #4), which do not have schedule II requirements. Additionally, there was no significant reduction in total pain medication prescribed after converting all agents to morphine equivalents.
In 2011, the Centers for Disease Control and Prevention declared overdose from prescription narcotics to be an epidemic in the United States (1). In an attempt to reduce misuse, the Drug Enforcement Administration changed the schedule of hydrocodone combination products from schedule III to schedule II on October 6, 2014, resulting in significant changes in the prescribing, handling, and distribution of these drugs. Currently, there are at least 93 formulations of hydrocodone in combination with acetaminophen or ibuprofen marketed as either analgesics or cough suppressants in the United States. Of these combinations, hydrocodone/acetaminophen products are by far the most popular formulation and were the most frequently prescribed drug from 2007 to 2011 (1). Despite the almost universal acknowledgment of the growing prescription drug abuse epidemic in the US, many health care providers worried that changing hydrocodone combination products would only result in increased administrative tasks without a substantial decrease in overall opiate abuse and overdose (2). Our study hypothesized that rescheduling of hydrocodone combination products would lead to a decrease in prescriptions for hydrocodone combination products; however, this would be offset by increases in other schedule III narcotic prescriptions such as tramadol and codeine/acetaminophen formulations.
METHODS
Fourteen Baylor Scott & White pharmacies encompassing a 200-mile radius in Central Texas were queried for narcotic prescription information from July 2014 through January 2015. Our study focused on the most commonly prescribed hydrocodone combination products, namely the hydrocodone/acetaminophen 5/325 mg (Norco 5/325) and 10/325 mg (Norco 10/325) formulations. We also obtained prescription information on several schedule III narcotic medications, including tramadol, codeine/acetaminophen 30/300 mg (Tylenol #3), and codeine/acetaminophen 60/300 mg (Tylenol #4). Prescription information on oral morphine sulfate was obtained as a control.
Pharmaceutical data from July 2014 through September 2014 before the rescheduling were then compared to data from November 2014 through January 2015 after rescheduling to evaluate trends in prescription drug usage. Statistical analysis using the Poisson test of means was used to compare the number of prescriptions as well as the quantity of medication dispensed between these time periods. This test was done for each of the individual medications as well as the overall total. Statistical significance was indicated by a P value < 0.05. Each medication was then converted to its morphine equivalent, and the total quantity of morphine equivalents dispensed in the 3-month period before the rescheduling was compared to that in the 3-month period after rescheduling.
RESULTS
Statistical analysis of the number of prescriptions received for each medication illustrated a 17% increase in tramadol, 597% increase in Tylenol #3, and 1056% increase in Tylenol #4 after federal rescheduling of hydrocodone combination products. In contrast, there was a 58% reduction in Norco 5/325, 34% reduction in Norco 10/325, and no statistically significant change in morphine sulfate (Table 1).
Table 1.
Number of prescriptions for each medication before and after federal rescheduling of hydrocodone combination products
| Drug | Total before | Rate before (/month) | Total after | Rate after (/month) | Rate ratio | % change | P value |
|---|---|---|---|---|---|---|---|
| Tramadol | 4463 | 1488 | 5199 | 1733 | 1.17 | 17% | <0.0001 |
| Tylenol #3 | 278 | 93 | 1938 | 646 | 6.97 | +597% | <0.0001 |
| Tylenol #4 | 32 | 11 | 370 | 124 | 11.56 | +1056% | <0.0001 |
| Norco 5/325 | 4708 | 1570 | 1976 | 659 | 0.42 | −58% | <0.0001 |
| Norco 10/325 | 6260 | 2087 | 4128 | 1376 | 0.66 | −34% | <0.0001 |
| Morphine sulf | 528 | 176 | 520 | 174 | 0.98 | −2% | 0.8288 |
| Total | 16,269 | 5423 | 14,131 | 4711 | 0.87 | −13% | <0.0001 |
Further review of the quantity of medications dispensed illustrated a 42% reduction in the quantity of dispensed Norco 5/325, 14% reduction in Norco 10/325, and 7% reduction in oral morphine sulfate after federal rescheduling. During the same period, however, there was a 9% increase in tramadol, 122% increase in Tylenol #3, and 828% increase in Tylenol #4, resulting in a very modest 6% net decrease in total quantity of narcotic medications filled (Table 2 and Figure 1). Additionally, when all narcotic prescriptions were converted to morphine equivalents, there was only a small 3% reduction in equivalents dispensed after federal rescheduling (Figure 2).
Table 2.
Quantity of pills dispensed for each medication before and after federal rescheduling of hydrocodone combination products
| Drug | Total before | Rate before (/month) | Total after | Rate after (/month) | Rate ratio | % Change | P value |
|---|---|---|---|---|---|---|---|
| Tramadol | 347,368 | 115,790 | 379,658 | 126,553 | 1.09 | +9% | <0.0001 |
| Tylenol #3 | 39,913 | 11,305 | 88,458 | 29,486 | 2.22 | +122% | <0.0001 |
| Tylenol #4 | 2,639 | 880 | 24,486 | 8,162 | 9.28 | +828% | <0.0001 |
| Norco 5/325 | 243,292 | 81,098 | 141,221 | 47,044 | 0.58 | −42% | <0.0001 |
| Norco 10/325 | 534,505 | 178,169 | 459,933 | 153,311 | 0.86 | −14% | <0.0001 |
| Morphine sulf | 51,260 | 17,087 | 47,676 | 15,892 | 0.93 | −7% | <0.0001 |
| Total | 1,218,977 | 406,326 | 1,141,432 | 380,478 | 0.94 | −6% | <0.0001 |
Figure 1.
