Abstract
Here we establish a neotype for Alatina alata (Reynaud, 1830) from the Dutch Caribbean island of Bonaire. The species was originally described one hundred and eighty three years ago as Carybdea alata in La Centurie Zoologique—a monograph published by René Primevère Lesson during the age of worldwide scientific exploration. While monitoring monthly reproductive swarms of A. alata medusae in Bonaire, we documented the ecology and sexual reproduction of this cubozoan species. Examination of forty six A. alata specimens and additional archived multimedia material in the collections of the National Museum of Natural History, Washington, DC revealed that A. alata is found at depths ranging from surface waters to 675 m. Additional studies have reported it at depths of up to 1607 m in the tropical and subtropical Atlantic Ocean. Herein, we resolve the taxonomic confusion long associated with A. alata due to a lack of detail in the original description and conflicting statements in the scientific literature. A new cubozoan character, the velarial lappet, is described for this taxon. The complete description provided here serves to stabilize the taxonomy of the second oldest box jellyfish species, and provide a thorough redescription of the species.
Keywords: Neotype, Carybdea alata, Carybdeida, cubomedusae, box jellyfish, Atlantic Ocean, aggregation, sexual reproduction, deep-sea, taxonomy
Introduction
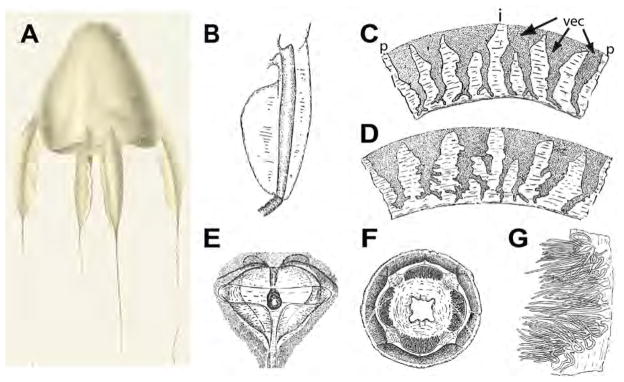
The winged box jellyfish Alatina alata (Cnidaria: Cubozoa: Carybdeida: Alatinidae) has appeared in the scientific literature (as Carybdea alata) many times (see Gershwin 2005 for list of citations) since its original description (by Reynaud) and illustration (by Prêtre; reproduced herein as Fig 1a) appeared in the extensive monograph by French naturalist René Primevère Lesson in 1830. Reynaud’s brief description gave no details about the collection events or the whereabouts of the specimen (or possibly multiple specimens), stating only that this box jellyfish “lives in the Atlantic Ocean”. Almost a century later, Bigelow (1918, 1938) redescribed A. alata (as C. alata) (original drawings reproduced herein as Fig 1b–g) from Jamaica and Cuba, effectively clarifying its identity in the Atlantic Ocean and the associated Gulf of Mexico and Caribbean Sea, where the name has since been in common usage (see additional reports by Arneson (1976) from Puerto Rico, Morandini (2003) from Brazil, Graham (1998, as C. alata var. grandis) and Larson et al. (1991) from the Gulf of Mexico). In the last decade, nine nominal species from various disparate localities around the world formerly recognized under the name C. alata (see Mayer 1910; Bigelow 1938) were revived within the newly established genus Alatina Gershwin 2005. As a result, Carybdea alata, under the new combination Alatina alata (Gershwin 2005), became the oldest available name within the genus.
FIGURE 1.
Line drawings of A. alata reproduced from Reynaud (1830) and Bigelow (1938) (as Carybdea alata). A. Whole body showing bell, tentacles and pedalia (reproduced from Reynaud (1830). B–G, Reproduced from Bigelow (1938). B. Pedalium, C. Velarial canals (BH=70 mm), D. Velarial canals (BH=90 mm), E. Rhopalial niche with T–shaped opening, F. Bell apex showing crescentric gastric phacellae, G. Dissected gastric phacella (comprising gastric cirri). Abbreviations: i=interradius (location of pedalium), p=perradius (location of frenulum), vec=velarial canal (three per quadrant, simple to branching).
With this work, we aim to stabilize the species name A. alata because it is the oldest species within its genus, the original description lacks detail, and no type material exists. Following a thorough examination of live and preserved material from several Atlantic localities, we provide a detailed redescription of A. alata and establish a neotype from a population in Bonaire, Dutch Caribbean. A. alata forms monthly aggregations at this locality, making regular collection and in situ observations feasible. We also report on its ovoviviparous mode of sexual reproduction, and bathymetric and geographic distribution in the Atlantic Ocean. This study sets the groundwork for future studies of Alatina species, which are notorious for their painful stings (see Yoshimoto & Yanagihara 2002 for detailed list of symptoms related to the debilitating Irukandji syndrome). Furthermore, Alatina is poised to emerge as a key cnidarian model organism, with the mitochondrial genome recently characterized (Kayal et al. 2012; Smith et al. 2012), and a nuclear genome assembly currently underway (Genbank accession PRJNA167165 and PRJNA41627).
Methods
Sampling location (from De Meyer 1997)
Bonaire is a small crescent–shaped island (approximately 40 km by 11 km, oriented NW to SE) surrounded by fringing coral reefs. It lies outside of the hurricane belt, but the exposed eastern side (windward) of the island experiences rough water conditions, in contrast to the protected western side (leeward) where only moderate swells occur. On the leeward side, the reef starts in the upper littoral zone and continues to a depth of 12 m where a steep slope of 20–50° leads to a flat at 25–55 m; and a second drop off descends to 250 m. Currents are slight with the predominate current moving northward on the leeward shore. Water temperatures range from 26–28 °C, salinity from 34–36 ppt. Maximum annual tide range is about 1 m, and the average range is 0.30 m during a lunar cycle.
Collection and lab culture
In 2008 the authors began documenting monthly swarms of A. alata medusae on the leeward side of the island of Bonaire (The Netherlands) during an initiative to describe the box jellyfish taxa of Bonaire (see also Collins et al. 2011). On June 24–25, 2011 (20:00–23:00), eight and nine days after the full moon respectively, live A. alata medusae were collected close to the surface about 25 m from shore off Karel’s Pier (Kralendijk, Bonaire) using a hand–held net (medusae were attracted to the lights around the perimeter of the pier). Males and females were put together in a bucket of seawater (~5 individuals per bucket). Subsequently (23:00–01:00), an additional 100 individuals that were stranded along the shore of Playa Lechi Beach were collected using gallon–sized Ziploc bags. Stranded medusae had truncated tentacles, diminished swimming ability, and disassociated pieces of opaque gonad material circulating in the gastro–vascular cavity. All medusae were transported to the Bonaire Research Station of The Council on International Educational Exchange (CIEE) several hours after collection. Blastulae were exuded from the manubria of the females, and became planulae within several hours. Planulae were reared for several days in glass bowls filled with filtered seawater in a climate–controlled room at approximately 27°C in the lab at CIEE. Live medusae were placed in an acrylic and silicone aquarium tank filled with filtered seawater, and photographed. Eleven adult medusae (collected off Karel’s Pier) were preserved in 8% Formalin for deposit at the National Museum of Natural History (NMNH) in Washington, D.C. To supplement our observations on live and preserved material from Bonaire, we examined a total of forty six museum specimens in the collection at the NMNH, as well as a video and photo material that we identified as A. alata. Using oil immersion light microscopy at 600× and 1000× magnification on live specimens (for methods see Yanagihara et al. 2002) and preserved specimens (for methods see Bentlage and Lewis 2012), the cnidome of A. alata was characterized.
