Abstract
VITAMIN B12 OR COBALAMIN DEFICIENCY occurs frequently (> 20%) among elderly people, but it is often unrecognized because the clinical manifestations are subtle; they are also potentially serious, particularly from a neuropsychiatric and hematological perspective. Causes of the deficiency include, most frequently, food-cobalamin malabsorption syndrome (> 60% of all cases), pernicious anemia (15%–20% of all cases), insufficent dietary intake and malabsorption. Food-cobalamin malabsorption, which has only recently been identified as a significant cause of cobalamin deficiency among elderly people, is characterized by the inability to release cobalamin from food or a deficiency of intestinal cobalamin transport proteins or both. We review the epidemiology and causes of cobalamin deficiency in elderly people, with an emphasis on food-cobalamin malabsorption syndrome. We also review diagnostic and management strategies for cobalamin deficiency.
Vitamin B12 or cobalamin deficiency occurs frequently among elderly patients,1 but it is often unrecognized or not investigated because the clinical manifestations are subtle. However, the potential seriousness of the complications (particularly neuropsychiatric and hematological)1,2,3,4 requires investigation of all patients who present with vitamin or nutritional deficiency. We summarize the current state of knowledge on cobalamin deficiency, with a particular focus on deficiency in elderly people.
In gathering information for this article, we systematically searched PubMed for articles published from 1990 to July 2003 (the search strategy is outlined in online Appendix 1 [www.cmaj.ca/cgi/content/full/171/3/251/DC1]). We have also included unpublished data from our working group, the Groupe d'étude des carences en vitamine B12 des Hôpitaux Universitaires de Strasbourg.
Defining cobalamin deficiency
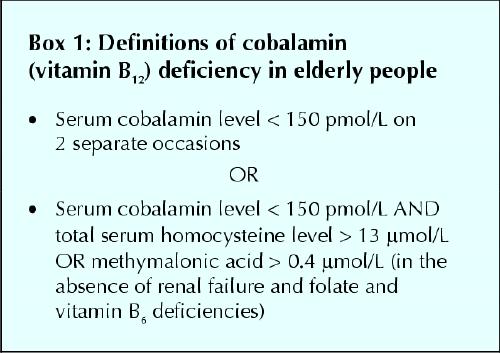
Cobalamin deficiency is defined in terms of the serum values of cobalamin and of homocysteine and methylmalonic acid, 2 components of the cobalamin metabolic pathway. High homocysteine levels (hyperhomocysteinemia) may also be caused by folate or vitamin B6 deficiencies, and these should be excluded as causes of cobalamin deficiency before a diagnosis is made. To obtain cutoff points of cobalamin serum levels, patients with known complications are compared with age-matched control patients without complications. Because different patient populations have been studied, several serum concentration definitions have emerged.5,6,7 Varying test sensitivities and specificities result from the lack of a precise “gold standard.” The definitions of cobalamin deficiency used in this review are shown in Box 1 Based in part on the work of Klee7 and in part on our own work,8 they are calculated for elderly patients. The first definition is simpler to interpret, but it requires that blood samples be drawn on 2 separate days.
Box 1.
New serum cobalamin (holotranscobalamin) assay kits have replaced older assay kits in most countries and should become the standard for testing.9
Epidemiology
Epidemiological studies show a prevalence of cobalamin deficiency of around 20% (between 5% and 60%, depending on the definition of cobalamin deficiency used in the study) in the general population of industrialized countries. The Framingham study demonstrated a prevalence of 12% among elderly people living in the community.10 Other studies focusing on elderly people, particularly those who are in institutions or who are sick, have suggested a higher prevalence: 30%–40%.11,12 However, these figures are questionable since they depend directly on normality thresholds selected by the authors. Using the definitions shown in Box 1, we found a prevalence of greater than 4.8% in a large group of patients in hospital between the ages of 65 and 98 (data submitted to the 47th Congress of the French National Society for Internal Medicine in Bordeaux, June 11–13, 1998).
Cobalamin metabolism and function
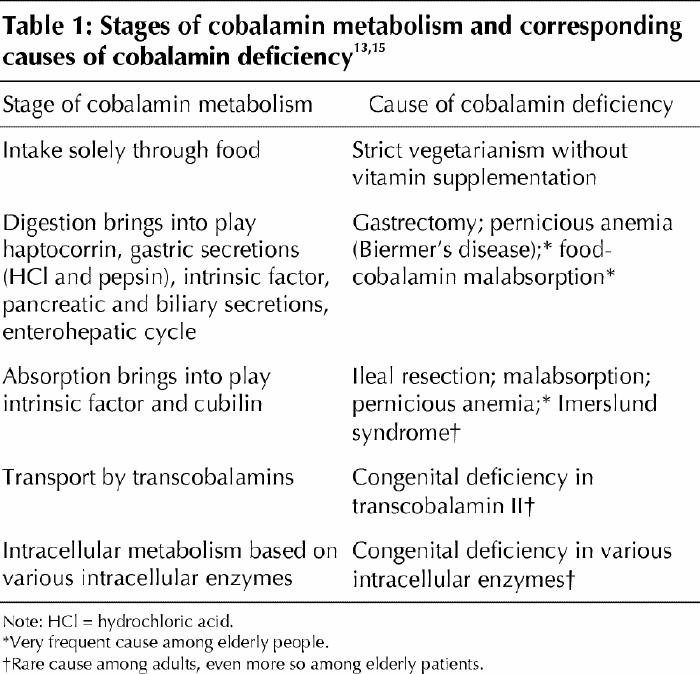
Cobalamin metabolism is complex and requires many processes, any one of which, if not present, may lead to cobalamin deficiency.4,13,14,15 The causes of cobalamin deficiency are shown in Fig. 1 and listed in Table 1.
