Abstract
Background
The incidence of hepatocellular carcinoma (hcc) and the complexity of its diagnosis and treatment are increasing. We estimated trends in net health care utilization, costs of care attributable to hcc in Ontario, and rate ratios of resource use at various stages of care.
Methods
This population-based retrospective cohort study identified hcc patients and non-cancer control subjects, and health care resource utilization between 2002 and 2009. Generalized estimating equations were then used to estimate net health care utilization (hcc patients vs. the matched control subjects) and net costs of care attributable to hcc. Generalized linear models were used to analyze rate ratios of resource use.
Results
We identified 2832 hcc patients and 2808 matched control subjects. In comparison with the control subjects, hcc patients generally used a greater number of health care services. Overall, the mean net cost of care per 30 patient–days (2013 Canadian dollars) attributable to outpatient visits and hospitalizations was highest in the pre-diagnosis (1 year before diagnosis), initial (1st year after diagnosis), and end-of-life (last 6 months before death, short-term survivors) phases. Mean net homecare costs were highest in the end-of-life phase (long-term survivors). In the end-of-life phase (short-term survivors), mean net costs attributable to outpatient visits and total services significantly increased to $14,220 from $1,547 and to $33,121 from $14,450 (2008–2009 and 2002–2003 respectively).
Conclusions
In hcc, our study found increasing resource use and net costs of care, particularly in the end-of-life phase among short-term survivors. Our findings offer a basis for resource allocation decisions in the area of cancer prevention and control.
Keywords: Costs, cost analyses, economics, end-of-life care, health care utilization, liver cancer, survivors
INTRODUCTION
Hepatocellular carcinoma (hcc) is the 6th most common cancer and the 2nd most frequent cause of cancer-related death worldwide, accounting for approximately 600,000 deaths each year 1. The incidence of hcc is increasing worldwide; more than 500,000 new cases occur annually, accounting for more than 5% of all cancers 1. Cirrhosis often precedes hcc, and major risk factors for hcc include hepatitis B and C infections, hiv co-infection 2, alcohol- and non-alcohol-induced liver disease (typically nonalcoholic steatohepatitis), diabetes, obesity, and smoking 3–8.
In Canada, the incidence of hcc has increased significantly both for men (3.6% annually between 1970 and 2007) and for women (2.4% annually between 1986 and 2007) 9. The increase in the hcc incidence since the mid-1980s is related to the aging Canadian population, the significant domestic burden of hepatitis C, and the ongoing trend in immigration from high-risk hcc countries where hepatitis B and C infections are endemic 1,10,11. Additionally, hcc-related mortality rates increased in both sexes between 2000 and 2009, and are likely to continue to increase given the increase in hcc incidence 9. Because of a low hcc surveillance rate and the fact that hcc is generally asymptomatic until very late in the progression of the disease 12,13, patients are often diagnosed at an advanced stage of the disease at the time of referral for treatment, leading to relatively short survival periods 9,14.
Over the years, hcc has imposed a substantial burden on the Canadian health care system 15. The rising incidence of hcc and cancer-related mortality has pointed to a need for additional health care services and resources to be allocated for prevention, screening, and diagnostic, therapeutic, and supportive care strategies in Canada. To inform policy decision-makers, the objectives of the present study were therefore to estimate trends in net health care utilization and costs of care attributable to hcc in Ontario between 2002 and 2009, as well as the relative risks (rrs) of health care utilization at various stages of care.
METHODS
Study Design and Population
This population-based retrospective cohort study considered all eligible patients 18 years of age and older who were diagnosed with hcc in Ontario between 1 January 2002 and 31 December 2009. The study design included 3 key components and used an incidence-based approach.
Component 1 was a phase-of-care approach to estimate health care utilization and costs 16–20 that divided each patient’s care into 3 discrete care phases: pre-diagnosis, initial, and end-of-life. The pre-diagnosis phase, often a resource-intensive component of cancer care episodes 21,22, was defined as the 12 months before diagnosis. That period was chosen so as also to capture any screening that might have occurred during that period. The initial phase was defined as the first 12 months after diagnosis. The end-of-life phase was defined as the 6 months preceding death, with that analysis including only patients who died during the study period (2002–2011). Depending on length of time from diagnosis to death, patients who died were stratified into short-term survivors (survived <6 months) and long-term survivors (survived ≥6 months). A hierarchal approach (end-of-life period > initial period) was used so that all phases were mutually exclusive. For example, if a patient died 10 months after diagnosis, 6 months was allotted to the end-of-life period, and 4 months, to the initial period.
Component 2 was an estimation of the mean net health care utilization attributable to hcc (difference between hcc patients and matched non-cancer control subjects in the mean number of health services used) and of the rr for health care utilization, overall and by year of diagnosis (“index year,” for the pre-diagnosis and initial phases) and year of death (for the end-of-life phase).
Component 3 was an estimation of the net cost of care attributable to hcc.
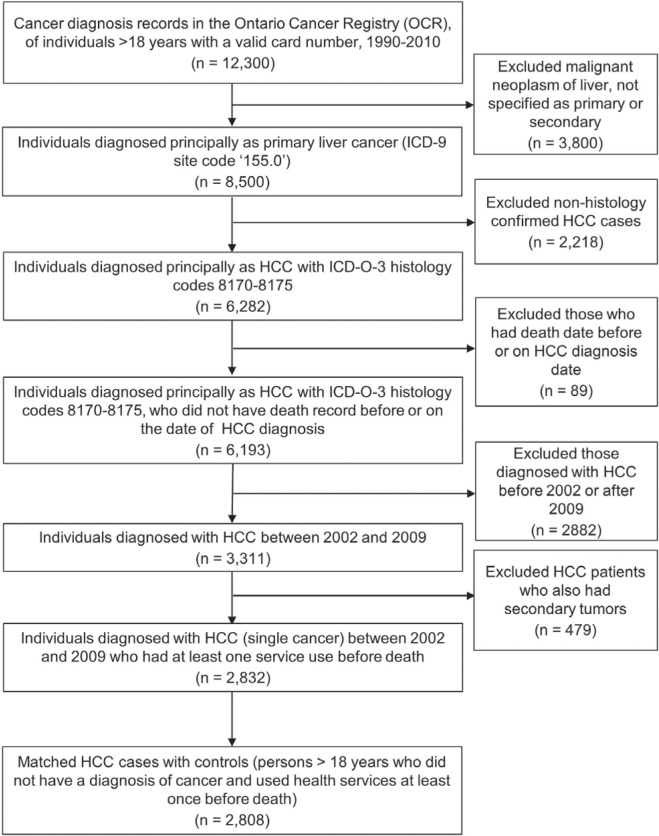
The Ontario Cancer Registry (ocr) 23 was used to create the study cohort. Figure 1 summarizes the selection criteria for the hcc patient sample. The site code 155.0 (International Classification of Diseases, 9th revision) and histology codes 8170–8175 (International Classification of Diseases for Oncology, 3rd edition) were used to identify primary hepatic neoplasms. All adult patients with hcc were followed from their date of diagnosis to date of death or until June 2011 (at least 18 months after diagnosis) to capture deaths. Patients were excluded if the hcc diagnosis was recorded on or after the date of death.
FIGURE 1.
Flowchart describing the selection of the study population. ICD = International Classification of Diseases; HCC = hepatocellular carcinoma; ICD-O = International Classification of Diseases for Oncology.
Potential control subjects were selected from a 5% random sample of the reference Ontario population database (Registered Persons Database), including residents of Ontario with unique health card numbers registered for the purpose of Ontario health insurance coverage and Ontario drug benefits provided through a universally funded health care system administered by the Ontario Ministry of Health and Long-Term Care. Eligible control subjects were individuals 18 years of age and older who did not have a diagnosis of cancer and who had used health services at least once before death. Although health care services vary in some respects, the system provides free access to hospital and emergency department (ed) visits, physician services, and homecare; copayments for long-term care placements; and copayments for prescription medications for individuals 65 years of age and older.
Ethics approval for the study was granted by the University of Toronto Health Sciences Research Ethics Board. Informed consent was not obtained because this secondary analysis accessed existing de-identified data; consent was therefore deemed to be neither feasible nor necessary.
Data Sources
The Ontario Ministry of Health and Long-Term Care routinely collects health administrative information for the approximately 13.6 million people resident in Ontario, Canada’s most populous province. Those data are housed at the Institute for Clinical Evaluative Sciences in several linked health care service utilization databases for Ontario.
The ocr is a population-based tumour registry that contains information on all new cases of cancer (except for non-melanoma skin cancers) diagnosed in Ontario since 1964. It captures about 95% of all cancers in the province and has been shown to be both accurate and reliable 24–27.
To estimate comorbidities, frequency and type of hospital admissions, length of stay, and in-hospital mortality, the cancer registry cohort was linked to the Discharge Abstract Database maintained by the Canadian Institute for Health Information. Where possible, hospitalization records from the date of diagnosis were used to assign each patient and control subject a baseline Charlson–Deyo comorbidity index. If patients did not have a hospitalization record at their diagnosis date, baseline comorbidity was determined by looking 2 years back into the hospitalization data to find the most recent hospitalization record; the comorbidity score from that hospitalization was then applied 15,28,29. The Charlson–Deyo comorbidity index at baseline was marked as “missing” if the individual had no hospitalization records at diagnosis or during the 2 years before diagnosis. Comorbidity was adjusted for each hospitalization after baseline.
Health care utilization and direct medical costs were determined from the perspective of the Ontario Ministry of Health and Long-Term Care. Health care utilization and costs associated with outpatient physician visits and laboratory tests were determined from the physician Claims History Database of the Ontario Health Insurance Plan. Emergency department visits and same-day surgery were determined using the National Ambulatory Care Reporting System database. Prescription medication use and costs were obtained from the Ontario Drug Benefit Program database. Client-level data for homecare services were obtained from the Ontario Home Care Administrative System (pre-2005) and the Ontario Home Care Database (post-2005).
Study Variables
The ocr includes data for the date of hcc diagnosis, age, sex, birth location, urban or rural residence, cause of death, and date of death.
Median neighbourhood household income was determined by linking patient postal codes found in the ocr to Canadian census data, which categorizes neighbourhoods into income quintiles. The least and most well-off 20% of neighbourhoods were respectively included within the 1st and the 5th quintiles 30.
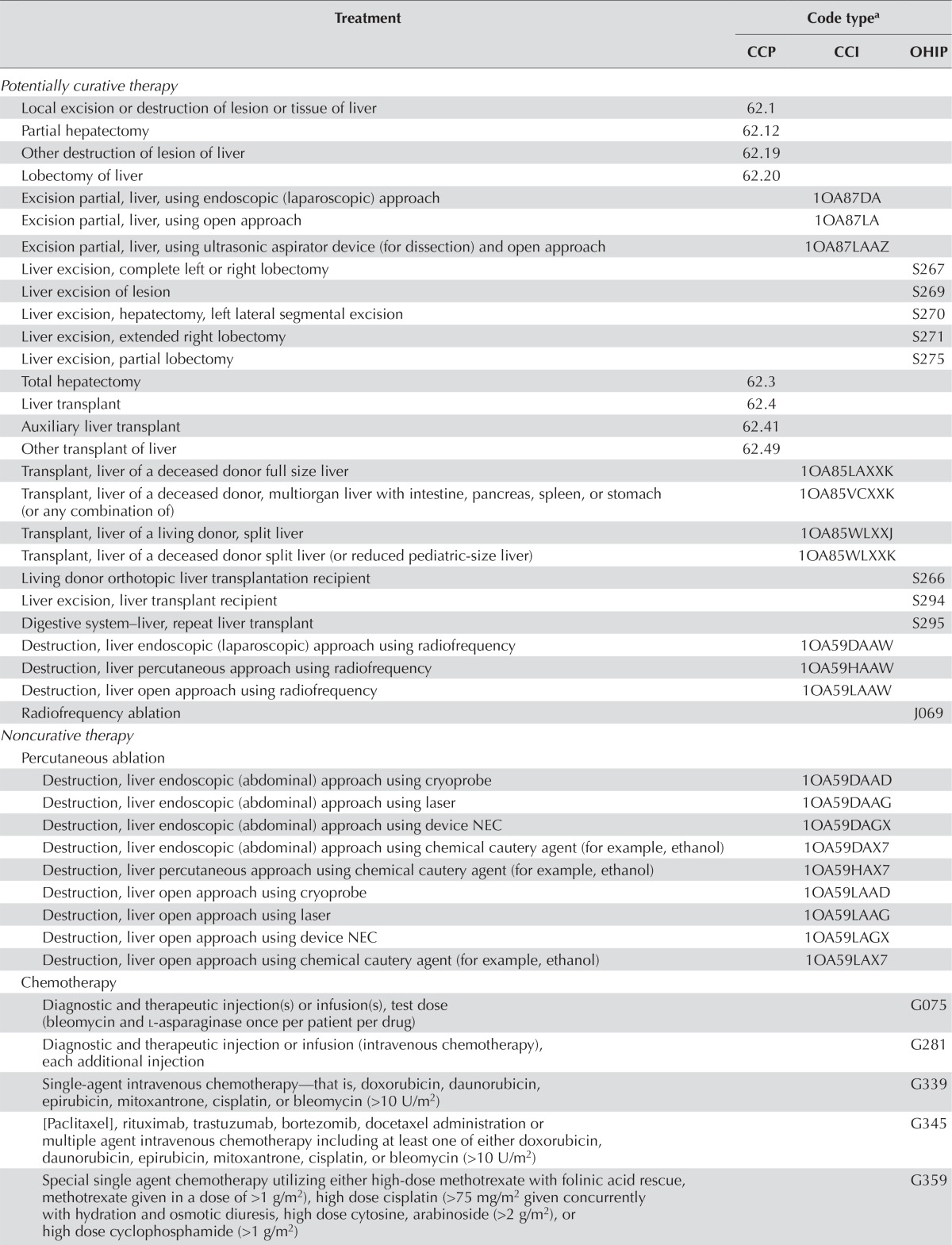
The Charlson–Deyo comorbidity index was calculated using methods previously described 31,32; an International Classification of Diseases (10th revision) coding algorithm was applied to the diagnostic field codes from the hospitalization data (excluding diagnoses for liver disease and metastatic cancer). Conditions were weighted and then totalled to provide an overall comorbidity index value for a given episode, which was then categorized into one of five groups (0, 1, 2, ≥3, or no hospitalization record), representing varying degrees of comorbidity as already described. Treatments for hcc—such as potentially curative treatment (surgical resection, liver transplantation, or radiofrequency ablation), noncurative treatment (chemotherapy, sorafenib, or transarterial chemoembolization), palliative care, and no treatment—were determined using databases maintained by the Canadian Institute for Health Information, the Ontario Health Insurance Plan, and the Ontario Drug Benefit Program. Sorafenib was approved by Health Canada in late 2007. The definitions of comorbidity and hcc treatments used were those established in previous studies 15,28,29. Table i presents the codes used to identify hcc treatments.
TABLE I.
Treatment procedures for patients with hepatocellular carcinoma
| Treatment | Code typea | ||
|---|---|---|---|
|
| |||
| CCP | CCI | OHIP | |
| Potentially curative therapy | |||
| Local excision or destruction of lesion or tissue of liver | 62.1 | ||
| Partial hepatectomy | 62.12 | ||
| Other destruction of lesion of liver | 62.19 | ||
| Lobectomy of liver | 62.20 | ||
| Excision partial, liver, using endoscopic (laparoscopic) approach | 1OA87DA | ||
| Excision partial, liver, using open approach | 1OA87LA | ||
| Excision partial, liver, using ultrasonic aspirator device (for dissection) and open approach | 1OA87LAAZ | ||
| Liver excision, complete left or right lobectomy | S267 | ||
| Liver excision of lesion | S269 | ||
| Liver excision, hepatectomy, left lateral segmental excision | S270 | ||
| Liver excision, extended right lobectomy | S271 | ||
| Liver excision, partial lobectomy | S275 | ||
| Total hepatectomy | 62.3 | ||
| Liver transplant | 62.4 | ||
| Auxiliary liver transplant | 62.41 | ||
| Other transplant of liver | 62.49 | ||
| Transplant, liver of a deceased donor full size liver | 1OA85LAXXK | ||
| Transplant, liver of a deceased donor, multiorgan liver with intestine, pancreas, spleen, or stomach (or any combination of) | 1OA85VCXXK | ||
| Transplant, liver of a living donor, split liver | 1OA85WLXXJ | ||
| Transplant, liver of a deceased donor split liver (or reduced pediatric-size liver) | 1OA85WLXXK | ||
| Living donor orthotopic liver transplantation recipient | S266 | ||
| Liver excision, liver transplant recipient | S294 | ||
| Digestive system–liver, repeat liver transplant | S295 | ||
| Destruction, liver endoscopic (laparoscopic) approach using radiofrequency | 1OA59DAAW | ||
| Destruction, liver percutaneous approach using radiofrequency | 1OA59HAAW | ||
| Destruction, liver open approach using radiofrequency | 1OA59LAAW | ||
| Radiofrequency ablation | J069 | ||
| Noncurative therapy | |||
| Percutaneous ablation | |||
| Destruction, liver endoscopic (abdominal) approach using cryoprobe | 1OA59DAAD | ||
| Destruction, liver endoscopic (abdominal) approach using laser | 1OA59DAAG | ||
| Destruction, liver endoscopic (abdominal) approach using device NEC | 1OA59DAGX | ||
| Destruction, liver endoscopic (abdominal) approach using chemical cautery agent (for example, ethanol) | 1OA59DAX7 | ||
| Destruction, liver percutaneous approach using chemical cautery agent (for example, ethanol) | 1OA59HAX7 | ||
| Destruction, liver open approach using cryoprobe | 1OA59LAAD | ||
| Destruction, liver open approach using laser | 1OA59LAAG | ||
| Destruction, liver open approach using chemical cautery agent (for example, ethanol) | 1OA59LAX7 | ||
| Chemotherapy | |||
| Diagnostic and therapeutic injection(s) or infusion(s), test dose (bleomycin and l-asparaginase once per patient per drug) | G075 | ||
| Diagnostic and therapeutic injection or infusion (intravenous chemotherapy), each additional injection | G281 | ||
| Single-agent intravenous chemotherapy—that is, doxorubicin, daunorubicin, epirubicin, mitoxantrone, cisplatin, or bleomycin (>10 U/m2) | G339 | ||
| [Paclitaxel], rituximab, trastuzumab, bortezomib, docetaxel administration or multiple agent intravenous chemotherapy including at least one of either doxorubicin, daunorubicin, epirubicin, mitoxantrone, cisplatin, or bleomycin (>10 U/m2) | G345 | ||
| Special single agent chemotherapy utilizing either high-dose methotrexate with folinic acid rescue, methotrexate given in a dose of >1 g/m2), high dose cisplatin (>75 mg/m2 given concurrently with hydration and osmotic diuresis, high dose cytosine, arabinoside (>2 g/m2), or high dose cyclophosphamide (>1 g/m2) | G359 | ||
| Single injection (for agents other than doxorubicin, cisplatin, bleomycin, or high dose methotrexate) | G381 | ||
| Supervision of chemotherapy (marrow suppressant) for malignant or autoimmune disease by telephone, monthly | G382 | ||
| Arteries–cannulation–chemotherapy–hepatic (TACE) | R776 | ||
| Supportive and palliative care | |||
| General or family practice, special palliative care consultation | A945 | ||
| Special palliative care consultation, hospital inpatient | C945 | ||
| Palliative care | C982 | ||
| Palliative care support, individual care, 0.5 hours or major part | K023 | ||
The CCI is the new national standard for classifying health care procedures. It is the companion classification system to the International Statistical Classification of Diseases and Related Health Problems, 10th revision, Canada, and replaces the CCP and the intervention portion of the International Classification of Diseases, 9th revision, Clinical Modification, in Canada. The CCP was originally developed by Statistics Canada in 1978 to meet Canadian needs for a procedural classification to be used in conjunction with the International Classification of Diseases, 9th revision.