Abbreviations
National Museum of Natural History, Smithsonian Institution catalogue number=USNM; BH=bell height, measured from tip of bell to velarial turnover; BW=bell width, the distance measured between two adjacent rhopalia.
Results
Systematics
Phylum Cnidaria Verrill, 1865
Subphylum Medusozoa Peterson, 1979
Class Cubozoa Werner, 1973
Order Carybdeida Gegenbaur, 1856
Family Alatinidae Gershwin, 2005
Genus Alatina Gershwin, 2005
Species Alatina alata (Reynaud, 1830)
TABLE 1.
| Body Part | Nematocyst Type | Length (μm) min–mean–max | SD | Width (μm) min–mean–max | SD | n | Fig No. |
|---|---|---|---|---|---|---|---|
| Tentacle tip | oval, heterotrichus microbasic p–euryteles | 19.6–24.5–30 | 3.59 | 10–12.58–20 | 2.45 | 49 | Fig 5a–e |
| Tentacle base | oval, heterotrichus microbasic p–euryteles | 20–23.7–30 | 4.71 | 10–11.8–20 | 3.49 | 16 | Fig 5a–e |
| Tentacle tip | small birhopaloids | 10.5–13.4–15.1 | 1.79 | 9.3–12.2–15.5 | 2.74 | 5 | Fig 5f |
| Tentacle base | small birhopaloids | 10–11.4–15.2 | 2.21 | 10–10.4–12.4 | 0.8 | 13 | Fig 5f |
| Bell warts | large, spherical holotrichous, isorhizas | 28.1–29.5–31 | 0.99 | 27.8–29.8–31.7 | 1.29 | 6 | Fig 5g–h |
| Velarial lappet warts | large, spherical holotrichous, isorhizas | 22.7–26.9–29.7 | 1.8 | 23.4–26.8–28.9 | 1.8 | 11 | Fig 5g–h |
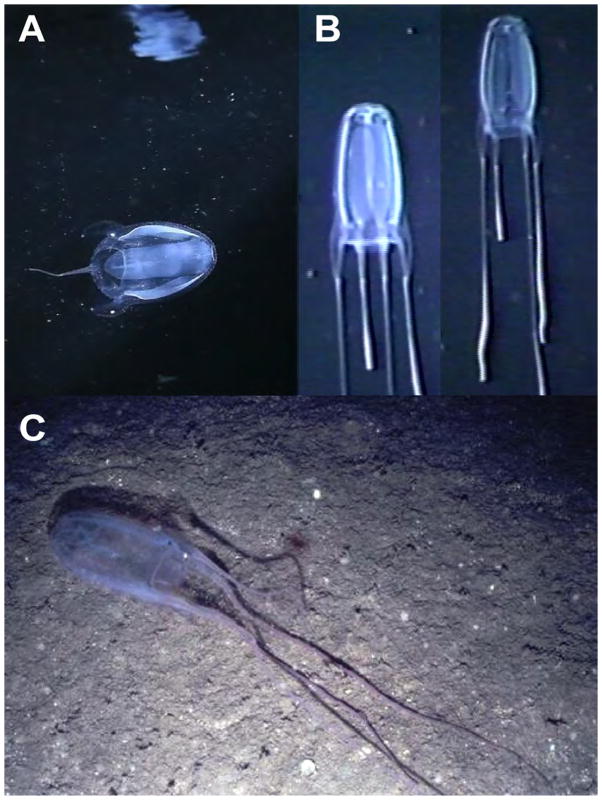
FIGURE 6.
Alatina alata in situ images. A. A. alata at a depth of 2 m from Kralendijk, Bonaire, The Netherlands. Reflection on the underside of the surface of the water seen above the medusa (frame grab from video by D. Karamehmedovic & A. Yanagihara). B. Series of digital frame grabs taken from video footage of a mature A. alata medusa actively ascending in the water column; filmed at a depth of 500–540 m, West off Gorda Cay, Bahamas, from Johnson Sea Link I manned submersible (video voucher USNM 1195809). B. Photograph of A. alata medusa oriented parallel to the ocean bottom, photographed by the Remotely Operated Vehicle SeaROVER at a depth of about 100 m in the Gulf of Mexico (USNM 1005621). No specimen was collected as a museum voucher for multimedia material.
Carybdea (medusa) alata
Reynaud, 1830 (in Lesson 1830, pl. 33, Fig 1a)
Marsupialis alata
Lesson, 1837, p. 9, n. 26; Lesson, 1843, p. 278
Tamoya alata
Agassiz, 1862, p. 174
Charybdea alata
Haeckel 1880, p. 441; p. 42; 1940a, p. 5
Carybdea alata
Mayer 1910, p. 508–510; Bigelow, 1918, p. 400; 1938, pp. 144–151, Text–Figs 11–16; Kramp, 1961, p. 304; Arneson 1976, pp. 36, Figs 1,2, Table 1,2, pl. I–V; Arneson and Cutress, 1976, pp. 227–236, Table 1, pl. I A–G; Cutress, 1971, p. 19, pl. 1; Larson, 1976, pp. 242; Larson et al. 1991, p. 313, Table 2; Humann & Deloach, 2002; Morandini, 2003, p. 15–17, Fig. 2; Gershwin 2005 pp. 501–523; Calder 2009, pp. 12, 13, Fig. 1; Bentlage 2010, p. 52; Bentlage et al. 2010, p. 498; Bentlage and Lewis, 2012, p. 2602
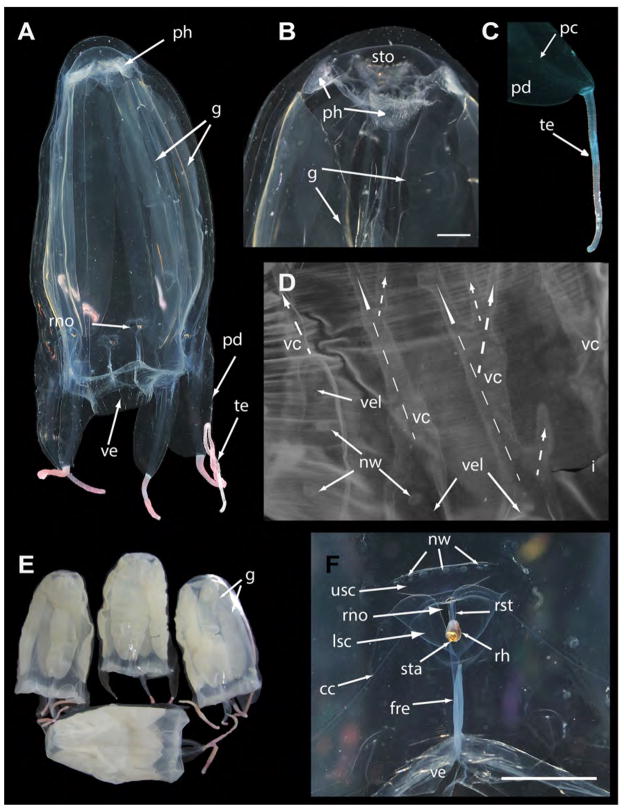
FIGURE 2.
