Abstract
Background
Colorectal cancer (crc) has a median diagnostic age of 68 years. Despite significant progress in chemotherapy (ctx) options, few data on outcomes or toxicity from ctx in patients 80 years of age and older are available. We investigated ctx in such patients with metastatic crc (mcrc), hypothesizing high rates of hospitalization and toxicity.
Methods
A retrospective chart review identified patients 80 years of age and older with mcrc who initiated ctx between 2005–2010 at our institution. Patient demographics and ctx data were collected. Endpoints included rates of hospitalization, ctx discontinuation because of toxicity, and overall survival.
Results
In 60 patients, ctx was initiated on 88 occasions. Median age in the cohort was 83 years; 52% were men; 72% lived with family; 53% had a modified Charlson comorbidity index of 2 or greater; and 31% were taking 6 or more prescription medications at baseline. At baseline, 33% of the patients were anemic (hemoglobin < 100 g/L), 36% had leukocytosis (white blood cells > 11×109/L), and 48% had renal impairment (estimated glomerular filtration rate < 60 mL/min/1.73 m2). In 53%, ctx was given as first-line treatment. The initial ctx dose was adjusted in 67%, and capecitabine was the most common chemotherapeutic agent (45%). In 19 instances (22%), the patient was hospitalized during or within 30 days of ctx; in 26 instances (30%), the ctx was discontinued because of toxicity, and in 48 instances (55%), the patient required at least 1 dose reduction, omission, or delay. Median overall survival was 17.8 months (95% confidence interval: 14.3 to 20.8 months).
Conclusions
In the population 80 years of age and older, ctx for mcrc is feasible; however, most recipients will require dose adjustments, and a significant proportion will be hospitalized or stop ctx because of toxicity. Prospective research incorporating geriatric assessment tools is required to better select these older patients for ctx.
Keywords: Chemotherapy, octogenarians, elderly patients, colorectal cancer
INTRODUCTION
Colorectal cancer (crc) is the 3rd most common cancer worldwide, representing 9.7% of all cases in 20121. In Canada, crc represents 15% of cancers in individuals more than 70 years of age, and the lifetime probability of developing crc increases with age, from 0.2% in 40- to 49-year-olds to 3.3% and 2.7% in 80- to 89-year-old men and women respectively2. In the United States, crc is diagnosed at a median age of 68 years, with 23.2% of new cases occurring in 75- to 84-year-olds and 12.1% in individuals more than 84 years of age3.
For decades, the only treatment for metastatic crc (mcrc) was single-agent fluoropyrimidine4. The advent of leucovorin with fluoropyrimidine doubled tumour response rates and prolonged overall survival (os)5. Since the early 2000s, multiple novel agents (irinotecan, oxaliplatin, bevacizumab, cetuximab, panitumumab, aflibercept, regorafenib) have been introduced for patients with mcrc. Triple therapy using leucovorin–fluoropyrimidine with irinotecan or oxaliplatin has become the standard for the first-line treatment of mcrc. Median survival reliably exceeds 2 years when such multiple-agent therapies are used, compared with a median survival of 5–6 months with best supportive care alone6–8. Metastatic crc is rarely curable, unless there are resectable isolated liver metastases, and therefore other factors such as quality of life should be considered in addition to survival in the mcrc setting.
Optimal chemotherapy in elderly patients, particularly those 80 years of age and older, is not well defined. Thus, given the advances in chemotherapy for mcrc and the growing incidence of mcrc in elderly patients, safety and tolerance data for that population are needed. As is well known, the many physiologic changes that occur with age—particularly decreased hepatic and renal function, decreased bone marrow reserve, and increased risk of cardiovascular disease—can affect tolerance for chemotherapy9,10. Patients over the age of 75 diagnosed with crc have a mean of 5 comorbid conditions11,12, further affecting their overall fitness for chemotherapy. Elderly patients enrolled in trials are often defined as being 65 or 70 years of age and older; few are 80 years of age and older13. The limited high-quality results available for patients in the latter age group come from pooled phase iii trial data, including trials in both the adjuvant and metastatic disease settings. Compared with their non-trial counterparts, enrolled patients 80 years of age and older are generally more fit and typically demonstrate good performance status [Eastern Cooperative Oncology Group (ecog) performance status (ps) 0 or 1]14,15.
The Canadian population is aging2,16. In 2011, 5 million Canadians (14.8%) were 65 years of age or older, and the proportion in that age group is expected to double by 2036. Octogenarian Canadians represented 1.9% of the population in 1982; that proportion had more than doubled to 4.1% by 2012, and a rise to 7.6% is predicted by 203617. Between 1982 and 2012, the age group that saw the largest population increase was the 85+ group (+250.1%), followed by the 80–84 group (+114.5%)17. As life expectancy increases, more cases of cancer will be seen in the octogenarian population, and consequently, those elderly patients will represent a larger proportion of chemotherapy candidates. However randomized controlled trials that include patients 80 years of age and older are rare, and evidence to guide treatment in this ever-increasing population is therefore scant.
Given the increasing age of the population, the increasing number of elderly patients being diagnosed with crc, and the expansion of systemic therapy options, we conducted the present study to investigate the feasibility and tolerability of systemic therapy in a group of mcrc patients 80 years of age and older. We also sought to identify factors that could be predictive for chemotherapy suitability.
METHODS
Patients
After receiving research ethics board approval, we conducted a retrospective chart review of patients 80 years of age and older who had started a course of cytotoxic chemotherapy from June 2005 to January 2010 at our institution. The results of the main study were reported by Sud et al.18, but for the present work, we specifically identified the subset of elderly mcrc patients, because they represented the largest single subgroup within an otherwise heterogeneous cohort18. Patients were eligible if they were 80 years of age or older at chemotherapy initiation.
Baseline data were collected: age, sex, height and weight, ecog ps, smoking history, baseline number of prescription medications, and living situation before chemotherapy. Scores on the Charlson comorbidity index (cci) were calculated using patient comorbidities with the exclusion of the primary cancer diagnosis (“modified cci”). Chemotherapy characteristics [regimen, intent and line of therapy, setting of first chemotherapy delivery (inpatient or outpatient), and whether the dose was adjusted before the first cycle] were recorded, as was baseline blood work [hemoglobin, white blood cell count, estimated glomerular filtration rate (egfr), albumin]. Patients were excluded if they received only biologic therapy (for example, bevacizumab, cetuximab).
