Abstract
Background
The Enhanced Recovery After Surgery (eras) colorectal guideline has been implemented widely across Alberta. Our study examined the clinical and cost impacts of eras on colon cancer patients across the province.
Methods
We first used both summary statistics and multivariate regression methods to compare, before and after guideline implementation, clinical outcomes (length of stay, complications, readmissions) in consecutive elective colorectal patients 18 or more years of age and in colon cancer and non-cancer patients treated at the Peter Lougheed Centre and the Grey Nuns Hospital between February 2013 and December 2014. We then used the differences in clinical outcomes for colon cancer patients, together with the average cost per hospital day, to estimate cost impacts.
Results
The analysis considered 790 patients (398 cancer and 392 non-cancer patients). Mean guideline compliance increased to 60% in cancer patients and 57% in non-cancer patients after eras implementation from 37% overall before eras implementation. From pre- to post-eras, mean length of stay declined to 8.4 ± 5 days from 9.5 ± 7 days in cancer patients, and to 6.4 ± 4 days from 8.8 ± 5.5 days in non-cancer patients (p = 0.0012 and p = 0.0041 respectively). Complications declined significantly in the renal, hepatic, pancreatic, and gastrointestinal groups (difference in proportions: 13% in cancer patients; p < 0.05). No significant change in the risk of readmission was observed. The net cost savings attributable to eras implementation ranged from $1,096 to $2,771 per cancer patient and from $3,388 to $7,103 per non-cancer patient.
Conclusions
Implementation of eras not only resulted in clinical outcome improvements, but also had a significant beneficial impact on scarce health system resources. The effect for cancer patients was different from that for non-cancer patients, representing an opportunity for further refinement and study.
Keywords: eras, cost savings, complications, readmissions, length of stay, colon cancer, benefits, guideline compliance
INTRODUCTION
The colorectal guideline from the Enhanced Recovery After Surgery (ERAS) Society (Kista, Sweden) has been widely studied internationally1–6, with most centres achieving significant outcome effects, including a reduction in length of stay [los (2.5 days on average)], a decline in complications5–8, and cost benefits to the system [mean savings of €1651 (US$2245) per patient]4,9,10. We recently used the Alberta Health Services ERAS Implementation Program to implement the eras guideline across Alberta, achieving similar health-system benefits11.
To the best of our knowledge, the effects of the ERAS Society’s colorectal guideline on patient outcomes and on health system economic benefits, comparing colon cancer patients with non-cancer patients, have yet to be reported. In the present study, we therefore aimed to evaluate the initial effects of the ERAS Society’s colorectal guideline on patient outcomes and health system costs in comparisons of colorectal surgical patients before and after guideline implementation, and of colon cancer patients with non-cancer patients.
METHODS
The Alberta Health Services ER AS Implementation Program began in February 2013 at the Peter Lougheed Centre and the Grey Nuns Hospital. Starting in February 2014, 4 other centres (Foothills Medical Centre, University of Alberta Hospital, Royal Alexandra Hospital, and Misericordia Community Hospital) joined the implementation program. The program methods have been described in detail elsewhere11. Using the definitions published by Gustafsson et al.12 and Nygren et al.13, compliance with the eras guideline was measured separately for each of its 22 elements. We hypothesized that, because cancer patients are generally sicker than non-cancer patients, overall compliance with the guideline was not similar for cancer and non-cancer patients, and therefore the effects of implementing the guideline would be different for cancer and non-cancer patients. Patients undergoing colorectal surgery for cancer were compared with those who underwent colorectal surgery for non-cancer indications.
Outcomes
The main outcome measures were los (number of days between the primary operation date and the discharge date), complications (grouped by eras Interactive Audit System categories: urologic, respiratory, infectious, cardiovascular, renal, hepatic, pancreatic, gastrointestinal, surgical, anesthetic, and psychiatric), and readmissions (readmission within 30 days after discharge). Demographic and clinical variables were also measured.