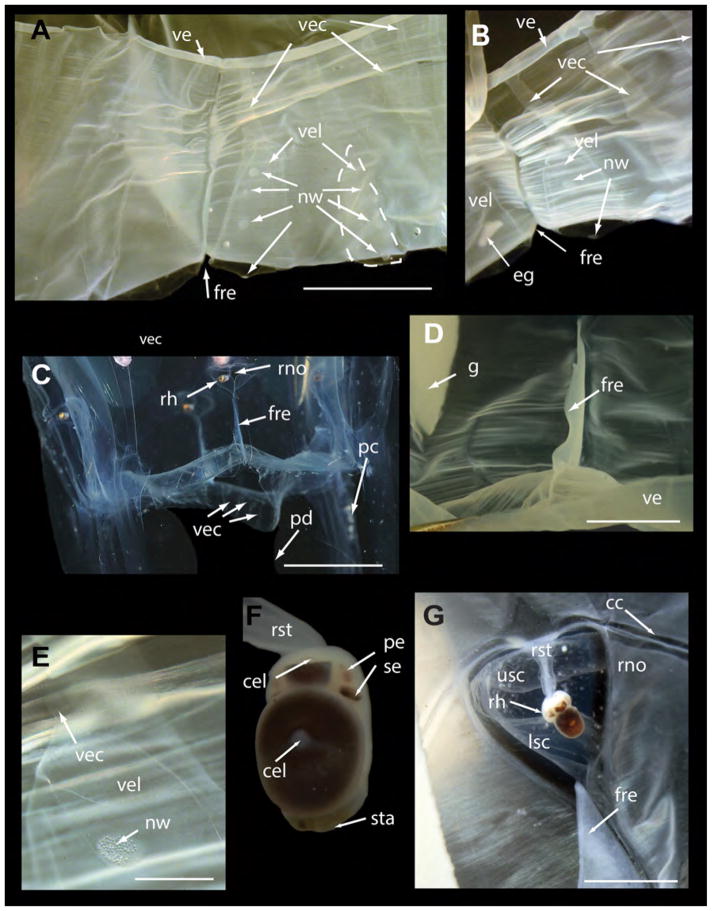
A. alata neotype (USNM 1195802 unless otherwise specified) from Kralendijk, Bonaire, The Netherlands. A. Mature female medusa (live), whole body (live, BW=40, BH=70). B. Apical portion showing stomach and crescentric gastric phacellae, visible in each corner, and wide central manubrium opening into subumbrella in live medusa. C. Pedalium and tentacle of live medusa. D. Velarial canals (3 per octant) in preserved medusa: each bears a row of 3 nematocyst warts on proximal first half to two thirds (on the velarial lappets) (dashed arrows indicate the main three velarial canals and branches extending from the main base of each canal). E. USNM 1195803–1195806, preserved mature medusae from the same locality as the neotype. F. T–shaped rhopalial niche opening with nematocyst warts on upper and lower scale coverings in live medusa. Live photographs by T. Peters (Fish Eye Photography, Bonaire) & A. Yanagihara. Abbreviations: cc=circular canal, fre=frenulum, g=gonads, i=interradius, lsc=lower scale of rhopalial niche covering, nw=nematocyst wart, ph=gastric phacellae (comprises gastric cirri), pc=pedalial canal, pd=pedalium, rh=rhopalium, rno=rhopalial niche opening, rst=rhopalial stalk, sta=statocyst, sto=stomach, te=tentacle, usc=upper scale of rhopalial niche covering. Scale bars: 5 mm (B & F).
Carybdea alata var. grandis
Graham 1998, pp 28–30;
Material examined
Neotype: USNM 1195802, 1 ind, female, BW 40 mm, BH 70 mm (live), BW 30 mm, BH 69 mm (preserved), 24 June 2011, Karel’s Pier, Kralendijke, Bonaire, The Netherlands, 12 09′ 06.37 N 6816′ 40.84 W, depth=surface.
Other material
Collected and identified by Lewis et al., depth=surface: USNM 1205450, 1 ind, female, BW 26 mm, BH 78 mm; USNM 1205449, 1 ind, female, BW 38 mm, BH 77 mm; USNM 1205448, 1 ind, male, BW 24 mm, BH 64 mm; USNM 1205447, 1 ind, female, BW 31 mm, BH 83 mm; USNM 1195807, 1 ind, female, BW 27 mm, BH 80 mm; USNM 1195806, 1 ind, male, BW 30 mm, BH 75 mm; USNM 1195805, 1 ind, male, BW 49 mm, BH 77 mm; USNM 1195804, 1 ind, male, BW 29 mm, BH 84 mm; USNM 1195803, 1 ind, female, BW 32 mm, BH 50 mm; USNM 1195801, 1 ind, female, BW 30 mm, BH 83 mm, 25 June 2011, Karel’s Pier, Kralendijke, Bonaire, The Netherlands, 12 09′ 06.37″ N 68 16′ 40.84″ W. Collected by Ross et al. and identified by Bentlage, B.: USNM 1131246, 1 ind, gonads absent, BW 22 mm, BH 47 mm, 28 Aug 2007, depth=98–133 m (bottom depth=468–595 m), Lease Block VK826, Gulf of Mexico, 29 09′ 34.99″ N 88 01′ 19.99″ W. USNM 1131245, 1 ind, gonads absent, BW 19 mm, BH 25 mm, 25 Aug 2007, depth=surface (bottom depth=2206–2282 m), Lease Block AT340, Gulf of Mexico, 27 38′ 38.00″ N 88 20′ 59.99″ W. Identified by Burnett, J.W.: USNM 94780, 1 ind, female, BW 24 mm, BH 55 mm, Feb 1992, Guantanamo Bay, Cuba, Caribbean Sea. Collected by U S Navy, identified by Larson, R. J.: USNM 58692, 1 ind, gonads absent, BW 17 mm, BH 30 mm, 3 June 1970, depth=55m, Ocean Acre Area, Off Bermuda, North Atlantic, 31 55′ 59.99 N 64 25′ 00.00 W. USNM 58691, 1 ind, gonads absent, BW 7 mm, BH 17 mm, 7 Sept 1968, depth=327–335 m, Ocean Acre Area, Off Bermuda, North Atlantic, 31 52′ 59.99 N 64 25′ 00.00 W. USNM 58655, 1 ind, male, BW 20 mm, BH 49 mm, 28 Oct 1967, depth=550–675 m, Ocean Acre Area, Off Bermuda, North Atlantic, 32 34′ 59.99 N 63 58′ 00.00 W. USNM 58316, 1 ind, gonads absent, BW 8 mm, BH 16 mm, 28 Oct 1967, Depth=55m, Ocean Acre Area, Off Bermuda, North Atlantic, 31 55′ 59.99″ N 64 25′ 00.00 W. USNM 54367, 1 ind, gonads absent, BW 8 mm, BH 22 mm, Apr 27 1969, depth=0–300 m, Open Ocean Area, Off Bermuda, North Atlantic, 31 55′ 00.00 N 67 57′ 00 W. USNM 54366, 1 ind, gonads absent, BW 16 mm, BH 55 mm, 25 Apr 1968, depth=350 m, Open Ocean Area, Off Bermuda, North Atlantic, 31 55′ 59.99 N 63 46′ 00.00 W. USNM 53694, Open Ocean Area, 1 ind, gonads absent, BW 5 mm, BH 9 mm, 6 Apr 1967, depth=224–298 m, Off Cape Hatteras, North Atlantic, 35 02′ 59.99 N 74 40′ 59.99 W. USNM 53659, 4 ind(s) gonads absent, BW 26 mm, BH 51 mm, BW 10 mm, BH 22 mm, BW 10 mm, BH 24 mm, BW 10 mm, BH 22.5 mm, 1 ind sex undetermined, BW 19 mm, BH 47 mm, 28 Sept 1965, depth=surface, Caracas, Caribbean Sea, 10 54′ 00 N 67 58′ 00.00 W. Identified by Larson: USNM 58211, 1 ind, female, BW 22 mm, BH 75 mm, 3 Apr 1978, Carrie Bow Cay, Lagoon, Dock, Belize, Caribbean Sea. USNM 58210, 1 ind, female, BW 25 mm, BH 52 mm, 25 Mar 1978, Carrie Bow Cay, Lagoon, Dock. USNM 54472, 4 ind(s), female 1, BW 16 mm, BH 58 mm, female 2, BW 18 mm, BH 64 mm, gonads absent, BW 17 mm, BH 32 mm, sex undetermined, BW 26 mm, BH 43 mm, 13 Oct 1974, Mona Island, Caribbean Sea, 18 04′ 0.00 N 67 52′ 59.99 W. USNM 54398, 5 ind(s), female, BW 8 mm, BH 17 mm, gonads absent, BW 7 mm, BH 19 mm, sex undetermined 1, BW 6 mm, BH 20 mm, sex undetermined 2, BW 8 mm, BH 16 mm, sex undetermined 3, BW 7 mm, BH 18 mm, 13 Oct 1974, Mona Island, Caribbean Sea, 18 04′ 00.00 N 67 52′ 59.99 W. Collected by Lea and identified by Larson, R.J.: USNM 56737, 1 ind, gonads absent, BW 10 mm, BH 20 mm, 11 Sept 1977, depth=0–90 m, Open Ocean Area, Off Delaware, North Atlantic, 37 20′ 41.99 N 69 10′ 23.99 W. USNM 56736, 1 ind, gonads absent, BW 10 mm, BH 17 mm, 17 Sept 1977, depth=0–50 m, Open Ocean Area, Off Delaware, North Atlantic, 37 18′ 24.00 N 66 51′ 24.00 W. USNM 56735, 1 ind, gonads absent, BW 6 mm, BH 8 mm, 10 Sept 1977, depth=0–150 m, Open Ocean Area, Off Delaware, North Atlantic, 37 49′ 59.99 N 67 25′ 23.99 