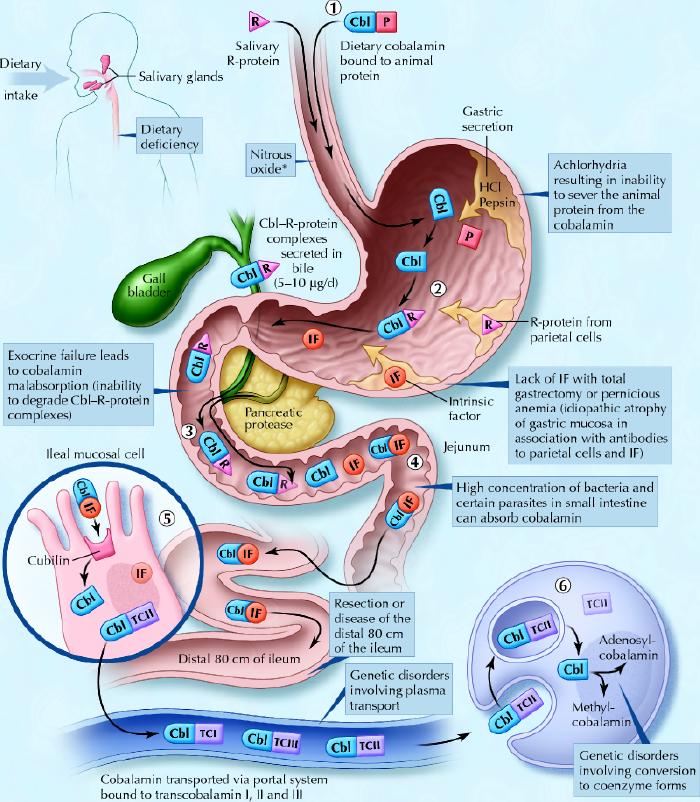
Fig. 1: Cobalamin metabolism and corresponding causes of deficiency. Causes of cobalamin deficiency are shown in blue. The metabolic pathway starts when (1) dietary cobalamin (Cbl), obtained through animal foods, enters the stomach bound to animal proteins (P). (2) Pepsin and hydrochloric acid (HCl) in the stomach sever the animal protein, releasing free cobalamin. Most of the free cobalamin is then bound to R-protein (R), which is released from the parietal and salivary cells. Intrinsic factor (IF) is also secreted in the stomach, but its binding to cobalamin is weak in the presence of gastric and salivary R-protein. (3) In the duodenum, dietary cobalamin bound to R-protein is joined by cobalamin–R-protein complexes that have been secreted in the bile. Pancreatic enzymes degrade both biliary and dietary cobalamin–R-protein complexes, releasing free cobalamin. (4) The cobalamin then binds with intrinsic factor. The cobalamin–intrinsic factor complex remains undisturbed until the distal 80 cm of the ileum, where (5) it attaches to mucosal cell receptors (cubilin) and the cobalamin is bound to transport proteins known as transcobalamin I, II and III (TCI, TCII and TCIII). Transcobalamin II, although it represents only a small fraction (about 10%) of the transcobalamins, is the most important because it is able to deliver cobalamin to all cells in the body. The cobalamin is subsequently transported systemically via the portal system. (6) Within each cell, the transcobalamin II–cobalamin complex is taken up by means of endocytosis and the cobalamin is liberated and then converted enzymatically into its 2 coenzyme forms, methylcobalamin and adenosylcobalamin (this process is shown in greater detail in Fig. 2).
*Nitrous oxide, a general anesthetic, causes multiple defects in cobalamin use, most of which are intracellular and clinically relevant only in people who have low or borderline-low serum cobalamin levels. Photo: Christine Kenney
Table 1
Once metabolized, cobalamin is a cofactor and coenzyme in many biochemical reactions, including DNA synthesis, methionine synthesis from homocysteine and conversion of propionyl into succinyl coenzyme A from methylmalonate, as shown in Fig. 2.
