Abstract
The use of exogenous proteins as intracellular probes and chemotherapeutic agents is in its infancy. A major hurdle has been the delivery of native proteins to an intracellular site of action. Herein, we report on a compact delivery vehicle that employs the intrinsic affinity of boronic acids for the carbohydrates that coat the surface of mammalian cells. In the vehicle, benzoxaborole is linked to protein amino groups via a “trimethyl lock”. Immolation of this linker is triggered by cellular esterases, releasing native protein. Efficacy is demonstrated by enhanced delivery of green fluorescent protein and a cytotoxic ribonuclease into mammalian cells. This versatile strategy provides new opportunities in chemical biology and pharmacology.
Graphical abstract
The delivery of proteins and other macromolecules to an intracellular site is made difficult by cellular membranes.1 Extensive efforts have led to the development of effective delivery systems that invoke cell-penetrating peptides,2–5 antibodies,6 ligands for natural receptors,7 dendrimers,8 functionalized polymers,9,10 liposomes,11 or nanoparticles.12,13 Extant strategies can, however, lead to adducts that are inapplicable in vivo, unstable in a physiological context, recalcitrant to biodegradation, or immunogenic.14
Boronic acids are physiologically benign Lewis acids that react spontaneously and reversibly with 1,2- and 1,3-diols to form five- and six-membered cyclic boronic esters, respectively.15,16 The dynamic covalent bonding of boronic acids/esters can facilitate the delivery of cargo into cells, which are coated with a diol-rich glycocalyx. To exploit that attribute, polymers, nanoparticles, and noncovalent assemblies have been decorated with phenylboronic acid and other arylboronic acids.17,18
Recently, we showed that boronic acids can be advantageous when conjugated directly to a protein.19 The ensuing formation of transient boronate esters with the glycocalyx enhances cellular delivery. To date, this approach has relied on the irreversible modification of the target protein, which can compromise activity20,19,10,21 or lead to immunogenicity.22,23 An ideal delivery system based on boronic acids (or any moiety) is “traceless” in its delivery of cargo.
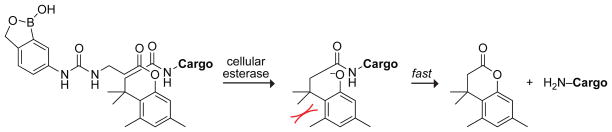
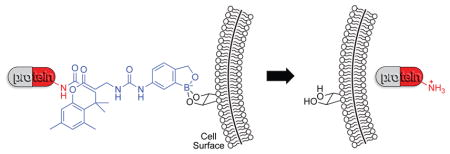
We sought to use a boronic acid and an immolative linker to promote the delivery of native proteins into a cell. As a boronic acid, we chose 2-hydroxymethylphenylboronic acid (benzoxaborole), which has higher affinity than does phenylboronic acid for the glycopyranosides that are abundant in the glycocalyx.24,19,18 As an immolative linker, we chose the o-hydroxydihydrocinnamic acid derivative known as the trimethyl lock (TML). After being triggered, the TML exhibits extremely high lactonization rates to release a cargo of interest (Scheme 1).25–29 The TML has been used for a wide variety of applications in chemistry and pharmacology,30 but not as an immolative linker on a protein. We chose ester hydrolysis as the means to trigger lactonization of the TML, as esterases are abundant inside, but not outside, of human cells31–33 and underlie the action of numerous prodrugs.34 We equipped our TML scaffold with an N-hydroxysuccinimide ester for chemoselective conjugation to amino groups,20 such as those at the N terminus and on the side chain of lysine residues, which have a ~6% abundance in proteins.35 Thus, our delivery vehicle (B-TML–NHS ester) has three modules: benzoxaborole, an esterase-activated TML linker, and an NHS ester (Figure 1A).
Scheme 1.
Figure 1.