W. Collected by Chase and Nicholson, identified by Larson, R.J.: USNM 54385, 2 ind(s), female 1, BW 30 mm, BH 80 mm, female 2, BW 34 mm, BH 100 mm, 4 Apr 1956, Freemans Bay, English Harbor, Antigua Island, Caribbean Sea. Collected and identified by Bigelow, H.B: USNM 42017, 1 ind, female, BW 29 mm, BH 78 mm, 30 Jan 1914, depth=0–100 m, Open Ocean Area, E of Cape Romain, North Atlantic, 32 32′ 59.99 N 72 13′ 59.99 W. USNM 41921, 1 ind, gonads absent, BW 17 mm, BH 37 mm, collected 21 Mar 1914, depth=surface, N of Little Bahama Bank, Bahamas, North Atlantic, 27 46′ 00.00 N 78 46′ 00.00 W. USNM 41920, 1 ind, gonads absent, BW 18 mm, BH 40 mm, 18 Mar 1914, depth=surface, N of Havana, Cuba, Caribbean Sea, 22 31′ 59.99 N 81 47′ 59.99 W. USNM 41919, 1 ind, gonads absent, BW 19 mm, BH 36 mm, 3 Mar 1914, depth=surface, W of Eleuthera Island, Bahamas, North Atlantic, 25 26′ 59.99 N 77 16′ 00.00 W.
Catalogued multimedia material (no specimen collected)
USNM 1195809, filmed by Harbison, R & Widde, E et al., identified by Larson, R (in Larson et al. 1991), still frame from a video voucher taken from the JSL manned submersible, 11 Nov 1989, depth=540 m (range=457–610 m), 26 04′ 00.00 N 77 32′ 59.99 W, Off Gordon Cay, 96 NM Off Rock Point. USNM 1005621, photographed by Continental Shelf Associates for BLM/MMS and Texas A & M University taken from ROV SeaROVER, identified by Lewis, C., photo voucher, Aug 1998, depth=96.5–108.7 m, 29 19′ 39 N 87 46′ 00.00 W, Mississippi, MMS Lease Block Destin Dome 661, MMS–MAPTEM/M3–4, Gulf of Mexico.
Neotype locality
Bonaire, Dutch Caribbean (Atlantic Ocean)
Diagnosis
Alatina with tall narrow bell, flared at base, tapering into truncated pyramid at apex; 4 crescentric gastric phacellae at interradial corners of stomach; 3 simple to palmate branching velarial canals per octant, each with a velarial lappet bearing a row of 3 to 4 nematocyst warts; 4 long wing–like (sensu Reynaud 1830) pedalia, each with a pink tentacle. Cnidome consisting of heterotrichous microbasic p–euryteles and small birhopaloids in tentacles, and large isorhizas in nematocysts warts.
Description (Figs 1–6, Table 1). Neotype Fig 2a–f.
Mature female specimen (BW 40 mm, BH 70 mm live; BW 30 mm, BH 69 mm preserved), with tall narrow bell flared at base (Fig 2a), tapering into truncated pyramid at apex. Each interradial corner bearing a pedalium: 3 of the 4 pedalia long and broad (approximately 15 mm wide) and wing–like, each bearing a pink tentacle (2a, c) about 2 mm in diameter, with bands of nematocysts along the entire length (Fig 2c, e). Fourth pedalium bearing a remnant of a tentacle (only several millimeters in height and width), much smaller and thinner than the other three (possibly damaged while being photographed together with other live specimens in an aquarium). Bell transparent and colorless in life (Fig 2a), translucent in fixed specimens (Fig 2e); exumbrella speckled with nematocyst warts. Stomach shallow (Fig 2b), lacking mesenteries. Manubrium short (2–3 mm long), wide and flat, with 4 mouth lips curled at the tips. Each of the four corners of the stomach housing a crescentric gastric phacella bearing about 20 gastric cirri (Fig 2a, b). A pair of leaf–like gonads, flanking each interradial septa and extending into the gastro–vascular cavity, filled with developing oocytes (Fig 2a, b). Adjacent gonads (i.e., ovaries) overlapping in the gastrovacular cavity, disassociated into large pieces following rupture due to internal fertilization event prior to preservation in formalin. Clumps of eggs found in various parts of the gastrovascular system (e.g., the velarial and pedalial canals, and the gastric pockets). Velarium wide, suspended by 4 perradial muscular brackets (frenulae) bracing the subumbrellar wall. Each octant bearing 3 simple to palmate branching velarial canals (variable with each octant), with the pair flanking the peradial frenulum simple to bifurcating, those flanking the pedalia at the interadius bifurcating as two main branches each with 3 to 4 distal branches (up to 7 branches in total), with the velarial canal in between bearing three distal branches (Fig 2d). Velarial canals in the two octants flanking the diminutive pedalium are not organized in the regular orderly fashion seen in the 6 other octants. Additionally, the velarium is torn near the base of the smaller pedalium, making it difficult to count the number of velarial canal branches there (N.B. the damaged pedalium is not readily apparent in the live photographs of the neotype specimen, and its small size may be exaggerated due to shrinkage following fixation in formalin). A new cubozoan character was discovered that appears to have been overlooked by previous workers. The structure which we call velarial lappets is found in sets of three in each octant overlaying the proximal two thirds of each velarial canal, each bearing a row of 3 to 4 nematocyst warts (Fig 2d). Four club–shaped rhopalia (Fig 2a), each situated just above the point where the frenulum connects to the subumbrella bearing 2 median lensed eyes, 2 lateral slit eyes, 2 lateral pit eyes, and 1 statocyst (Fig 2f). Rhopaliar niche opening T–shaped (sensu Gershwin 2005), with a single upper scale and 2 lower scales (Fig 2f). Nematocyst warts are scattered over the entire exumbrella and bell apex; occurring in rows varying in number along the pedalial keel and the rhopalial niche scales (Fig 2f). Cnidome characterized below.