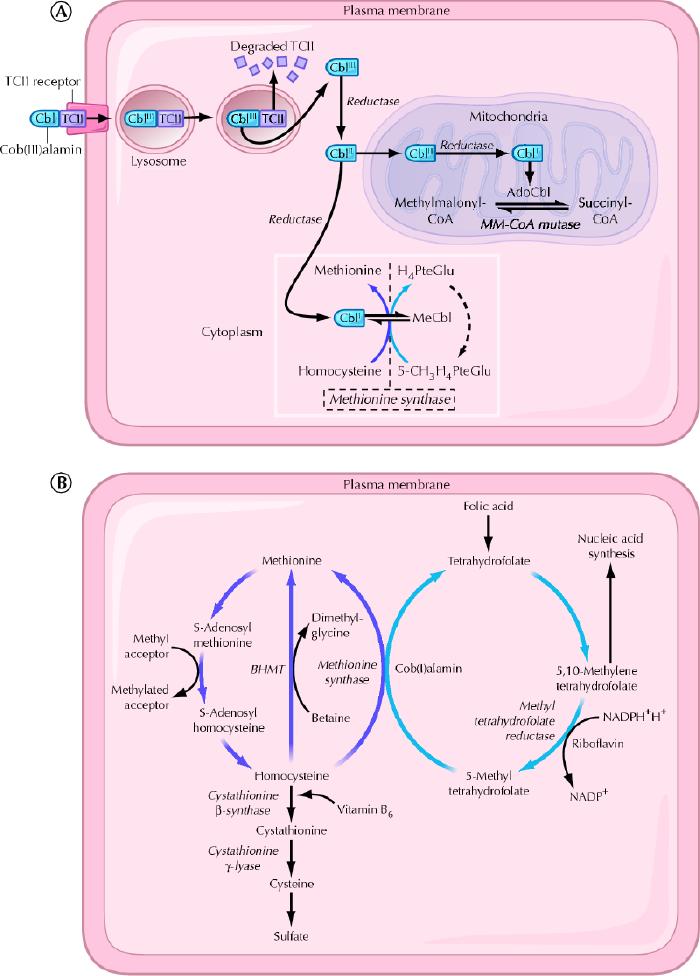
Fig. 2: A. Cellular uptake and processing of cobalamin. Cobalamin (Cbl) bound to the transport protein transcobalamin II (TCII) enters cells by means of transcobalamin II receptor-mediated endocytosis. Lysosomal enzymes degrade the transcobalamin II, thereby freeing the cobalamin. Cob(III)alamin (CBLIII) represents the most oxidized form of cobalamin, and cob(II)alamin (CBLII) and cob(I)alamin (CBLI) represent reduced forms. In the mitochondria, cobalamin is converted to adenosylcobalamin (AdoCbl), a coenzyme involved in the conversion of methylmalonyl-CoA (MM-CoA) to succinyl-CoA. In the cytoplasm, cobalamin functions as a coenzyme for the reaction catalyzed by methionine synthase. PteGlu = folic acid, MeCbl = methylcobalamin. B. Intracytoplasmic biochemical pathways involving cobalamin. BHMT = betaine-homocysteine S-methyltransferase, NADP = nicotinamide adenine dinucleotide phosphate, NADPH = reduced form of NADP. Photo: Christine Kenney
A typical Western diet contributes 3–30 μg of cobalamin per day toward the estimated daily requirement of 2–5 μg that is recommended by the American Society of Geriatry,16 the US Food and Drug Administration and the Association Française de Sécurité Sanitaire des Aliments. Reserves, which are primarily hepatic, are significant (> 1.5 mg). The 5- to 10-year delay between the onset of insufficient intake and the development of clinical illness is a direct result of the hepatic stores and the enterohepatic cycle, whereby cobalamin is excreted in bile and then reabsorbed in the small intestine (see Fig. 1).4,13 Between 1% and 5% of free cobalamin (and crystalline cobalamin) is absorbed along the entire intestine by passive diffusion, which explains the mechanism underlying oral treatment of deficiencies associated with pernicious anemia and food-cobalamin malabsorption.4,17,18
In a clinical setting, cobalamin absorption is examined (imperfectly) by the Schilling test.4,8 The test is currently performed as follows: patients are given 1000 μg of cyanocobalamin intramuscularly at day 1 to saturate the intestinal mucosal cells, followed by 1000 μg of free 58Cocyanocobalamin orally on day 2. Excess cobalamin, which is not absorbed, is excreted, and the patient's urine is collected for 24 hours (from day 2 to day 3) and the percentage of labelled cyanocobalamin is determined. Abnormally low levels of cobalamin in the collected urine indicate cases of malabsorption or pernicious anemia; normal levels indicate dietary deficiency, food-cobalamin malabsorption or hereditary cobalamin metabolism deficiencies.
