Abstract
A 37-year-old woman with known glioblastoma multiforme was admitted for treatment of new deep vein thrombosis. Anion gap and plasma lactate levels were found to be elevated. Magnetic resonance imaging of the brain showed a stable, advanced glioblastoma multiforme. All causes of lactic acidosis, including infections and medications, were ruled out. Aggressive tumors have been shown to produce lactate levels in minute quantities in their microenvironment, which helps them metastasize and evade immune response and even radiation.
Malignancy is a known cause of elevated lactate, although it is rarely recognized in nonhematologic cancers. We present a case of chronically elevated lactate levels in a patient with glioblastoma multiforme (GBM), a circumstance not reported previously.
CASE DESCRIPTION
A 37-year-old woman with GBM and a history of ventriculoperitoneal shunt, obesity, and glucocorticoid-induced diabetes mellitus was admitted for treatment of newly found deep vein thrombosis in her right leg. Except for leg swelling, the patient was asymptomatic. An anion gap of 20 was found, but her serum bicarbonate level was 25 mmol/L. The plasma lactate level was 5.5 mmol/L and ketones were negative. The blood urea nitrogen was 17 mg/dL and creatinine was 1 mg/dL. Liver function tests were also within normal limits. An arterial blood gas revealed a pH of 7.40, partial pressure of carbon dioxide of 42 mm Hg, oxygen saturation of 99%, bicarbonate level of 27 mmol/L, base excess of 1, and partial pressure of oxygen of 100 mm Hg on 2 L oxygen by nasal cannula. Her anion gap had been elevated for approximately a year. During the entire time her serum bicarbonate levels were within normal range. Serial lactate levels and arterial blood gas during the same hospital admission revealed persistently elevated lactate levels. Magnetic resonance (MR) imaging of the brain revealed the glioblastoma to be stable compared with prior imaging (Figure). She was not receiving chemotherapy for GBM due to the advanced stage of her disease; her treatment consisted of dexamethasone, acetaminophen/oxycodone, and newly started low-molecular-weight heparin for deep vein thrombosis. She died from her GBM.
Figure.
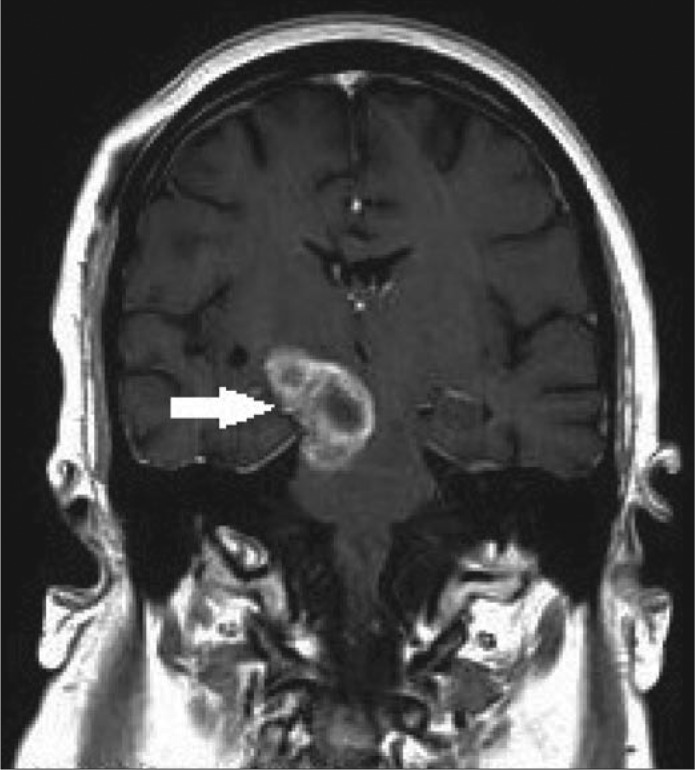
MRI of the brain showing glioblastoma multiforme in the right brain stem.
DISCUSSION
High lactate levels in malignancies are associated with poor prognosis (1). The pathogenesis of lactate production in tumor cells is not well understood (2). Tumorigenesis leads to alteration of metabolic switch in genes regulating glycolysis, such as PI3K, mTOR, KRAS, EGF, and HIF-1α, causing increased glucose uptake and lactate formation by cancer cells (1). This is caused by aerobic glycolysis or the Warburg effect. In 1925, Warburg showed that blood in veins from tumors had more lactate than the arteries feeding them (3) independent of glucose uptake or hypoxia (4). Several oncogenes and tumor suppressor genes are involved in the switch from oxidative phosphorylation to glycolysis. The phosphoinositide 3-kinase (PI3K)/AKT genetic pathway, which is a proto-oncogene, promotes the switch from oxidative phosphorylation to glycolysis. Similarly, antioncogenes such as p53/TIGAR have been shown to inhibit the Warburg effect, thus preventing tumor progression (5). Studies are investigating the role of PI3K-Akt-mTOR inhibitors as treatment for GBM (6). Although the Warburg effect is most commonly seen in hematologic malignancies, it has also been observed in solid malignancies (7).