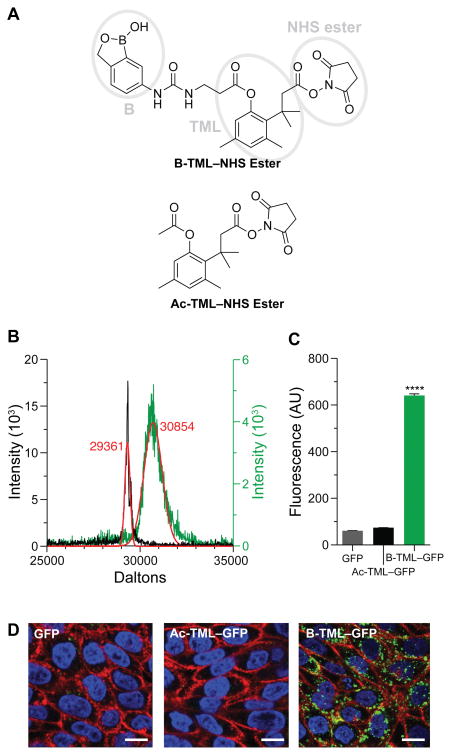
Cellular internalization of B-TML–labeled GFP. (A) Structures of B-TML–NHS ester and Ac-TML–NHS ester. Ellipses denote the three distinct modules within B-TML–NHS ester. (B) MALDI–TOF mass spectra of B-TML–GFP (green), conjugated to ~3 boronic acid moieties per molecule, and the same protein after exposure to CHO K1 cell lysate and purification (gray). Expected m/z: GFP, 29361; each B-TML moiety, 450. (C) Flow cytometry analysis of CHO K1 cells incubated with 10 μM unlabeled GFP, GFP labeled with a control vehicle (Ac-TML), or GFP labeled with the boronate vehicle (B-TML) for 4 h (p < 0.0001). (D) Confocal microscopy of CHO K1 cells grown as in panel C. Cells were stained with WGA-594 (red) and Hoechst 33342 (blue). Scale bars: 10 μm.
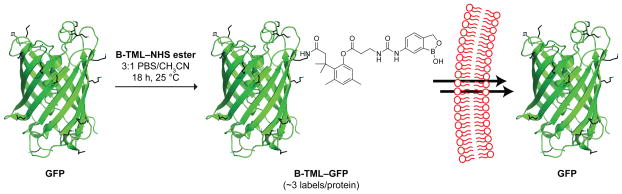
We synthesized B-TML–NHS ester convergently in 10 steps by extending a known procedure.36 Then, we characterized its ability to enhance the cellular internalization of a green fluorescent protein (GFP) (Scheme 2), which has distinctive fluorescence and an inability to enter mammalian cells.37 Overnight incubation at ambient temperature with 100-fold excess of B-TML–NHS ester in 3:1 PBS/acetonitrile yielded 3 ± 1 labels per protein (Figures 1B and S1). The number of labels in the B-TML–GFP conjugate did not decrease after a month of storage in PBS (Figure S2), consistent with the stability observed for other TML conjugates.38–40 Labeling was, however, “bioreversible”. Incubation with a lysate from Chinese hamster ovary (CHO) K1 cells removed all of the labels from B-TML–GFP (Figure 1B).
Scheme 2.
Next, we compared the uptake of B-TML–GFP and unlabeled GFP by CHO K1 cells. After a 4-h incubation, we observed a dramatic increase in the cellular uptake of B-TML–GFP (Figure 1C). The fluorescence in microscopy images was largely punctate, suggesting that B-TML–GFP was taken up via an endosomal pathway (Figure 1D). Co-localization of this bright punctate staining with a stain for transferrin was consistent with this conclusion (Figure S3). After a 24-h incubation, some cytosolic staining was observed, suggestive of endosomal escape (Figure S4).