CCP = Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures; CCI = Canadian Classification of Health Interventions; OHIP = Ontario Health Insurance Plan; NEC = not elsewhere classified; TACE = transarterial chemoembolization.
The categories of health care utilization included family physician visits, specialist visits, ed visits, acute inpatient hospitalizations, same-day surgery, prescription medications, homecare use, and total services (the sum of the numbers for all health care utilization types). For primary care and specialist visits, health care utilization was determined by using physician and laboratory service fee codes to estimate outpatient costs (physician services and other fee-for-service practitioner services). If a patient had multiple service billings from specialists or a family physician on the same day, only 1 unique visit was counted for that day. Similarly, 1 unique homecare use was counted when there were multiple records of homecare services provided to a patient on the same day. For each phase of care, we estimated the number of health services used per 30 patient–days of follow-up, while accounting for the varying length of follow-up for each patient within each phase. The length of follow-up for each patient within each phase was calculated using the hierarchal approach already described and taking into consideration whether the patient had died by the end of study follow-up and, if dead, the length of time from date of diagnosis to death. For example, if a patient did not die during study follow-up, the patient would have 1 year in the initial phase, but would not be included in the end-of-life analysis. If a patient died at least 1 year after diagnosis, the first 365 days from diagnosis would be allotted to the initial phase, and the remaining period (from the 366th day from diagnosis) to death would be allotted to the end-of-life phase. If a patient died within 1 year after diagnosis (for instance, 10 months after diagnosis), the lengths of the follow-up periods allotted to the initial and end-of-life phases would be 4 and 6 months, respectively, and if a patient died 5 months after diagnosis, that patient would have 5 months in the end-of-life phase, but would not contribute any length of follow-up to the initial phase.
The categories of health care costs included outpatient visits, ed visits, acute inpatient hospitalizations, same-day-surgery, prescription medications, homecare visits, and total services. The costs of outpatient visits were estimated using the available 2008 unit cost for each physician and laboratory service fee code. The main costs of hospitalization, ed visits, and same-day-surgery for a particular year were estimated using the Resource Intensity Weight methodology developed by the Canadian Institute for Health Information 33–36. To determine person-level costs for the hcc patients, we calculated unit costs (for example, hospitalization-specific cost per weighted case multiplied by the individual’s resource intensity weight for a given hospitalization). Paralleling the calculation of net health care utilization attributable to hcc, we calculated the total costs of care and the lengths of follow-up periods (in days) for patients and control subjects within each phase of care, taking into consideration whether the individual had died by the end of the study period.
Matching Patients and Control Subjects
Matching on sociodemographic and clinical factors associated with resource use was performed as detailed by Thein et al. 15. Propensity scores were derived by fitting a logistic model with hcc status as the dependent variable and the index year or year of death, age, sex, urban or rural residence, income quintile, Charlson–Deyo comorbidity index, and interaction between age and comorbidity as the independent variables.
Matching for each cohort used two sets of patients and control subjects: Cohort 1 included all incident patients, who were matched 1:1 to control subjects to estimate utilization for the pre-diagnosis and initial phases. Cohort 2 included all patients who died (classified as short-term or long-term survivors). To estimate utilization for the end-of-life phase, short-term and long-term survivors were separately matched 1:1 to control subjects who had died. Each patient was matched to the closest non-cancer control subject who met these criteria: age ± 10 years at the index date; same sex; same index year (for Cohort 1) or same year of death (for Cohort 2); same Charlson–Deyo comorbidity index; and a propensity score within a caliper width of 0.2 standard deviation 15.
Statistical Analysis
Differences in sociodemographic and clinical characteristics of hcc patients by year of diagnosis (2002–2003, 2004–2005, 2006–2007, and 2008–2009) were examined using the chi-square test and Fisher exact test, as appropriate. In addition, sociodemographic and clinical information, including age, sex, urban or rural residence, income quintile, Charlson–Deyo comorbidity index, index year, and death year are presented for matched and unmatched patients and control subjects. All statistical analyses were performed using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.).
Estimation of Health Care Utilization Attributable to HCC
To account for the matched study design, generalized estimating equations were used to estimate net health care utilization per 30 patient–days attributable to hcc for each care phase [mean with 95% confidence interval (ci)], adjusting for age, sex, urban or rural residence, income quintile, and Charlson–Deyo comorbidity index. Generalized linear models were used to analyze the rr for health care utilization, comparing hcc patients with matched control subjects, specifying a negative binomial distribution and a log-link function, and also adjusting for the same covariates. The rr was reported because that measure provides valuable insights into the differences in health care utilization between patients and control subjects on a relative scale. Mean net resource utilization and rr were determined for the overall study period (2002–2009) and by year of diagnosis (2002–2003, 2004–2005, 2006–2007, 2008–2009) for the pre-diagnosis and initial phases, and by year of death (in 2-year subgroups) for the end-of-life phase.
Estimation of Health Care Costs Attributable to HCC
To account for the matched study design, generalized estimating equations were used to estimate the mean (95% ci) net costs of care attributable to hcc per 30 patient–days, adjusting for age, sex, urban or rural residence, income quintile, and Charlson–Deyo comorbidity index. To account for inflation, the Statistics Canada Consumer Price Index for health care and personal items for Ontario 37 was used to adjust all costs to 2013 Canadian dollars. As for health care utilization, the results are reported by year of diagnosis for the pre-diagnosis and initial phases, and by year of death for the end-of-life phase.
RESULTS
Study Population Characteristics
Overall, 2832 patients in the ocr were identified as having a primary diagnosis of hcc between 2002 and 2009 (Table ii). The number of hcc cases increased to 841 in 2008–2009 from 570 in 2002–2003. Comorbidity (one or more diseases) also increased to 46.1% from 40.5% (p = 0.004). Radiofrequency ablations in the year after diagnosis increased significantly to 21.9% in 2008–2009 from 6.3% in 2002–2003 (p < 0.001); however, surgical resections decreased to 15.3% from 21.2% (p = 0.007). In addition, the use of sorafenib increased to 13.9% in 2008–2009 from 6.2% in 2006–2007 (p < 0.001).
TABLE II.
Baseline characteristics of patients diagnosed with hepatocellular carcinoma by year of diagnosis, 2002–2009
| Variable | Year of diagnosisa | p Value | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | 2002–2003 | 2004–2005 | 2006–2007 | 2008–2009 | ||
| Patients (n) | 2832 | 570 | 661 | 760 | 841 | |
| Age group [n (%)] | ||||||
| <60 Years | 1089 (38.5) | 219 (38.4) | 273 (41.3) | 285 (37.5) | 312 (37.1) | |
| 60–69 Years | 735 (26.0) | 145 (25.4) | 172 (26.0) | 205 (27.0) | 213 (25.3) | |
| 70–79 Years | 742 (26.2) | 168 (29.5) | 162 (24.5) | 186 (24.5) | 226 (26.9) | |
| ≥80 Years | 266 (9.4) | 38 (6.7) | 54 (8.2) | 84 (11.1) | 90 (10.7) | 0.070 |
| Male sex [n (%)] | 2238 (79.0) | 451 (79.1) | 512 (77.5) | 602 (79.2) | 673 (80.0) | 0.681 |
| Residence [n (%)] | ||||||
| Rural | 220 (7.8) | 38 (6.7) | 62 (9.4) | 44 (5.8) | 76 (9.0) | |
| Urban | 2609 (92.1) | 532 (93.3) | 599 (90.6) | 713 (93.8) | 765 (91.0) | |
| Missing | — (0.1) | 0 | 0 | — (0.4) | 0 | 0.010b |
| Income quintile [n (%)] | ||||||
| Q1 (lowest) | 727 (25.7) | 150 (26.3) | 179 (27.1) | 180 (23.7) | 218 (25.9) | |
| Q2 | 628 (22.2) | 113 (19.8) | 143 (21.6) | 187 (24.6) | 185 (22.0) | |
| Q3 | 565 (20.0) | 125 (21.9) | 146 (22.1) | 134 (17.6) | 160 (19.0) | |
| Q4 | 477 (16.8) | 103 (18.1) | 104 (15.7) | 125 (16.5) | 145 (17.2) | |
| Q5 (highest) | 422 (14.9) | 78 (13.7) | 84 (12.7) | 128 (16.8) | 132 (15.7) | 0.091b |
| Missing | 13 (0.5) | — (0.2) | — (0.8) | 6 (0.8) | — (0.1) | |
| Charlson–Deyo comorbidity index [n (%)] | ||||||
| 0 | 1159 (40.9) | 243 (42.6) | 304 (46.0) | 275 (36.2) | 337 (40.1) | |
| 1 | 612 (21.6) | 113 (19.8) | 121 (18.3) | 188 (24.7) | 190 (22.6) | |
| 2 | 340 (12.0) | 60 (10.5) | 72 (10.9) | 90 (11.8) | 118 (14.0) | |
| ≥3 | 292 (10.3) | 58 (10.2) | 69 (10.4) | 85 (11.2) | 80 (9.5) | |
| No hospitalization record | 429 (15.2) | 96 (16.8) | 95 (14.4) | 122 (16.1) | 116 (13.8) | 0.013 |
| Stage at diagnosis [n (%)] | ||||||
| Early (stages 0–I) | 236 (10.4) | 36 (5.5) | 79 (10.4) | 121 (14.4) | ||
| Intermediate (stage II) | 322 (14.2) | 63 (9.5) | 109 (14.3) | 150 (17.8) | ||
| Advanced (stages III–IV) | 668 (29.5) | 160 (24.2) | 229 (30.1) | 279 (33.2) | ||
| Unknown | 1036 (45.8) | 402 (60.8) | 343 (45.1) | 291 (34.6) | <0.001 | |
| Type of treatment [n (%)] | ||||||
| Surgical resection | 480 (17.0) | 121 (21.2) | 118 (17.9) | 112 (14.7) | 129 (15.3) | 0.007 |
| Liver transplantation | 381 (13.5) | 70 (12.3) | 93 (14.1) | 113 (14.9) | 105 (12.5) | 0.412 |
| Radiofrequency ablation | 339 (12.0) | 36 (6.3) | 40 (6.1) | 79 (10.4) | 184 (21.9) | <0.001 |
| Sorafenibc | 181 (6.4) | 8 (1.4) | 9 (1.4) | 47 (6.2) | 117 (13.9) | <0.001 |
| Chemotherapy | 349 (12.3) | 88 (15.4) | 90 (13.6) | 73 (9.6) | 98 (11.7) | 0.009 |
| Transarterial chemoembolization | 215 (7.6) | 30 (5.3) | 53 (8.0) | 62 (8.2) | 70 (8.3) | 0.135 |
| Percutaneous ethanol injection | 36 (1.3) | 14 (2.5) | 12 (1.8) | 7 (0.9) | — (0.4) | 0.002b |
| Palliative care | 1294 (45.7) | 255 (44.7) | 307 (46.4) | 354 (46.6) | 378 (45.0) | 0.852 |
| No treatment | 708 (25.0) | 162 (28.4) | 187 (28.3) | 193 (25.4) | 166 (19.7) | <0.001 |
Counts less than 6 are suppressed.
By Fisher exact test.
Approved by Health Canada in late 2007.
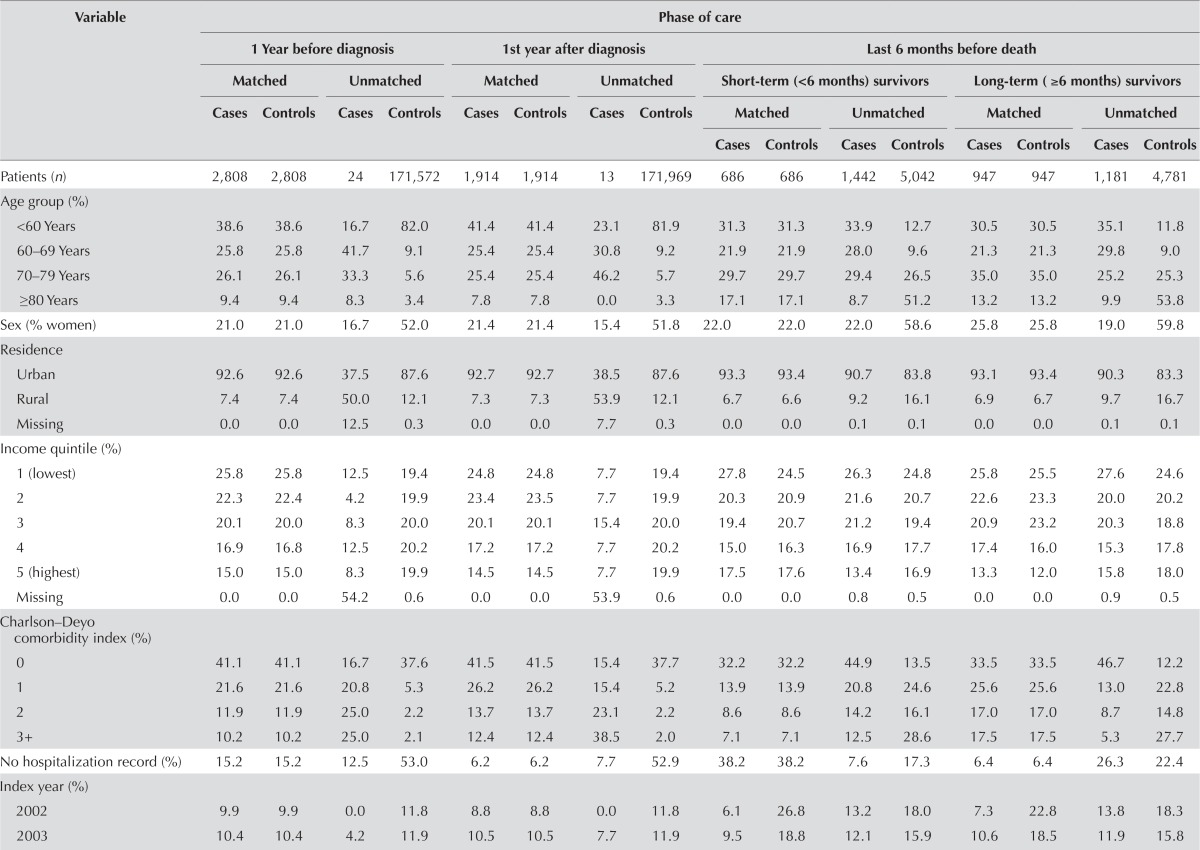
For the pre-diagnosis phase, 2808 of 2832 patients were able to be matched to control subjects; for the initial phase, 1914 of 1927 could be matched; and for the end-of-life phase, 686 of 902 (short-term survivors) and 947 of 1226 (long-term survivors) could be matched. Patients in the pre-diagnosis and initial phases were able to be closely matched to non-cancer controls; however, many patients who contributed time to the end-of-life phase could not be matched to suitable controls (Table iii).
TABLE III.