Other material
Medusae measurements are given as min–mean–max values, and were made on preserved specimens unless otherwise stated.
In addition to the neotype, forty five A. alata specimens were examined (BH=8–48–100 mm, BW=5–20–49 mm). Bells tall, narrow truncated pyramids like the neotype (Fig 1a; 4g, 6a–c). Four long wing–like pedalia (Fig 1b; 3c; 6a–c), each with a single highly contractile tentacle, round in cross–section, ‘fluorescent’ pink in both live and preserved specimens, with numerous bands of nematocysts along the length (Fig 5a). Wide velarium (Fig 1c, d; 3a–d), suspended by 4 perradial muscular brackets (frenulae) bracing the subumbrellar wall (Fig 3a–d). Twenty four simple to branching velarial canals, 3 per octant, extending from the gastro–vascular space of the bell into the velarium (Fig 1c, d; 3a–c & e). Velarial canals flanking perradial frenulae simple, bifurcating distally, or giving rise to side branches; those flanking interradial pedalia splitting into two distinct sub–branches in first half to two–thirds of main branch; subsequent branching increasing in complexity (on average 4 sub–branches per primary branch; maximum 10), making it difficult to trace velarial canal tips back to the original branch, and often giving the appearance of greater than 3 primary velarial canals per octant (Fig 1c, d; 3a, b). Bifurcating velarial canals in individuals as small as BH=8 mm; some canals with three branches in individuals of BH=16 mm. Larger medusae generally displaying more highly branched velarial canals; wide variation exists between each octant in a single individual (1 to 5 branches seen per canal). Velarial lappets are thick gelatinous pouch–like structures comprising the proximal 50–75% of each velarial canal (24 total; 3 per octant), each bearing a row of 3–4 nematocyst warts (Fig 3a, b, e), function unknown. Bell transparent and colorless in life (Fig 4g, 6a–c), translucent in fixed specimens, speckled with nematocyst warts (nematocyst warts in older material were often lacking, and may have rubbed off in collection gear or after many years in preservation fluids, despite velarial lappet warts being still recognizable in most specimens).
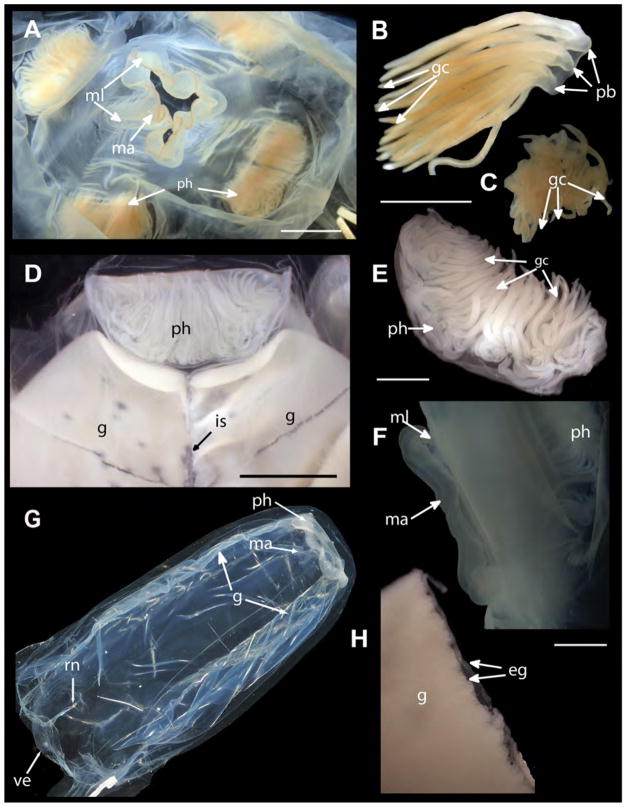
FIGURE 4.
Alatina alata specimens, preserved and live (all except A–C from Kralendijk, Bonaire (The Netherlands) the same locality as the neotype). A. USNM 53659, oral view of manubrium and four crescentric gastric phacellae in each corner of the stomach (BW=19 mm, BH=47 mm). B. USNM 53659, dissected tuft of gastric cirri from crescentric gastric phacella. C. USNM 53659, dissected gastric cirri, appearing as central mass in stomach of developing medusa (BW=10 mm, BH=22 mm). D. USNM 1195807, gastric phacellae and adjacent ripe paired gonads in preserved female medusa. E. USNM 1195807, dissected crescentric gastric phacella from mature medusa. F. USNM 1195806, lateral view of manubrium with mouth lips curled up. G. Live mature medusa, gonads beginning to rupture following a reproductive swarming episode (live, BW=60, BH=90). H. USNM 1195807, gonads dissected from mature female, bilayer of mature oocytes visible along adradial point of rupture. Live photographs by T. Peters (Fish Eye Photography Bonaire) and A. Yanagihara. (Fish Eye Photography, Bonaire). Abbreviations: eg=eggs, gc=gastric cirri, ma=manubrium, ml=mouth lips, pb=primary branch, ph=gastric phacellae (comprises gastric cirri), is=interradial septum. Scale bars: 5 mm (A & D), 2mm (B, C, E, F, H).
FIGURE 3.
Alatina alata from Kralendijk, Bonaire, The Netherlands (same location as neotype). A. & B. USNM 1195807, velarial canals (3 per octant) in preserved medusa, simple to branching (one outlined with dotted line in Fig 3A). Each canal bears a row of 3 nematocyst warts on the proximal first half to two thirds, i.e., on the velarial lappets. A clump of disassociated eggs is seen within the velarial canal on the left. C. USNM 1195802 (neotype), velarium of live medusa, partially pushed out revealing velarial canals. D. USNM 1195806, double layered frenulum anchoring the subumbrella to velarium at perradius. E. USNM 1195807, nematocyst wart on velarial lappet filled with isorhiza nematocysts. F. USNM 1195804, rhopalium dissected from preserved medusa, bears two median complex eyes, two upper lateral pit eyes, and two lower lateral slit eyes. G. USNM 1195806, subumbrella view of rhopalial window of preserved medusa, outline of rhopalial niche opening visible. Live photographs by T. Peters (Fish Eye Photography, Bonaire) & A. Yanagihara. Abbreviations: cel=complex eye lens, fre=frenulum, g=gonads, nw=nematocyst wart, pc=pedalial canal, pe=pit eye, pd=pedalium, rh=rhopalium, rno=rhopalial niche opening, rst=rhopalial stalk, se=slit eye, sta=statocyst, usc=upper scale of rhopalial niche covering. ve=velarium, vec=velarial canal, vel=velarial lappet. Scale bars: 5 mm (A & D), 10 mm (C), 1 mm (E), 3 mm (G).
FIGURE 5.
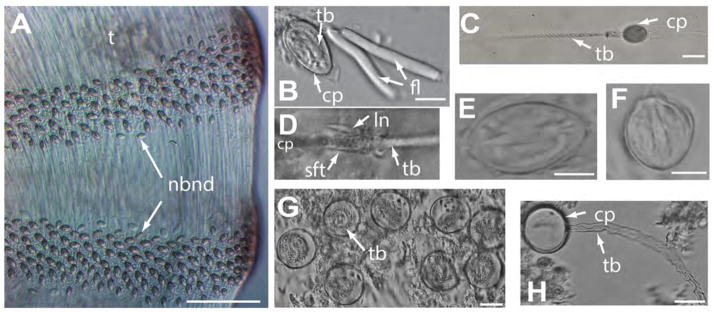
Cnidome of Alatina alata (all extracted from preserved material unless specified otherwise). A. Nematocyst bands along the length of the tentacle of a live specimen collected in Bonaire (June 2013). B. & E. Undischarged, microbasic heterotrichus p–euryteles extracted from tentacles; filaments associated with euryteles in B (USNM 1195807). C. Discharged microbasic heterotrichus p–eurytele extracted from tentacles of live medusa. D. Lancet contiguous with discharged tubule of microbasic heterotrichus p–eurytele extracted from tentacles (USNM 1195805) F. Small undischarged birhopaloid found in tentacles of preserved specimen USNM 1195807. G. Large undischarged holotrichus isorhizas found in nematocysts warts (i.e., bell warts & velarial lappet warts (USNM 1195802, neotype). H. Large discharged holotrichus isorhizas found in bell warts (USNM 1195802). H. Abbreviations: cp=capsule, fl=filaments, ln=lancet, nbnd=nematocyst band, sft=shaft, tb=tubule. Scale bars: ~150 μm (A), 10 μm (B, E, F), 25 μm (C), 20 μm (G, H).