Sample Size Estimate
Given a within-group standard deviation of 8.2 days (calculated from historical data), a confidence level of 0.05, and a power of 80%, an estimated total sample size of 270 patients was expected to detect a mean difference of 2 days (estimated from historical data) between cancer and non-cancer patients. In the same vein, given a standard deviation of 11 days (also estimated from historical data, all patients included), an estimated total sample size of 475 patients was expected to detect a mean difference of 2.5 days5,6. Using the rule of at least 10 observations per predictor variable in multiple regression models14,15, the foregoing sample size permitted the use of regression models that included all potential confounding factors for readmissions as an outcome variable.
Data Analyses
Wilcoxon tests were used for comparisons of cancer with non-cancer patients and of pre- and post-eras patients for age, body mass index, and overall eras compliance. Chi-square tests with post-hoc Bonferroni corrections were used for comparing multinomial variables. Patients were grouped into five 3-month time intervals, and those intervals were used to compare los for the pre- and post-eras patients. A log-binomial regression model was used to compute the relative risk (rr) for 30-day post-discharge readmission, adjusted for potential confounding factors (surgical approach, American Society of Anesthesiologists physical status classification16, surgery type, sex, smoking status, alcohol consumption, and diabetes status). Chi-square tests were used to compare the proportions of patients who developed at least 1 complication (by complications group) during their primary hospital stay.
Cost Impact Analysis
A cost impact analysis, using a hospital perspective, examined primary los (that is, for surgery), 30-day post-discharge readmissions, and readmission los, to determine potential differences in costs for cancer patients pre- and post-eras and also for non-cancer patients preand post-eras. To calculate the net cost impact, we subtracted the eras intervention costs, including labour or coordination and licensing fees. Differences in los and in readmission likelihood were estimated as already described. Cost impacts were estimated by applying the unit cost of an inpatient hospital day to the differences for patients pre- and post-eras. Unit cost of an inpatient hospital day ($1,114–$2,106) was estimated using the Alberta hospital discharge abstract database and the case-mix groups for colorectal surgery. The case-mix groups are “colostomy or enterostomy,” “open large-intestine or rectum resection without colostomy” (planned and unplanned), “endoscopic large-intestine or rectum resection without colostomy,” “repair or fixation and other moderate intervention on lower gastrointestinal tract,” “minor lower gastrointestinal intervention,” and “digestive malignancy.” The unit cost included patient-specific drug and supply costs, salaries, medical and surgical supplies, administration and support services, but not the costs of physician services and pharmaceuticals. All costs were converted to 2014 Canadian dollars using the Consumer Price Index.
Ethics approval was obtained from the research ethics boards at the University of Calgary and the University of Alberta.
RESULTS
The study considered 790 colorectal surgical patients from the two original sites, consisting of 398 patients who underwent surgery for primary adenocarcinoma (68 pre- and 330 post-eras) and 392 non-cancer patients (48 pre- and 344 post-eras). The balance of demographic characteristics for pre- and post-eras patient was good for sex, age, body mass index, American Society of Anesthesiologists class, and main procedure (Table i). However, significantly more men than women had a cancer diagnosis. As expected, cancer patients were significantly older than non-cancer patients: more of the cancer patients were at least 64 years of age (59% vs. 40% for the non-cancer patients).
TABLE I.