Baseline characteristics of matched cases (patients with hepatocellular carcinoma) and controls (non-cancer control subjects) and of unmatched cases and controls by phase of care, 2002–2009
| Variable | Phase of care | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 1 Year before diagnosis | 1st year after diagnosis | Last 6 months before death | ||||||||||||||
|
|
|
|
||||||||||||||
| Matched | Unmatched | Matched | Unmatched | Short-term (<6 months) survivors | Long-term (≥6 months) survivors | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Matched | Unmatched | Matched | Unmatched | |||||
|
|
|
|
|
|||||||||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |||||||||
| Patients (n) | 2,808 | 2,808 | 24 | 171,572 | 1,914 | 1,914 | 13 | 171,969 | 686 | 686 | 1,442 | 5,042 | 947 | 947 | 1,181 | 4,781 |
| Age group (%) | ||||||||||||||||
| <60 Years | 38.6 | 38.6 | 16.7 | 82.0 | 41.4 | 41.4 | 23.1 | 81.9 | 31.3 | 31.3 | 33.9 | 12.7 | 30.5 | 30.5 | 35.1 | 11.8 |
| 60–69 Years | 25.8 | 25.8 | 41.7 | 9.1 | 25.4 | 25.4 | 30.8 | 9.2 | 21.9 | 21.9 | 28.0 | 9.6 | 21.3 | 21.3 | 29.8 | 9.0 |
| 70–79 Years | 26.1 | 26.1 | 33.3 | 5.6 | 25.4 | 25.4 | 46.2 | 5.7 | 29.7 | 29.7 | 29.4 | 26.5 | 35.0 | 35.0 | 25.2 | 25.3 |
| ≥80 Years | 9.4 | 9.4 | 8.3 | 3.4 | 7.8 | 7.8 | 0.0 | 3.3 | 17.1 | 17.1 | 8.7 | 51.2 | 13.2 | 13.2 | 9.9 | 53.8 |
| Sex (% women) | 21.0 | 21.0 | 16.7 | 52.0 | 21.4 | 21.4 | 15.4 | 51.8 | 22.0 | 22.0 | 22.0 | 58.6 | 25.8 | 25.8 | 19.0 | 59.8 |
| Residence | ||||||||||||||||
| Urban | 92.6 | 92.6 | 37.5 | 87.6 | 92.7 | 92.7 | 38.5 | 87.6 | 93.3 | 93.4 | 90.7 | 83.8 | 93.1 | 93.4 | 90.3 | 83.3 |
| Rural | 7.4 | 7.4 | 50.0 | 12.1 | 7.3 | 7.3 | 53.9 | 12.1 | 6.7 | 6.6 | 9.2 | 16.1 | 6.9 | 6.7 | 9.7 | 16.7 |
| Missing | 0.0 | 0.0 | 12.5 | 0.3 | 0.0 | 0.0 | 7.7 | 0.3 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 |
| Income quintile (%) | ||||||||||||||||
| 1 (lowest) | 25.8 | 25.8 | 12.5 | 19.4 | 24.8 | 24.8 | 7.7 | 19.4 | 27.8 | 24.5 | 26.3 | 24.8 | 25.8 | 25.5 | 27.6 | 24.6 |
| 2 | 22.3 | 22.4 | 4.2 | 19.9 | 23.4 | 23.5 | 7.7 | 19.9 | 20.3 | 20.9 | 21.6 | 20.7 | 22.6 | 23.3 | 20.0 | 20.2 |
| 3 | 20.1 | 20.0 | 8.3 | 20.0 | 20.1 | 20.1 | 15.4 | 20.0 | 19.4 | 20.7 | 21.2 | 19.4 | 20.9 | 23.2 | 20.3 | 18.8 |
| 4 | 16.9 | 16.8 | 12.5 | 20.2 | 17.2 | 17.2 | 7.7 | 20.2 | 15.0 | 16.3 | 16.9 | 17.7 | 17.4 | 16.0 | 15.3 | 17.8 |
| 5 (highest) | 15.0 | 15.0 | 8.3 | 19.9 | 14.5 | 14.5 | 7.7 | 19.9 | 17.5 | 17.6 | 13.4 | 16.9 | 13.3 | 12.0 | 15.8 | 18.0 |
| Missing | 0.0 | 0.0 | 54.2 | 0.6 | 0.0 | 0.0 | 53.9 | 0.6 | 0.0 | 0.0 | 0.8 | 0.5 | 0.0 | 0.0 | 0.9 | 0.5 |
| Charlson–Deyo comorbidity index (%) | ||||||||||||||||
| 0 | 41.1 | 41.1 | 16.7 | 37.6 | 41.5 | 41.5 | 15.4 | 37.7 | 32.2 | 32.2 | 44.9 | 13.5 | 33.5 | 33.5 | 46.7 | 12.2 |
| 1 | 21.6 | 21.6 | 20.8 | 5.3 | 26.2 | 26.2 | 15.4 | 5.2 | 13.9 | 13.9 | 20.8 | 24.6 | 25.6 | 25.6 | 13.0 | 22.8 |
| 2 | 11.9 | 11.9 | 25.0 | 2.2 | 13.7 | 13.7 | 23.1 | 2.2 | 8.6 | 8.6 | 14.2 | 16.1 | 17.0 | 17.0 | 8.7 | 14.8 |
| 3+ | 10.2 | 10.2 | 25.0 | 2.1 | 12.4 | 12.4 | 38.5 | 2.0 | 7.1 | 7.1 | 12.5 | 28.6 | 17.5 | 17.5 | 5.3 | 27.7 |
| No hospitalization record (%) | 15.2 | 15.2 | 12.5 | 53.0 | 6.2 | 6.2 | 7.7 | 52.9 | 38.2 | 38.2 | 7.6 | 17.3 | 6.4 | 6.4 | 26.3 | 22.4 |
| Index year (%) | ||||||||||||||||
| 2002 | 9.9 | 9.9 | 0.0 | 11.8 | 8.8 | 8.8 | 0.0 | 11.8 | 6.1 | 26.8 | 13.2 | 18.0 | 7.3 | 22.8 | 13.8 | 18.3 |
| 2003 | 10.4 | 10.4 | 4.2 | 11.9 | 10.5 | 10.5 | 7.7 | 11.9 | 9.5 | 18.8 | 12.1 | 15.9 | 10.6 | 18.5 | 11.9 | 15.8 |
| 2004 | 10.7 | 10.7 | 8.3 | 12.3 | 10.2 | 10.2 | 7.7 | 12.3 | 10.2 | 15.5 | 12.6 | 16.1 | 10.6 | 15.8 | 12.9 | 16.0 |
| 2005 | 12.6 | 12.6 | 16.7 | 12.4 | 12.0 | 12.0 | 23.1 | 12.4 | 13.1 | 13.4 | 13.3 | 13.6 | 11.9 | 12.4 | 14.2 | 13.8 |
| 2006 | 13.0 | 13.0 | 8.3 | 12.6 | 13.4 | 13.4 | 7.7 | 12.6 | 13.4 | 10.5 | 12.4 | 11.8 | 13.7 | 12.5 | 11.9 | 11.4 |
| 2007 | 13.7 | 13.7 | 41.7 | 12.9 | 13.9 | 13.9 | 30.8 | 12.9 | 15.6 | 5.7 | 12.6 | 9.9 | 15.2 | 7.3 | 12.3 | 9.8 |
| 2008 | 13.4 | 13.4 | 8.3 | 13.1 | 14.1 | 14.1 | 7.7 | 13.1 | 14.4 | 6.1 | 11.2 | 8.3 | 14.8 | 6.6 | 10.2 | 8.3 |
| 2009 | 16.4 | 16.4 | 12.5 | 13.1 | 17.2 | 17.2 | 15.4 | 13.1 | 17.6 | 3.2 | 12.6 | 6.6 | 16.0 | 4.2 | 12.9 | 6.6 |
| Death year (%) | ||||||||||||||||
| 2002 | — | — | — | — | — | — | — | — | 6.1 | 6.1 | 6.5 | 1.4 | 1.5 | 1.5 | 10.3 | 2.1 |
| 2003 | — | — | — | — | — | — | — | — | 9.5 | 9.5 | 7.6 | 3.4 | 4.0 | 4.0 | 11.6 | 4.1 |
| 2004 | — | — | — | — | — | — | — | — | 10.2 | 10.2 | 9.5 | 5.0 | 6.1 | 6.1 | 12.6 | 5.5 |
| 2005 | — | — | — | — | — | — | — | — | 13.1 | 13.1 | 10.3 | 6.5 | 8.5 | 8.5 | 13.5 | 7.1 |
| 2006 | — | — | — | — | — | — | — | — | 13.4 | 13.4 | 12.1 | 9.3 | 10.7 | 10.7 | 14.1 | 9.6 |
| 2007 | — | — | — | — | — | — | — | — | 15.6 | 15.6 | 10.8 | 11.0 | 12.5 | 12.5 | 12.3 | 11.3 |
| 2008 | — | — | — | — | — | — | — | — | 14.4 | 14.4 | 12.6 | 12.8 | 15.3 | 15.3 | 11.4 | 12.5 |
| 2009 | — | — | — | — | — | — | — | — | 17.6 | 17.6 | 15.1 | 16.0 | 18.5 | 18.5 | 13.8 | 15.8 |
| 2010 | — | — | — | — | — | — | — | — | 0.0 | 0.0 | 9.8 | 17.9 | 14.5 | 14.5 | 0.3 | 16.0 |
| 2011 | — | — | — | — | — | — | — | — | 0.0 | 0.0 | 5.7 | 16.8 | 8.6 | 8.6 | 0.1 | 16.0 |
In the cohort used for the pre-diagnosis phase analysis, 571 patients (20.3%) and 2362 control subjects (84.1%) died during study follow-up. In the cohort used for the initial phase analysis, 571 patients (29.8%) and 1628 control subjects (85.1%) died during study follow-up.
Health Care Utilization Attributable to HCC
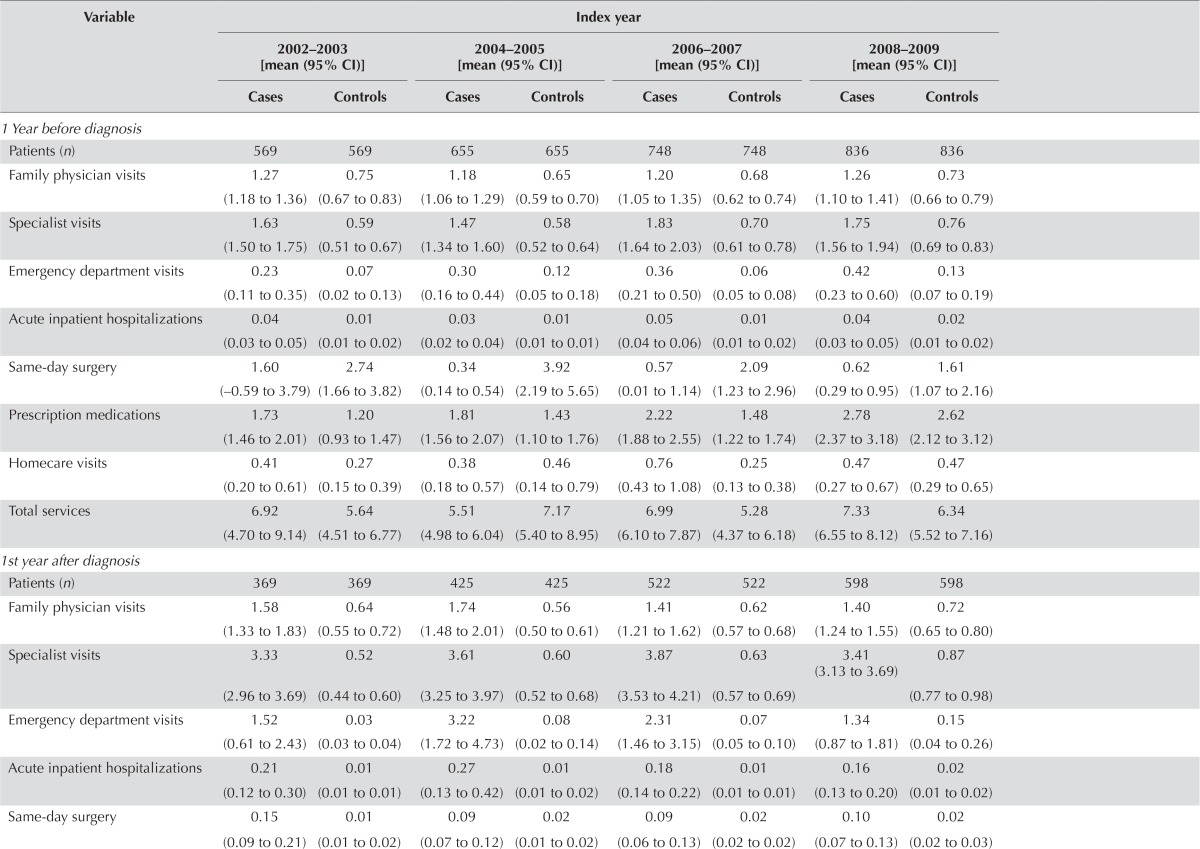
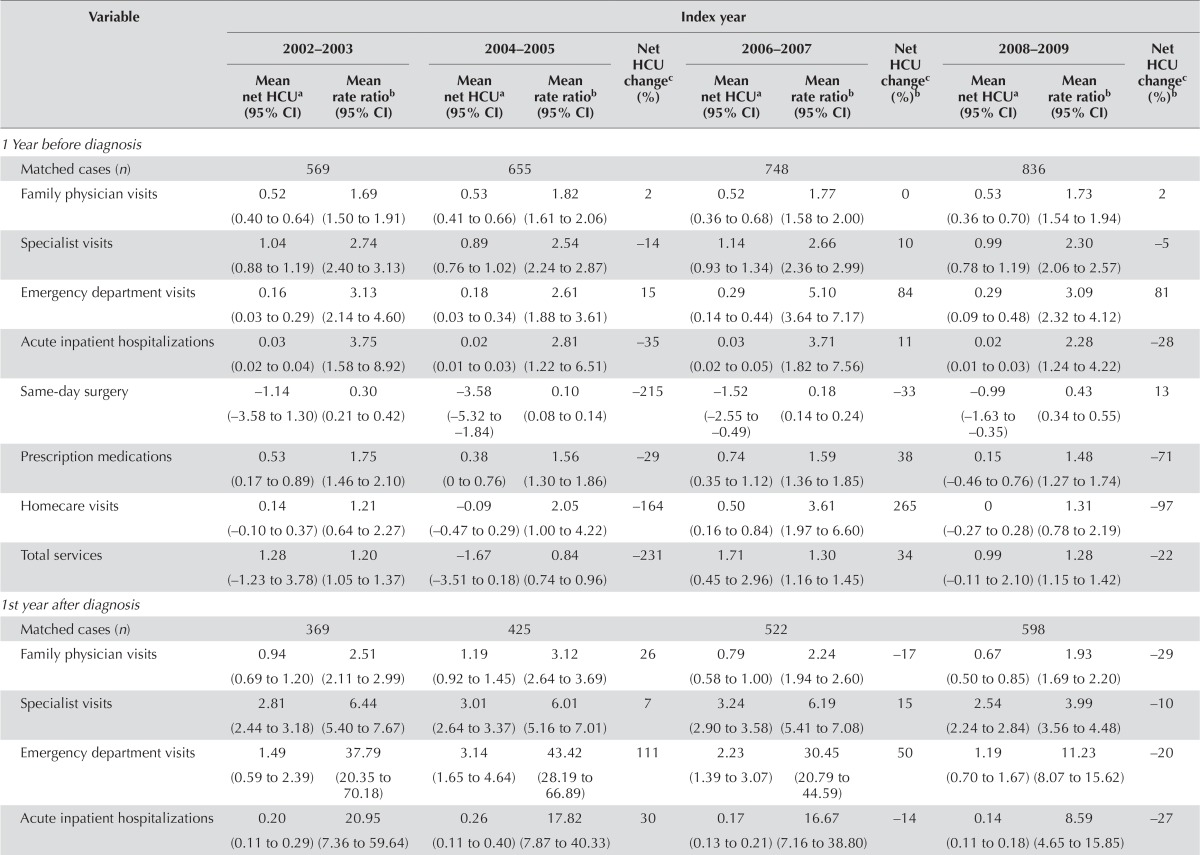
Tables iv–v present the mean utilization and costs per 30 patient–days for matched hcc patients and control subjects for various sources of care, by care phase and index or death year.
TABLE IV.