Causes of cobalamin deficiency
In elderly patients, cobalamin deficiency is caused primarily by food-cobalamin malabsorption and pernicious anemia. Cobalamin deficiency caused by dietary deficiency or malabsorption (Fig. 1, Table 1) is rarer.1,8,11 In our studies, in which we followed a total of more than 200 patients with a proven cobalamin deficiency, food-cobalamin malabsorption accounted for about 60%–70% of the cases among elderly patients, and pernicious anemia accounted for 15%–20% of the cases.14,19,20,21 Other causes included dietary deficiency (< 5%), malabsorption (< 5%) and hereditary cobalamin metabolism diseases (< 1%).
Food-cobalamin malabsorption
First described by Carmel in 1995,22 food-cobalamin malabsorption syndrome is characterized by the inability to release cobalamin from food or from intestinal transport proteins (Table 1), particularly in the presence of hypochlorhydria, where the absorption of “unbound” cobalamin remains normal. As various studies have shown,14,22,23 this syndrome is defined by cobalamin deficiency in the presence of sufficient food-cobalamin intake and a negative Schilling test, where the latter rules out malabsorption or pernicious anemia (Box 2). In theory — because the test is rarely practical in clinical settings — the indisputable evidence of food-cobalamin malabsorption comes from using a modified Schilling test, which uses radioactive cobalamin bound to animal proteins (e.g., salmon, trout) and reveals malabsorption when the results of a standard Schilling test are normal.4,22
Box 2.

Food-cobalamin malabsorption is caused primarily by gastric atrophy. Over 40% of patients older than 80 years have gastric atrophy that may or may not be related to Helicobacter pylori infection.11,24 Other factors that contribute to food-cobalamin malabsorption in elderly people include chronic carriage of H. pylori and intestinal microbial proliferation (which can be caused by antibiotic treatment);25 long-term ingestion of biguanides (metformin)26,27 and antacids, including H2-receptor antagonists and proton pump inhibitors28,29 (particularly among patients with Zollinger–Ellison syndrome30); chronic alcoholism; surgery or gastric reconstruction (e.g., bypass surgery for obesity); partial pancreatic exocrine failure;4,14 and Sjögren's syndrome31 (Box 2).
Pernicious anemia
Pernicious anemia, or Biermer's disease, is a classic cause of cobalamin deficiency and one of the most frequent among elderly patients: 20%–50% of cases according to 2 studies32,33 and more than 15% in our patient series.14,19,20,21 Pernicious anemia is an autoimmune disease characterized by the destruction of the gastric mucosa, especially fundal mucosa, by a primarily cell-mediated process.34 Gastric secretions are neutral to slightly acidic even in the presence of gastrin (which normally increases acidity) and contain little or no intrinsic factor.13,32,34 The disease is also characterized by the presence of 2 antibodies, particularly in plasma and gastric secretions: few people who do not have the disease have antibodies (specificity 98%). However, only about 50% of patients with pernicious anemia will have anti-intrinsic factor antibodies (sensitivity 50%). Anti-gastric parietal cell antibodies, which target the H+/K+ adenosine triphosphatase alpha and beta subunits, can also be measured in the serum; sensitivity is higher (> 90%), but specificity is much lower (50%).32,34 Moderate hypergastrinemia, and sometimes major hypergastrinemia (levels of up to 4 to 8 times above normal), has also been associated with pernicious anemia. Owing to gastric atrophy with hypochlorhydria, patients have a feedback hypergastrinemia with hyperplasia of antral G cells. Hypergastrinemia is suggestive but not pathognomonic of pernicious anemia (sensitivity > 80%, specificity < 50%).32,33 A positive Schilling test (with the addition of a test for anti-intrinsic factor antibodies) virtually confirms the diagnosis (specificity > 99%).21,32
From a clinical perspective, pernicious anemia is associated with many autoimmune disorders, including vitiligo, dysthyroidia, Addison's disease and Sjögren's syndrome.21,32 It is also associated with an increased frequency of gastric neoplasms: adenocarcinomas, lymphomas and carcinoid tumours.32,33 Most experts recommend that patients with pernicious anemia undergo endoscopic surveillance every 3 to 5 years with multiple biopsies, even in the absence of macroscopic lesions.21 This practice has recently revealed the near absence of mucosal H. pylori in patients with the disease.21
Dietary deficiency
Intake or nutritional deficiency of cobalamin is rare among healthy adults in industrialized countries, even among elderly people: less than 5% in our experience.14 It is limited to rare instances of patients on strict vegetarian diets and people who are already malnourished, such as elderly patients, patients in institutions or patients in psychiatric hospitals.16,35 Studies of the dietary intake of elderly people in the United States have shown that up to 50% may have an insufficient intake of cobalamin (< 2 μg/d).16 Such studies, however, are extremely difficult to conduct because they rely mainly on dietary histories.8 Moreover, even if present, dietary deficiency does not result in symptomatic cobalamin deficiency until hepatic reserves are exhausted.