The reported line of therapy accounts for all previous lines of systemic treatment regardless of intent. If therapy was paused for 4 or more months, resumption of the same regimen constituted a new line. If patients received more than 1 line of chemotherapy after 80 years of age, each chemotherapy initiation was recorded as a separate instance.
Outcomes
Co-primary endpoints included chemotherapy dose reductions, omissions, or delays greater than 1 week; therapy discontinuation because of toxicity; and hospitalizations exceeding 24 hours if they occurred within 30 days of chemotherapy delivery. Secondary outcomes included the number of blood or platelet transfusions received within 30 days of chemotherapy, and os calculated per the date of the last clinical encounter or death (from chart or regional obituaries).
Statistical Analysis
The primary analysis was descriptive, reported as percentages. The co-primary endpoints were rates of hospitalization, of therapy discontinuation because of toxicity, and of dose reductions, omissions, or delays. Univariate and multivariate logistic regression tests were performed. Median os was calculated using the Kaplan–Meier method. All statistical analyses were performed using the SAS software application (version 9.2: SAS Institute, Cary, NC, U.S.A.).
RESULTS
Demographics
Between June 2005 and January 2010, 60 patients meeting the inclusion criteria initiated chemotherapy on 88 occasions (Table i). Age range was 80–92 years (median: 83 years). Most patients were men (52%), and the average body mass index was 25.1 kg/m2. Most patients lived with family (72%); 23% lived alone, and 2%, in a residential facility. The ecog ps was known in 79 of the 88 instances, with a score of 0 or 1 being recorded in 60% of those instances. The patient’s modified cci score was 2 or greater in 53% of the instances. Almost one third of the cohort (31%) was taking 6 or more medications at baseline. Anemia (hemoglobin < 100 g/L) was noted in 33%; leukocytosis [white blood cell (wbc) count > 11×109/L], in 37%; and impaired renal function (egfr < 60 mL/min/1.73 m2), in 48%.
TABLE I.
Patient characteristics
| Characteristic | Value |
|---|---|
| Age (years) | |
| Median | 83 |
| Range | 80–92 |
| Age group (%) | |
| <85 Years | 69.3 |
| ≥85 Years | 30.7 |
| ECOG performance status (%) | |
| 0–1 | 60.2 |
| 2 | 20.5 |
| 3–4 | 9.1 |
| Unknown | 10.2 |
| Medications (%) | |
| <6 | 69.3 |
| ≥6 | 30.7 |
| Smoking history (%) | |
| Never-smoker | 42.9 |
| Ex-smoker | 53.6 |
| Current smoker | 3.6 |
| Unknown | 4.5 |
| Score on the modified CCI (%) | |
| 0 | 23.9 |
| 1 | 22.8 |
| ≥2 | 53.4 |
| Living situation (%) | |
| Alone | 22.8 |
| With family | 71.6 |
| Retirement or nursing home | 2.3 |
| Unknown | 3.4 |
| Hemoglobin (%) | |
| <100 g/L | 33.0 |
| ≥100 g/L | 67.0 |
| White blood cells (%) | |
| >11×109/L | 36.7 |
| ≤11×109/L | 63.3 |
| eGFR (%) | |
| <60 mL/min/1.73 m2 | 47.8 |
| ≥60 mL/min/1.73 m2 | 52.2 |
ECOG = Eastern Cooperative Oncology Group; CCI = Charlson comorbidity index; eGFR = estimated glomerular filtration rate.
Chemotherapy
The most common systemic therapies used were single-agent capecitabine (45%) and irinotecan (22%, Table ii). The goal of therapy was palliative in 95% of the cohort, and 98% initiated treatment as outpatients. More than half the treatment instances (53%) constituted first-line therapy, and 27 patients received more than 1 line of systemic therapy. Dose adjustments at first cycle occurred in 67% of chemotherapy instances.
TABLE II.
Chemotherapy characteristics
| Characteristic | Value (%) |
|---|---|
| Treatment goal | |
| Palliative | 95.5 |
| Adjuvant | 3.4 |
| Neoadjuvant | 1.1 |
| Concurrent chemoradiation | 0 |
| Curative | 0 |
| Treatment line | |
| First | 53.4 |
| Second | 34.1 |
| Third | 9.1 |
| Fourth or more | 3.4 |
| Multiple lines | |
| Yes | 59.1 |
| No | 40.9 |
| Dose adjustment at first cycle | |
| Yes | 67.1 |
| No | 32.9 |
| Chemotherapy type | |
| Capecitabine | 45.5 |
| Irinotecan | 21.6 |
| FOLFOX | 10.2 |
| XELOX | 9.1 |
| FOLFIRI | 6.9 |
| Fluorouracil | 2.3 |
| Carboplatin–gemcitabine | 1.1 |
| XELIRI | 1.1 |
| IFL | 1.1 |
| Raltitrexed | 1.1 |
FOLFOX = 5-fluorouracil–oxaliplatin–leucovorin; XELOX = capecitabine–oxaliplatin; FOLFIRI = 5-fluorouracil–irinotecan–leucovorin; XELIRI = capecitabine–irinotecan; IFL = irinotecan–5-fluorouracil–leucovorin.
Outcomes
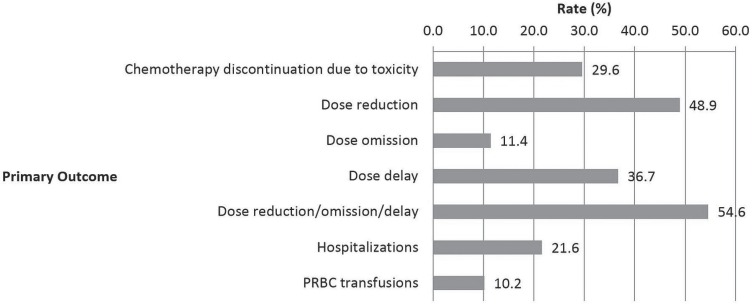
Chemotherapy was discontinued because of toxicity in 30% of the instances. A dose reduction occurred in 49%, a dose omission in 11%, and a dose delay in 37% (Figure 1). Overall, 55% instances involved at least 1 dose reduction (excluding an initial dose reduction at first cycle), omission, or delay after initiation of treatment. In fewer than one third of the instances (31%) was the course of chemotherapy completed without a dose reduction, omission, or delay, or discontinuation because of toxicity. Hospitalization during or within 30 days of chemotherapy occurred in 22% of the instances. Blood transfusions were given in 10% of the treatment instances.