Health care utilization per 30 patient–days for matched cases (hepatocellular carcinoma patients) and controls (non-cancer control subjects) by index year, 2002–2009, and by death year, 2002–2011
| Variable | Index year | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 2002–2003 [mean (95% CI)] | 2004–2005 [mean (95% CI)] | 2006–2007 [mean (95% CI)] | 2008–2009 [mean (95% CI)] | |||||
|
|
|
|
|
|||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| 1 Year before diagnosis | ||||||||
| Patients (n) | 569 | 569 | 655 | 655 | 748 | 748 | 836 | 836 |
| Family physician visits | 1.27 (1.18 to 1.36) | 0.75 (0.67 to 0.83) | 1.18 (1.06 to 1.29) | 0.65 (0.59 to 0.70) | 1.20 (1.05 to 1.35) | 0.68 (0.62 to 0.74) | 1.26 (1.10 to 1.41) | 0.73 (0.66 to 0.79) |
| Specialist visits | 1.63 (1.50 to 1.75) | 0.59 (0.51 to 0.67) | 1.47 (1.34 to 1.60) | 0.58 (0.52 to 0.64) | 1.83 (1.64 to 2.03) | 0.70 (0.61 to 0.78) | 1.75 (1.56 to 1.94) | 0.76 (0.69 to 0.83) |
| Emergency department visits | 0.23 (0.11 to 0.35) | 0.07 (0.02 to 0.13) | 0.30 (0.16 to 0.44) | 0.12 (0.05 to 0.18) | 0.36 (0.21 to 0.50) | 0.06 (0.05 to 0.08) | 0.42 (0.23 to 0.60) | 0.13 (0.07 to 0.19) |
| Acute inpatient hospitalizations | 0.04 (0.03 to 0.05) | 0.01 (0.01 to 0.02) | 0.03 (0.02 to 0.04) | 0.01 (0.01 to 0.01) | 0.05 (0.04 to 0.06) | 0.01 (0.01 to 0.02) | 0.04 (0.03 to 0.05) | 0.02 (0.01 to 0.02) |
| Same-day surgery | 1.60 (−0.59 to 3.79) | 2.74 (1.66 to 3.82) | 0.34 (0.14 to 0.54) | 3.92 (2.19 to 5.65) | 0.57 (0.01 to 1.14) | 2.09 (1.23 to 2.96) | 0.62 (0.29 to 0.95) | 1.61 (1.07 to 2.16) |
| Prescription medications | 1.73 (1.46 to 2.01) | 1.20 (0.93 to 1.47) | 1.81 (1.56 to 2.07) | 1.43 (1.10 to 1.76) | 2.22 (1.88 to 2.55) | 1.48 (1.22 to 1.74) | 2.78 (2.37 to 3.18) | 2.62 (2.12 to 3.12) |
| Homecare visits | 0.41 (0.20 to 0.61) | 0.27 (0.15 to 0.39) | 0.38 (0.18 to 0.57) | 0.46 (0.14 to 0.79) | 0.76 (0.43 to 1.08) | 0.25 (0.13 to 0.38) | 0.47 (0.27 to 0.67) | 0.47 (0.29 to 0.65) |
| Total services | 6.92 (4.70 to 9.14) | 5.64 (4.51 to 6.77) | 5.51 (4.98 to 6.04) | 7.17 (5.40 to 8.95) | 6.99 (6.10 to 7.87) | 5.28 (4.37 to 6.18) | 7.33 (6.55 to 8.12) | 6.34 (5.52 to 7.16) |
| 1st year after diagnosis | ||||||||
| Patients (n) | 369 | 369 | 425 | 425 | 522 | 522 | 598 | 598 |
| Family physician visits | 1.58 (1.33 to 1.83) | 0.64 (0.55 to 0.72) | 1.74 (1.48 to 2.01) | 0.56 (0.50 to 0.61) | 1.41 (1.21 to 1.62) | 0.62 (0.57 to 0.68) | 1.40 (1.24 to 1.55) | 0.72 (0.65 to 0.80) |
| Specialist visits | 3.33 (2.96 to 3.69) | 0.52 (0.44 to 0.60) | 3.61 (3.25 to 3.97) | 0.60 (0.52 to 0.68) | 3.87 (3.53 to 4.21) | 0.63 (0.57 to 0.69) | 3.41 (3.13 to 3.69) | 0.87 (0.77 to 0.98) |
| Emergency department visits | 1.52 (0.61 to 2.43) | 0.03 (0.03 to 0.04) | 3.22 (1.72 to 4.73) | 0.08 (0.02 to 0.14) | 2.31 (1.46 to 3.15) | 0.07 (0.05 to 0.10) | 1.34 (0.87 to 1.81) | 0.15 (0.04 to 0.26) |
| Acute inpatient hospitalizations | 0.21 (0.12 to 0.30) | 0.01 (0.01 to 0.01) | 0.27 (0.13 to 0.42) | 0.01 (0.01 to 0.02) | 0.18 (0.14 to 0.22) | 0.01 (0.01 to 0.01) | 0.16 (0.13 to 0.20) | 0.02 (0.01 to 0.02) |
| Same-day surgery | 0.15 (0.09 to 0.21) | 0.01 (0.01 to 0.02) | 0.09 (0.07 to 0.12) | 0.02 (0.01 to 0.02) | 0.09 (0.06 to 0.13) | 0.02 (0.02 to 0.02) | 0.10 (0.07 to 0.13) | 0.02 (0.02 to 0.03) |
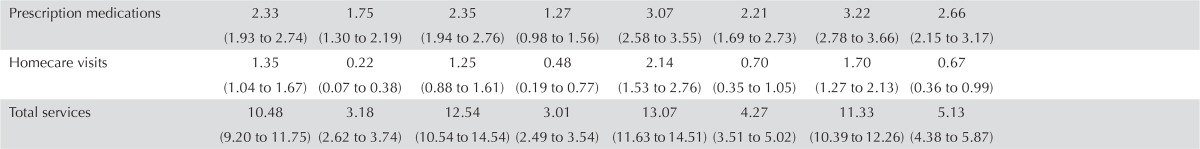
| Prescription medications | 2.33 (1.93 to 2.74) | 1.75 (1.30 to 2.19) | 2.35 (1.94 to 2.76) | 1.27 (0.98 to 1.56) | 3.07 (2.58 to 3.55) | 2.21 (1.69 to 2.73) | 3.22 (2.78 to 3.66) | 2.66 (2.15 to 3.17) |
| Homecare visits | 1.35 (1.04 to 1.67) | 0.22 (0.07 to 0.38) | 1.25 (0.88 to 1.61) | 0.48 (0.19 to 0.77) | 2.14 (1.53 to 2.76) | 0.70 (0.35 to 1.05) | 1.70 (1.27 to 2.13) | 0.67 (0.36 to 0.99) |
| Total services | 10.48 (9.20 to 11.75) | 3.18 (2.62 to 3.74) | 12.54 (10.54 to 14.54) | 3.01 (2.49 to 3.54) | 13.07 (11.63 to 14.51) | 4.27 (3.51 to 5.02) | 11.33 (10.39 to 12.26) | 5.13 (4.38 to 5.87) |
| Death year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 2002–2003 [mean (95% CI)] | 2004–2005 [mean (95% CI)] | 2006–2007 [mean (95% CI)] | 2008–2009 [mean (95% CI)] | 2010–2011 [mean (95% CI)] | ||||||
|
|
|
|
|
|
||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
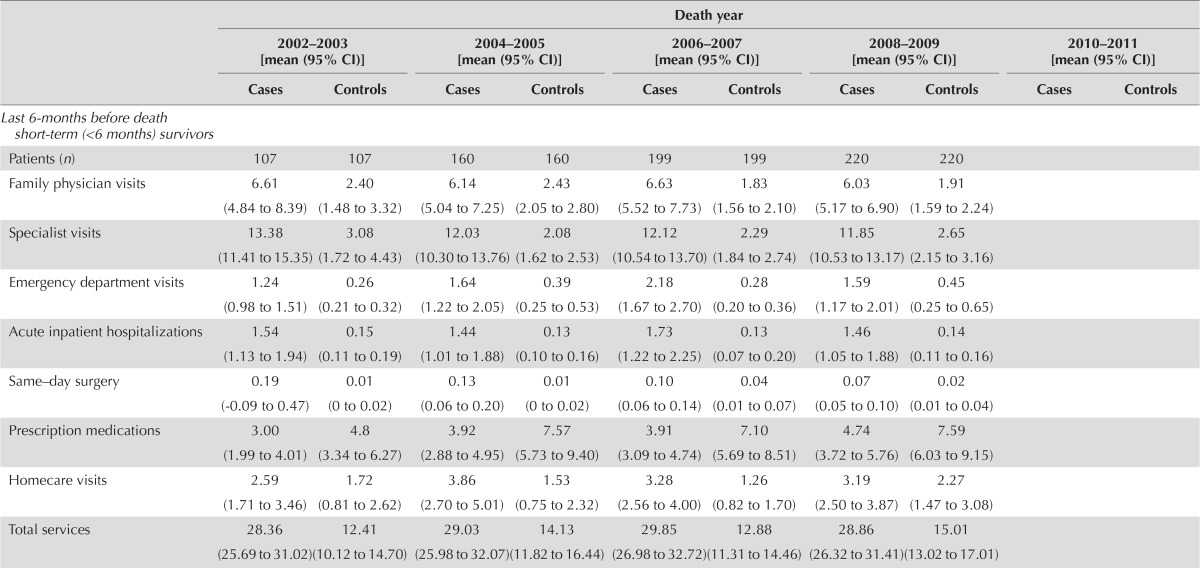
| Last 6-months before death short-term (<6 months) survivors | ||||||||||
| Patients (n) | 107 | 107 | 160 | 160 | 199 | 199 | 220 | 220 | ||
| Family physician visits | 6.61 (4.84 to 8.39) | 2.40 (1.48 to 3.32) | 6.14 (5.04 to 7.25) | 2.43 (2.05 to 2.80) | 6.63 (5.52 to 7.73) | 1.83 (1.56 to 2.10) | 6.03 (5.17 to 6.90) | 1.91 (1.59 to 2.24) | ||
| Specialist visits | 13.38 (11.41 to 15.35) | 3.08 (1.72 to 4.43) | 12.03 (10.30 to 13.76) | 2.08 (1.62 to 2.53) | 12.12 (10.54 to 13.70) | 2.29 (1.84 to 2.74) | 11.85 (10.53 to 13.17) | 2.65 (2.15 to 3.16) | ||
| Emergency department visits | 1.24 (0.98 to 1.51) | 0.26 (0.21 to 0.32) | 1.64 (1.22 to 2.05) | 0.39 (0.25 to 0.53) | 2.18 (1.67 to 2.70) | 0.28 (0.20 to 0.36) | 1.59 (1.17 to 2.01) | 0.45 (0.25 to 0.65) | ||
| Acute inpatient hospitalizations | 1.54 (1.13 to 1.94) | 0.15 (0.11 to 0.19) | 1.44 (1.01 to 1.88) | 0.13 (0.10 to 0.16) | 1.73 (1.22 to 2.25) | 0.13 (0.07 to 0.20) | 1.46 (1.05 to 1.88) | 0.14 (0.11 to 0.16) | ||
| Same–day surgery | 0.19 (−0.09 to 0.47) | 0.01 (0 to 0.02) | 0.13 (0.06 to 0.20) | 0.01 (0 to 0.02) | 0.10 (0.06 to 0.14) | 0.04 (0.01 to 0.07) | 0.07 (0.05 to 0.10) | 0.02 (0.01 to 0.04) | ||
| Prescription medications | 3.00 (1.99 to 4.01) | 4.8 (3.34 to 6.27) | 3.92 (2.88 to 4.95) | 7.57 (5.73 to 9.40) | 3.91 (3.09 to 4.74) | 7.10 (5.69 to 8.51) | 4.74 (3.72 to 5.76) | 7.59 (6.03 to 9.15) | ||
| Homecare visits | 2.59 (1.71 to 3.46) | 1.72 (0.81 to 2.62) | 3.86 (2.70 to 5.01) | 1.53 (0.75 to 2.32) | 3.28 (2.56 to 4.00) | 1.26 (0.82 to 1.70) | 3.19 (2.50 to 3.87) | 2.27 (1.47 to 3.08) | ||
| Total services | 28.36 (25.69 to 31.02) | 12.41 (10.12 to 14.70) | 29.03 (25.98 to 32.07) | 14.13 (11.82 to 16.44) | 29.85 (26.98 to 32.72) | 12.88 (11.31 to 14.46) | 28.86 (26.32 to 31.41) | 15.01 (13.02 to 17.01) | ||
| Last 6-months before death long-term (≥6 months) survivors | ||||||||||
| Patients (n) | 52 | 52 | 138 | 138 | 219 | 219 | 320 | 320 | 218 | 218 |
| Family physician visits | 3.57 (2.84 to 4.29) | 2.51 (1.58 to 3.44) | 3.34 (2.86 to 3.81) | 3.02 (2.59 to 3.44) | 2.95 (2.58 to 3.32) | 2.36 (2.02 to 2.71) | 3.08 (2.78 to 3.37) | 2.41 (2.08 to 2.74) | 3.57 (2.84 to 4.29) | 2.51 (1.58 to 3.44) |
| Specialist visits | 3.82 (3.06 to 4.58) | 2.84 (2.20 to 3.49) | 4.61 (3.95 to 5.26) | 3.72 (3.04 to 4.39) | 4.05 (3.61 to 4.50) | 3.04 (2.60 to 3.48) | 3.71 (3.41 to 4.01) | 3.23 (2.81 to 3.65) | 3.82 (3.06 to 4.58) | 2.84 (2.20 to 3.49) |
| Emergency department visits | 0.78 (0.43 to 1.14) | 0.54 (0.05 to 1.02) | 1.33 (1.01 to 1.66) | 0.73 (0.37 to 1.08) | 1.09 (0.89 to 1.30) | 0.45 (0.30 to 0.61) | 0.70 (0.59 to 0.82) | 0.58 (0.38 to 0.78) | 0.78 (0.43 to 1.14) | 0.54 (0.05 to 1.02) |
| Acute inpatient hospitalizations | 0.21 (0.16 to 0.26) | 0.23 (0.17 to 0.30) | 0.26 (0.22 to 0.30) | 0.24 (0.20 to 0.28) | 0.25 (0.22 to 0.28) | 0.22 (0.16 to 0.28) | 0.26 (0.23 to 0.28) | 0.22 (0.16 to 0.28) | 0.21 (0.16 to 0.26) | 0.23 (0.17 to 0.30) |
| Same–day surgery | 0.07 (0 to 0.15) | 0.03 (0 to 0.05) | 0.06 (0.04 to 0.08) | 0.02 (0.01 to 0.04) | 0.07 (0.04 to 0.11) | 0.03 (0.02 to 0.05) | 0.05 (0.04 to 0.07) | 0.04 (0.02 to 0.05) | 0.07 (0 to 0.15) | 0.03 (0 to 0.05) |
| Prescription medications | 4.70 (3.83 to 5.57) | 4.77 (2.68 to 6.86) | 5.03 (4.02 to 6.03) | 6.96 (5.09 to 8.82) | 4.93 (4.00 to 5.87) | 9.42 (7.71 to 11.14) | 6.23 (5.32 to 7.13) | 9.13 (7.57 to 10.68) | 4.70 (3.83 to 5.57) | 4.77 (2.68 to 6.86) |
| Homecare visits | 3.70 (2.20 to 5.19) | 2.34 (1.15 to 3.54) | 3.64 (2.81 to 4.47) | 2.14 (1.23 to 3.05) | 4.88 (3.98 to 5.78) | 1.63 (1.05 to 2.21) | 4.81 (4.08 to 5.53) | 2.51 (1.81 to 3.21) | 3.70 (2.20 to 5.19) | 2.34 (1.15 to 3.54) |
| Total services | 16.77 (14.57 to 18.97) | 13.24 (10.23 to 16.25) | 18.21 (16.62 to 19.80) | 16.79 (14.45 to 19.14) | 18.16 (16.58 to 19.74) | 17.13 (15.12 to 19.13) | 18.79 (17.46 to 20.11) | 18.07 (16.16 to 19.98) | 16.77 (14.57 to 18.97) | 13.24 (10.23 to 16.25) |
CI = confidence interval.
TABLE V.