Rhopaliar niche opening T–shaped (sensu Gershwin 2005), with a single upper scale and 2 lower scales enclosing the rhopalial niche (Fig 1e). Four club–shaped rhopalia, each with 2 median lensed eyes, 2 lateral slit eyes, 2 lateral pit eyes, and 1 statocyst (Fig 3f, g). All eyes are conspicuous in newly collected material from Bonaire, but in older preserved museum specimens the lens eyes are discolored (having a brown tinge), and bilateral paired pigmented pit eyes and slit eyes are faded to absent, leaving only the complex lens eyes visible. This may be an artifact of prolonged exposure to fixatives.
Stomach shallow, lacking mesenteries; manubrium short (2–3 mm long), wide and flat, 4 mouth lips curled at the tips (Fig 4a, f). Four crescentric gastric phacellae, 1 in each corner of the shallow stomach, each with 6–24 basal trunks branching several times at the base, giving rise to up to 100 terminal filaments (gastric cirri) in larger specimens (Fig 1f, g; 4a, b, d–g). Smaller individuals (BH<20 mm) possessing long filaments (6–8 basal trunks) extending from each corner into the stomach, giving appearance of a central mass (Fig 4c). Eight narrow leaf–like gonads extend in pairs within the gastric pockets, along either side of the interradial septa; filled with developing sperm or eggs in mature males and females (Fig 4d, g, h). We examined preserved individuals with gonads (BH=16–61–100, BW=6–23–34, n=23), as well as preserved medusae without gonads (BH=8–32–88, BW=5–15–32, n=22). All medusae < BH=16 mm lacked gonads, as did many larger medusae (n=18). Gonads presumably are shed in mature medusae following a spawning event (see Arneson 1976). Gonads, translucent in live medusae (Fig 4g; 6a, b), turning cream to pale amber in spawning medusae; and opaque in fixed material. Ripe gonads overlap along the perradial plane, becoming increasingly pleated (Fig 4g).
Cnidome Table 1; Fig 5a–h. Nematocyst measurements are provided as min–mean–max; L=length of capsule in μm, W=width of capsule at widest point in μm, SD=standard deviation, n=number of nematocysts measured. Nematocyst identification follows Mariscal (1974), Östman (2000), and Collins et al. (2011).
Bands of nematocysts found along the entire length of the tentacles primarily contain oval, heterotrichus microbasic p–euryteles (Table 1, Fig 5a–e) both at tentacle tips, and tentacle base, but more abundant in tentacle tips. Discharged tubules sometimes possessed intact characteristic lancet (Fig 5d) contiguous to the shaft (see Yanagihara et al. 2002 for description of lancet). Rod–like filaments (Fig 5 b, c) are seen attached to euryteles from the tentacles. Small birhopaloids (Table 1, Fig 5f) found both in tentacle tips and tentacle base, but more abundant in tentacle base. Large, spherical holotrichous, isorhizas (Table 1, Fig 5g–h) found in exumbrellar (bell) warts and velarial lappet warts. Isorhizas (n=2) were occasionally seen in tissue from tentacle base. Only a single nematocyst was found associated with the gastric cirri: a small birhopaloid was found within tissue of the gastric cirri (L 17.4 μm, W 12.1 μm). No nematocysts were found associated with the manubrium despite thorough examination.
Sexual reproduction and early life history
Both male and female gonads are comprised of a bilayer matrix (Fig. 4H). During spawning events witnessed in buckets in the CIEE lab, male gonads became cloudy, and ruptured in several spots along the distal axis releasing spermatozoa into the gastro–vascular cavity, which were then shed into the surrounding water via the manubrium. As females took up sperm into the gastro–vascular system, gonads became opaque, and like in males, they ruptured in several spots, and spherical eggs were ovulated into the gastric sacs, which were by then saturated with sperm (based on microscopic observations of contents). Within several hours embryos were seen circulating throughout the entire gastro–vascular system of the female medusae (in and out of tentacles, velarial canals, etc.). Blastulae were exuded, which developed into planulae that settled as polyps after several days. Later developmental stages were not observed in this study. All but two of the medusae collected during the swarming episode in this study had ruptured gonads with mature ova or spermatozoa spilling into the gastric sacs. In the two exceptions (USNM 1205449 and USNM 1205450), gonads were present as thin strips along the interradial septa, despite the large size of the medusae (BH=77 mm and BH=78 mm). Examination of an excised portion of the preserved gonads from these individuals using oil emersion light microscopy (100× objective (i.e., 1000× magnification) revealed no mature gametes.
Distribution and diet
In this study A. alata medusae were mostly observed in shallow waters near shore (Fig 6a), but the species is also encountered offshore in deeper waters in the Atlantic Ocean. For instance, seven of the museum specimens examined were collected at discreet depths between 55 m and 675 m. Additionally, we obtained video footage of a mature A. alata medusa swimming at a depth of about 540 m West off Gorda Cay, Bahamas (USNM 1195809) (Fig 6b), and an in situ photo voucher taken from the ROV SeaROVER (USNM 1005621) depicting A. alata close to bottom at a depth of about 100 m in the Gulf of Mexico (Fig 6c). Among the USNM material, four A. alata lots were collected in open–net trawls between the surface and depths of up to 300 m. Fourteen were collected on the surface, in shallow and open ocean localities where bottom depth was up to 2282 m. The remaining 22 lots observed in this study had no associated depth record. Live medusae collected from Bonaire had hyperiid amphipods in their subumbrella; some museum specimens examined contained euphausids and small caridean shrimps in the stomach or subumbrella, and one individual had pelagic polycheate worms lodged in three of the six velarial canals in a quadrant, but most medusae collected had empty stomachs.
Discussion
At the time of Alatina alata’s original description, Carybdea marsupialis (Linnaeus 1758) was the only other cubozoan species known, and only a few morphological characters had been established for delineating the species. It may be argued that it is impossible to know that the specimens described herein as Alatina alata refer to the species that Reynaud referred to when he first described it as Carybdea alata in Lesson (1830). In the following discussion we recount why we are convinced that specimens from the Dutch Caribbean Island of Bonaire are appropriate for establishing a neotype for Alatina alata (Reynaud 1830), and ultimately stabilizing the species name. According to the definition and rules set forth under Article 75 of the International Code for Zoological Nomenclature (ICZN 1999): “A neotype is the name–bearing type of a nominal species–group taxon designated under conditions specified in this Article when no name–bearing type specimen (i.e., holotype, lectotype, syntype or prior neotype) is believed to be extant and an author considers that a name–bearing type is necessary to define the nominal taxon objectively”.