FIGURE 1.
Primary outcomes: rates of dose adjustments, hospitalizations, and blood transfusions. PRBC = packed red blood cells.
At the end of data collection, the patients in 77% of the treatment instances had died (46 patients). The median os duration was 17.8 months [95% confidence interval (ci): 14.3 to 20.8 months]. On univariable and multivariable analyses, patients with anemia were less likely to survive [odds ratio (or): 0.58; 95% ci: 0.34 to 0.10; p = 0.05; and or: 0.29; 95% ci: 0.10 to 0.82; p = 0.02 respectively). On multivariable analysis, patients who received 2 or more lines of chemotherapy experienced a significantly longer os (or: 1.82; 95% ci: 1.10 to 3.02; p = 0.02; Table iii).
TABLE III.
Factors associated with overall survival
| Factor | Comparator | Analysis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Univariable | Multivariable | ||||||
|
|
|
||||||
| Estimate | 95% CL | p Value | Estimate | 95% CL | p Value | ||
| Age ≥85 years | <85 Years | 0.88 | 1.50, 0.51 | 0.63 | 0.72 | 0.37, 1.40 | 0.33 |
| Male sex | Female | 1.13 | 0.66, 1.91 | 0.66 | 1.00 | 0.53, 1.89 | 0.99 |
| Hemoglobin < 100 g/L | ≥100 g/L | 0.56 | 0.34, 1.00 | 0.05 | 0.29 | 0.10, 0.82 | 0.02 |
| White blood cells > 11×109/L | ≤11×109/L | 0.67 | 0.39, 1.13 | 0.13 | 1.68 | 0.57, 4.93 | 0.35 |
| eGFR < 60 mL/min/1.73 m2 | ≥60 mL/min/1.73 m2 | 1.05 | 0.63, 1.74 | 0.86 | 1.01 | 0.51, 1.99 | 0.98 |
| Palliative therapy | Others | 1.88 | 0.56, 6.28 | 0.30 | 2.38 | 0.45, 12.52 | 0.31 |
| Modified CCI score ≥ 2 | <2 | 1.31 | 0.79, 2.19 | 0.29 | 1.57 | 0.80, 3.07 | 0.19 |
| ≥6 Medications | 0–5 Medications | 0.94 | 0.58, 1.50 | 0.78 | 1.16 | 0.67, 2.00 | 0.60 |
| First dose adjusted | Unadjusted | 1.34 | 0.81, 2.22 | 0.25 | 1.20 | 0.68, 2.13 | 0.54 |
| Second-line or greater chemotherapy | First-line | 1.52 | 0.95, 2.42 | 0.08 | 1.82 | 1.10, 3.02 | 0.02 |
CL = confidence limits; eGFR = estimated glomerular filtration rate; CCI = Charlson comorbidity index.
Baseline factors (age, sex, hemoglobin < 100 g/L, wbcs > 11×109/L, egfr < 60 mL/min/1.73 m2, modified cci ≥ 2, baseline medications ≥ 6, dose adjustment at first dose, and number of therapy lines received) were tested against our co-primary outcomes: rate of hospitalization; therapy discontinuation because of toxicity; and dose reductions, omissions, or delays.
Hospitalizations were not associated with any of the factors studied in the univariable analysis (Table iv). On multivariable analysis, hospitalizations were more common in patients 85 years of age and older (or: 4.24; 95% ci: 0.93 to 19.38; p = 0.06) and in male patients (or: 5.89; 95% ci: 1.17 to 29.66; p = 0.03).
TABLE IV.
Factors associated with hospitalization
| Factor | Comparator | Analysis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Univariable | Multivariable | ||||||
|
|
|
||||||
| Estimate | 95% CL | p Value | Estimate | 95% CL | p Value | ||
| Age ≥ 85 years | <85 Years | 2.55 | 0.80, 8.14 | 0.11 | 4.24 | 0.93, 19.38 | 0.06 |
| Male sex | Female | 3.24 | 0.89, 11.73 | 0.07 | 5.89 | 1.17, 29.66 | 0.03 |
| Hemoglobin < 100 g/L | ≥100 g/L | 0.44 | 0.13, 1.51 | 0.19 | 0.17 | 0.02, 1.18 | 0.07 |
| White blood cells > 11×109/L | ≤11×109/L | 0.71 | 0.23, 2.17 | 0.55 | 4.07 | 0.55, 29.95 | 0.17 |
| eGFR < 60 mL/min/1.73 m2 | ≥60 mL/min/1.73 m2 | 2.00 | 0.63, 6.36 | 0.24 | 1.85 | 0.54, 6.40 | 0.33 |
| Palliative therapy | Others | 0.25 | 0.03, 1.93 | 0.18 | 0.11 | 0.01, 1.10 | 0.06 |
| Modified CCI score ≥ 2 | <2 | 1.26 | 0.42, 3.78 | 0.68 | 0.81 | 0.20, 3.32 | 0.77 |
| ≥6 Medications | 0–5 Medications | 1.43 | 0.45, 4.55 | 0.55 | 1.75 | 0.42, 7.37 | 0.44 |
| Adjusted first dose | Unadjusted | 1.13 | 0.37, 3.48 | 0.83 | 1.97 | 0.52, 7.52 | 0.32 |
| Second-line or greater chemotherapy | First-line | 0.98 | 0.36, 2.68 | 0.97 | 1.23 | 0.36, 4.24 | 0.74 |
CL = confidence limits; eGFR = estimated glomerular filtration rate; CCI = Charlson comorbidity index.