Cobalamin malabsorption
Gastrectomy and surgical resection of the terminal small intestine have been the most common causes of cobalamin malabsorption in elderly people.4,9 However, as we have shown, these causes have become rare (< 5%),14 owing mainly to the decreasing frequency of the operations. Total gastrectomies and most partial gastrectomies eliminate both the only source of intrinsic factor and, especially, gastric acidity. In the absence of gastric acidity, cobalamin malabsorption is associated with intraluminal microbial proliferation (or “blind loop syndrome”)13,14 and can be corrected with antibiotics.25
Other causes of cobalamin malabsorption that are rarely encountered in elderly people (< 2% in our practice)14 include disorders that result in damage to the last 80 cm of the small intestine mucosa, which is the site of elective cobalamin absorption: Crohn's disease, lymphomas, tuberculosis, amyloidosis, scleroderma, Whipple's disease, and even celiac disease,4,7 or ingestion of colchicine or chole styr a -mine.7,36 Agammaglobulinemia, AIDS (because of the associated microbial proliferation) and Diphyllobothrium infections may also cause cobalamin deficiency in elderly people.4
Even rarer, as we have reported,14 is deficiency in the exocrine function of the pancreas following chronic pancreatitis (which is usually caused by alcoholism) or a pancreatectomy.11,19,36
Hereditary cobalamin metabolism diseases
These hereditary diseases may cause deficiency in cubilin (as with Imerslund syndrome) or transcobalamin II and, more rarely, deficiency in intracellular enzymes that are involved in the signal transduction chain in cells, for example in methylating pathways.4,9 These deficiencies appear in newborns and therefore do not involve elderly patients.
Clinical manifestations
The primary clinical manifestations of cobalamin deficiency are described in Table 2 They are highly polymorphic and of varying severity, ranging from milder conditions, such as the common sensory neuropathy and isolated anomalies of macrocytosis and hypersegmentation of neutrophils, to severe disorders, including combined sclerosis of the spinal cord, hemolytic anemia and even pancytopenia.2,4,14,37
Table 2
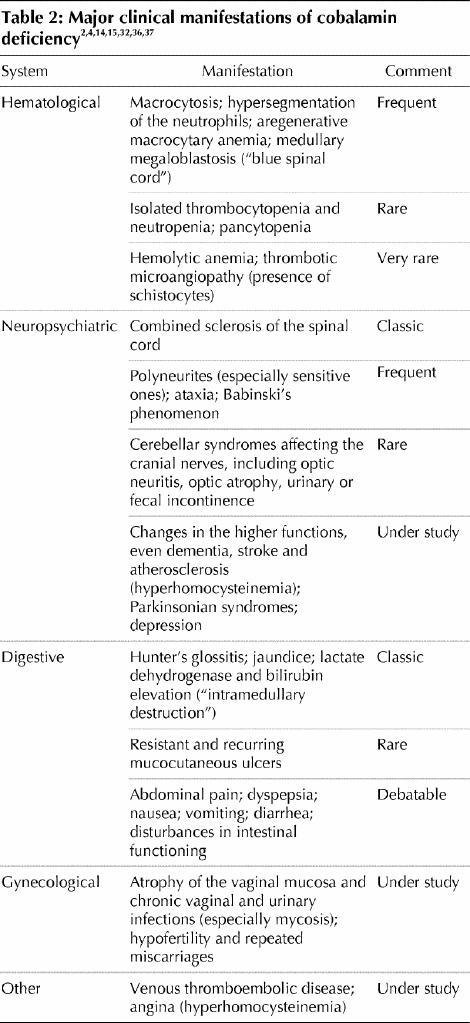
Among the classic manifestations are Hunter's glossitis, which causes the lingual papillae to atrophy, making the tongue look smooth and shiny, and neuroanemic syndrome. This syndrome includes combined sclerosis of the spinal cord and megaloblastic anemia with subacute combined degeneration of the spinal cord, which causes deep lemniscal sensory disturbances and pyramidal motor disturbances (resulting in advanced forms of spastic ataxia) that are possibly associated with cerebellar or sphincter disturbances.2,4
Typical neurological manifestions include polyneuritis (particularly sensory in the distal extremities), ataxia and positive Babinski reflexes. Cerebral syndromes, including optic neuritis, optic atrophy and urinary and fecal incontinence, are rarer.
Cobalamin deficiency appears to be more common among patients who have a variety of chronic neurological conditions such as dementia, Alzheimer's disease, stroke, Parkinson's disease and depression, although it is unclear if these are causal relationships.4,38 Our own work, in which we administered cobalamin to patients with dementia, did not result in any improvement.8 Other studies have shown similar results.18,39 At this time a causal role of cobalamin in these conditions remains speculative.
