Abstract
Hemoglobin SE disease was first described during the 1950s as a relatively benign microcytosis, but increasing prevalence has revealed a predisposition towards vasoocclusive sickling. Recognition of SE hemoglobinopathies’ potential complications is crucial so medical measures can be utilized to avoid multiorgan injury.
Microcytosis is a common finding on a peripheral blood smear that can reflect a variety of hematologic issues. We demonstrate the significance of identifying the etiology of a microcytosis by describing a patient whose underlying blood disorder resulted in multiorgan failure and death.
CASE PRESENTATION
A 52-year-old black woman with known chronic obstructive pulmonary disease, hepatitis C, and recurrent pulmonary embolism presented with a 3-day history of abdominal pain, chest discomfort, nonproductive cough, and dyspnea. Home medications included inhaled bronchodilators and rivaroxaban. Her blood pressure was 143/64 mm Hg; heart rate, 126 beats/minute; and respirations, 20 breaths/minute. Oxygen saturation was 91% on 5L nasal cannula. Her lungs were clear, and the physical exam was normal except for tenderness over the rectus abdominis. Laboratory results revealed normal electrolytes and liver enzymes but an elevated creatinine of 1.9 mg/dL and lactic acid of 3.2 mmol/L. Leukocytosis was present at 21 K/uL, with a platelet count of 122 K/uL. A microcytosis (68 fL) was noted with a hemoglobin of 13 g/dL. Chest radiograph and abdominal ultrasound disclosed cholelithiasis and a chronic right-sided pulmonary embolism. Broad-spectrum antibiotics, intravenous fluids, bronchodilators, and intravenous steroids were initiated.
Twelve hours later, the patient developed respiratory distress with coarse breath sounds. Blood gas after intubation revealed a pH of 7.43 and a partial pressure of oxygen of 58 (50% fraction of inspired oxygen). A repeat computed tomography scan did not show any new pulmonary emboli or infiltrates. Shortly afterwards, the patient entered pulseless electrical activity but recovered after cardiopulmonary resuscitation. Her hemoglobin rapidly decreased to 6 g/dL, requiring packed red blood cell support without any gross evidence of bleeding. The prothrombin and partial prothrombin time were normal with a fibrinogen level of 281 mg/dL. The patient required multiple vasopressors and continuous renal replacement therapy for worsening hyperkalemia. All cultures remained negative, and the patient went into asystole 26 hours after admission. Laboratory values revealed a hemoglobin of 11.8 g/dL and 1+ schistocytes but no sickled erythrocytes. Iron studies disclosed a serum iron level of 50 μg/dL, total iron binding capacity of 294 μg/dL, and ferritin level of 50 ng/mL.
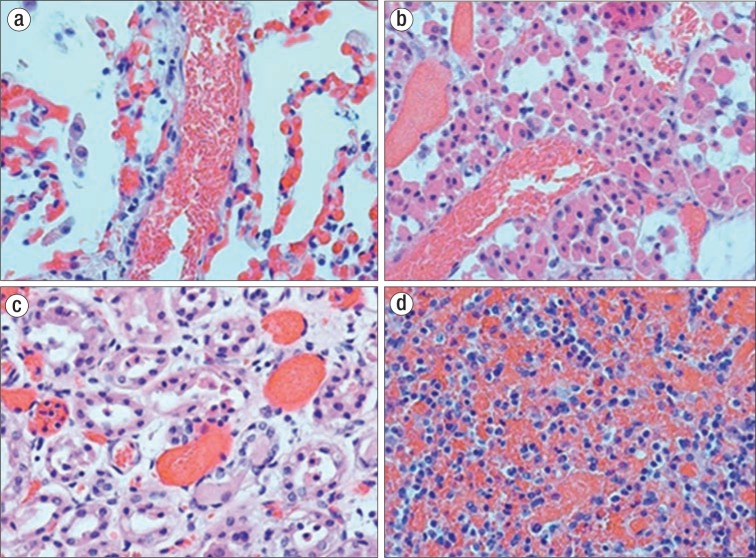
At autopsy, the pulmonary hilar and peripheral arteries were free of grossly apparent thromboemboli. On microscopic examination, the alveolar septal capillaries were congested, with small and larger vessels packed with sickled erythrocytes (Figure 1). In addition, sickled erythrocyte congestion was seen in the pituitary gland, liver, spleen, colon, stomach, and urinary system (Figure 1). Hemoglobin electrophoresis revealed 8% hemoglobin E, 25% hemoglobin S, 64% hemoglobin A, and 3% hemoglobin A2, consistent with a transfused patient with hemoglobin SE disease. It was concluded that this 52-year-old woman died of a cardiac arrhythmia secondary to chronic lung disease and vasoocclusion with intravascular ischemia.