On univariable analysis, patients with a modified cci less than 2 were significantly less likely to discontinue therapy because of toxicity (Table v; or: 0.34; 95% ci: 0.16 to 0.97; p = 0.04). Higher egfr was also associated with a lower rate of discontinuation, although that association did not reach statistical significance (or: 0.42; 95% ci: 0.17 to 1.01; p = 0.05). On multivariable analysis, no factors were associated with discontinuation of chemotherapy because of toxicity. On multivariable analysis, patients who needed no dose reductions, omissions, or delays and who did not discontinue chemotherapy because of toxicity were more likely to have a wbc count exceeding 11×109/L (or: 16.81; 95% ci: 1.60 to 176.48; p = 0.02).
TABLE V.
Factors associated with chemotherapy discontinuation because of toxicity
| Factor | Comparator | Analysis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Univariable | Multivariable | ||||||
|
|
|
||||||
| Estimate | 95% CL | p Value | Estimate | 95% CL | p Value | ||
| Age ≥ 85 years | <85 Years | 1.83 | 0.70, 4.79 | 0.22 | 4.12 | 0.91, 18.75 | 0.07 |
| Male sex | Female | 1.66 | 0.68, 4.00 | 0.26 | 2.38 | 0.75, 7.57 | 0.14 |
| Hemoglobin < 100 g/L | ≥100 g/L | 0.87 | 0.34, 2.20 | 0.77 | 2.11 | 0.09, 47.32 | 0.64 |
| White blood cells > 11×109/L | ≤11×109/L | 0.70 | 0.29, 1.65 | 0.41 | 0.53 | 0.03, 10.41 | 0.68 |
| eGFR < 60 mL/min/1.73 m2 | ≥60 mL/min/1.73 m2 | 0.42 | 0.17, 1.01 | 0.05 | 0.36 | 0.11, 1.13 | 0.08 |
| Palliative therapy | Others | 1.34 | 0.14, 13.29 | 0.80 | 0.91 | 0.11, 7.51 | 0.93 |
| Modified CCI score ≥ 2 | <2 | 0.34 | 0.16, 0.97 | 0.04 | 0.51 | 0.20, 1.32 | 0.17 |
| ≥6 Medications | 0–5 Medications | 1.31 | 0.51, 3.38 | 0.58 | 2.13 | 0.77, 5.86 | 0.15 |
| Adjusted first dose | Unadjusted | 0.65 | 0.23, 1.78 | 0.40 | 0.72 | 0.20, 2.60 | 0.62 |
| Second-line or greater chemotherapy | First-line | 1.38 | 0.58, 3.33 | 0.47 | 1.51 | 0.52, 4.39 | 0.45 |
CL = confidence limits; eGFR = estimated glomerular filtration rate; CCI = Charlson comorbidity index.
Dose reductions, omissions, and delays were not associated with any of the factors studied in either univariable or multivariable analysis (Table vi).
TABLE VI.
Factors associated with chemotherapy dose reduction, omission, or delay
| Factor | Comparator | Analysis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Univariable | Multivariable | ||||||
|
|
|
||||||
| Estimate | 95% CL | p Value | Estimate | 95% CL | p Value | ||
| Age ≥ 85 years | <85 Years | 1.27 | 0.50, 3.23 | 0.61 | 0.58 | 0.19, 1.81 | 0.35 |
| Male sex | Female | 0.64 | 0.28, 1.49 | 0.30 | 0.60 | 0.20, 1.86 | 0.38 |
| Hemoglobin < 100 g/L | ≥100 g/L | 0.83 | 0.30, 2.33 | 0.72 | 1.63 | 0.25, 10.50 | 0.61 |
| White blood cells > 11×109/L | ≤11×109/L | 0.73 | 0.26, 2.04 | 0.55 | 0.34 | 0.05, 2.14 | 0.25 |
| eGFR < 60 mL/min/1.73 m2 | ≥60 mL/min/1.73 m2 | 1.16 | 0.50, 2.72 | 0.73 | 1.33 | 0.46, 3.91 | 0.60 |
| Palliative therapy | Others | 0.40 | 0.04, 4.02 | 0.43 | 0.91 | 0.07, 11.76 | 0.94 |
| Modified CCI score ≥ 2 | <2 | 1.98 | 0.83, 4.73 | 0.13 | 2.44 | 0.85, 7.00 | 0.10 |
| ≥6 medications | 0–5 Medications | 0.74 | 0.30, 1.82 | 0.52 | 0.58 | 0.19, 1.81 | 0.35 |
| Adjusted first dose | Unadjusted | 0.62 | 0.24, 1.62 | 0.33 | 0.70 | 0.23, 2.16 | 0.53 |
| Second-line or greater chemotherapy | First-line | 0.55 | 0.23, 1.35 | 0.19 | 0.46 | 0.17, 1.27 | 0.14 |
CL = confidence limits; eGFR = estimated glomerular filtration rate; CCI = Charlson comorbidity index.
DISCUSSION
Our study of mcrc patients 80 years of age and older revealed high rates of chemotherapy discontinuation because of toxicity (30%) and of chemotherapy dose reductions, omissions, or delays (49%, 11%, and 37% respectively). Hospitalization within 30 days of chemotherapy was high at 22%, and more common in men and in patients 85 years of age and older. The median os in the cohort was 17.8 months. These results are of important clinical relevance and should, at the very least, lead physicians to carefully consider the pros and cons of chemotherapy in this population group, which has already undergone selection bias to be receiving chemotherapy.
Elderly patients are often underrepresented in clinical trials14,19,20, and patients 70 years of age and older constituted fewer than 20% of the subjects in important crc studies21,22. Many observational studies include patients with nonmetastatic stage iii crc, and their “elderly” patients are defined as 65–80 years of age. Given the limited studies available, we chose to study only octogenarian mcrc patients.