Demographics and clinical characteristics of the study patients
| Variable | Patient groupa | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| ERAS implementation | p value | With cancer | p Value | |||
|
|
|
|||||
| Before | After | Yes | No | |||
| Patients (n) | 117 | 673 | — | 398 | 392 | — |
| Sex [n (%)] | ||||||
| Men | 58 (50) | 377 (56) | 0.2288 | 238 (60) | 197 (50) | 0.0062 |
| Women | 58 (50) | 296 (44) | 0.2288 | 159 (40) | 195 (50) | 0.0062 |
| Unknown | 1 (1) | 0 | — | 1 (0) | 0 | — |
| BMI [n patients (median value)] | 109 (27) | 662 (27) | 0.8611 | 388 (28) | 383 (27) | 0.5382 |
| Age [n patients (median years)] | 115 (63) | 674 (64) | 0.4953 | 397 (66) | 392 (60) | <0.0001 |
| Age group [n (%)] | ||||||
| ≤33 Years | 2 (2) | 34 (5) | 0.2471 | 1 (1) | 34 (9) | <0.0001 |
| 34–43 Years | 6 (5) | 39 (6) | 0.7922 | 13 (3) | 32 (8) | 0.0030 |
| 44–53 Years | 21 (18) | 95 (14) | 0.2599 | 48 (12) | 68 (17) | 0.0358 |
| 54–63 Years | 32 (27) | 166 (25) | 0.6550 | 98 (25) | 100 (26) | 0.7736 |
| ≥64 Years | 55 (47) | 339 (50) | 0.5662 | 236 (59) | 158 (40) | <0.0001 |
| Unknown | 1 (1) | 0 | — | 1 (0) | 0 | — |
| Surgical approach [n (%)] | ||||||
| Laparoscopy | 51 (44) | 349 (52) | 0.3860 | 211 (53) | 189 (48) | 1.0000 |
| Open surgery | 52 (44) | 198 (29) | 0.0092 | 149 (37) | 100 (25) | 0.0023 |
| Stoma approach | 3 (3) | 65 (10) | 0.0460 | 3 (0.8) | 65 (17) | <0.0001 |
| Converted | 11 (9) | 55 (8) | 1.0000 | 35 (9) | 31 (8) | 1.0000 |
| Unknown | 0 | 7 (1) | — | 0 | 7 (2) | — |
| Main procedure [n (%)] | ||||||
| Intestinal | 63 (54) | 355 (53) | 1.0000 | 218 (55) | 200 (51) | 0.8719 |
| Rectal | 43 (37) | 204 (30) | 0.4331 | 174 (44) | 74 (19) | <0.0001 |
| Revision | 10 (9) | 114 (17) | 0.0700 | 6 (1) | 118 (30) | <0.0001 |
| ASA class [n (%)] | ||||||
| 1, 2, or 3 | 110 (94) | 661 (98) | 0.4987 | 392 (98) | 379 (97) | 0.2962 |
| 4 or 5 | 2 (2) | 7 (1) | 0.4987 | 3 (1) | 6 (1) | 0.2962 |
| Unknown | 5 (4) | 5 (1) | — | 3 (1) | 7 (2) | — |
Because of missing data, the sum of the n values for a particular variable might not match the total population. Because of rounding, some percentages might not total to exactly 100%.
BMI = body mass index; ASA = American Society of Anesthesiologists.
Compliance
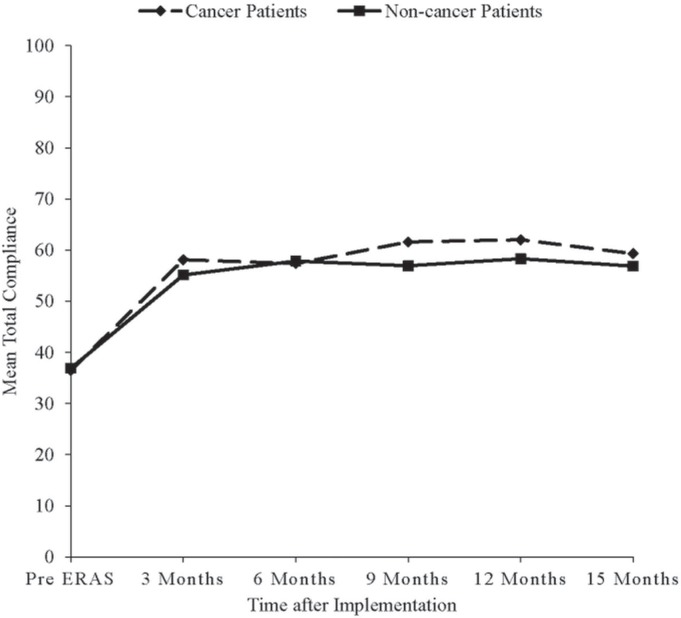
Pre-eras, mean total guideline compliance was 37% in cancer and non-cancer patients alike. Post-eras, mean total compliance was significantly higher in cancer patients (60%), than in non-cancer patients (57%, p = 0.0024). That difference in compliance began after the first 6 months of eras implementation and continued up to the 15th month of implementation (Figure 1). After the start of eras implementation, mean total compliance increased rapidly during the first 3 months of implementation; it then stayed almost constant thereafter for cancer and non-cancer patients alike (Figure 1). Before eras, as expected, mean total compliance was similar for patients undergoing open (38%) and laparoscopic surgery (36%, p = 0.3042). However, post-eras, mean total compliance was significantly higher for patients undergoing laparoscopic surgery (61%) than for those undergoing open surgery (56%, p < 0.0001).