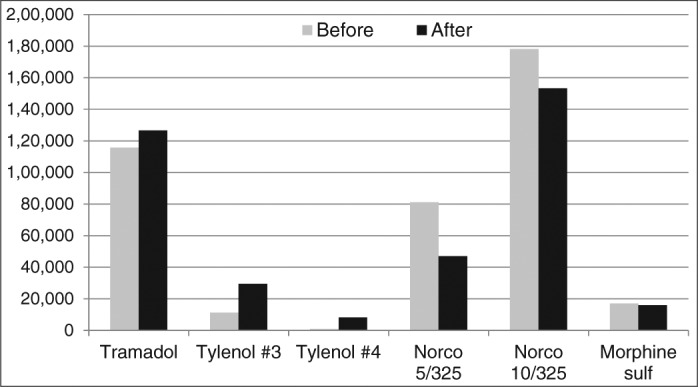
Average quantity of medications dispensed per month before and after rescheduling of hydrocodone combination products.
Figure 2.
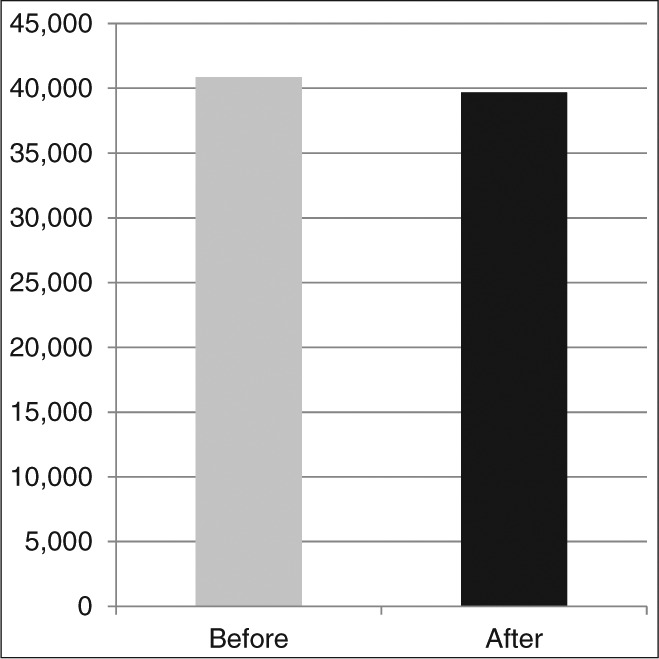
Total morphine equivalents dispensed before and after rescheduling of hydrocodone combination products.
DISCUSSION
While the rescheduling of hydrocodone combination products resulted in a reduced number of prescriptions for both the Norco 5/325 and Norco 10/325 formulations, this was offset by a dramatic increase in schedule III narcotic analgesics including tramadol, Tylenol #3, and Tylenol #4. With the significant rise in alternative prescriptions, there was only a slight change in the quantity of morphine equivalents prescribed before and after the federal rescheduling. Although schedule III medications are considered to have a lower potential for abuse than schedule II medications, many health care providers are less familiar with these medications and are therefore less equipped to handle potential side effects. Additionally, multiple studies have demonstrated an extreme variance in tramadol's effectiveness and side effects within the general population, with ultra-rapid CYP2D6 enzyme metabolizers having increased toxicity and slow CYP2D6 enzyme metabolizers having fewer analgesic effects (3).
Our study illustrates an important and evolving trend in narcotic prescription habits after federal rescheduling of hydrocodone combination products and highlights the need for further research on effective means for controlling prescription drug abuse in the United States. A recently released study demonstrated a modest decrease in opioid prescriptions following the initiation of Florida's Prescription Drug Monitoring Program, or so-called “pill mill laws.” Rather than increase legislation on specific medications, this legislation focused primarily on clinics with the highest rates of narcotic prescriptions per month. The new legislation required these clinics to register with the state, have a physician owner, and maintain an electronic, statewide database (4). Additionally, the Food and Drug Administration (FDA) introduced a number of other initiatives to discourage opioid abuse, including voluntary educational programs for providers on how to recognize abuse, funding for abuse-deterrent narcotic formulations, and packaging and labeling changes on current narcotic formulations (5).
Though prescription drug abuse is an undeniable problem in the United States, this study demonstrates several shortcomings of the federal rescheduling of hydrocodone combination products. While several other state and FDA regulations show promise, there is little published data regarding the effectiveness of specific drug policies (4). With approximately 44 deaths due to prescription drug overdose each day, increased research and a multifaceted approach are essential in combating the prescription drug abuse epidemic.
References
- 1.Traynor K. FDA advisers support rescheduling of hydrocodone products. Am J Health Syst Pharm. 2013;70(5):383–384. doi: 10.2146/news130017. [DOI] [PubMed] [Google Scholar]
- 2.Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II. Final rule. Fed Regist. 2014;79(163):49661–49682. [PubMed] [Google Scholar]
- 3.DePriest AZ. Puet BL. Holt AC. Roberts A. Cone EJ. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115–145. [PubMed] [Google Scholar]
- 4.Rutkow L. Chang HY. Daubresse M. Webster DW. Stuart EA. Alexander GC. Effect of Florida's prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern Med. 2015;175(10):1642–1649. doi: 10.1001/jamainternmed.2015.3931. [DOI] [PubMed] [Google Scholar]
- 5.Covvey JR. Recent developments toward the safer use of opioids, with a focus on hydrocodone. Res Social Adm Pharm. 2015;11(6):901–908. doi: 10.1016/j.sapharm.2015.02.001. [DOI] [PubMed] [Google Scholar]