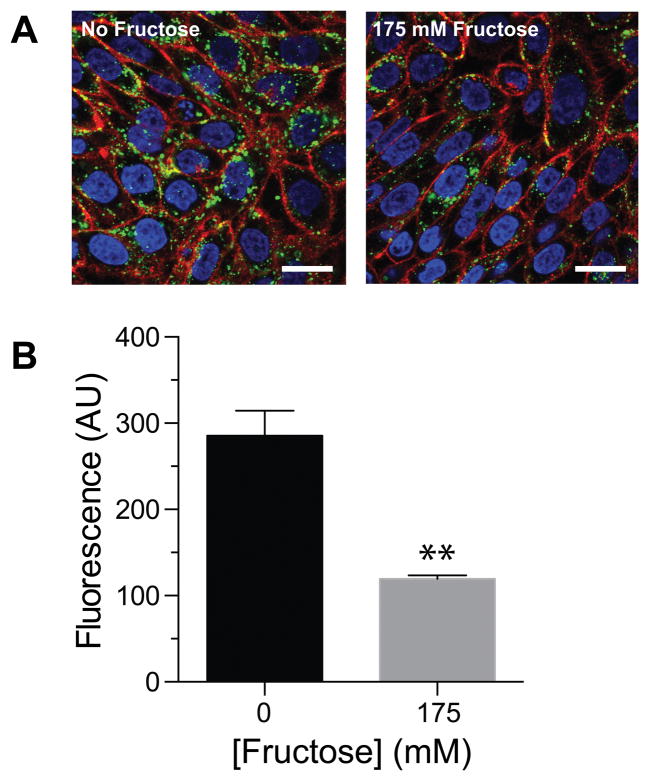
To confirm that the boronic acid moiety was responsible for the difference in cellular entry, we performed two control experiments. First, we modified GFP with a vehicle (Ac-TML–NHS ester) that lacks the benzoxaborole functionality (Figure 1A), yielding a level of labeling similar to that from B-TML–NHS ester (Figure S1). When incubated with cells for 4 h, Ac-TML–GFP was taken up comparably to unlabeled GFP rather than to B-TML–GFP (Figures 1C and 1D). These data indicate that the enhanced delivery upon treatment with B-TML–NHS ester is not due to the mere modification of lysine residues or to interactions with the TML portion of B-TML. Next, we repeated the cellular uptake experiments with B-TML–GFP in the presence of fructose, which has a Ka of 336 M−1 for benzoxaborole.19 We observed a significant decrease in GFP uptake in the presence of fructose, apparent with both confocal microscopy and flow cytometry (Figures 2A and 2B). Again, these data indict the boronic acid portion of B-TML–GFP as being responsible for cellular uptake.
Figure 2.
Effect of fructose on the cellular internalization of B-TML–labeled GFP. (A) Confocal microscopy of B-TML–GFP (10 μM) preincubated with PBS or 175 mM fructose for 30 min, then used to treat CHO K1 cells for 4 h. Cells were stained with WGA-594 (red) and Hoechst 33342 (blue). Scale bars: 20 μm. (B) Flow cytometry analysis of CHO K1 cells treated as in panel A (p < 0.01).