Costa per 30 patient–days for matched cases (hepatocellular carcinoma patients) and controls (non-cancer control subjects), by index year, 2002–2009, and by death year, 2002–2011
| Variable | Index year | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 2002–2003 [mean $ (95% CI)] | 2004–2005 [mean $ (95% CI)] | 2006–2007 [mean $ (95% CI)] | 2008–2009 [mean $ (95% CI)] | |||||
|
|
|
|
|
|||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
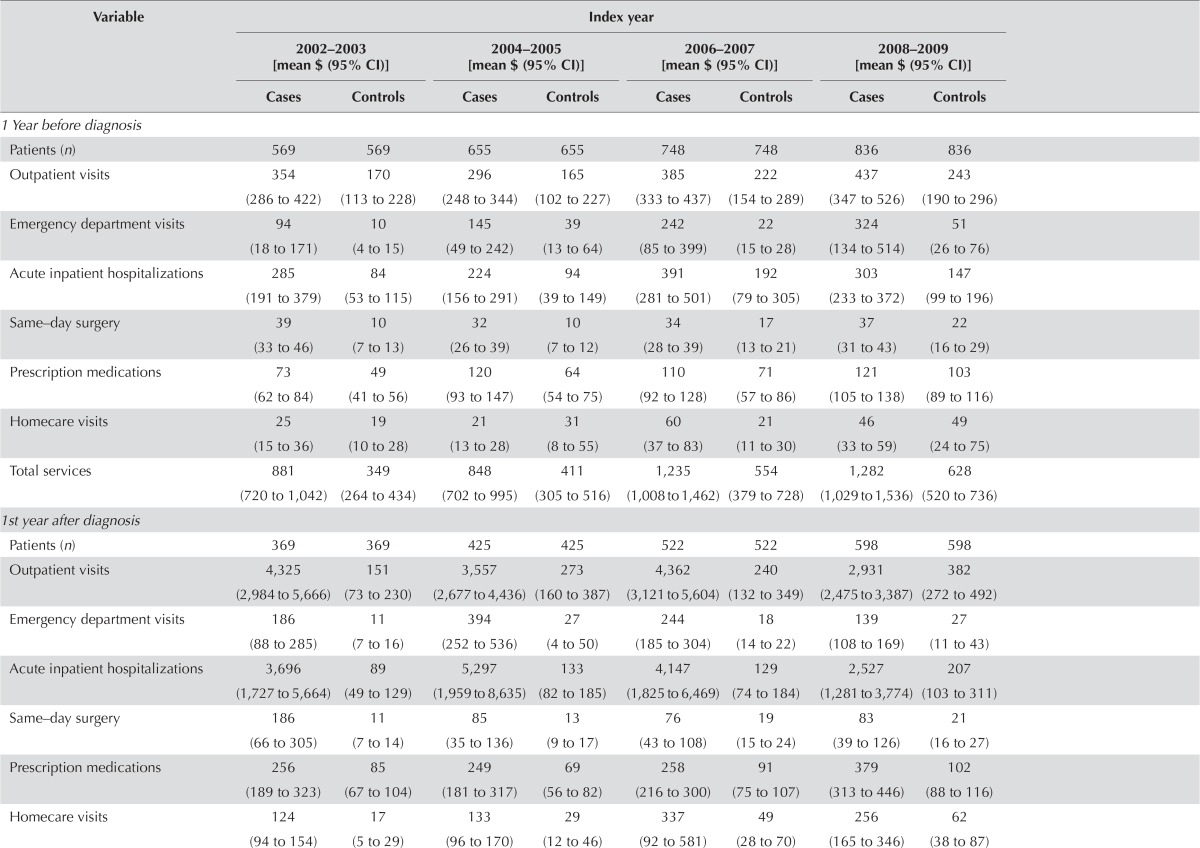
| 1 Year before diagnosis | ||||||||
| Patients (n) | 569 | 569 | 655 | 655 | 748 | 748 | 836 | 836 |
| Outpatient visits | 354 (286 to 422) | 170 (113 to 228) | 296 (248 to 344) | 165 (102 to 227) | 385 (333 to 437) | 222 (154 to 289) | 437 (347 to 526) | 243 (190 to 296) |
| Emergency department visits | 94 (18 to 171) | 10 (4 to 15) | 145 (49 to 242) | 39 (13 to 64) | 242 (85 to 399) | 22 (15 to 28) | 324 (134 to 514) | 51 (26 to 76) |
| Acute inpatient hospitalizations | 285 (191 to 379) | 84 (53 to 115) | 224 (156 to 291) | 94 (39 to 149) | 391 (281 to 501) | 192 (79 to 305) | 303 (233 to 372) | 147 (99 to 196) |
| Same–day surgery | 39 (33 to 46) | 10 (7 to 13) | 32 (26 to 39) | 10 (7 to 12) | 34 (28 to 39) | 17 (13 to 21) | 37 (31 to 43) | 22 (16 to 29) |
| Prescription medications | 73 (62 to 84) | 49 (41 to 56) | 120 (93 to 147) | 64 (54 to 75) | 110 (92 to 128) | 71 (57 to 86) | 121 (105 to 138) | 103 (89 to 116) |
| Homecare visits | 25 (15 to 36) | 19 (10 to 28) | 21 (13 to 28) | 31 (8 to 55) | 60 (37 to 83) | 21 (11 to 30) | 46 (33 to 59) | 49 (24 to 75) |
| Total services | 881 (720 to 1,042) | 349 (264 to 434) | 848 (702 to 995) | 411 (305 to 516) | 1,235 (1,008 to 1,462) | 554 (379 to 728) | 1,282 (1,029 to 1,536) | 628 (520 to 736) |
| 1st year after diagnosis | ||||||||
| Patients (n) | 369 | 369 | 425 | 425 | 522 | 522 | 598 | 598 |
| Outpatient visits | 4,325 (2,984 to 5,666) | 151 (73 to 230) | 3,557 (2,677 to 4,436) | 273 (160 to 387) | 4,362 (3,121 to 5,604) | 240 (132 to 349) | 2,931 (2,475 to 3,387) | 382 (272 to 492) |
| Emergency department visits | 186 (88 to 285) | 11 (7 to 16) | 394 (252 to 536) | 27 (4 to 50) | 244 (185 to 304) | 18 (14 to 22) | 139 (108 to 169) | 27 (11 to 43) |
| Acute inpatient hospitalizations | 3,696 (1,727 to 5,664) | 89 (49 to 129) | 5,297 (1,959 to 8,635) | 133 (82 to 185) | 4,147 (1,825 to 6,469) | 129 (74 to 184) | 2,527 (1,281 to 3,774) | 207 (103 to 311) |
| Same–day surgery | 186 (66 to 305) | 11 (7 to 14) | 85 (35 to 136) | 13 (9 to 17) | 76 (43 to 108) | 19 (15 to 24) | 83 (39 to 126) | 21 (16 to 27) |
| Prescription medications | 256 (189 to 323) | 85 (67 to 104) | 249 (181 to 317) | 69 (56 to 82) | 258 (216 to 300) | 91 (75 to 107) | 379 (313 to 446) | 102 (88 to 116) |
| Homecare visits | 124 (94 to 154) | 17 (5 to 29) | 133 (96 to 170) | 29 (12 to 46) | 337 (92 to 581) | 49 (28 to 70) | 256 (165 to 346) | 62 (38 to 87) |
| Total services | 8,789 (5,713 to 11,866) | 375 (263 to 487) | 9,734 (5,780 to 13,688) | 553 (398 to 707) | 9,453 (5,709 to 13,196) | 558 (410 to 707) | 6,338 (4,893 to 7,782) | 813 (618 to 1,008) |
| Death year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 2002–2003 [mean $ (95% CI)] | 2004–2005 [mean $ (95% CI)] | 2006–2007 [mean $ (95% CI)] | 2008–2009 [mean $ (95% CI)] | 2010–2011 [mean $ (95% CI)] | ||||||
|
|
|
|
|
|
||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
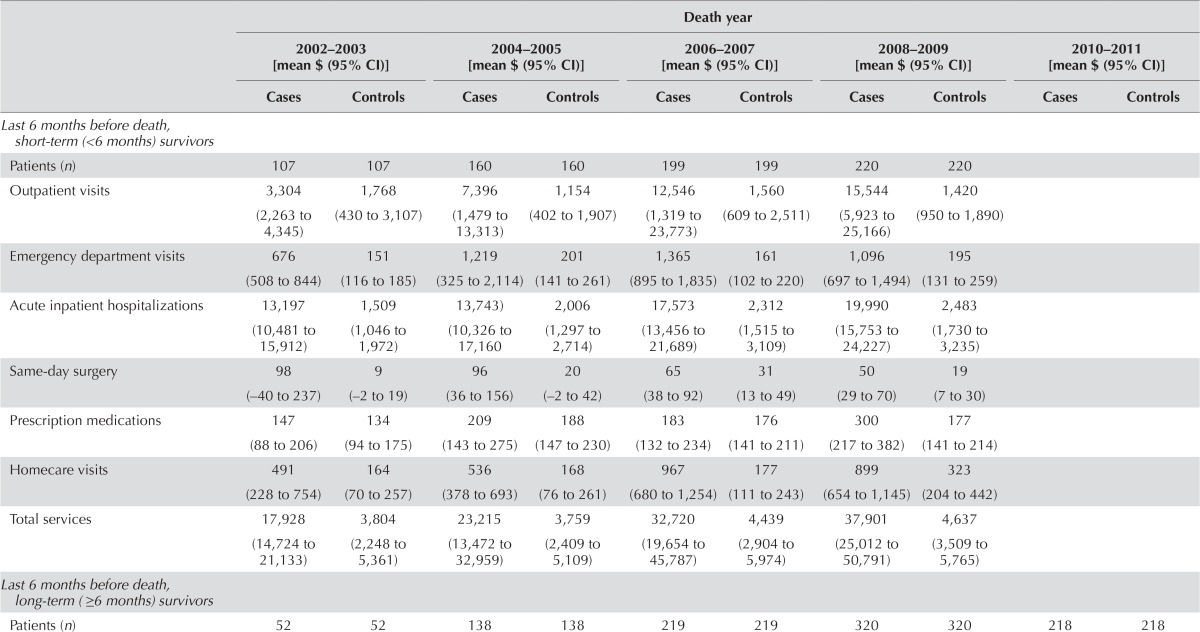
| Last 6 months before death, short-term (<6 months) survivors | ||||||||||
| Patients (n) | 107 | 107 | 160 | 160 | 199 | 199 | 220 | 220 | ||
| Outpatient visits | 3,304 (2,263 to 4,345) | 1,768 (430 to 3,107) | 7,396 (1,479 to 13,313) | 1,154 (402 to 1,907) | 12,546 (1,319 to 23,773) | 1,560 (609 to 2,511) | 15,544 (5,923 to 25,166) | 1,420 (950 to 1,890) | ||
| Emergency department visits | 676 (508 to 844) | 151 (116 to 185) | 1,219 (325 to 2,114) | 201 (141 to 261) | 1,365 (895 to 1,835) | 161 (102 to 220) | 1,096 (697 to 1,494) | 195 (131 to 259) | ||
| Acute inpatient hospitalizations | 13,197 (10,481 to 15,912) | 1,509 (1,046 to 1,972) | 13,743) (10,326 to 17,160 | 2,006 (1,297 to 2,714) | 17,573 (13,456 to 21,689) | 2,312 (1,515 to 3,109) | 19,990 (15,753 to 24,227) | 2,483 (1,730 to 3,235) | ||
| Same-day surgery | 98 (−40 to 237) | 9 (−2 to 19) | 96 (36 to 156) | 20 (−2 to 42) | 65 (38 to 92) | 31 (13 to 49) | 50 (29 to 70) | 19 (7 to 30) | ||
| Prescription medications | 147 (88 to 206) | 134 (94 to 175) | 209 (143 to 275) | 188 (147 to 230) | 183 (132 to 234) | 176 (141 to 211) | 300 (217 to 382) | 177 (141 to 214) | ||
| Homecare visits | 491 (228 to 754) | 164 (70 to 257) | 536 (378 to 693) | 168 (76 to 261) | 967 (680 to 1,254) | 177 (111 to 243) | 899 (654 to 1,145) | 323 (204 to 442) | ||
| Total services | 17,928 (14,724 to 21,133) | 3,804 (2,248 to 5,361) | 23,215 (13,472 to 32,959) | 3,759 (2,409 to 5,109) | 32,720 (19,654 to 45,787) | 4,439 (2,904 to 5,974) | 37,901 (25,012 to 50,791) | 4,637 (3,509 to 5,765) | ||
| Last 6 months before death, long-term (≥6 months) survivors | ||||||||||
| Patients (n) | 52 | 52 | 138 | 138 | 219 | 219 | 320 | 320 | 218 | 218 |
| Outpatient visits | 1,362 (209 to 2,516) | 1,261 (298 to 2,223) | 1,090 (746 to 1,434) | 2,350 (1,213 to 3,487) | 1,460 (1,012 to 1,909) | 2,228 (1,304 to 3,152) | 1,032 (743 to 1,321) | 1,442 (1,043 to 1,842) | 1,409 (833 to 1,985) | 1,407 (901 to 1,913) |
| Emergency department visits | 277 (150 to 404) | 262 (92 to 432) | 458 (353 to 563) | 358 (226 to 491) | 403 (324 to 482) | 256 (178 to 335) | 273 (228 to 319) | 267 (185 to 350) | 11 (3 to 18) | 10 (3 to 17) |
| Acute inpatient hospitalizations | 2,291 (1,099 to 3,484) | 2,247 (1,448 to 3,046) | 2,614 (2,067 to 3,160) | 3,295 (2,405 to 4,185) | 3,191 (2,585 to 3,796) | 3,842 (2,906 to 4,779) | 2,105 (1,820 to 2,390) | 3,104 (2,364 to 3,844) | 310 (−10 to 630) | 105 (8 to 202) |
| Same-day surgery | 42 (2 to 83) | 60 (−26 to 146) | 32 (18 to 46) | 33 (6 to 59) | 60 (25 to 96) | 38 (11 to 65) | 27 (17 to 37) | 39 (10 to 68) | 1 (0 to 3) | 0 (0 to 0) |
| Prescription medications | 345 (214 to 476) | 169 (97 to 241) | 275 (203 to 347) | 188 (152 to 224) | 281 (205 to 357) | 253 (191 to 315) | 489 (396 to 581) | 204 (173 to 235) | 412 (301 to 523) | 215 (167 to 263) |
| Homecare visits | 480 (257 to 704) | 202 (85 to 320) | 441 (326 to 556) | 269 (136 to 402) | 754 (608 to 900) | 214 (151 to 278) | 745 (642 to 848) | 367 (251 to 483) | 417 (301 to 534) | 190 (120 to 260) |
| Total services | 4,818 (2,656 to 6,981) | 4,218 (2,812 to 5,625) | 4,930 (4,143 to 5,718) | 6,514 (4,647 to 8,380) | 6,172 (5,209 to 7,136) | 6,859 (5,215 to 8,504) | 4,693 (4,238 to 5,148) | 5,446 (4,367 to 6,526) | 2,581 (1,898 to 3,264) | 1,952 (1,438 to 2,467) |
In 2013 Canadian dollars.
CI = confidence interval.
Utilization by Resource Type
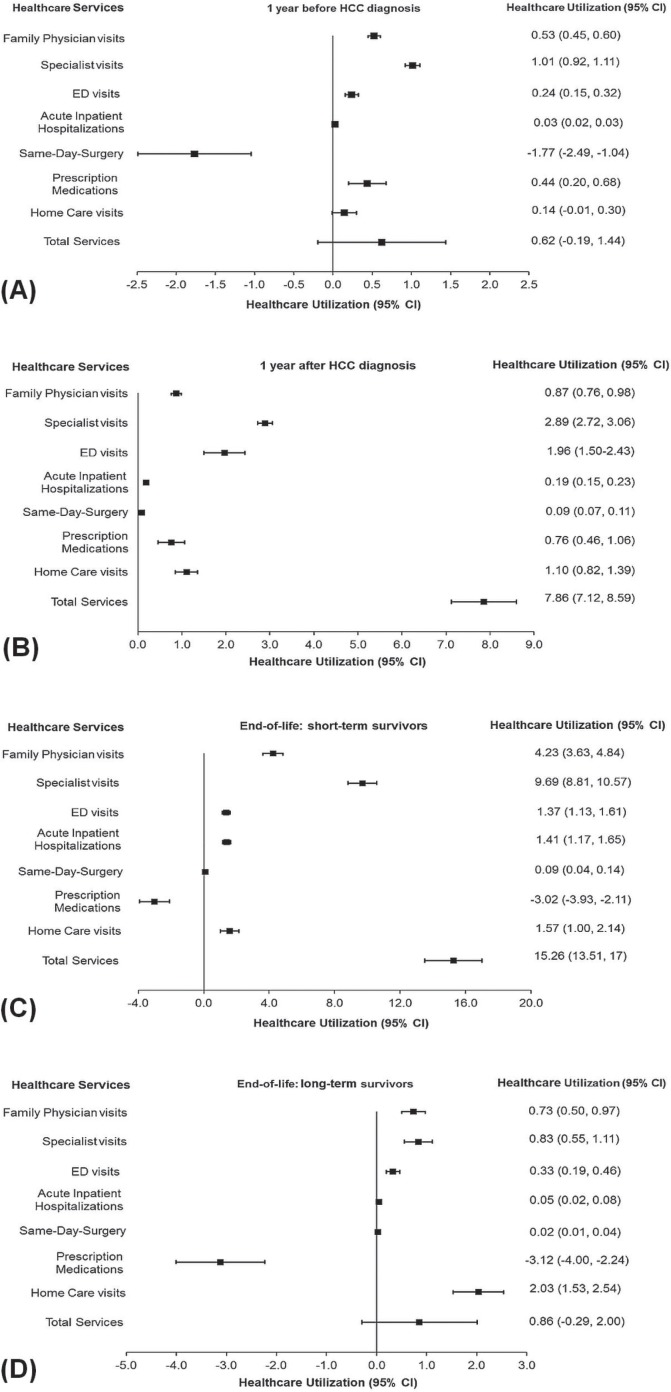
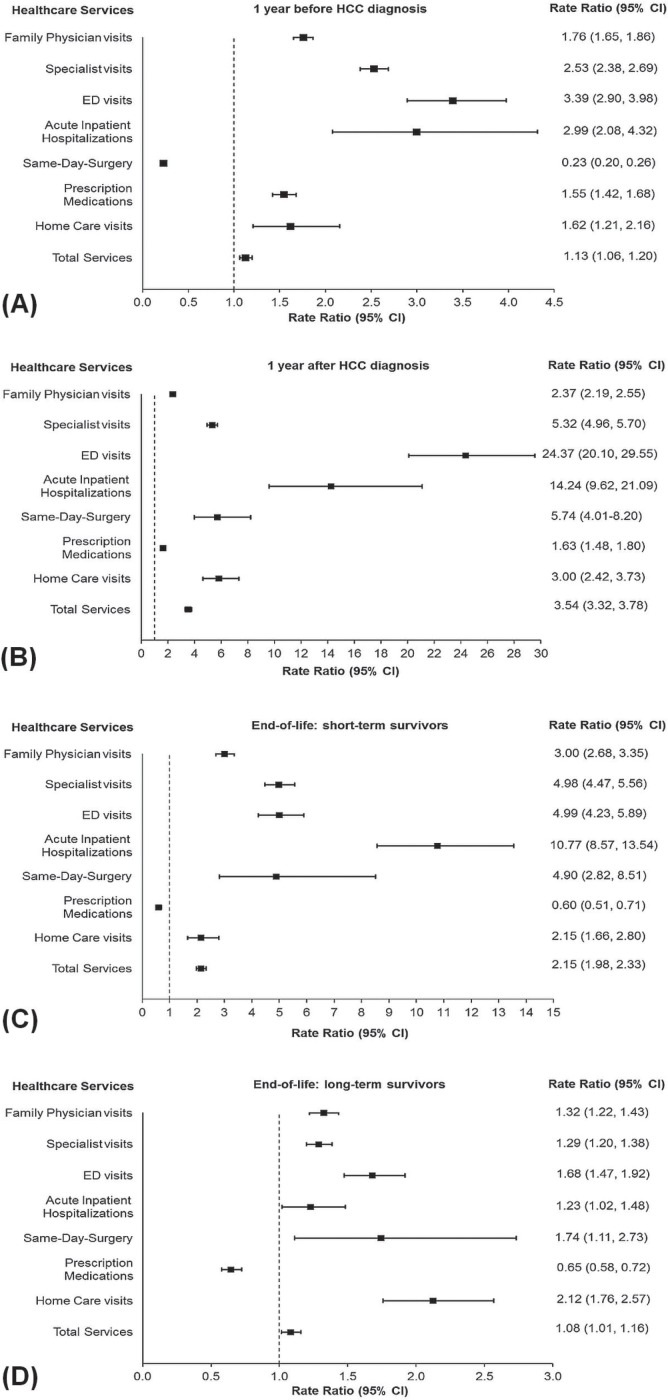
Figures 2 and 3 show the net values attributable to hcc from a comparison of the overall mean number of health care visits and of the rrs for resource use per 30 patient–days by hcc patients and by non-cancer control subjects during the various phases of care (2002–2009 or 2002–2011). In general, hcc patients received a greater number of health care services (Figures 2 and 3); exceptions were same-day surgery during the pre-diagnosis phase (utilization: −1.77; 95% ci: −2.49 to −1.04; rr: 0.23; 95% ci: 0.20 to 0.26) and prescription medications during the end-of-life phase for short-term survivors (utilization: −3.02; 95% ci: −3.93 to −2.11; rr: 0.60; 95% ci: 0.51 to 0.71) and for long-term survivors (utilization: −3.12; 95% ci: −4.00 to −2.24; rr: 0.65; 95% ci: 0.58 to 0.72).