FIGURE 1.
Change in overall compliance with the Enhanced Recovery After Surgery (ERAS) program over time, by site.
LOS
Length of stay declined significantly from pre- to post-eras, in cancer and non-cancer patients alike (Table ii). The los reduction maintained a steady trend from the 3rd month of implementation onward (pre-eras being the comparator). At month 15 post-eras, los declined significantly to a median of 5 days from a pre-eras median of 7 days in cancer patients and to a median of 4 days from a pre-eras median of 5.5 days in non-cancer patients. Pre-eras, the mean los of 9.5 days for cancer patients was not significantly different from the mean los of 8.8 days for non-cancer patients (p = 0.2944); however, post-eras, the mean los of 8.4 days for cancer patients was significantly different from the mean los of 6.4 days for non-cancer patients. The los reduction was higher for patients undergoing laparoscopic surgery [median los: 5 days pre-eras (mean: 7.2 days); 4 days post-eras (mean: 5.4 days); p = 0.0001] than for those undergoing open surgery [median los: 8 days pre-eras (mean: 10 days); 7 days post-eras (mean: 10.3 days); p = 0.1050].
TABLE II.
Change in length of stay over time, after implementation of the Enhanced Recovery After Surgery (ERAS) program
| Time since implementation and patient group | Pts (n) | Length of stay (days) | p Value | |
|---|---|---|---|---|
|
| ||||
| Mean | Median | |||
| Before ERAS | ||||
| Cancer patients | 68 | 9.5±11.5 | 7.0 | |
| Non-cancer patients | 48 | 8.8±7.3 | 5.5 | |
| After ERAS | ||||
| Overall | ||||
| Cancer patients | 330 | 8.4±12.7 | 5.0 | 0.0012 |
| Non-cancer patients | 344 | 6.4±8.2 | 4.0 | 0.0041 |
| 3 Months | ||||
| Cancer patients | 73 | 9.2±14.9 | 5.0 | 0.0439 |
| Non-cancer patients | 73 | 6.7±7.9 | 4.0 | 0.0266 |
| 6 Months | ||||
| Cancer patients | 66 | 8.9±8.9 | 6.0 | 0.4298 |
| Non-cancer patients | 69 | 7.5±14.4 | 4.0 | 0.0024 |
| 9 Months | ||||
| Cancer patients | 69 | 8.1±15.9 | 5.0 | 0.0003 |
| Non-cancer patients | 81 | 6.6±5.5 | 4.0 | 0.0586 |
| 12 Months | ||||
| Cancer patients | 64 | 7.9±13.0 | 5.0 | 0.0011 |
| Non-cancer patients | 63 | 5.4±3.3 | 5 | 0.0170 |
| 15 Months | ||||
| Cancer patients | 58 | 7.8±8.1 | 5.0 | 0.0051 |
| Non-cancer patients | 58 | 5.8±4.3 | 4.0 | 0.0339 |
Pts = patients.
Complications
Although the proportion of patients who developed at least 1 complication declined in cancer patients to a post-eras proportion of 46% from a pre-eras proportion of 57%, the difference was nonsignificant (Table iii). Similarly, in non-cancer patients, the decline to a post-eras proportion of 44% from a pre-eras proportion of 56% was nonsignifi-cant. However, in cancer patients, a significant decline in the proportion of patients who developed at least 1 complication was observed in the renal, hepatic, pancreatic, and gastrointestinal complications groups. Post-eras, the difference in the proportions of non-cancer and cancer patients who developed at least 1 complication [2.0%; 95% confidence interval (ci): −5.5% to 9.6%] was nonsignificant (p = 0.5974).
TABLE III.