The precise type locality of A. alata is unknown, and can never be positively determined. Reynaud’s original description of this species in Lesson’s (1830) La Centurie Zoologique states only that “this medusa inhabits the Atlantic Ocean.” The “Centurie”, also known under the title “Choix d’animaux rares, nouveaux ou imparfaitement connus” or “Selection of rare, new or imperfectly known animals” was dedicated to Geoffroy-Saint-Hilaire, then professor at what is now the National Museum of Natural History (Paris, France) (Lesson 1830). In the preface, Lesson explains that the name Centurie was chosen because the intent was to publish 100 plates “drawn from nature” (i.e., still life) from the many collections in the Paris museum (ranging from terrestrial mammals, to birds, and marine invertebrates) (see Lesson 1830). However, due to political events of the time, only 80 plates were compiled to produce the Centurie, which was distributed in 20 issues over a 15–month period from 1830 to 1831 (see Postscript in Lesson 1830). The colored sketches in the plates are almost exclusively attributed to Prêtre (see inscription on front cover of Lesson 1830), but some illustrations were also done by Lesson, as indicated in signatures below each plate.
Many animals depicted in the Centurie were little–known species redescribed by Lesson, or other well–known naturalists (e.g., Cuvier, Peron, Smith, Geoffroy-Saint-Hilaire) (e.g., see Lesson 1830, Plate 2, pp. 14–17, etc.), who emphasized the necessity of an accompanying color plate to enrich each description (see Lesson 1830, Preface). Additionally, a number of new species were described from specimens collected by Lesson and others aboard the vessels La Coquille (1822–1825) and L’Astrolabe (1826–1829) as part of the combined French Scientific World Tour throughout the Pacific and Atlantic oceans (to Madagascar, New Guinea, Australia, Brazil, Mexico; see Lesson 1830; Bauchot et al. 1990 for additional localities and details). Some of the specimens were collected by Reynaud aboard the corvette La Chevrette to South Africa and India (1827–1828) and described by him or other specialists (e.g., see Lesson 1830, Plate 1, pp. 11–13). Twelve of the 80 descriptions in the Centurie are of new species described by Reynaud (see Lesson 1830, plates 1, 15, 17, 20, 23, 25, 28, 33, 34, 37 and accompanying descriptions). Five of these species are described from specific localities in India, and one from Cap de Bonne–Espérance in South Africa. The remaining six species, including A. alata (as C. alata), are reported simply from the Atlantic Ocean suggesting that A. alata was observed in an undocumented locality or in several localities throughout the Atlantic Ocean. The entries in the Centurie are not compiled in chronological order or by geographic vicinity, and there is no date associated with the description of A. alata. Thus there is no way to determine where Reynaud was when he witnessed and described this new species. There is doubt about whether a specimen was ever collected for A. alata, as Reynaud doesn’t mention one, unlike Lesson who specifies that many of his descriptions are of museum specimens adorning the galleries, or those found in the collection of the Paris museum and other museums (Lesson 1830). Furthermore, during a recent visit to the collections of the Paris museum we were unable to recover any A. alata specimens from the Atlantic Ocean.
Reynaud’s new species was quickly adopted by other workers at the time (Lesson 1843; Agassiz 1862). In the absence of Reynaud’s material, Haeckel (1880) reported on A. alata (as Carybdea alata) from a specimen he received through Wilhelm Bleek from South Africa. In addition, he placed Reynaud’s observation of A. alata into the South Atlantic without further explanation, even though Reynaud did not specify where in the Atlantic the medusa he described as Carybdea alata was collected. Furthermore, Haeckel (1880) described two additional species of Alatina from the tropical belt of the Atlantic, specifically A. pyramis from the Antilles and A. obeliscus from the Cape Verde Islands. Specimens of both species had been deposited in the now defunct Museum Goddefroy, Germany and their whereabouts are unknown, A. pyramis had not been recognized for about a century until its resurrection by Gershwin (2005), who believes it would be recognizable by its “frilly lips and non–bifurcating velarial canals if ever reencountered. Its description appears distinct from the material we describe herein as A. alata, even though Haeckel reports A. pyramis from a region of the Atlantic adjacent to our proposed neotype locality for A. alata. A. obeliscus was described from the tropical Western Atlantic (as C. obeliscus), but has been considered unrecognizable (Gershwin 2005), and we concur.
Gershwin (2005) suggested that a neotype should be established for A. alata when suitable material from the South Atlantic became available, presumably following Haeckel’s (1880) reference to the South Atlantic. However, as detailed above, the specific locality of the original material cannot be confidently determined. A. alata has appeared in the literature for 183 years, and forty six USNM specimens exist documenting its morphology and distribution in the Western Atlantic (including the Gulf of Mexico and Caribbean Sea). Our investigations of these specimens convince us that the Western Atlantic specimens belong to the same species described by Reynaud in 1830 as C. alata. Our redescription of A. alata from the island of Bonaire, Dutch Caribbean is provided with the purpose of stabilizing the identity of A. alata whose taxonomy in the absence of type material has been problematic and the cause of much confusion in cubozoan taxonomic studies. Thus by fixing the name to a neotype from Bonaire where fresh material can reliably be obtained, we hope to encourage future studies of the Alatina group.
Acknowledgments
We are thankful to the Bonaire CIEE Research Station staff (Dr. Rita Peachey, Caren Eckrich) for allowing us use of the laboratory during our stay in Bonaire; additional collectors Lauren and Cinde Wirth, Esther van Blerk, Jen, Brevan and Ayla Collins; Andy Ames; Geoff Keel and Katie Ahlfeld of the Smithsonian Museum Support Center (MSC) who helped with specimen curation, and Cheryl Bright (MSC Invertebrate Zoology Collections Manager) for hosting us during our work in the collections; Dr. Mike Vecchione of NOAA NMFS–NSL for notifying us of the in situ video footage; John Reed (Fort Pierce) and Dr. Richard Harbison (WHOI) for providing details of the video log; NMNH Library staff and the Biodiversity Heritage Library who provided us with access to rare and old manuscripts; STINAPA Bonaire Manager Kalli De Meyer and Dr. Frank van Slobbe of DROB; Bonaire Marine Park Manager Ramon de Leon who gave us collection permits and letters of authorization. Finally, we extend a special thanks to Karel of Karel’s Pier, Kralendjik, for allowing our group to sample from the pier on which his restaurant is located during the annual Jellyfish Jamboree on Bonaire, The Netherlands. Jonathan Lawley assisted with photographing preserved specimens. We are also thankful to the reviewers of an earlier draft of this manuscript who provided excellent advice for improvement, and to the Zootaxa editors.
Contributor Information
CHERYL LEWIS, Email: clames1@umd.edu.
BASTIAN BENTLAGE, Email: bastian.bentlage@gmail.com.
ANGEL YANAGIHARA, Email: ayanagih@hawaii.edu.
WILLIAM GILLAN, Email: william.gillan@palmbeachschools.org.
JOHAN VAN BLERK, Email: blerkbonaire@hotmail.com.
DANIEL P. KEIL, Email: dkeil@umd.edu.
ALEXANDRA E. BELY, Email: abely@umd.edu.
ALLEN G. COLLINS, Email: collinsa@si.edu.