FIGURE 2.
Net health care utilization attributable to hepatocellular carcinoma (HCC) patients (A) at 1 year before diagnosis (2002–2009), (B) during the 1st year after diagnosis (2002–2009), (C) during the last 6 months before death for short-term (<6 months) survivors (2002–2011), and (D) during the last 6 months before death for long-term (≥6 months) survivors (2002–2011). Net health care utilization was calculated as the difference between the mean number of health care services attributed to HCC patients and to propensity-score-matched non-cancer control subjects. Values are expressed as means with 95% confidence intervals per 30 patient–days. Error bars indicate the 95% confidence intervals.
FIGURE 3.
Rate ratios for resource use attributable to hepatocellular carcinoma (HCC) patients (A) at 1 year before diagnosis (2002–2009), (B) during the 1st year after diagnosis (2002–2009), (C) during the last 6 months before death for short-term (<6 months) survivors (2002–2011), and (D) during the last 6 months before death for long-term (≥6 months) survivors (2002–2011). Rate ratios are shown for HCC patients compared with propensity-score-matched non-cancer control subjects. Rates were measured from 2002 to 2009 or to 2011 (inclusive), assuming a negative binomial distribution. Error bars indicate 95% confidence intervals.
Compared with the non-cancer control subjects, hcc patients made a substantially higher number of specialist visits during the end-of-life phase for short-term survivors (average utilization: 9.69 visits; 95% ci: 8.81 to 10.57 visits), during the initial phase (utilization: 2.89 visits; 95% ci: 2.72 to 3.06 visits), and during the pre-diagnosis phase (utilization: 1.01 visits; 95% ci: 0.92 to 1.11 visits). The number of family physician visits made by hcc patients was highest during the end-of-life phase for short-term survivors (utilization: 4.23 visits; 95% ci: 3.63 to 4.84 visits). During the initial phase, utilization was 0.87 visits (95% ci: 0.76 to 0.98 visits), and during the end-of-life phase for long-term survivors, it was 0.73 visits (95% ci: 0.50 to 0.97 visits). The number of homecare visits was highest during the end-of-life phase for long-term survivors (utilization: 2.03 visits; 95% ci: 1.53 to 2.54 visits). During the end-of-life phase for short-term survivors, utilization was 1.57 visits (95% ci: 1.00 to 2.14 visits), and during the initial phase, it was 1.10 visits (95% ci: 0.82 to 1.39 visits; Figure 2).
The hcc patients made ed visits at 24.37 times (rr) the rate of the non-cancer control subjects during the initial phase (95% ci: 20.10 to 29.55), at 4.99 times the control rate during the end-of-life phase for short-term survivors (95% ci: 4.23 to 5.89), and at 3.39 times the control rate during the pre-diagnosis phase (95% ci: 2.90 to 3.98). In addition, hcc patients were hospitalized at 14.24 times (rr) the control rate during the initial phase (95% ci: 9.62 to 21.09), at 10.77 times the control rate during the end-of-life phase for short-term survivors (95% ci: 8.57 to 13.54), and at 2.99 times the control rate during the pre-diagnosis phase (95% ci: 2.08 to 4.32). Lastly, hcc patients received same-day surgery services at 5.74 times (rr) the control rate during the initial phase (95% ci: 4.01 to 8.20), at 4.90 times the control rate during the end-of-life phase for short-term survivors (95% ci: 2.82 to 8.51), and at 1.74 times the control rate during the end-of-life phase for long-term survivors (95% ci: 1.11 to 2.73; Figure 3). In a comparison of health care utilization by short-term and long-term survivors during the end-of-life phase, rates of health care utilization by the short-term survivors were significantly higher for all services with the exception of homecare visits and prescription medications, for which service use was not significantly different.
Trends by Type of Resources
Tables vi–viii present trends in health care utilization over time (to 2008–2009 and to 2010–2011 from 2002–2003). The analysis of trends over time showed that health care utilization numbers attributable to hcc remained relatively consistent for all phases of care; an exception was hospitalizations, which increased 573% to 0.10 (95% ci: 0.07 to 0.14) hospitalizations per 30 patient–days in 2010–2011 from −0.02 (95% ci: −0.09 to 0.05) hospitalizations per 30 patient–days in 2002–2003 during the end-of-life phase for long-term survivors. At the same time, net prescription medications use by hcc patients decreased −4434% to −3.64 (95% ci: −5.50 to −1.77) from 0.08 (95% ci: −2.03 to 2.19; Table viii) per 30 patient–days. Similarly, the rrs for resource use remained relatively consistent over time for all phases of care. Exceptions occurred in the initial phase, in which specialist visits decreased [to a 2008–2009 rr of 3.99 (95% ci: 3.56 to 4.48) from a 2002–2003 rr of 6.44 (95% ci: 5.40 to 7.67), representing a change of −38%], as did ed visits [to rr 11.23 (95% ci: 8.07 to 15.62) from rr 37.79 (95% ci: 20.35 to 70.18), for a change of −70%] and total services [to rr 2.71 (95% ci: 2.42 to 3.04) from rr 3.92 (95% ci: 3.37 to 4.56), for a change of −31%; Table vi]; and in the end-of-life phase for long-term survivors, in which prescription medication use decreased over time [to a 2010–2011 rr of 0.64 (95% ci: 0.51 to 0.80; Table viii) from a 2002–2003 rr of 1.35 (95% ci: 0.89 to 2.05), for a change of −52%].
TABLE VI.
Net health care utilization per 30 patient–days and rate ratio of resource use attributable to hepatocellular carcinoma care, by index year of service, 2002–2009
| Variable | Index year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 2002–2003 | 2004–2005 | Net HCU changec (%) | 2006–2007 | Net HCU changec(%)b | 2008–2009 | Net HCU changec (%)b | |||||
|
|
|
|
|
||||||||
| Mean net HCUa (95% CI) | Mean rate ratiob (95% CI) | Mean net HCUa (95% CI) | Mean rate ratiob (95% CI) | Mean net HCUa (95% CI) | Mean rate ratiob (95% CI) | Mean net HCUa (95% CI) | Mean rate ratiob (95% CI) | ||||
| 1st year after diagnosis | |||||||||||
| Matched cases (n) | 569 | 655 | 748 | 836 | |||||||
| Family physician visits | 0.52 (0.40 to 0.64) | 1.69 (1.50 to 1.91) | 0.53 (0.41 to 0.66) | 1.82 (1.61 to 2.06) | 2 | 0.52 (0.36 to 0.68) | 1.77 (1.58 to 2.00) | 0 | 0.53 (0.36 to 0.70) | 1.73 (1.54 to 1.94) | 2 |
| Specialist visits | 1.04 (0.88 to 1.19) | 2.74 (2.40 to 3.13) | 0.89 (0.76 to 1.02) | 2.54 (2.24 to 2.87) | −14 | 1.14 (0.93 to 1.34) | 2.66 (2.36 to 2.99) | 10 | 0.99 (0.78 to 1.19) | 2.30 (2.06 to 2.57) | −5 |
| Emergency department visits | 0.16 (0.03 to 0.29) | 3.13 (2.14 to 4.60) | 0.18 (0.03 to 0.34) | 2.61 (1.88 to 3.61) | 15 | 0.29 (0.14 to 0.44) | 5.10 (3.64 to 7.17) | 84 | 0.29 (0.09 to 0.48) | 3.09 (2.32 to 4.12) | 81 |
| Acute inpatient hospitalizations | 0.03 (0.02 to 0.04) | 3.75 (1.58 to 8.92) | 0.02 (0.01 to 0.03) | 2.81 (1.22 to 6.51) | −35 | 0.03 (0.02 to 0.05) | 3.71 (1.82 to 7.56) | 11 | 0.02 (0.01 to 0.03) | 2.28 (1.24 to 4.22) | −28 |
| Same-day surgery | −1.14 (−3.58 to 1.30) | 0.30 (0.21 to 0.42) | −3.58 (−5.32 to −1.84) | 0.10 (0.08 to 0.14) | −215 | −1.52 (−2.55 to −0.49) | 0.18 (0.14 to 0.24) | −33 | −0.99 (−1.63 to −0.35) | 0.43 (0.34 to 0.55) | 13 |
| Prescription medications | 0.53 (0.17 to 0.89) | 1.75 (1.46 to 2.10) | 0.38 (0 to 0.76) | 1.56 (1.30 to 1.86) | −29 | 0.74 (0.35 to 1.12) | 1.59 (1.36 to 1.85) | 38 | 0.15 (−0.46 to 0.76) | 1.48 (1.27 to 1.74) | −71 |
| Homecare visits | 0.14 (−0.10 to 0.37) | 1.21 (0.64 to 2.27) | −0.09 (−0.47 to 0.29) | 2.05 (1.00 to 4.22) | −164 | 0.50 (0.16 to 0.84) | 3.61 (1.97 to 6.60) | 265 | 0 (−0.27 to 0.28) | 1.31 (0.78 to 2.19) | −97 |
| Total services | 1.28 (−1.23 to 3.78) | 1.20 (1.05 to 1.37) | −1.67 (−3.51 to 0.18) | 0.84 (0.74 to 0.96) | −231 | 1.71 (0.45 to 2.96) | 1.30 (1.16 to 1.45) | 34 | 0.99 (−0.11 to 2.10) | 1.28 (1.15 to 1.42) | −22 |
| 1st year after diagnosis | |||||||||||
| Matched cases (n) | 369 | 425 | 522 | 598 | |||||||
| Family physician visits | 0.94 (0.69 to 1.20) | 2.51 (2.11 to 2.99) | 1.19 (0.92 to 1.45) | 3.12 (2.64 to 3.69) | 26 | 0.79 (0.58 to 1.00) | 2.24 (1.94 to 2.60) | −17 | 0.67 (0.50 to 0.85) | 1.93 (1.69 to 2.20) | −29 |
| Specialist visits | 2.81 (2.44 to 3.18) | 6.44 (5.40 to 7.67) | 3.01 (2.64 to 3.37) | 6.01 (5.16 to 7.01) | 7 | 3.24 (2.90 to 3.58) | 6.19 (5.41 to 7.08) | 15 | 2.54 (2.24 to 2.84) | 3.99 (3.56 to 4.48) | −10 |
| Emergency department visits | 1.49 (0.59 to 2.39) | 37.79 (20.35 to 70.18) | 3.14 (1.65 to 4.64) | 43.42 (28.19 to 66.89) | 111 | 2.23 (1.39 to 3.07) | 30.45 (20.79 to 44.59) | 50 | 1.19 (0.70 to 1.67) | 11.23 (8.07 to 15.62) | −20 |
| Acute inpatient hospitalizations | 0.20 (0.11 to 0.29) | 20.95 (7.36 to 59.64) | 0.26 (0.11 to 0.40) | 17.82 (7.87 to 40.33) | 30 | 0.17 (0.13 to 0.21) | 16.67 (7.16 to 38.80) | −14 | 0.14 (0.11 to 0.18) | 8.59 (4.65 to 15.85) | −27 |
| Same-day surgery | 0.14 (0.08 to 0.20) | 11.56 (4.50 to 29.71) | 0.08 (0.05 to 0.10) | 5.97 (2.62 to 13.64) | −44 | 0.07 (0.04 to 0.11) | 4.65 (2.39 to 9.06) | −46 | 0.08 (0.05 to 0.11) | 4.36 (2.42 to 7.86) | −44 |
| Prescription medications | 0.58 (0 to 1.16) | 1.55 (1.24 to 1.94) | 1.08 (0.60 to 1.56) | 2.12 (1.72 to 2.61) | 85 | 0.85 (0.25 to 1.46) | 1.98 (1.65 to 2.39) | 46 | 0.56 (−0.08 to 1.19) | 1.43 (1.21 to 1.70) | −5 |
| Homecare visits | 1.13 (0.79 to 1.47) | 12.13 (6.62 to 22.20) | 0.77 (0.31 to 1.23) | 5.70 (3.30 to 9.83) | −32 | 1.44 (0.86 to 2.03) | 6.30 (4.14 to 9.58) | 28 | 1.02 (0.55 to 1.50) | 6.84 (4.43 to 10.55) | −9 |
| Total services | 7.29 (5.92 to 8.66) | 3.92 (3.37 to 4.56) | 9.52 (7.50 to 11.55) | 4.75 (4.11 to 5.49) | 31 | 8.80 (7.39 to 10.22) | 3.88 (3.44 to 4.38) | 21 | 6.20 (5.11 to 7.29) | 2.71 (2.42 to 3.04) | −15 |
Difference between the mean number of health services allocated to patients with hepatocellular carcinoma and to matched non-cancer control subjects.
Estimated by modelling count, using negative binomial regression. Control subjects constituted the reference population.
Compared with 2002–2003.
HCU = health care utilization; CI = confidence interval.
TABLE VIII.
Net health care utilization per 30 patient–days and rate ratio of resource use attributable to hepatocellular carcinoma care at end-of-lifea among long-term survivorsb by death year of service, 2002–2011
| Variable | Death year | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| 2002–2003 | 2004–2005 | Net HCU changee (%) | 2006–2007 | Net HCU changee (%) | 2008–2009 | Net HCU changee (%) | 2010–2011 | Net HCU changee (%) | ||||||
|
|
|
|
|
|
||||||||||
| Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | |||||
| Matched cases (n) | 52 | 138 | 219 | 320 | 218 | |||||||||
| Family physician visits | 0.85 (−0.16 to 1.87) | 1.44 (1.04 to 2.00) | 0.32 (−0.25 to 0.90) | 1.11 (0.92 to 1.34) | −62 | 0.59 (0.08 to 1.09) | 1.28 (1.08 to 1.51) | −31 | 0.65 (0.23 to 1.07) | 1.29 (1.12 to 1.48) | −24 | 1.17 (0.70 to 1.64) | 1.61 (1.36 to 1.90) | 37 |
| Specialist visits | 0.95 (0 to 1.90) | 1.31 (1.01 to 1.70) | 1.04 (0.26 to 1.82) | 1.37 (1.15 to 1.64) | 9 | 1.03 (0.47 to 1.59) | 1.37 (1.18 to 1.59) | 9 | 0.47 (−0.03 to 0.98) | 1.15 (1.01 to 1.31) | −50 | 1.04 (0.47 to 1.61) | 1.40 (1.21 to 1.63) | 10 |
| Emergency department visits | 0.31 (−0.28 to 0.91) | 1.82 (1.00 to 3.32) | 0.58 (0.12 to 1.05) | 2.14 (1.52 to 3.02) | 86 | 0.64 (0.39 to 0.90) | 2.46 (1.89 to 3.20) | 105 | 0.13 (−0.11 to 0.36) | 1.34 (1.06 to 1.69) | −59 | 0.17 (−0.05 to 0.40) | 1.41 (1.07 to 1.86) | −44 |
| Acute inpatient hospitalizations | −0.02 (−0.09 to 0.05) | 0.93 (0.40 to 2.14) | 0.02 (−0.03 to 0.07) | 1.10 (0.68 to 1.77) | 208 | 0.04 (−0.03 to 0.10) | 1.17 (0.79 to 1.72) | 269 | 0.04 (−0.02 to 0.11) | 1.19 (0.87 to 1.64) | 290 | 0.10 (0.07 to 0.14) | 1.60 (1.06 to 2.41) | 573 |
| Same-day surgery | 0.04 (−0.02 to 0.10) | 2.23 (0.27 to 18.31) | 0.04 (0.01 to 0.06) | 2.61 (0.71 to 9.57) | −1 | 0.04 (0.01 to 0.08) | 2.35 (0.96 to 5.76) | 11 | 0.01 (−0.01 to 0.03) | 1.38 (0.65 to 2.90) | −62 | 0.01 (−0.01 to 0.02) | 1.17 (0.42 to 3.30) | −87 |
| Number of prescription medications | 0.08 (−2.03 to 2.19) | 1.35 (0.89 to 2.05) | −1.87 (−3.85 to 0.12) | 0.75 (0.56 to 1.00) | −2326 | −4.51 (−6.37 to −2.65) | 0.53 (0.42 to 0.66) | −5479 | −2.89 (−4.54 to −1.24) | 0.69 (0.56 to 0.84) | −3545 | −3.64 (−5.50 to −1.77) | 0.64 (0.51 to 0.80) | −4434 |
| Homecare visits | 1.44 (−0.43 to 3.31) | 2.59 (1.18 to 5.68) | 1.36 (0.10 to 2.62) | 2.06 (1.25 to 3.38) | −5 | 3.22 (2.16 to 4.29) | 3.80 (2.67 to 5.39) | 124 | 2.35 (1.37 to 3.33) | 2.04 (1.49 to 2.79) | 63 | 0.91 (0.09 to 1.74) | 1.72 (1.04 to 2.82) | −37 |
| Total services | 3.62 (−0.06 to 7.30) | 1.39 (1.08 to 1.78) | 1.46 (−1.22 to 4.14) | 1.13 (0.97 to 1.31) | −60 | 1.01 (−1.37 to 3.39) | 1.10 (0.96 to 1.25) | −72 | 0.75 (−1.48 to 2.98) | 1.08 (0.96 to 1.21) | −79 | −0.24 (−2.42 to 1.95) | 1.02 (0.90 to 1.17) | −107 |
Last 6 months before death.