Proportion of cancer patients who developed at least 1 complication, by complication group
| Complication | Patients [n (%)] | Difference in proportion (%) | 95% CI | p Value | |
|---|---|---|---|---|---|
|
| |||||
| Before ERAS | After ERAS | ||||
| Overall | 39 (57) | 153 (46) | 11 | −2 to 24 | 0.1032 |
| Respiratory | 8 (12) | 23 (7) | 5 | −3 to 13 | 0.2117 |
| Infectious | 8 (12) | 31 (9) | 3 | −6 to 11 | 0.5081 |
| Cardiovascular | 5 (7) | 15 (5) | 2 | −4 to 9 | 0.3590 |
| Gastrointestinala | 27 (40) | 89 (27) | 13 | 0.2 to 25 | 0.0353 |
| Surgical | 18 (26) | 59 (18) | 8 | −3 to 20 | 0.1024 |
| Epidural–related | 1 (1) | 6 (2) | −1 | −4 to 3 | 1.0000 |
| Psychiatric | 14 (21) | 66 (20) | 1 | −10 to 11 | 0.9122 |
Renal, hepatic, pancreatic, and other gastrointestinal.
ERAS = Enhanced Recovery After Surgery program; CI = confidence interval.
Overall, only 15 reoperations were performed, 1 in a pre-eras patient, and 14 in post-eras patients. The decline in the proportion of patients who developed at least 1 complication, pre-eras to post-eras, was significant neither in patients undergoing laparoscopic surgery (difference in proportions: 4%; p = 0.5804) nor in patients undergoing open surgery (difference in proportions: 14%; p = 0.0697).
Readmissions
For cancer patients, 30-day post-discharge readmissions occurred 11 times (16%) pre-eras and 33 times (10%) post-eras. Comparing pre-eras with post-eras cancer patients, the risk of readmission declined nonsignificantly (adjusted rr: 1.65; 95% ci: 0.88 to 3.09; p = 0.1172). In 338 non-cancer patients, 29 readmissions occurred, and the rr of 0.78 resulting from a comparison of the readmission risk between non-cancer patients and cancer patients was nonsignificant (95% ci: 0.51 to 1.19; p = 0.7830). Comparing post-eras with pre-eras patients, the risk of 30-day read-mission was similar for those undergoing laparoscopic surgery (rr: 0.7081; 95% ci: 0.2843 to 1.7633; p = 0.4584) and open surgery (rr: 0.6295; 95% ci: 0.3392 to 1.1682).
Costs
For colon cancer patients, eras was associated with a decline in the primary los by 1.1 days per patient, equating to 363 hospital days. The likelihood of readmission was reduced by 6.2%, equating to 20 prevented readmissions and 180 hospital days. For patients that were readmitted, eras was associated with a decline in the readmission los by 0.4 days per patient, equating to 14 hospital days. The total estimated gross cost saving was in the $620,498–$1,173,042 range. The total cumulative intervention cost for eras during the analysis period was $258,741. The net cost saving from eras was therefore in the $361,757–$914,301 range ($1,096–$2,771 per patient, Table iv).
TABLE IV.
Cost impact of implementing the Enhanced Recovery After Surgery (ERAS) programa
| Variable | Pts (n) | Reduction attributable to ERAS | Total savings | |
|---|---|---|---|---|
|
| ||||
| Average | Total | |||
| LOS, primary | (days) | (days) | ($) | |
| Cancer patients | 330 | 1.1 | 363 | 404,382 to 764,478 |
| Non-cancer patients | 344 | 2.4 | 826 | 920,164 to 1,739,556 |
| Readmissions prevented | (%) | (n) | ||
| Cancer patients | 330 | 6.2 | 20 | |
| Non-cancer patients | 344 | 6.2 | 21 | |
| LOS prevented | (daysb) | (days) | ($) | |
| Cancer patients | 20 | 9 | 180 | 200,520 to 379,080 |
| Non-cancer patients | 21 | 13.4 | 282 | 314,148 to 593,892 |
| LOS, readmission | (days) | (days) | ($) | |
| Cancer patients | 33 | 0.4 | 14 | 15,596 to 29,484 |
| Non-cancer patients | 29 | 6.1 | 177 | 197,178 to 372,762 |
| TOTAL | ($) | |||
| Cancer patients | 620,498 to 1,173,042 | |||
| Non-cancer patients | 1,435,161 to 2,713,150 | |||
| ERAS implementation cost | ($) | |||
| Cancer patients | 258,741 | |||
| Non-cancer patients | 269,718 | |||
| Net cost-savings | ($) | |||
| Cancer patients | 361,757 to 914,301 | |||
| Non-cancer patients | 1,165,443 to 2,443,432 | |||
| Net cost-savings per patient | ($) | |||
| Cancer patients | 1,096 to 2,771 | |||
| Non-cancer patients | 3,388 to 7,103 | |||
| Breakeven point | (surgeries) | |||
| Cancer patients | 93 to 236 | |||
| Non-cancer patients | 38 to 80 | |||
Cost per hospital day: $1,114–$2,106.