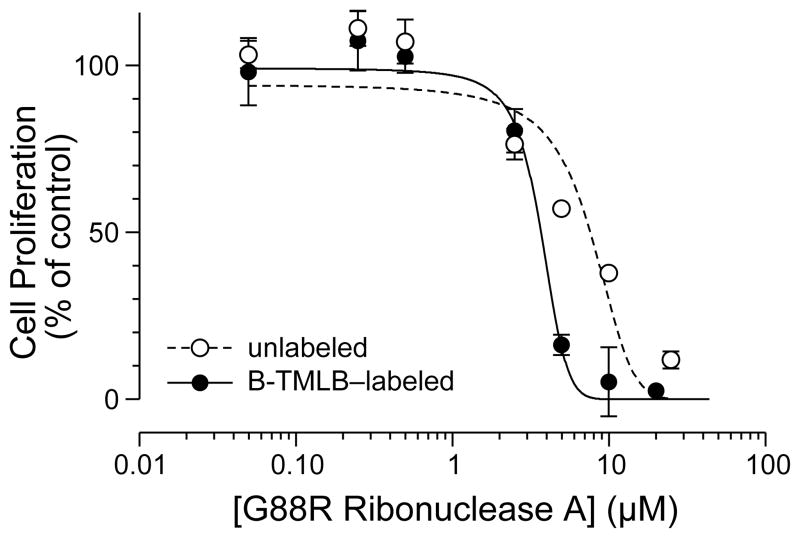
Finally, we sought to test the efficacy of B-TML as a delivery vehicle to the cytosol. To do so, we employed an enzymic cytotoxin—the G88R variant of ribonuclease A, which must reach cytosolic RNA to manifest its toxic activity.41,42 After labeling the ribonuclease by the same procedure used to label GFP, we observed an average of 1.6 ± 0.7 labels per molecule of protein (Figure S5). This lower labeling is consistent with GFP (19 lysine residues) having more amino groups than does the ribonuclease (12 lysine residues). Again, we found that the labeling was bioreversible, as incubation with a CHO K1 cell lysate removed all of the labels (Figure S6). Finally, we assayed the ability of B-TML–ribonuclease and unlabeled ribonuclease to inhibit the proliferation of K-562 cells, which are derived from a human myelogenous leukemia line. We found that the pendant boronic acids resulted in a decrease in the IC50 value (Figure 3), indicative of more cytotoxin reaching the cytosol.
Figure 3.
Effect of B-TML–labeling on the inhibition of K-562 cell proliferation by a ribonuclease. Unlabeled G88R ribonuclease A, IC50 = (6.4 ± 0.1) μM; B-TML–labeled G88R ribonuclease A, IC50 = (3.5 ± 0.8) μM. Each data point represents the mean ± SE for three separate experiments, each performed in duplicate.
We conclude that covalent modification of proteins with B-TML–NHS ester can increase their ability to enter mammalian cells. Importantly, this modification is traceless, as cellular esterase activity restores the proteins to their unmodified state. This bioreversibility of our delivery vehicle provides new opportunities. The sulfhydryl groups of cysteine residues have long been used for this purpose because their mixed disulfides suffer reduction within the cytosol.43 Recently, we found that appropriately tuned diazo compounds can esterify protein carboxyl groups, providing a second type of bioreversible modification.44,45 In this work, we report on a bioreversible modification of protein amino groups that is distinct from others46–48 in its reliance on enzymatic catalysis. With its traceless utility in promoting cellular uptake, B-TML–NHS ester provides new opportunities in chemical biology and pharmacology.
Supplementary Material
Acknowledgments
We are grateful to Dr. Ismet Çağlar Tanrikulu (University of Wisconsin–Madison) for technical advice. K.A.A. was supported by a predoctoral fellowship from the PhRMA Foundation and Molecular and Cellular Pharmacology Training Grant T32 GM008688 (NIH). J.E.L. was supported by a National Science Foundation Graduate Research Fellowship. This work was supported by grants R01 GM044786 and R01 CA073808 (NIH), and made use of the National Magnetic Resonance Facility at Madison, which is supported by Grant P41 GM103399 (NIH), and the University of Wisconsin Carbone Cancer Center, which is supported by Grant P30 CA014520 (NIH).
Footnotes
The authors declare no competing financial interest.