Lactate is a powerful signaling molecule in tumor cells and has been shown to promote cell motility (8) and enhance cell migration (9). Increased lactate levels can induce resistance to radiation and chemotherapy (10). This resistance results from the antioxidant properties of lactate, which neutralize reactive oxygen species produced by ionizing radiation to induce DNA/RNA damage to tumor cells. Lactate also promotes tumor angiogenesis by inducing expression of vascular endothelial growth factor (11). In mouse models, intraperitoneal injections of lactate were able to increase metastasis and vascularity in an alveolar soft tissue sarcoma, specifically within the cranial vault, which contained the highest concentration of lactate (12). Lactate mediates immune escape by activating memory T cells (13), inhibiting monocytes (14), and inactivating cytokine release (8).
Thus far, there have been no reported cases of lactic acid production from a GBM significant enough to be detected in blood. However, noninvasive spectroscopic imaging modalities have shown that GBM does produce lactic acid in its microenvironment, which is associated with poor survival and advanced disease (15). The case discussed above describes an unusual picture of chronically elevated lactate without acidosis. All hypoxic and nonhypoxic causes of elevated lactate levels were eliminated, suggesting that the elevation was likely due to GBM. GBM cell lines have also been shown to overexpress lactate dehydrogenase A (LDHA), which is required for anaerobic glycolysis in normal cells but increases lactate production and the rate of glycolysis through the Warburg effect in cancer cells (16). Inhibition of LDHA promotes apoptosis, decreases cell growth, migration, and invasion, and is currently being investigated as a potential therapeutic target (17). More research is required to establish the role of LDHA in normal brain cells before such targets can be further investigated in GBM. Studies correlating MR spectroscopy and blood lactate levels in a cohort of GBM patients are needed to determine if LDHA can be used as a marker for tumor progression, prognosis, and response to therapy.
References
- 1.Brizel DM. Schroeder T. Scher RL. Walenta S. Clough RW. Dewhirst MW. Mueller-Klieser W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(2):349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 2.Andersen LW. Mackenhauer J. Roberts JC. Berg KM. Cocchi MN. Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O. Wind F. Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks GA. Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc. 1986;45(13):2924–2929. [PubMed] [Google Scholar]
- 5.Vander Heiden MG. Cantley LC. Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan QW. Weiss WA. Inhibition of PI3K-Akt-mTOR signaling in glioblastoma by mTORC1/2 inhibitors. Methods Mol Biol. 2012;821:349–359. doi: 10.1007/978-1-61779-430-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Imad T. El Khoury L. Geara AS. Warburg's effect on solid tumors. Saudi J Kidney Dis Transpl. 2014;25(6):1270–1277. doi: 10.4103/1319-2442.144266. [DOI] [PubMed] [Google Scholar]
- 8.Hirschhaeuser F. Sattler UG. Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71(22):6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 9.Goetze K. Walenta S. Ksiazkiewicz M. Kunz-Schughart LA. Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39(2):453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 10.Sattler UG. Meyer SS. Quennet V. Hoerner C. Knoerzer H. Fabian C. Yaromina A. Zips D. Walenta S. Baumann M. Mueller-Klieser W. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol. 2010;94(1):102–109. doi: 10.1016/j.radonc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Végran F. Boidot R. Michiels C. Sonveaux P. Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71(7):2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin ML. Jin H. Straessler K. Smith-Fry K. Zhu JF. Monument MJ. Grossman A. Randall RL. Capecchi MR. Jones KB. Modeling alveolar soft part sarcomagenesis in the mouse: a role for lactate in the tumor microenvironment. Cancer Cell. 2014;26(6):851–862. doi: 10.1016/j.ccell.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer K. Gottfried E. Kreutz M. Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60(3):425–431. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottfried E. Kunz-Schughart LA. Ebner S. Mueller-Klieser W. Hoves S. Andreesen R. Mackensen A. Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 15.Saraswathy S. Crawford FW. Lamborn KR. Pirzkall A. Chang S. Cha S. Nelson SJ. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J Neurooncol. 2009;91(1):69–81. doi: 10.1007/s11060-008-9685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J. Zhu S. Tong J. Hao H. Yang J. Wang Y. Suppression of lactate dehydrogenase A compromises tumor progression by downregulation of the Warburg effect in glioblastoma. Neuroreport. 2016;27(2):110–115. doi: 10.1097/WNR.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valvona CJ. Fillmore HL. Nunn PB. Pilkington GJ. The regulation and function of lactate dehydrogenase A: therapeutic potential in brain tumor. Brain Pathol. 2016;26(1):3–17. doi: 10.1111/bpa.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]