Chemotherapy is often prescribed and used differently in the geriatric population. A population-based study (n = 27,805)23 showed that, despite proven survival benefits, adjuvant chemotherapy was given to only 30% of stage iii colon cancer patients 75 years of age and older; 68% of 50- to 74-year-olds received such therapy. Alam and colleagues24 found age to be the strongest discriminating factor in determining whether patients 75 years of age and older with colon cancer (n = 445) received chemotherapy. Di Bartolomeo et al.25 suggested that age-related differences in treatment regimens can be attributed to “higher refusal rates, declining of functional and mental status, hospital volume and socioeconomic factors ... perceive[d] lowering of treatment efficacy and tolerance with advancing age.” Their review also states that phase iii randomized controlled trials focused on elderly patients do not assess the safety and efficacy of specific systemic therapies. Older patients with advanced colon cancer have significantly more comorbidities, reported at 60% in those 70 years of age and older compared with 35% in those younger than 7026,27. In our study, the patient in 53% of the treatment instances had a modified cci score of 2 or greater, indicating at least 1 comorbidity in addition to metastatic disease. Interestingly, our patients with a modified cci score of 2 or greater were also more likely to discontinue chemotherapy because of toxicity. The rate of chemotherapy discontinuation in our study was high at 30%, but other factors (age, sex, hemoglobin < 100 g/L, wbcs > 11×109/L, egfr < 60 mL/min/1.73 m2, baseline medications ≥ 6, dose adjustment at first dose, and number of therapy lines received) were not associated with that result. Reddy et al.28 studied 33 crc patients 80 years of age and older, reporting that 12% discontinued therapy because of toxicity. The prospective phase iii trial of chemotherapy management in mcrc patients 74 years of age and older conducted by Shayne et al.29 did not note any chemotherapy discontinuation because of toxicity. It is important to note that chemotherapy might also be refused by the patient as a treatment option to begin with, or after initiation29. Our cohort consisted only of patients who agreed to initiate chemotherapy; we did not investigate the possibility of patient refusal as a reason for chemotherapy discontinuation.
We also noted high rates of therapy dose reductions, omissions, or delays. Dose reductions at first cycle occurred in 67% of treatment instances, and further dose reductions were noted in 49% of cases. Shayne and colleagues29 showed that about half their elderly cancer patients received chemotherapy at doses less than 85% of standard. The study of crc patients 80 years of age and older by Reddy et al.28 noted that all their patients required dose reductions. Other studies of crc patients 70 years of age and older noted varying rates of dose reductions between 46% and 63%30,31.
Chemotherapy dosing can also vary based on factors other than age—for example, hepatic and renal function32. In our study, capecitabine was the most commonly used therapy, and therefore the reductions in dosage might accord with the published adjusted renal doses rather than be attributable to age alone. In addition to dose reductions, we also recorded dose omissions and delays because of toxicity. Rates of chemotherapy toxicities in elderly crc patients range widely in the few available studies, with grade 3 or 4 toxicities reported in 12%–65% of patients22,30,31, making it difficult to compare our results with the available data.
Chemotherapy with multiple agents has become the standard of care in mcrc; however, data on the outcomes with multiple-agent chemotherapy in elderly mcrc patients are limited and contentious30,33,34. Combination therapy with folfox (5-fluorouracil–leucovorin–oxaliplatin) or folfiri (irinotecan–5-fluorouracil–leucovorin) is often considered in the first-line treatment of unresectable mcrc35. Our analysis noted that the greatest proportion of patients (67%) were being treated with capecitabine or irinotecan monotherapy. There is emerging evidence that, compared with upfront combination chemotherapy, a sequential monotherapy treatment strategy could be advantageous in elderly and frail patients with mcrc. A recent meta-analysis by Asmis and colleagues36 noted a greater response rate and progression-free survival (pfs) and an os benefit with upfront combination therapy; however, the os benefit was small and of questionable clinical benefit (hazard ratio: 0.92; 95% ci: 0.86 to 0.99; p = 0.02).
Notwithstanding its benefits, upfront combination chemotherapy led to lower quality-of-life scores and higher rates of hematologic toxicities such as neutropenia, and of nonhematologic toxicities such as nausea, vomiting, and diarrhea. In fact, one trial in the meta-analysis37 specifically included elderly and frail patients and noted significantly more diarrhea and sensory neuropathy in the combination arm and more hand–foot syndrome in the monotherapy arm despite upfront dose reductions for both arms. Interestingly, 13% of the focus2 trial population was more than 80 years of age, and despite an upfront dose reduction, 49% of patients required further dose reduction or discontinuation by 12 weeks of treatment37. In addition, focus2 found no significant pfs or os benefit from upfront combination therapy in its population of frail and elderly mcrc patients37. Ultimately, the option of monotherapy sequencing (with fewer side effects) and an option to eventually escalate to combination therapy might be the best option in a palliative population in whom quality of life and reduction of the toxicity burden takes precedence over a small survival benefit.
With new targeted therapies, the median os for patients with mcrc is close to 29 months after diagnosis38–43. Our cohort had a mean survival of 17.8 months despite the fact that 67% of their treatment instances involved monotherapy regimens. There is evidence that doublet chemotherapy fails to provide an os benefit compared with single-agent 5-fluorouracil, while engendering increased rates of toxicity as demonstrated in the meta-analysis by Landre and colleagues44, who compared doublet chemotherapy with 5-fluorouracil monotherapy in elderly patients more than 75 years of age. However, there is also clear evidence that elderly patients can obtain a survival benefit from simpler combination regimens. The avex trial added bevacizumab to capecitabine and found that pfs was significantly longer with that combination than with capecitabine monotherapy in elderly patients who were not candidates for irinotecan- or oxaliplatin-based regimens45. Moreover, when combination chemotherapy is initiated in elderly patients, its efficacy is comparable to that in younger patients, as noted by similar response rates and pfs with folfiri use in patients more than 70 years of age compared with those 70 years of age and younger46. In fact, studies investigating the effects of age and comorbidity on survival have identified a significant correlation of comorbidity score with survival and a correlation of age with comorbidity burden, but no effect of increasing age on survival47. A retrospective study by Aparicio and colleagues48 in patients 75 years of age and older (n = 110) showed, as other independent risk factors for poor survival, the presence of metastasis (hazard ratio: 3.9; 95% ci: p = 0.005) and cci score greater than 3 (hazard ratio: 28.9; 95% ci: 2.5 to 335.6; p = 0.001). In our cohort, modified cci scores of 2 or greater and 5 or greater did not affect os, and all our patients had metastatic disease. We did not specifically record which comorbidities in the cci score were most common, because our focus was on whether higher scores led to poorer outcomes. We recognize that certain comorbidities could have been associated with particular outcomes—an issue that should be addressed in future studies.