Procedures for the synthesis of B-TML–NHS ester and Ac-TML–NHS ester, their use in protein modification, analyses of cellular internalization, and Figures S1–S6. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fu A, Tang R, Hardie J, Farkas ME, Rotello VM. Promises and pitfalls of intracellular delivery of proteins. Bioconjugate Chem. 2014;25:1602–1608. doi: 10.1021/bc500320j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fonesca SB, Pereira MP, Kelley SO. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev. 2009;61:953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Milletti F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov Today. 2012;17:850–860. doi: 10.1016/j.drudis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Bechara C, Sagan S. Cell-penetrating peptides: 20 Years later, where do we stand? FEBS Lett. 2013;587:1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Brock R. The uptake of arginine-rich cell-penetrating peptides: Putting the puzzle together. Bioconjugate Chem. 2014;25:863–868. doi: 10.1021/bc500017t. [DOI] [PubMed] [Google Scholar]
- 6.Sievers EL, Senter PD. Antibody–drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015;14:203–219. doi: 10.1038/nrd4519. [DOI] [PubMed] [Google Scholar]
- 8.Cloninger MJ. Biological applications of dendrimers. Curr Opin Chem Biol. 2002;6:742–748. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]
- 9.Chu DSH, Schellinger JG, Shi J, Convertine AJ, Stayton PS, Pun SH. Application of living free radical polymerization for nucleic acid delivery. Acc Chem Res. 2012;45:1089–1099. doi: 10.1021/ar200242z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, Leroux JC, Gauthier MA. Releasable conjugation of polymers to proteins. Bioconjugate Chem. 2015;26:1172–1181. doi: 10.1021/bc500611k. [DOI] [PubMed] [Google Scholar]
- 11.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Chou LYT, Ming K, Chan WCW. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 13.Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2015;10:1–13. doi: 10.3109/1061186X.2015.1051049. [DOI] [PubMed] [Google Scholar]
- 14.Onoue S, Yamada S, Chan HK. Nanodrugs: Pharmacokinetics and safety. Int J Nanomed. 2014;9:1025–1037. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch LI, Fyles TM, James TD. Binary and ternary phenylboronic acid complexes with saccharides and Lewis bases. Tetrahedron. 2004;60:11175–11190. [Google Scholar]
- 16.Peters JA. Interactions between boric acid derivatives and saccharides in aqueous media: Structures and stabilities of resulting esters. Coord Chem Rev. 2014;268:1–22. [Google Scholar]
- 17.Ma R, Shi L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Polym Chem. 2014;5:1503–1518. [Google Scholar]
- 18.Adamczyk-WoŸniak A, Blrys KM, Sporzyński A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem Rev. 2015;115:5224–5247. doi: 10.1021/cr500642d. [DOI] [PubMed] [Google Scholar]
- 19.Ellis GA, Palte MJ, Raines RT. Boronate-mediated biologic delivery. J Am Chem Soc. 2012;134:3631–3634. doi: 10.1021/ja210719s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baslé E, Joubert N, Pucheault M. Protein chemical modification on endogenous amino acids. Chem Biol. 2010;17:213–227. doi: 10.1016/j.chembiol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Boutureira O, Bernardes GJ. Advances in chemical protein modification. Chem Rev. 2015;115:2174–2195. doi: 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- 22.De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99 doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bérubé M, Dowlut M, Hall DG. Benzoboroxoles as efficient glycopyranoside-binding agents in physiological conditions: Structure and selectivity of complex formation. J Org Chem. 2008;73:6471–6479. doi: 10.1021/jo800788s. [DOI] [PubMed] [Google Scholar]
- 25.Milstien S, Cohen LA. Concurrent general-acid and general-base catalysis of esterfication. J Am Chem Soc. 1970;92:4377–4382. [Google Scholar]
- 26.Danforth C, Nicholson AW, James JC, Loudon GM. Steric acceleration of lactonization reactions: An analysis of “stereopopulation control”. J Am Chem Soc. 1976;98:4275–4281. [Google Scholar]