Figure 1.
Sickling shown at autopsy. (a) Spleen: Microocclusive sickling within splenic sinusoids. (b) Lung: Microocclusive sickling within alveolar capillaries and septal vessels. (c) Kidney: Microocclusive sickling with acute tubular necrosis. (d) Pituitary gland: Disseminated microocclusive sickling.
Table 1.
Laboratory results
| Test | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Hemoglobin (g/dL) | 13.4 | 6.1, 9.2 | 11.8 |
| Hematocrit | 39% | 18%, 27% | 36% |
| Mean corpuscular volume (fL) | 66.7 | 65.9 | N/A |
| Schistocytes on peripheral smear | 1+ | 1+ | 1+ |
| Potassium (mmol/L) | 4.4 | 6.1, 7.6 | 8.3 |
| Lactic acid (mmol/L) | 5.7 | 3.2 | N/A |
DISCUSSION
Microcytic anemia is based on having a mean corpuscular volume <80 fL. Small erythrocytes are primarily related to abnormalities in the production of globin or the heme components of hemoglobin. This can result from iron deficiency, chronic inflammation, iron-resistant iron deficiency anemia, and sideroblastic anemia alpha/beta thalassemia (1). Thus, the differential for microcytosis is broad, but expansion is essential to include variant hemoglobinopathies.
Two of the world's most common variant hemoglobins are hemoglobin S and hemoglobin E. The amino acid substitution of glutamine for valine in the beta chain resulting in hemoglobin S is most prevalent in people of African, Caribbean, and South American descent. In contrast, the mutation in which lysine is substituted for glutamine at position 26 of the beta chain results in hemoglobin E (2). It is most common in the “hemoglobin E triangle” of Cambodia, Laos, and Thailand but is also present in the remainder of Southeast Asia. Patients who are homozygotes or heterozygotes for hemoglobin E are typically asymptomatic. Typical hemoglobin levels range from 10.5 to 12 g/dL, while mean corpuscular volumes range from 65 to 80 fL. The evolution of these hemoglobinopathies stems from resistance against malarial disease (3). Despite the geographical dissimilarities, population migrations and interracial relationships have resulted in the emergence of SE hemoglobinopathy. The first case was described in Turkey during 1957, but an increasing incidence of the disease has been documented (4). Hemoglobin electrophoresis demonstrates a hemoglobin S level of 62% ± 7% and a hemoglobin E level of 33% ± 4% (4). Although SE hemoglobinopathy is considered relatively benign, retrospective studies have revealed that patients with it develop complications of vasoocclusive sickling, including acute pain syndromes, avascular necrosis of long bones, splenic infarction, and sickle cell retinopathy (5).
Our patient presented with acute pain symptoms and microcytosis despite the absence of an anemia. Iron studies did not suggest iron deficiency. The patient's acute respiratory failure and hypoxia initiated her vasoocclusive sickling, which was demonstrated by sickled erythrocyte congestion in the lungs, liver, spleen, colon, stomach, and urinary system. The presence of microscopic pulmonary capillary obstruction due to sickle crisis explains the patient's imminent hypoxia and repeated cardiac arrests. Thus, recognition of SE disease's potential complications is crucial so that exchange transfusions can be utilized to avoid the risk of multiorgan injury.
References
- 1.DeLoughery TG. Microcytic anemia. N Engl J Med. 2014;371(14):1324–1331. doi: 10.1056/NEJMra1215361. [DOI] [PubMed] [Google Scholar]
- 2.Masiello D. Heeney MM. Adewoye AH. Eung SH. Luo HY. Steinberg MH. Chui DH. Hemoglobin SE disease: a concise review. Am J Hematol. 2007;82(7):643–649. doi: 10.1002/ajh.20847. [DOI] [PubMed] [Google Scholar]
- 3.Chotivanich K. Udomsangpetch R. Pattanapanyasat K. Chierakul W. Simpson J. Looareesuwan S. White N. Hemoglobin E: a balanced polymorphism protective against high parasitemias and thus severe P falciparum malaria. Blood. 2002;100(4):1172–1176. [PubMed] [Google Scholar]
- 4.Knox-Macaulay HH. Ahmed MM. Gravell D. Al-Kindi S. Ganesh A. Sickle cell-haemoglobin E (HbSE) compound heterozygosity: a clinical and haematological study. Int J Lab Hematol. 2007;29(4):292–301. doi: 10.1111/j.1365-2257.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 5.Vichinsky E. Hemoglobin E syndromes. Hematology (Am Soc Hematol Educ Program) 2007;12(4):79–83. doi: 10.1182/asheducation-2007.1.79. [DOI] [PubMed] [Google Scholar]