In addition to comorbidity and metastatic disease, ps is believed to have a significant effect on survival. In an analysis of patients being treated for mcrc with first-line chemotherapy, Crosara Teixeira and colleagues49 demonstrated that ecog ps was directly associated with os, median survival duration being 18.4 months for chemotherapy-treated patients with an ecog score of 0–1, 10.8 months for those with an ecog ps of 2, and 6.8 months for those with an ecog ps of 3–4. Considering that a large proportion of the patients in our study had an ecog ps of 0–1, and that more than half had a modified cci score of 2 or greater, the resulting median os of 17.8 months is quite good in this older—and conceivably frailer—population. Although it is difficult to make direct cross-trial comparisons, the focus trial37 reported a median os of 10–12.4 months depending on the treatment initiated, and a median os of 16.8–20.7 months was noted in the avex trial patients45. Thus, despite upfront dose reductions and the dose alterations noted in our analysis, the median os in our cohort falls into line with published evidence.
“Ageing is a progressive decline of multiple organ functions, with an increased prevalence of comorbidity conditions ... influencing treatment decision-making due to decrease of life expectancy and tolerance to chemotherapy”25. Many geriatric screening and assessment tools have been developed. The activities of daily living (adl) tool50 and the instrumental adl tool51 were not found to be useful in predicting chemotherapy toxicity27, but only 34 patients were enrolled in that prospective study. Comorbidities have been shown to play an important prognostic role in determining survival and to affect treatment benefit. In our study, we used the cci score in logistic regression analyses of our primary outcomes, revealing a lower rate of chemotherapy discontinuation because of toxicity in patients with modified cci scores of less than 2. The lack of other associations in our study alludes the need for a more comprehensive tool. Di Bartolomeo and colleagues25 suggested that the comprehensive geriatric assessment provides “the best estimates of individual functional reserve.”
In addition to comorbidities, acute hospitalizations in the elderly population are common, and rates increase with age. In 2004–2005, Canadians 60 years of age and older represented 43% of hospitalizations and 60% of total hospital days52. Cancer was the 4th leading cause of hospitalization in those 65 years of age and older53. In Canada, the hospitalization rate is higher in men older than 6554. In our study, patients in 20% of treatment instances required hospitalization within 30 days of chemotherapy delivery, and hospitalization was more common in men and in patients 85 years of age and older. The hospitalization rate in our study is within the range described in other publications. Reddy and colleagues28 reported a treatment-related hospitalization rate of 73% in their retrospective study of 33 elderly crc patients. A retrospective study by Schrag et al.55 of 6262 crc patients 65 years of age and older reported increasing chemotherapy-related hospitalizations with age, with a rate of 13% in those aged 85–89. Notably, we did not record the specific cause for admission, but we made every effort to distinguish therapy-related from non-therapy-related admissions.
Hospitalizations in elderly patients are not only more common, they are often detrimental. One of the most detrimental effects is loss of independence, especially in adls. Covinsky and his team56 studied 2293 hospitalized patients 70 years of age and older and found that the frequency of adl decline increased with age, from 38% in those 80–84 years of age up to 63% in those 90 years of age or older. In addition, they reported that failure to recover the loss of adl function increased significantly with age56. Similarly, a prospective cohort study by Wu et al.57 of 804 admitted patients 80 years of age and older showed that 42% of patients without dependencies at baseline developed one or more limitations 2 months after admission, and 41% continued to have limitations 12 months later.
Another effect of hospitalization is delirium, reported in 10%–15% of seniors at admission, and developing in 15%–25% during hospitalization53. Delirium results in a higher fall risk and longer length of stay58 and is often difficult to treat because the underlying cause can be multifactorial. Other complications that can arise from hospitalization of vulnerable patients include adverse events related to medications, nosocomial infections, malnutrition, dehydration, immobilization, and pressure ulcers59.
Limitations of our research include small sample size and the retrospective nature of the data collection. In some cases, data such as ecog ps scores and laboratory results were missing. Another example of the limitations of retrospective research is found in the capture of toxicities. We could reliably capture only objective measures (blood values, transfusions, hospitalizations, etc.) because routine clinical notes varied widely in how other toxicities were recorded, and they rarely used the type of standardized reporting criteria that are seen in a clinical trial setting. Doses of chemotherapy were not compared, nor were specific descriptions and grading of toxicities noted. Patients were excluded if they received biologic therapies, because the initial study included a heterogeneous sample of solid tumour types, stages, and treatment intents. As a result, we could not account for the effect that biologic agents can have on outcomes when given in conjunction with chemotherapy. We did not record the specific causes of hospitalization, but only admissions longer than 24 hours in duration and within 30 days of chemotherapy provision—efforts that were meant to ensure that only chemotherapy-related admissions were included. However, causes for admission can often be multifactorial. Capturing that information in future studies would be useful in guiding chemotherapy monitoring in elderly patients. Finally, no control group was included for comparison—for example, a cohort 80 years of age and older who did not receive chemotherapy either because of refusal or other reasons (such as frailty).
After this study, an important next step is to develop a geriatric assessment tool to identify elderly patients suitable for chemotherapy. A prospective study of the mcrc population 80 years of age and older, including a control group, would be useful to highlight other factors such as patient perceptions and wishes with respect to chemotherapy and related outcomes. Close monitoring of patients to assess tolerance for and toxicities from various therapies and dosing regimens is also needed.
CONCLUSIONS
Our study revealed relatively high rates of adverse outcomes in the population 80 years of age and older receiving chemotherapy for mcrc. Feliu and colleagues22 conclude from their review that, for elderly patients with advanced crc, “age itself should not be a contraindication to rule out the chemotherapy administration, even for second or third line treatment.” Given the limited evidence in this area, the aging population, and the consequent increased crc incidence, development of a simpler and comprehensive, yet effective geriatric assessment scale is essential to optimize clinical decision-making for elderly mcrc patients.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: TA has received grants for outside submitted work from Sanofi, Roche, and Pfizer, and fellowship funding from Roche. TA has also acted as a consultant for Sanofi, Amgen, and Roche.