Cobalamin deficiency can go undetected for several years, during which time the neuropsychiatric manifestations may become irreversible (and worsen with folate supplementation).2,4,15,32,36
Diagnostic process
The diagnostic process for cobalamin deficiency is detailed in Fig. 3. This process is intended to avoid invasive, systematic or unnecessary explorations, such as a myelogram, which in occasional cases is the only way to rule out hemopathies. In the presence of megaloblastosis on the blood smear and equivocal cobalamin serum levels, the diagnosis can be securely made only by showing degeneration of the spinal cord.14
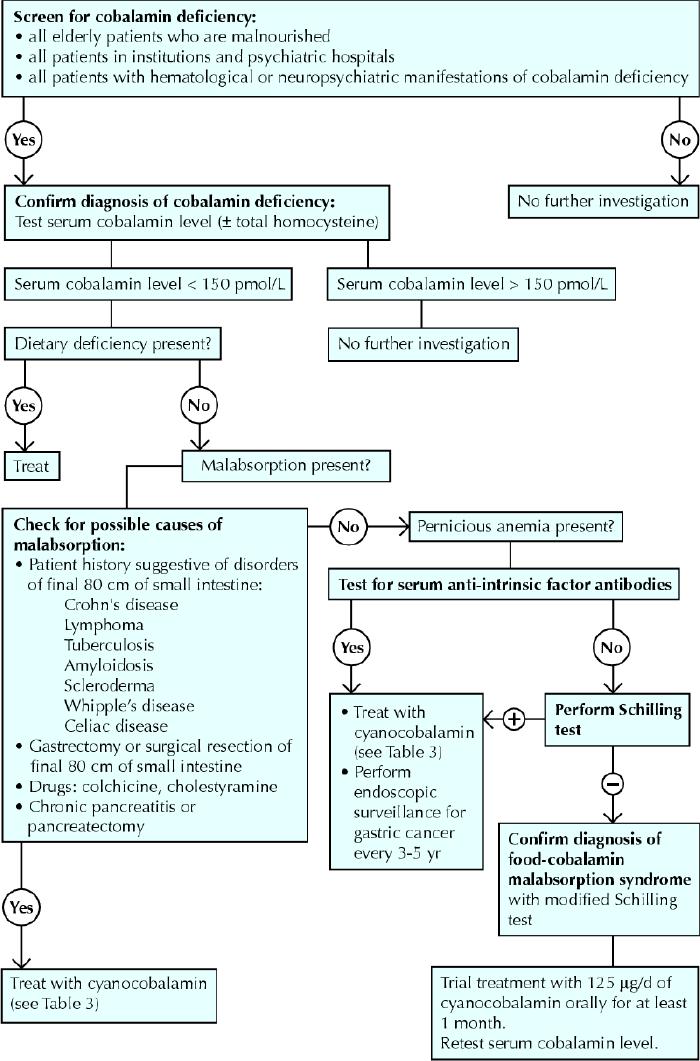
Fig. 3: Diagnostic process for cobalamin deficiency. Photo: Christine Kenney
All people over 65 years of age who are malnourished, all people in institutions or psychiatric hospitals, and all people with hematological or neuropsychiatric manifestations of cobalamin deficiency as reported in Table 2 should have their serum cobalamin levels measured.
Therapeutic management
The classic treatment of cobalamin deficiency, particularly when the cause is not dietary deficiency, has been parenteral administration — usually by intramuscular injection — of the vitamin (in the form of cyanocobalamin and, more rarely, hydroxocobalamin).1,17,18,32 In France, the recommended practice to build up the tissue stores of the vitamin quickly and correct serum cobalamin hypovitaminosis, particularly in the case of pernicious anemia, involves administration of 1000 μg of cobalamin per day for 1 week, followed by 1000 μg per week for 1 month and then by 1 injection of the same dose once per month, normally for the rest of the patient's life (Table 3).32,33 In other Western countries, dosages of 100–1000 μg per day are used.17
Table 3
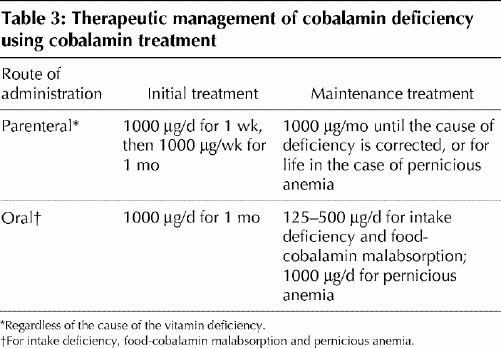
In cases other than those caused by nutritional deficiency, alternative routes of administration have recently been proposed: oral17,18,39,40,41,42 or nasal.43,44 Our working group has developed an effective oral treatment of food-cobalamin malabsorption40,41 and pernicious anemia45 using crystalline cobalamin (cyanocobalamin). However, until the procedure is validated, the following may be proposed: ongoing supplementation until associated disorders are corrected (e.g., by halting the ingestion of the offending medication or exogenosis, or by treating H. pylori infection or pancreatic exocrine failure), lifelong administration or, where applicable, sequential administration as shown in Table 3.46
Supplementary Material
Footnotes
This article has been peer reviewed.