Survived 6 months or more.
Difference between the mean number of health services allocated to patients with hepatocellular carcinoma and to matched non-cancer control subjects.
Estimated by modelling count, using negative binomial regression. Control subjects constituted the reference population.
Compared with 2002–2003.
HCU = health care utilization; CI = confidence interval.
TABLE VII.
Net health care utilization per 30 patient–days and rate ratio of resource use attributable to hepatocellular carcinoma care at end-of-lifea among short-term survivorsb by death year of service, 2002–2009
| Variable | Death year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 2002–2003 | 2004–2005 | Net HCU changee (%) | 2006–2007 | Net HCU changee (%) | 2008–2009 | Net HCU changee (%) | |||||
|
|
|
|
|
||||||||
| Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | Mean net HCUc (95% CI) | Mean rate ratiod (95% CI) | ||||
| Matched cases (n) | 107 | 160 | 199 | 220 | |||||||
| Family physician visits | 4.10 (2.05 to 6.15) | 2.75 (2.03 to 3.73) | 3.72 (2.59 to 4.84) | 2.50 (2.01 to 3.11) | −9 | 4.75 (3.62 to 5.87) | 3.43 (2.79 to 4.22) | 16 | 4.12 (3.17 to 5.07) | 3.15 (2.60 to 3.82) | 0 |
| Specialist visits | 10.39 (8.09 to 12.69) | 4.88 (3.74 to 6.37) | 9.91 (8.05 to 11.77) | 6.04 (4.78 to 7.64) | −5 | 9.91 (8.19 to 11.63) | 5.26 (4.31 to 6.43) | −5 | 9.14 (7.68 to 10.61) | 4.82 (3.97 to 5.86) | −12 |
| Emergency department visits | 1.00 (0.73 to 1.27) | 4.82 (3.18 to 7.32) | 1.23 (0.80 to 1.66) | 4.18 (3.04 to 5.73) | 23 | 1.92 (1.39 to 2.44) | 7.77 (5.60 to 10.79) | 92 | 1.16 (0.69 to 1.63) | 4.16 (3.08 to 5.60) | 16 |
| Acute inpatient hospitalizations | 1.42 (0.98 to 1.86) | 9.98 (5.87 to 16.98) | 1.30 (0.87 to 1.74) | 10.35 (6.43 to 16.66) | −8 | 1.61 (1.09 to 2.14) | 11.98 (7.73 to 18.58) | 14 | 1.33 (0.91 to 1.75) | 10.36 (6.92 to 15.49) | −6 |
| Same-day surgery | 0.20 (−0.11 to 0.50) | 16.31 (1.95 to 136.60) | 0.12 (0.05 to 0.19) | 12.67 (2.56 to 62.69) | −38 | 0.06 (0.01 to 0.10) | 2.35 (1.02 to 5.38) | −71 | 0.05 (0.02 to 0.08) | 3.13 (1.17 to 8.40) | −75 |
| Number of prescription medications | −2.04 (−3.82 to −0.26) | 0.62 (0.39 to 0.99) | −3.62 (−5.68 to −1.56) | 0.52 (0.36 to 0.74) | −78 | −3.18 (−4.59 to −1.76) | 0.56 (0.42 to 0.75) | −56 | −2.89 (−4.72 to −1.06) | 0.68 (0.51 to 0.92) | −42 |
| Homecare visits | 0.82 (−0.36 to 2.00) | 2.98 (1.37 to 6.50) | 2.36 (0.92 to 3.79) | 3.94 (2.17 to 7.16) | 189 | 2.01 (1.17 to 2.86) | 3.19 (1.97 to 5.16) | 147 | 1.04 (−0.01 to 2.09) | 1.62 (1.03 to 2.56) | 27 |
| Total services | 15.69 (12.18 to 19.20) | 2.42 (2.01 to 2.91) | 14.89 (11.05 to 18.73) | 2.14 (1.79 to 2.55) | −5 | 17.02 (13.95 to 20.10) | 2.34 (2.03 to 2.71) | 9 | 13.90 (10.57 to 17.24) | 2.01 (1.72 to 2.34) | −11 |
Last 6 months before death.
Survived less than 6 months.
Difference between the mean number of health services allocated to patients with hepatocellular carcinoma and to matched non-cancer control subjects.
Estimated by modelling count, using negative binomial regression. Control subjects constituted the reference population.
Compared with 2002–2003.
HCU = health care utilization; CI = confidence interval.
Health Care Costs Attributable to HCC
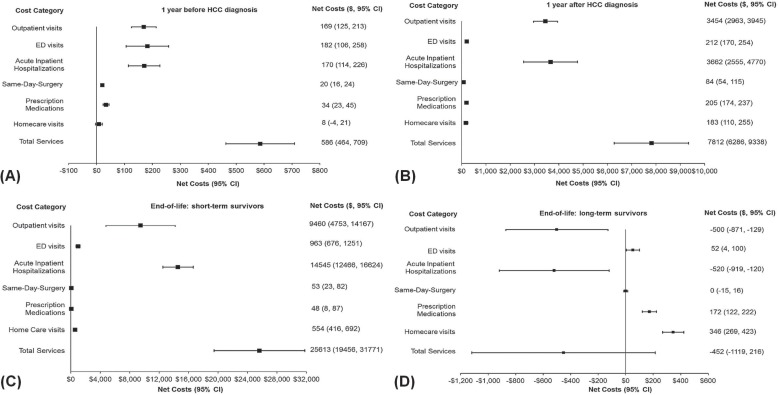
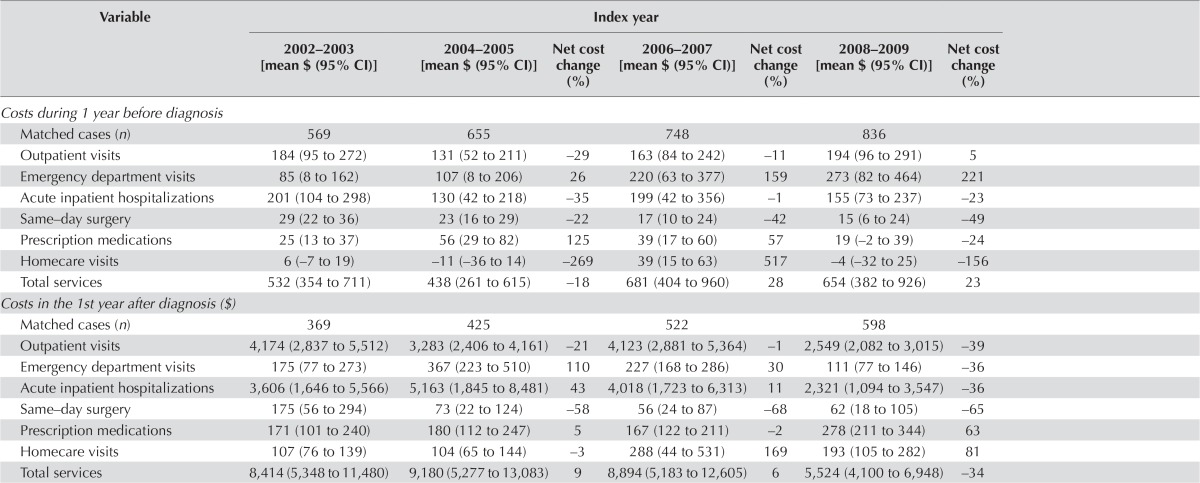
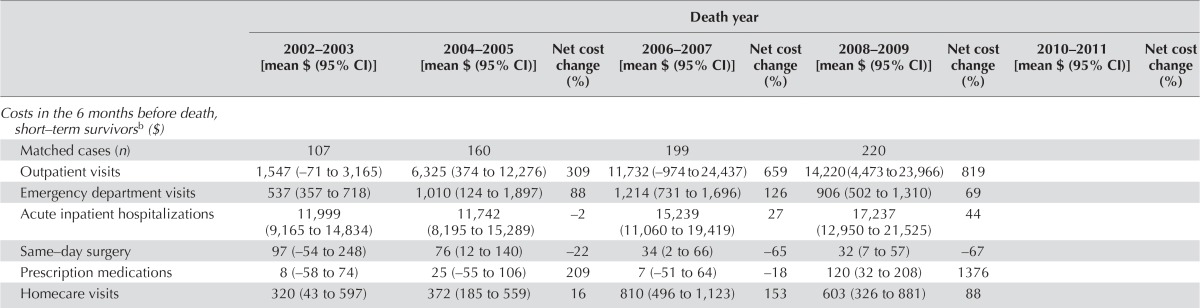
Figure 4 presents the overall mean net cost of care per 30 patient–days attributable to hcc for each type of service, and the net cost of all services for each phase of care (2002–2009 or 2002–2011). Table ix summarizes trends in the net costs of care attributable to hcc over time (to 2008–2009 or 2010–2011 from 2002–2003)—that is, the estimate of the difference in costs for hcc patients compared with non-cancer control subjects over time. Overall, the mean net costs per 30 patient–days of outpatient visits and hospitalizations were the highest in the pre-diagnosis, initial, and end-of-life for short-term survivors phases [Figure 4(A–C)]. Mean net homecare costs were highest during the end-of-life for long-term survivors phase [Figure 4(D)].
FIGURE 4.
Mean net cost of care attributable to hepatocellular carcinoma (HCC), by cost category, (A) at 1 year before diagnosis (2002–2009), (B) during the 1st year after diagnosis (2002–2009), (C) during the last 6 months before death for short-term (<6 months) survivors (2002–2011), and (D) during the last 6 months before death for long-term (≥6 months) survivors (2002–2011). Net costs were generated using generalized estimating equations. Values are expressed as means with 95% confidence intervals per 30 patient–days and reflect 2013 Canadian dollars. Error bars indicate the 95% confidence intervals. CI = confidence interval; ED = emergency department.
TABLE IX.
Net cost of carea attributable to hepatocellular carcinoma per 30 patient–days by cost category, disease phase, and index year or death year of service
| Variable | Index year | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2002–2003 [mean $ (95% CI)] | 2004–2005 [mean $ (95% CI)] | Net cost change (%) | 2006–2007 [mean $ (95% CI)] | Net cost change (%) | 2008–2009 [mean $ (95% CI)] | Net cost change (%) | |
| Costs during 1 year before diagnosis | |||||||
| Matched cases (n) | 569 | 655 | 748 | 836 | |||
| Outpatient visits | 184 (95 to 272) | 131 (52 to 211) | −29 | 163 (84 to 242) | −11 | 194 (96 to 291) | 5 |
| Emergency department visits | 85 (8 to 162) | 107 (8 to 206) | 26 | 220 (63 to 377) | 159 | 273 (82 to 464) | 221 |
| Acute inpatient hospitalizations | 201 (104 to 298) | 130 (42 to 218) | −35 | 199 (42 to 356) | −1 | 155 (73 to 237) | −23 |
| Same−day surgery | 29 (22 to 36) | 23 (16 to 29) | −22 | 17 (10 to 24) | −42 | 15 (6 to 24) | −49 |
| Prescription medications | 25 (13 to 37) | 56 (29 to 82) | 125 | 39 (17 to 60) | 57 | 19 (−2 to 39) | −24 |
| Homecare visits | 6 (−7 to 19) | −11 (−36 to 14) | −269 | 39 (15 to 63) | 517 | −4 (−32 to 25) | −156 |
| Total services | 532 (354 to 711) | 438 (261 to 615) | −18 | 681 (404 to 960) | 28 | 654 (382 to 926) | 23 |
| Costs in the 1st year after diagnosis ($) | |||||||
| Matched cases (n) | 369 | 425 | 522 | 598 | |||
| Outpatient visits | 4,174 (2,837 to 5,512) | 3,283 (2,406 to 4,161) | −21 | 4,123 (2,881 to 5,364) | −1 | 2,549 (2,082 to 3,015) | −39 |
| Emergency department visits | 175 (77 to 273) | 367 (223 to 510) | 110 | 227 (168 to 286) | 30 | 111 (77 to 146) | −36 |
| Acute inpatient hospitalizations | 3,606 (1,646 to 5,566) | 5,163 (1,845 to 8,481) | 43 | 4,018 (1,723 to 6,313) | 11 | 2,321 (1,094 to 3,547) | −36 |
| Same−day surgery | 175 (56 to 294) | 73 (22 to 124) | −58 | 56 (24 to 87) | −68 | 62 (18 to 105) | −65 |
| Prescription medications | 171 (101 to 240) | 180 (112 to 247) | 5 | 167 (122 to 211) | −2 | 278 (211 to 344) | 63 |
| Homecare visits | 107 (76 to 139) | 104 (65 to 144) | −3 | 288 (44 to 531) | 169 | 193 (105 to 282) | 81 |
| Total services | 8,414 (5,348 to 11,480) | 9,180 (5,277 to 13,083) | 9 | 8,894 (5,183 to 12,605) | 6 | 5,524 (4,100 to 6,948) | −34 |
| Death year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 2002–2003 [mean $ (95% CI)] | 2004–2005 [mean $ (95% CI)] | Net cost change (%) | 2006–2007 [mean $ (95% CI)] | Net cost change (%) | 2008–2009 [mean $ (95% CI)] | Net cost change (%) | 2010–2011 [mean $ (95% CI)] | Net cost change (%) | |
| Costs in the 6 months before death, short–term survivorsb($) | |||||||||
| Matched cases (n) | 107 | 160 | 199 | 220 | |||||
| Outpatient visits | 1,547 (−71 to 3,165) | 6,325 (374 to 12,276) | 309 | 11,732 (−974 to 24,437) | 659 | 14,220 (4,473 to 23,966) | 819 | ||
| Emergency department visits | 537 (357 to 718) | 1,010 (124 to 1,897) | 88 | 1,214 (731 to 1,696) | 126 | 906 (502 to 1,310) | 69 | ||
| Acute inpatient hospitalizations | 11,999 (9,165 to 14,834) | 11,742 (8,195 to 15,289) | −2 | 15,239 (11,060 to 19,419) | 27 | 17,237 (12,950 to 21,525) | 44 | ||
| Same−day surgery | 97 (−54 to 248) | 76 (12 to 140) | −22 | 34 (2 to 66) | −65 | 32 (7 to 57) | −67 | ||
| Prescription medications | 8 (−58 to 74) | 25 (−55 to 106) | 209 | 7 (−51 to 64) | −18 | 120 (32 to 208) | 1376 | ||
| Homecare visits | 320 (43 to 597) | 372 (185 to 559) | 16 | 810 (496 to 1,123) | 153 | 603 (326 to 881) | 88 | ||
| Costs in the 6 months before death,long−term survivorsc($) | |||||||||
| Matched cases (n) | 52 | 138 | 219 | 320 | 218 | ||||
| Outpatient visits | −46 (−1,649 to 1,557) | −1,232 (−2,313 to −150) | −2587 | −761 (−1,776 to 254) | −1560 | −426 (−888 to 36) | −829 | −18 (−780 to 743) | 60 |
| Emergency department visits | 40 (−171 to 250) | 93 (−68 to 254) | 133 | 148 (43 to 254) | 272 | 7 (−86 to 100) | −82 | 1 (−9 to 12) | −97 |
| Acute inpatient hospitalizations | −34 (−1,394 to 1,325) | −573 (−1,492 to 345) | −1564 | −639 (−1,751 to 474) | −1753 | −1,003 (−1,744 to −263) | −2812 | 204 (−135 to 543) | 691 |
| Same–day surgery | −30 (−132 to 71) | −1 (−28 to 25) | 96 | 22 (−20 to 64) | 173 | −12 (−42 to 19) | 62 | 1 (0 to 3) | 104 |
| Prescription medications | 175 (39 to 311) | 95 (7 to 183) | −46 | 28 (−71 to 126) | −84 | 284 (191 to 378) | 63 | 200 (79 to 320) | 14 |
| Homecare visits | 283 (49 to 518) | 138 (−42 to 318) | −51 | 537 (377 to 697) | 89 | 385 (232 to 538) | 36 | 230 (98 to 361) | −19 |
| Total services | 390 (−2,197 to 2,977) | −1,480 (−3,239 to 278) | −480 | −671 (−2,525 to 1,184) | −272 | −764 (−1,868 to 340) | −296 | 612 (−252 to 1,477) | 57 |
Generated using generalized estimating equations. Values reflect 2013 Canadian dollars.
Survived less than 6 months.
Survived 6 months or more.