REFERENCES
- 1.World Cancer Research Fund International (wcrf) Home > Cancer Facts and Figures > Worldwide data [Web page] London, UK: WCRF; 2012. [Available at: http://www.wcrf.org/int/cancer-facts-figures/worldwide-data; cited 15 April 2015] [Google Scholar]
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. [Google Scholar]
- 3.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci), Surveillance, Epidemiology, and End Results Program . SEER Stat Fact Sheets: Colon and Rectum Cancer [Web page] Bethesda, MD: NCI; n.d. [Available at: http://seer.cancer.gov/statfacts/html/colorect.html; cited 15 April 2015]. [Google Scholar]
- 4.Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer—a tale of two drugs: implications for biochemical modulation. J Clin Oncol. 1997;15:368–81. doi: 10.1200/JCO.1997.15.1.368. [DOI] [PubMed] [Google Scholar]
- 5.Thirion P, Michiels S, Pignon JP, et al. on behalf of the Meta-Analysis Group in Cancer Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766–75. doi: 10.1200/JCO.2004.03.104. [Erratum in: J Clin Oncol 2005;23:1337–8] [DOI] [PubMed] [Google Scholar]
- 6.Nordic Gastrointestinal Tumor Adjuvant Therapy Group Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. J Clin Oncol. 1992;10:904–11. doi: 10.1200/JCO.1992.10.6.904. [DOI] [PubMed] [Google Scholar]
- 7.Scheithauer W, Rosen H, Komek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–5. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000;321:531–5. doi: 10.1136/bmj.321.7260.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part i. Cancer J. 2005;11:449–60. doi: 10.1097/00130404-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Sehl M, Sawhney R, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part ii. Cancer J. 2005;11:461–73. doi: 10.1097/00130404-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123–34. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2123::AID-CNCR6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Yancik R, Ries LA. Cancer in older persons. Magnitude of the problem—how do we apply what we know? Cancer. 1994;74(suppl):1995–2003. doi: 10.1002/1097-0142(19941001)74:7+<1995::AID-CNCR2820741702>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Howard DH, Kauh J, Lipscomb J. The value of new chemotherapeutic agents for metastatic colorectal cancer. Arch Intern Med. 2010;170:537–42. doi: 10.1001/archinternmed.2010.36. [DOI] [PubMed] [Google Scholar]
- 14.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 15.Murphy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials—race, sex, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 16.Employment and Social Development Canada . Canadians in Context – Aging Population [archived Web page] Ottawa, ON: Government of Canada; 2015. [Available at: http://www4.hrsdc.gc.ca/.3ndic.1t.4r@-eng.jsp?iid=33; cited 15 April 2015] [Google Scholar]
- 17.Statistics Canada . Section 2: Population by age and sex [Web page] Ottawa, ON: Statistics Canada; 2013. [Available at: http://www.statcan.gc.ca/pub/91-215-x/2012000/part-partie2-eng.htm; cited 15 April 2015] [Google Scholar]
- 18.Sud S, Lai P, Zhang T, Clemons M, Wheatley-Price P. Chemotherapy in the oldest old: the feasibility of delivering cytotoxic therapy to patients 80 years old and older. J Geriatr Oncol. 2015;6:395–400. doi: 10.1016/j.jgo.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–23. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol. 2008;67:255–62. doi: 10.1016/j.critrevonc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Honecker F, Köhne C, Bokemeyer C. Colorectal cancer in the elderly: is palliative chemotherapy of value? Drugs Aging. 2003;20:1–11. doi: 10.2165/00002512-200320010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Feliu J, Sereno M, Castro JD, Belda C, Casado E, González-Barón M. Chemotherapy for colorectal cancer in the elderly: whom to treat and what to use. Cancer Treat Rev. 2009;35:246–54. doi: 10.1016/j.ctrv.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Abraham A, Habermann EB, Rothenberger DA, et al. Adjuvant chemotherapy for stage iii colon cancer in the oldest old: results beyond clinical guidelines. Cancer. 2013;119:395–403. doi: 10.1002/cncr.27755. [DOI] [PubMed] [Google Scholar]
- 24.Alam M, Gabriel G, Barton M, Eek R. Discriminating factors in treatment decisions for chemotherapy in elderly patients with colorectal cancer. Cancer Forum. 2008;32 [Available online at: http://cancerforum.org.au/forum/2008/march/discriminating-factors-in-treatment-decisions-for-chemotherapy-in-elderly-patients-with-colorectal-cancer; cited 24 March 2016] [Google Scholar]
- 25.Di Bartolomeo M, Pietrantonio F, Biondani P, de Braud F. Adjuvant treatment of colorectal cancer in the elderly: where do we come from and where are we going? J Solid Tumors. 2012;2:38–46. doi: 10.5430/jst.v2n3p38. [DOI] [Google Scholar]
- 26.De Marco MF, Janssen-Heijnen ML, van der Heijden LH, Coebergh JW. Comorbidity and colorectal cancer according to subsite and stage: a population-based study. Eur J Cancer. 2000;36:95–9. doi: 10.1016/S0959-8049(99)00221-X. [DOI] [PubMed] [Google Scholar]
- 27.Daniele B, Rosati G, Tambaro R, et al. First-line chemotherapy with fluorouracil and folinic acid for advanced colorectal cancer in elderly patients: a phase ii study. J Clin Gastroenterol. 2003;36:228–33. doi: 10.1097/00004836-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Reddy N, Yu J, Fakih MG. Toxicities and survival among octogenarians and nonagenarians with colorectal cancer treated with chemotherapy or concurrent chemoradiation therapy. Clin Colorectal Cancer. 2007;6:362–6. doi: 10.3816/CCC.2007.n.005. [DOI] [PubMed] [Google Scholar]
- 29.Shayne M, Culakova E, Poniewierski MS, et al. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer. 2007;110:1611–20. doi: 10.1002/cncr.22939. [DOI] [PubMed] [Google Scholar]
- 30.Figer A, Perez-Staub N, Carola E, et al. folfox in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the optimox1 study. Cancer. 2007;110:2666–71. doi: 10.1002/cncr.23091. [DOI] [PubMed] [Google Scholar]
- 31.Hochster HS, Luo W, Popa EC, et al. Phase ii study of uracil–tegafur with leucovorin in elderly (≥75 years old) patients with colorectal cancer: ecog 1299. J Clin Oncol. 2007;25:5397–402. doi: 10.1200/JCO.2006.10.4521. [DOI] [PubMed] [Google Scholar]