Contributors: Emmanuel Andrès and Noureddine H. Loukili contributed to the conception and design of the study, collection, assembly of data and Internet search, interpretation and analysis of data and drafting and revisions of the article. Emmanuel Andrès and Anne E. Perrin conducted the statistical analysis of the studies we have conducted. All authors gave final approval of the version submitted to be published.
Competing interests: None declared.
Correspondence to: Prof. Emmanuel Andrès, Department of Internal Medicine, Medical Clinic B, Strasbourg University Hospitals, 1 place de l'Hôpital, 67 091 Strasbourg cedex, France; fax (3)33 88 11 62 62; emmanuel.andres@chru-strasbourg.fr
References
- 1.Matthews JH. Cobalamin and folate deficiency in the elderly. Baillères Clin Haematol 1995;54:245-53. [DOI] [PubMed]
- 2.Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood 1990;76:871-81. [PubMed]
- 3.Reynolds EH. Neurological aspects of folate and vitamin B12 metabolism. Clin Haematol 1976;5:661-96. [PubMed]
- 4.Carmel R. Current concepts in cobalamin deficiency. Annu Rev Med 2000;51: 357-75. [DOI] [PubMed]
- 5.Snow C. Laboratory Diagnosis of vitamin B12 and folate deficiency. A guide for the primary care physician. Arch Intern Med 1999;159:1289-98. [DOI] [PubMed]
- 6.Zittoun J, Zittoun R. Modern clinical testing strategies in cobalamin and folate deficiency. Semin Hematol 1999;36:35-46. [PubMed]
- 7.Klee GG. Cobalamin and folate evaluation: measurements of methylmalonic acid and homocystein vs vitamin B12 and folate. Clin Chem 2000;46:1277-83. [PubMed]
- 8.Andrès E, Perrin AE, Kraemer JP, Goichot B, Demangeat C, Ruellan A, et al. Anémies par carence en vitamine B12 chez le sujet âgé de plus de 75 ans: nouveaux concepts. À propos de 20 observations. Rev Med Interne 2000;21:946-55. [DOI] [PubMed]
- 9.Markle HV. Cobalamin. Crit Rev Clin Lab Sci 1996;33:247-356. [DOI] [PubMed]
- 10.Lindenbaum J, Rosenberg IH, Wilson PWF, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994;60:2-11. [DOI] [PubMed]
- 11.Pautas E, Chérin P, De Jaeger C, Godeau P. Carence en vitamine B12 chez le sujet âgé. Presse Med 1999;28:1767-70. [PubMed]
- 12.Van Asselt DZ, Blom HJ, Zuiderent R, Wevers RA, Jakobs C, van den Broek WJ, et al. Clinical significance of low cobalamin levels in older hospital patients. Neth J Med 2000;57:41-9. [DOI] [PubMed]
- 13.Nicolas JP, Guéant JL. Absorption, distribution et excrétion de la vitamine B12. Ann Gastroenterol Hepatol (Paris) 1994;30:270-6,281-2. [PubMed]
- 14.Andrès E, Perrin AE, Demangeat C, Kurtz JE, Vinzio S, Grunenberger F, et al. The syndrome of food-cobalamin malabsorption revisited in a department of internal medicine. A monocentric cohort study of 80 patients. Eur J Intern Med 2003;14:221-6. [DOI] [PubMed]
- 15.Swain R. An update of vitamin B12 metabolism and deficiency states. J Fam Pract 1995;41:595-600. [PubMed]
- 16.Russel RM. Vitamin requirements in old age. Age Nutrition 1992;3:20-3.