References
- Agassiz L. Contributions to the natural history of the United States of America. IV. Little Brown; Boston: 1862. [accessed 2 Oct 2013]. p. 380.p. 19. Available from: http://www.biodiversitylibrary.org/item/54510#page/9/mode/1up. [Google Scholar]
- Arneson AC. MS Thesis. University of Puerto Rico, Mayaguez, Commonwealth of Puerto Rico; USA: 1976. Life history of Carybdea alata Reynaud, 1830 (Cubomedusae) p. 43. [Google Scholar]
- Arneson AC, Cutress CE. Life history of Carybdea alata Reynaud, 1830 (Cubomedusae) In: Mackie GO, editor. Coelenterate Ecology and Behavior. Plenum Press; New York, NY: 1976. pp. 227–236. [Google Scholar]
- Bauchot ML, Daget J, Bauchot R. L’ichthyologie en France au début du XIXe siècle. L’Histoire naturelle des Poissons de Cuvier et Valenciennes. Bulletin du Muséum national d’Histoire naturelle, Paris, section A. 1990;(supplément 12):3–142. http://dx.doi.org/10.3366/anh.1993.20.1.139a.
- Bentlage B. Carybdea alata auct. (Cubozoa): rediscovery of the Alatina grandis type. Zootaxa. 2010;2713:52–54. [Google Scholar]
- Bentlage B, Cartwright P, Yanagihara AA, Lewis C, Richards GS, Collins AG. Evolution of box jellyfish (Cnidaria: Cubozoa), a group of highly toxic invertebrates. Proceedings of the Royal Society B. 2010;277:493–501. doi: 10.1098/rspb.2009.1707. http://dx.doi.org/10.1098/rspb.2009.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentlage B, Lewis C. An illustrated key and synopsis of the families and genera of carybdeid box jellyfishes (Cnidaria: Cubozoa: Carybdeida), with emphasis on the “Irukandji family (Carukiidae) Journal of Natural History. 2012;41–42:2595–2620. http://dx.doi.org/10.1080/00222933.2012.717645. [Google Scholar]
- Bigelow HB. Some Medusae and Siphonophora from the western Atlantic. [accessed 14 Nov. 2013];Bulletin of the Museum of Comparative Zoology of Harvard College. 1918 62:363–442. pls 1–8. Available from: http://www.biodiversitylibrary.org/item/21150#page/463/mode/1up. [Google Scholar]
- Bigelow HB. Plankton of the Bermuda oceanographic expeditions. VII. Medusae taken during the years 1929 and 1930. Zoologica Scientific Contributions of the New York Zoological Society. 1938;23:99–189. [PubMed] [Google Scholar]
- Collins AG, Bentlage B, Gillan W, Lynn TH, Morandini AC, Marques AC. Naming the Bonaire banded box jelly, Tamoya ohboya, n. sp. (Cnidaria: Cubozoa: Carybdeida: Tamoyidae) Zootaxa. 2011;2753:53–68. [Google Scholar]
- De Meyer K. Bonaire, Netherlands Antilles. Coastal Region and Small Island (CSI) [accessed 2 Oct 2013];Environment and development in coastal regions and in small islands. Paper 3. 1997 Available from http://www.unesco.org/csi/pub/papers/demayer.htm.
- Gershwin L. Carybdea alata auct. and Manokia stiasnyi, reclassification to a new family with description of a new genus and two new species. Memoirs of the Queensland Museum. 2005;52:501–523. [Google Scholar]
- Graham WM. First report of Carybdea alata var. grandis (Reynaud 1830) (Cnidaria: Cubozoa) from the Gulf of Mexico. Gulf of Mexico Science. 1998;1:28–30. [Google Scholar]
- Haeckel E. System der Acraspeden – Zweite Hälfte des Systems der Medusen. Denkschriften der Medizinisch–Naturwissenschaftlichen Gesellschaft zu Jena. Germany: 1880. p. 82. [Google Scholar]
- Humann P, Deloach N. Reef creature Identification: Florida, Caribbean, Bahamas. 2. New World Publications Inc; 2002. p. 420. [Google Scholar]
- ICZN. International Code of Zoological Nomenclature, 4th edition. London: The International Commission on Zoological Nomenclature; 1999. [accessed 23 September 2013]. Available from: http://www.iczn.org/code. [Google Scholar]
- Kayal E, Bentlage B, Collins AG, Kayal M, Pirro S, Lavrov DV. Evolution of linear mitochondrial genomes in Cnidaria. Genome Biology and Evolution. 2012;1:1–12. doi: 10.1093/gbe/evr123. http://dx.doi.org/10.1093/gbe/evr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramp PL. Synopsis of the Medusae of the World. Journal of the Marine Biological Association of the United Kingdom. 1961;40:1–469. http://dx.doi.org/10.1017/s0025315400007347. [Google Scholar]
- Larson RJ. Cubomedusa: feeding – functional morphology, behavior and phylogenetic Position. In: Mackie GO, editor. Coelenterate ecology and behavior. Plenunm Press; New York: 1976. pp. 237–245. [Google Scholar]
- Larson RJ, Mills CE, Harbison GR. Western Atlantic midwater hydrozoan and scyphozoan medusae: in situ studies using manned submersibles. Hydrobiologia. 1991;216–217(1) [Google Scholar]
- Lesson RP, editor. Centurie Zoologique. Levrault; Paris, France: 1830. p. 244. [Google Scholar]
- Lesson RP. Prodrome d’une monographie des Méduses. Extraite d’une Histoire manuscrite des méduses, en 3 volumes 4e avec 200 planches coloriées, ouvrage entièrement termin. Rochefort. 1837:63. 200 pls. [Google Scholar]
- Lesson RP. Historie naturelle des zoophytes. Acalèphes. Encyclopédique de Roret; Paris: 1843. p. 663. [Google Scholar]
- Linnaeus C. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. Tomus I. Holmiae (Laurentii Salvii) 1758:i–iv. 824. [Google Scholar]
- Mariscal RN. Nematocysts. In: Muscatine L, Lenhoff HM, editors. Coelenterate biology–reviews and new perspectives. Academic Press; New York: 1974. pp. 129–178. [Google Scholar]
- Mayer AG. Medusae of the World. Vol. III. The Scyphomedusae. Carnegie Institution of Washington Publication. 1910;109(3):499–735. [Google Scholar]
- Morandini AC. Deep–Sea medusae (Cnidaria: Cubozoa, Hydrozoa and Scyphozoa) from the coast of Bahia (Western south Atlantic, Brazil) Mitteilungen aus dem hamburgischen zoologischen Museum und Institut. 2003;100:13–25. [Google Scholar]
- Östman C. A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Mills CE, Boero F, Migotto A, Gili JM, editors. Trends in hydrozoan biology–IV. Scientia Marina. 2000;64(Supl. 1):31–46. http://dx.doi.org/10.3989/scimar.2000.64s131.
- Reynaud M. Carybdea alata n. sp. In: Lesson RP, editor. Centurie Zoologique. Levrault; Paris (France): 1830. p. 95. Pl 33. [Google Scholar]
- Smith DR, Kayal E, Yanagihara AA, Collins AG, Pirro S, Keeling PJ. First complete mitochondrial genome sequence from a box jellyfish reveals a highly fragmented linear architecture and insights into telomere evolution. Genome Biology and Evolution. 2011;4(1):52–58. doi: 10.1093/gbe/evr127. http://dx.doi.org/10.1093/gbe/evr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara AA, Kuroiwa JMY, Oliver LM, Chung JJ, Kunkel DD. Ultrastructure of a novel eurytele nematocyst of Carybdea alata Reynaud (Cubozoa, Cnidaria) Cell and Tissue Research. 2002;308:307–318. doi: 10.1007/s00441-002-0545-8. http://dx.doi.org/10.1007/s00441-002-0545-8. [DOI] [PubMed] [Google Scholar]
- Yoshimoto CM, Yanagihara AA. Cnidarian (coelenterate) envenomations in Hawai’i improve following heat application. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:300–303. doi: 10.1016/s0035-9203(02)90105-7. http://dx.doi.org/10.1016/s0035-9203(02)90105-7. [DOI] [PubMed] [Google Scholar]