- 32.Cassidy J, Twelves C, Van Cutsem E, et al. on behalf of the Capecitabine Colorectal Cancer Study Group First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566–75. doi: 10.1093/annonc/mdf089. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–91. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 34.Arkenau HT, Graeven U, Kubicka S, et al. on behalf of the aio Colorectal Study Group Oxaliplatin in combination with 5-fluorouacil/leucovorin or capecitabine in elderly patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2008;7:60–4. doi: 10.3816/CCC.2008.n.009. [DOI] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Fort Washington, PA: NCCN; 2015. Ver 22016. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (free registration required); cited 31 March 2015] [DOI] [PubMed] [Google Scholar]
- 36.Asmis T, Berry S, Cosby R, Chan K, Coburn N, Rother M. Strategies of sequential therapies in unresectable metastatic colorectal cancer: a meta-analysis. Curr Oncol. 2014;21:318–28. doi: 10.3747/co.21.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seymour MT, Thompson LC, Wasan HS, et al. on behalf of the focus2 Investigators and the National Cancer Research Institute Colorectal Cancer Clinical Studies Group Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (mrc focus2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–59. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giacchetti S, Perpoint B, Zidani N, et al. Phase iii multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–47. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 39.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan and oxaliplatin combinations in patients with previously untreated metastatic colorectal. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 41.Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage ii and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30:3353–60. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 42.André T, Boni C, Mounedji-Boudiaf L, et al. on behalf of the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (mosaic) investigators Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 43.Venook AP, Niedzwiecki D, Lenz HJ, et al. calgc/swog 80405: phase iii trial of irinotecan/5-fu/leucovorin (folfiri) or oxaliplatin/5-fu/leucovorin (mfolfox6) with bevacizumab (bv) or cetuximab (cet) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (mcrc) [abstract LBA3] J Clin Oncol. 2014;32 [Available online at: http://meetinglibrary.asco.org/content/126013-144; cited 24 March 2016] [Google Scholar]
- 44.Landre T, Uzzan B, Nicolas P, et al. Doublet chemotherapy vs. single-agent therapy with 5fu in elderly patients with metastatic colorectal cancer: a meta-analysis. Int J Colorectal Dis. 2015;30:1305–10. doi: 10.1007/s00384-015-2296-5. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham D, Lang I, Marcuello E, et al. on behalf of the avex study investigators Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (avex): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–85. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 46.Jackson NS, Barrueco J, Soufi-Mahjoubi R, et al. Comparing safety and efficacy of first-line irinotecan/fluoropyrimidine combinations in elderly versus nonelderly patients with metastatic colorectal cancer: findings from the bolus, infusional, or capecitabine with Camptosar–celecoxib study. Cancer. 2009;115:2617–29. doi: 10.1002/cncr.24305. [DOI] [PubMed] [Google Scholar]
- 47.Asmis T, Powell E, Karapetis CS, et al. Comorbidity, age and overall survival in cetuximab-treated patients with advanced colorectal cancer (acrc)—results from ncic ctg co.17: a phase iii trial of cetuximab versus best supportive care. Ann Oncol. 2011;22:118–26. doi: 10.1093/annonc/mdq309. [DOI] [PubMed] [Google Scholar]
- 48.Aparicio T, Navazesh A, Boutron I, et al. Half of elderly patients routinely treated for colorectal cancer receive a sub-standard treatment. Crit Rev Oncol Hematol. 2009;71:249–57. doi: 10.1016/j.critrevonc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Crosara Teixeria M, Marques DF, Ferrari AC, et al. The effects of palliative chemotherapy in metastatic colorectal cancer patients with an ecog performance status of 3 and 4. Clin Colorectal Cancer. 2015;14:52–7. doi: 10.1016/j.clcc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Katz S. Assessing self maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–7. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 51.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 52.Canadian Institute for Health Information (cihi) Inpatient Hospitalizations and Average Length of Stay Trends in Canada, 2003–2004 and 2004–2005. Ottawa, ON: CIHI; 2005. [Available online at: https://secure.cihi.ca/free_products/hmdb_analysis_in_brief_e.pdf; cited 2 May 2015] [Google Scholar]
- 53.Public Health Agency of Canada (phac) Leading Causes of Deaths and Hospitalizations in Canada [Web page] Ottawa, ON: PHAC; 2008. [Available at: http://www.phac-aspc.gc.ca/publicat/lcd-pcd97/index-eng.php; cited 2 May 2015] [Google Scholar]
- 54.Health Canada . Canada’s Aging Population. Ottawa, ON: Minister of Public Works and Government Services Canada; 2002. [Available online at: http://publications.gc.ca/collections/Collection/H39-608-2002E.pdf; cited 15 April 2015] [Google Scholar]
- 55.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage iii colon cancer. J Natl Cancer Inst. 2001;93:850–7. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 56.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–8. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 57.Wu AW, Yasui Y, Alzola C, et al. Predicting functional status outcomes in hospitalized patients aged 80 years and older. J Am Geriatr Soc. 2000;48(suppl):S6–15. doi: 10.1111/j.1532-5415.2000.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 58.Public Health Agency of Canada (phac) The Chief Public Health Officer’s Report on The State of Public Health in Canada 2010. Ottawa, ON: Her Majesty the Queen in Right of Canada; 2010. [Available online at: http://www.phac-aspc.gc.ca/cphorsphc-respcacsp/2010/fr-rc/pdf/cpho_report_2010_e.pdf; cited 2 May 2015] [Google Scholar]
- 59.Walsh KA, Bruza JM. Review: hospitalization of the elderly. Ann Longterm Care. 2007;15:18–23. [Google Scholar]