- 27.Wang B, Nicolaou MG, Liu S, Borchard RT. Structural analysis of a facile lactonization system facilitated by a “trimethyl lock”. Bioorg Chem. 1996;5:39–49. [Google Scholar]
- 28.Nicolaou MG, Wolfe JL, Schowen RL, Borchard RT. Facilitated intramolecular conjugate addition of amides of 3-(3″,6″-dioxo-2″,4″-dimethyl-1″,4″-cyclohexadienyl)-3,3-dimethylpropionic acid. 2. Kinetics of degradation. J Org Chem. 1996;61:6633–6638. doi: 10.1021/jo961069l. [DOI] [PubMed] [Google Scholar]
- 29.Jung ME, Piizzi G. gem-Disubstituent effect: Theoretical basis and synthetic applications. Chem Rev. 2005;105:1735–1766. doi: 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]
- 30.Levine MN, Raines RT. Trimethyl lock: A trigger for molecular release in chemistry, biology, and pharmacology. Chem Sci. 2012;3:2412–2420. doi: 10.1039/C2SC20536J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- 32.Lavis LD. Ester bonds in prodrugs. ACS Chem Biol. 2008;3:203–206. doi: 10.1021/cb800065s. [DOI] [PubMed] [Google Scholar]
- 33.Fukami T, Yokoi T. The emerging role of human esterases. Drug Metab Pharmacokinet. 2012;27:466–477. doi: 10.2133/dmpk.dmpk-12-rv-042. [DOI] [PubMed] [Google Scholar]
- 34.Testa B, Mayer JM. Hydrolysis in Drug and Prodrug Metabolism: Chemistry, Biochemistry, and Enzymology. Verlag Helvetica Chimica Acta; Zürich, Switzerland: 2003. [Google Scholar]
- 35.McCaldon P, Argos P. Oligopeptide biases in protein sequences and their use in predicting protein coding regions in nucleotide sequences. Proteins. 1988;4:99–122. doi: 10.1002/prot.340040204. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald RB, Choe YH, Conover CD, Shum K, Wu D, Royzen M. Drug delivery systems based on trimethyl lock lactonization: Poly(ethylene glycol) prodrugs of amino-containing compounds. J Med Chem. 2000;43:475–487. doi: 10.1021/jm990498j. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs SM, Raines RT. Arginine grafting to endow cell permeability. ACS Chem Biol. 2007;2:167–170. doi: 10.1021/cb600429k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavis LD, Chao T-Y, Raines RT. Fluorogenic label for biomolecular imaging. ACS Chem Biol. 2006;1:252–260. doi: 10.1021/cb600132m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson RJ, Chao TY, Lavis LD, Raines RT. Cytotoxic ribonucleases: The dichotomy of Coulombic forces. Biochemistrry. 2007;46:10308–10316. doi: 10.1021/bi700857u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turcotte RF, Lavis LD, Raines RT. Onconase cytotoxicity relies on the distribution of its positive charge. FEBS J. 2009;276:4270–4281. doi: 10.1111/j.1742-4658.2009.07098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leland PA, Schultz LW, Kim BM, Raines RT. Ribonuclease A variants with potent cytotoxic activity. Proc Natl Acad Sci USA. 1998;95:10407–10412. doi: 10.1073/pnas.95.18.10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomax JE, Eller CH, Raines RT. Rational design and evaluation of mammalian ribonuclease cytotoxins. Methods Enzymol. 2012;502:273–290. doi: 10.1016/B978-0-12-416039-2.00014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbert HF. Molecular and cellular aspects of thiol–disulfide exchange. Adv Enzymol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 44.McGrath NA, Andersen KA, Davis AKF, Lomax JE, Raines RT. Diazo compounds for the bioreversible esterification of proteins. Chem Sci. 2015;6:752–755. doi: 10.1039/c4sc01768d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mix KA, Raines RT. Optimized diazo scaffold for protein esterification. Org Lett. 2015;17:2358–2361. doi: 10.1021/acs.orglett.5b00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixon HBF, Perham RN. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968;109:312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M, Sun S, Neufeld CI, Perez-Ramirez B, Xu Q. Reactive oxygen species-responsive protein modification and its intracellular delivery for targeted cancer therapy. Angew Chem Int Ed. 2014;63:13444–13448. doi: 10.1002/anie.201407234. [DOI] [PubMed] [Google Scholar]
- 48.Cal PM, Frade RF, Cordeiro C, Gois PM. Reversible lysine modification on proteins by using functionalized boronic acids. Chemistry. 2015;21:8182–8187. doi: 10.1002/chem.201500127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.