- 17.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood 1998;92: 1191-8. [PubMed]
- 18.Lane LA, Rojas-Fernandez C. Treatment of vitamin B12 deficiency anemia: oral versus parenteral therapy. Ann Pharmacother 2002;36:1268-72. [DOI] [PubMed]
- 19.Andrès E, Goichot B, Schlienger JL. Food-cobalamin malabsorption: a usual cause of vitamin B12 deficiency. Arch Intern Med 2000;160:2061-2. [DOI] [PubMed]
- 20.Andrès E, Kaltenbach G, Perrin AE, Kurtz JE, Schlienger JL. Food-cobalamin malabsorption in the elderly. Am J Med 2002;113:351-2. [DOI] [PubMed]
- 21.Loukili NH, Noel E, Blaison G, Goichot B, Kaltenbach G, Rondeau M, et al. Données actuelles sur la maladie de Biermer. A propos d'une étude rétrospective de 49 patients. Rev Med Interne. In press. [DOI] [PubMed]
- 22.Carmel R. Malabsorption of food-cobalamin. Baillieres Clin Haematol 1995; 8: 639-55. [DOI] [PubMed]
- 23.Andrès E, Noel E, Kaltenbach G, Perrin AE, Vinzio S, Goichot B, et al. Carences en vitamine B12 avec test de Schilling normal ou syndrome de non-dissociation de la vitamine B12 de ses protéines porteuses chez le sujet âgé. Étude de 60 patients. Rev Med Interne 2003;24:218-23. [DOI] [PubMed]
- 24.Carmel R, Aurangzeb I, Ojan D. Associations of food-cobalamin malabsorption with ethnic origin, age, Helicobacter pylori infection, and serum markers of gastritis. Am J Gastroenterol 2001;96:63-70. [DOI] [PubMed]
- 25.Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gulsen M, et al. Helicobacter pylori — Is it a novel causative agent in Vitamin B12 deficiency? Arch Intern Med 2000;160:1349-53. [DOI] [PubMed]
- 26.Bauman WA, Shaw S, Javatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 2000;23:1227-31. [DOI] [PubMed]
- 27.Andrès E, Noel E, Goichot B. Metformin-associated vitamin B12 deficiency. Arch Intern Med 2002;162:2251-2. [DOI] [PubMed]
- 28.Howden CW. Vitamin B12 levels during prolonged treatment with proton pump inhibitors. J Clin Gastroenterol 2000;30:29-33. [DOI] [PubMed]
- 29.Andrès E, Noel E, Ben Abdelghani M. Vitamin B12 deficiency associated with chronic acid suppression therapy. Ann Pharmacother 2003;37:1730. [DOI] [PubMed]
- 30.Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger–Ellison syndrome. Am J Med 1998;104:422-30. [DOI] [PubMed]
- 31.Andrès E, Goichot B, Perrin AE, Vinzio S, Demangeat C, Schlienger JL. Sjögren's syndrome: a potential new cause of mild cobalamin deficiency. Rheumatology(Oxford) 2001;40:1196-7. [DOI] [PubMed]
- 32.Lee GR, Herbert V. Pernicious anemia. In: Lee GR, Foerster J, Lukens J, Paraskevas F, Greer JP, Rodgers GM, editors. Wintrobe's clinical hematology. 10th ed. Philadelphia: Williams and Wilkins; 1999. p. 941-78.
- 33.Pruthi RK, Tefferi A. Pernicious anemia revisited. Mayo Clin Proc 1994; 69: 144-50. [DOI] [PubMed]
- 34.Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med 1997; 337: 1441-8. [DOI] [PubMed]
- 35.Herbert V. Staging vitamin B-12 (cobalamin) status in vegetarians. Am J Clin Nutr 1994;59:1213S-22S. [DOI] [PubMed]
- 36.Dharmarajan TS, Adiga GU, Norkus EP. Vitamin B12 deficiency. Recognizing subtle symptoms in older adults. Geriatrics 2003;58:30-8. [PubMed]
- 37.Andrès E, Noel E, Maloisel F. Hematological findings in the syndrome of food-cobalamin malabsorption. Am J Med 2003;115:592. [DOI] [PubMed]
- 38.Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging 2002; 6: 254-60. [PubMed]
- 39.Andrès E, Kaltenbach G. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging 2003;7:309-10. [PubMed]
- 40.Andrès E, Kaltenbach G, Noel E, Noblet-Dick M, Perrin AE, Vogel T, et al. Efficacy of short-term oral cobalamin therapy for the treatment of cobalamin deficiencies related to food-cobalamin malabsorption. A study of 30 patients. Clin Lab Haematol 2003;25:161-6. [DOI] [PubMed]
- 41.Andrès E, Kurtz JE, Perrin AE, Maloisel F, Demangeat C, Goichot B, et al. Oral cobalamin therapy for the treatment of patients with food-cobalamin malabsorption. Am J Med 2001;111:126-9. [DOI] [PubMed]
- 42.Elia M. Oral or parenteral therapy for B12 deficiency. Lancet 1998;352:1721-2. [DOI] [PubMed]
- 43.Slot WB, Merkus FW, Van Deventer SJ, Tytgat GN. Normalization of plasma vitamin B12 concentration by intranasal hydroxocobalamin in vitamin B12-deficient patients. Gastroenterology 1997;113:430-3. [DOI] [PubMed]
- 44.Van Asselt DZ, Merkus FW, Russel FG, Hoefnagels WH. Nasal absorption of hydroxocobalamin in healthy elderly adults. Br J Clin Pharmacol 1998; 45: 83-6. [DOI] [PMC free article] [PubMed]
- 45.Kaltenbach G, Noblet-Dick M, Barnier-Figue G, Berthel M, Kuntzmann F, Andrès E. Early normalization of low vitamin B12 levels by oral cobalamin therapy in three older patients with pernicious anemia. J Am Geriatr Soc 2002;50:1914-5. [DOI] [PubMed]
- 46.Andrès E, Noel E, Kaltenbach G. Comment: treatment of vitamin B12 deficiency anemia: oral versus parenteral therapy. Ann Pharmacother 2002;36: 1810. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