For hcc patients, the net average total health care cost per 30 patient–days was $586 (95% ci: $464 to $709) in the pre-diagnosis phase, which increased in the initial phase to $7,812 (95% ci: $6,286 to $9,338) and increased markedly in the end-of-life phase for short-term survivors to $25,613 (95% ci: $19,456 to $31,771), but which decreased substantially in the end-of-life phase for long-term survivors to −$452 (95% ci: −$1,119 to $216; Figure 4).
Outpatient visits accounted for approximately 29% of the net total cost during the pre-diagnosis phase [$169 (95% ci: $125 to $213)], 44% during the initial phase [$3,454 (95% ci: $2,963 to $3,945)], and 37% during the end-of-life phase for short-term survivors [$9,460 (95% ci: $4,753 to $14,167)]. Those costs contributed 11% in cost savings (fewer costs than were incurred by control subjects) during the end-of-life phase for long-term survivors [−$500 (95% ci: −$871 to −$129)]. The net total cost of outpatient visits was highest during the end-of-life phase for short-term survivors.
Visits to the ed accounted for approximately 31% of the net total cost during the pre-diagnosis phase [$182 (95% ci: $106 to $258)], 3% during the initial phase [$212 (95% ci: $170 to $254)], and 4% during the end-of-life phase for short-term survivors [$963 (95% ci: $676 to $1,251)]. Such visits contributed 12% during the end-of-life phase for long-term survivors [$52 (95% ci: $4 to $100)]. Short-term survivors in the end-of-life phase incurred the highest costs associated with ed visits.
Hospitalizations accounted for approximately 29% of the net total cost during the pre-diagnosis phase [$170 (95% ci: $114 to $226)], 47% during the initial phase [$3,662 (95% ci: $2,555 to $4,770)], and 57% during the end-of-life phase for short-term survivors [$14,545 (95% ci: $12,466 to $16,624)]. They contributed 15% in cost savings during the end-of-life phase for long-term survivors [−$520 (95% ci: −$919 to −$120)]. Short-term survivors in the end-of-life phase incurred the highest costs associated with hospitalizations.
Mean net costs attributable to outpatient visits and total services significantly increased to $14,220 (95% ci: $4,473 to $23,966) in 2008–2009 from $1,547 (95% ci: −$71 to $3,165) in 2002–2003 and to $33,121 (95% ci: $19,966 to $46,275) in 2008–2009 from $14,450 (95% ci: $10,872 to $18,027) in 2002–2003 respectively, during the end-of-life phase for short-term survivors.
DISCUSSION
Our study demonstrated that in all phases of care, compared with non-cancer control subjects, hcc patients used a greater number of health care services (exceptions were same-day surgery during the pre-diagnosis phase and prescription medications during the end-of-life phase for both short-term and long-term survivors). Mean net costs attributable to outpatient visits and total services significantly increased in 2008–2009 from 2002–2003 by 819% and 129% respectively in the end-of-life phase for short-term survivors. Those increases might reflect increases in the intensity of treatment with newer technologies—in particular, radiofrequency ablation (increased by a factor of 5) and sorafenib (increased by a factor of 15). Overall, health care utilization and costs attributable to hcc were high for specialist and outpatient visits, ed visits, and hospitalizations in the pre-diagnosis, initial, and end-of-life phases for short-term survivors, which could represent the increasing costs associated with hcc diagnosis. In many cases, hcc does not present with any severe symptoms until very late in the course of the disease 9,12,13. Thus, the resource utilization and costs incurred are significant even before diagnosis.
Very few studies have determined differences in health care resource utilization between cancer patients and matched non-cancer control subjects over time. Studies from Denmark 21 and the United States 22 using matched patients and control subjects have reported that the pre-diagnosis phase is a resource-intensive component in cancer care episodes, with large-factor increases in the use of general practice visits, diagnostic investigations, ed visits, and hospital services. In addition, the Danish study 21 reported a marked use of hospital services in the year after diagnosis.
The high treatment costs for hcc 15 and the complexity in managing the disease present both financial and clinical challenges. The increasing prevalence of cirrhosis and its complications in Canada 38 means that surveillance, diagnosis, and care for individuals with hcc is further complicated. Furthermore, the very early stage of hcc—for example, a single asymptomatic lesion measuring less than 2 cm in diameter, with no vascular or distant metastasis—is difficult to diagnose 12. Although ultrasound surveillance of populations at risk for hcc has been considered by the hepatology community both in Canada and internationally to be the standard of care, such surveillance has not been widely promoted by Canadian health agencies 39–43.
Given the ineffectiveness or low rates of community surveillance for cirrhosis or identification of patients at high risk for hcc, the incidence of hcc continues to increase 14. Consequently, demand for screening, diagnosis, care, and curative treatment is also increasing. Outpatient visits contributed a significant proportion of the net health care utilization and costs incurred in the pre-diagnosis care phase, the initial care phase, and the end-of-life care phase for short-term survivors, presenting significant policy implications given the national shortage of liver disease specialists.
With respect to gastroenterologists, Canada has, at 1.83 per 100,000 population, one of the lowest specialist- to-population ratios in the G8 countries, and that ratio is expected to drop by one third as current gastroenterologists approach retirement age over the next 5 years 44,45. Wait times for referred individuals are substantial, and according to one review, fewer than 33% of patients referred for a probable cancer were seen by a gastroenterologist within the target wait time 45. Compounding that difficulty is the fact that only a minority of gastroenterologists routinely see patients with liver disease 46. Lack of timely care could therefore affect the survival prognosis for patients with hcc and could increase the number of ed visits and hospitalizations. Because ed visits represent a significant burden to the health care system, the likely contribution to the patient load resulting from hcc emphasizes the need for prevention and early diagnosis.
This retrospective matched case–control population- based study had a large sample size and used rigorous propensity scoring to match hcc patients with non-cancer control subjects by sociodemographic characteristics and comorbidity. It therefore provides a comprehensive and accurate estimation of the net health care utilization and net costs of care in Ontario between 2002 and 2009. It builds on previous work by calculating net health care utilization and costs, exploring a longer pre-diagnosis care phase to capture routine surveillance for diagnosis, and reporting on cost trends over time. It also includes analyses of important contributors to costs—namely, hospitalizations, drugs, physician services, and homecare services, which account for the largest health care expenditures.
The study has some limitations, however.
First, hcc stage data in the ocr to differentiate utilization by cancer stage are limited, and cancer treatment, health care utilization, and costs can vary by stage at diagnosis 47,48. Classification of malignant tumours based on TNM staging [extent of the tumour (T), extent of spread to the lymph nodes (N), and presence of metastasis (M) 49,50] was used in the ocr from 2004 onwards; the most common stage grouping (45.8%) was “unknown”; 29.5% of cases were designated advanced-stage (iii–iv), 14.2% were designated intermediate-stage (ii), and 10.4% were designated early-stage (0–i). The 5-year relative survival rate for liver cancer in Canada is 20% 9, and yet that rate varies with both age and stage at diagnosis. Nevertheless, compared with long-term survivors, short-term survivors, who can be hypothesized to have more aggressive–stage disease, had significantly higher health care utilization and costs during the end-of-life phase.
Second, the Ontario Drug Benefit Program covers only patients 65 years of age and older or patients with special circumstances. Thus, the estimated cost of prescription medications is an underestimate. Nevertheless, prescription medication costs were higher for hcc patients than for non-cancer control subjects in all care phases.
Third, we were not able to estimate indirect costs (including caregiver time costs; out-of-pocket costs; costs of lost production because of short-term absence from work, permanent disability, and death before 65 years of age), which are important for reaching an understanding of the cost of an illness to society and patients.
Fourth, because of the relatively small sample of patients in the end-of-life phase, we were not able to conduct subgroup analyses or examine predictors of increased costs and health care system utilization. Our cohort is now several years old, and some secular trends in practice could have occurred; however, all current modalities of treatment, including sorafenib, were available by the end of our study period.
Finally, a generalized linear model specifying a negative binomial distribution was assumed for all health services utilization. However, that model might not have been completely appropriate for every service analysis, given that, in some situations, a large proportion of the control subjects used no services. The resulting differences might have contributed to the large rrs reached in the negative binomial regression.
CONCLUSIONS
Our study found increasing resource use and net costs of care during the study period for hcc patients, particularly for short-term survivors during the end-of-life phase. The combination of the growing hcc incidence in Canada and possible new, but expensive, future treatments mean that hcc will continue to place a significant burden on the health care system. Additional information that will contribute to effective prevention and early detection is needed so that the costs incurred from late diagnosis and the terminal period of life can be remediated. The information presented here can assist in policy decision-making with respect to resource allocation for cancer prevention and control, and can serve as a foundation for economic and health outcomes evaluations to improve survival and reduce costs within the context of the health care system.
ACKNOWLEDGMENTS
The authors thank Matthew Kumar, Nelson Chong, and Refik Saskin from the Institute for Clinical and Evaluative Sciences (ices) for data linkage. The authors are also grateful to Katrina Chan from the Ontario Institute for Cancer Research (oicr) and Cancer Care Ontario (cco) for assistance in data acquisition.
This study was supported through provision of data by ices and cco, and through funding support to ices from an annual grant by the Ministry of Health and Long-Term Care and oicr. The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ices, cco, oicr, or the Government of Ontario is intended or should be inferred. HHT received a New Investigator Award from the oicr Health Services Research Program at the Dalla Lana School of Public Health, University of Toronto.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Nunnari G, Berretta M, Pinzone MR, et al. Hepatocellular carcinoma in hiv positive patients. Eur Rev Med Pharmacol Sci. 2012;16:1257–70. [PubMed] [Google Scholar]
- 3.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–19. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–8. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–64. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 8.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of hcc and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–8.e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. [Google Scholar]
- 10.Dyer Z, Peltekian K, van Zanten SV. Review article: the changing epidemiology of hepatocellular carcinoma in Canada. Aliment Pharmacol Ther. 2005;22:17–22. doi: 10.1111/j.1365-2036.2005.02504.x. [DOI] [PubMed] [Google Scholar]
- 11.Pocobelli G, Cook LS, Brant R, Lee SS. Hepatocellular carcinoma incidence trends in Canada: analysis by birth cohort and period of diagnosis. Liver Int. 2008;28:1272–9. doi: 10.1111/j.1478-3231.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 13.Tejeda-Maldonado J, Garcia-Juarez I, Aguirre-Valadez J, et al. Diagnosis and treatment of hepatocellular carcinoma: an update. World J Hepatol. 2015;7:362–76. doi: 10.4254/wjh.v7.i3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalili K, Menezes R, Yazdi LK, et al. Hepatocellular carcinoma in a large Canadian urban centre: stage at treatment and its potential determinants. Can J Gastroenterol Hepatol. 2014;28:150–4. doi: 10.1155/2014/561732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thein HH, Isaranuwatchai W, Campitelli MA, et al. Health care costs associated with hepatocellular carcinoma: a population-based study. Hepatology. 2013;58:1375–84. doi: 10.1002/hep.26231. [DOI] [PubMed] [Google Scholar]
- 16.Baker MS, Kessler LG, Urban N, Smucker RC. Estimating the treatment costs of breast and lung cancer. Med Care. 1991;29:40–9. doi: 10.1097/00005650-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Taplin SH, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–26. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 18.Brown ML, Riley GF, Potosky AL, Etzioni RD. Obtaining long-term disease specific costs of care: application to Medicare enrollees diagnosed with colorectal cancer. Med Care. 1999;37:1249–59. doi: 10.1097/00005650-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from seer–Medicare data. Med Care. 2002;40:IV-104–17. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 20.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 21.Christensen KG, Fenger-Gron M, Flarup KR, Vedsted P. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis—a population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res. 2012;12:224. doi: 10.1186/1472-6963-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornbrook MC, Fishman PA, Ritzwoller DP, Elston-Lafata J, O’Keeffe-Rosetti MC, Salloum RG. When does an episode of care for cancer begin? Med Care. 2013;51:324–9. doi: 10.1097/MLR.0b013e3182731277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall S, Schulze K, Groome P, Mackillop W, Holowaty E. Using cancer registry data for survival studies: the example of the Ontario Cancer Registry. J Clin Epidemiol. 2006;59:67–76. doi: 10.1016/j.jclinepi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Holowaty E. The Ontario Cancer Registry: a registry with almost completely automated data collection. In: Black RJ, Simonato L, Storm HH, Démaret E, editors. Automated Data Collection in Cancer Registry. Lyon, France: IARC Press; 1998. pp. 39–44. IARC Technical Reports, no. 32. [Google Scholar]
- 25.Holowaty EJ, Moravan V, Lee G, Chong N, Dale D. A Reabstraction Study to Estimate the Completeness and Accuracy of Data Elements in the Ontario Cancer Registry: A Report to Health Canada. Toronto, ON: Ontario Cancer Registry; 1996. [Google Scholar]
- 26.Clarke EA, Marrett LD, Kreiger N. Cancer registration in Ontario: a computer approach. IARC Sci Publ. 1991:246–57. [PubMed] [Google Scholar]
- 27.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture–recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41:495–501. doi: 10.1016/0895-4356(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 28.Jembere N, Campitelli MA, Sherman M, et al. Influence of socioeconomic status on survival of hepatocellular carcinoma in the Ontario population; a population-based study, 1990–2009. PLoS One. 2012;7:e40917. doi: 10.1371/journal.pone.0040917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thein HH, Campitelli MA, Yeung LT, Zaheen A, Yoshida EM, Earle CC. Improved survival in patients with viral hepatitis-induced hepatocellular carcinoma undergoing recommended abdominal ultrasound surveillance in Ontario: a population-based retrospective cohort study. PLoS One. 2015;10:e0138907. doi: 10.1371/journal.pone.0138907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkins R. PCCF+ Version 4D User’s Guide. Ottawa, ON: Health Analysis and Measurement Group, Statistics Canada; 2004. [Available online at: http://data.library.utoronto.ca/datapub/codebooks/cstdli/pccf_health/pccf4d/msword-pccf4d.pdf; cited 12 March 2015] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. J Clin Epidemiol. 1992;45:613–19. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs P, Yim R. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2009. Using Canadian Administrative Databases to Derive Economic Data for Health Technology Assessments. [Available online at: http://www.cadth.ca/media/pdf/H0483_Canadian_Admin_Databases_mg_e.pdf; cited 19 June 2011] [Google Scholar]
- 34.Pink GH, Bolley HB. Physicians in health care management: 3. Case mix groups and resource intensity weights: an overview for physicians. CMAJ. 1994;150:889–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Pink GH, Bolley HB. Physicians in health care management: 4. Case mix groups and resource intensity weights: physicians and hospital funding. CMAJ. 1994;150:1255–61. [PMC free article] [PubMed] [Google Scholar]
- 36.Canadian Institute for Health Information (cihi) Acute Care Grouping Methodologies: From Diagnosis Related Groups to Case Mix Groups Redevelopment. Ottawa, ON: CIHI; 2004. [Available online at: https://secure.cihi.ca/free_products/Acute_Care_Grouping_Methodologies2004_e.pdf; cited 2 September 2015] [Google Scholar]
- 37.Statistics Canada . Consumer Price Index, by province (Canada) [Web page] Ottawa, ON: Statistics Canada; n.d. [Available at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ09a-eng.htm; cited 29 January 2015] [Google Scholar]
- 38.Myers RP, Ramji A, Bilodeau M, Wong S, Feld JJ. An update on the management of hepatitis C: consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterol. 2012;26:359–75. doi: 10.1155/2012/947676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman M, Bain V, Villeneuve JP, et al. The management of chronic viral hepatitis: a Canadian consensus conference 2004. Can J Gastroenterol. 2004;18:715–28. doi: 10.1155/2004/201031. [DOI] [PubMed] [Google Scholar]
- 40.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asmis T, Balaa F, Scully L, et al. Diagnosis and management of hepatocellular carcinoma: results of a consensus meeting of The Ottawa Hospital Cancer Centre. Curr Oncol. 2010;17:6–12. doi: 10.3747/co.v17i2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruix J, Sherman M, on behalf of the American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.European Association for the Study of the Liver and the European Organisation for Research and Treatment of Cancer easl–eortc clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Moayyedi P, Tepper J, Hilsden R, Rabeneck L. International comparisons of manpower in gastroenterology. Am J Gastroenterol. 2007;102:478–81. doi: 10.1111/j.1572-0241.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 45.Leddin D, Armstrong D, Borgaonkar M, et al. The 2012 sage wait times program: Survey of Access to Gastroenterology in Canada. Can J Gastroenterol. 2013;27:83–9. doi: 10.1155/2013/143018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bain VG, Wong WW, Greig PD, Yoshida EM, on behalf of the Canadian Association of Gastroenterology Hepatology and the Canadian gastroenterologist: interest, attitudes and patterns of practice: results of a national survey from the Canadian Association of Gastroenterology. Can J Gastroenterol. 2003;17:25–9. doi: 10.1155/2003/709303. [DOI] [PubMed] [Google Scholar]
- 47.Yabroff KR, Kim Y. Time costs associated with informal care-giving for cancer survivors. Cancer. 2009;115(suppl):4362–73. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 48.Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21:281–93. doi: 10.3747/co.21.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleming ID. ajcc/tnm cancer staging, present and future. J Surg Oncol. 2001;77:233–6. doi: 10.1002/jso.1101. [DOI] [PubMed] [Google Scholar]
- 50.Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK, on behalf of the American Joint Committee on Cancer, editors. AJCC Cancer Staging Atlas: A Companion to the Seventh Editions of the AJCC Cancer Staging Manual and Handbook. 2nd ed. New York, NY: Springer Science and Business Media; 2012. [DOI] [Google Scholar]