Average LOS per re-admission before ERAS implementation.
Pts = patients; LOS = length of stay.
For non-cancer patients, eras was associated with a decline in the primary los by 2.4 days per patient, a reduction in the likelihood of readmission by 6.2%, and a decline in the readmission los by 6.1 days per patient. Those improvements resulted in a net cost saving in the $1,165,443–$2,443,432 range ($3,388–$7,103 per patient, Table iv).
DISCUSSION
Implementation of the eras colorectal guideline in colon cancer patients in Alberta resulted in an improvement in clinical outcomes, which translated directly into significant cost savings to the health care system.
Our results show that, after accounting for intervention costs, the declines in los, complications, and readmissions generated a net cost saving in the range of $1,096–$2,771 per cancer patient and $3,388–$7,103 per non-cancer patient. Accordingly, when eras is scaled, the magnitude of the cost saving within and between other types of surgeries will likely be substantial.
Our results also show that, compared with the youngest age group, the oldest age group, consisting mostly of cancer patients, stay significantly longer in hospital. The shortest los (mean: 5.1 days; median: 4 days) was observed in the youngest age group (≤33 years), which constituted 9% of non-cancer patients and only 1% of cancer patients. The dependency of los on patient age, as illustrated here, is consistent with findings reported by Cakir et al.17, Hendry et al.18, and Delaney et al.19, who concluded that age is an independent determinant of los, with younger patients being more likely than their older counterparts to reap higher benefits from eras implementation. That observation partly explains why the cost saving attributable to eras implementation was higher for the non-cancer patients than for the cancer patients in the present study. In addition, colon cancer patients are typically more likely to require open surgeries, as noted in our study and reported in randomized controlled trials20–23, translating into longer los and therefore a lower cost saving. A fuller economic evaluation, evaluating the economic impact of eras both on the colorectal experience and on other surgery types (as the approach is scaled) is forthcoming. The early results presented here signal a significantly beneficial impact on scarce health system resources and warrant the attention of senior health system decision-makers.
In the present study, we also observed a significant reduction in los from pre- to post-eras in patients undergoing laparoscopic surgery; however, los did not significantly change in patients undergoing open surgery. We do not know if that observation relates to the fact that post-eras compliance was significantly different for the laparoscopic and open surgery patients. Similar results have been reported by other researchers23,24.
Our study had a number of strengths. First, it was based on a large sample of consecutive patients. Second, it addresses a system-wide provincial initiative to change surgical care according to high-level scientific evidence, thereby supporting the generalizability of the findings. Third, it used a real-time interactive audit system for guideline implementation and data collection, with real-time data checks that helped to maintain the high quality of the data. The data were collected by well-trained nurse clinicians with in-depth knowledge of all clinical aspects of colorectal surgery and the outcomes measured in our study.
The study is limited by the fact that eligible patients of colorectal surgeons who did not participate in the eras protocol were not analyzed. However, those patients represent only a very small proportion of eligible patients overall. There is no reason to postulate that the benefits of eras implementation would have been different in those patients. Although there were significant differences in age and sex between the cancer and non-cancer patients at baseline, the improvement in patient outcomes was independent of sex, and although los was age-dependent, the effect of age on los disappeared when cancer and non-cancer patients were considered separately. Although the eras Society recommends a pre-eras sample size of at least 50 patients for each centre, we included 130 pre-eras patients from the two centres analyzed. Still, the imbalance in the pre- and post-eras sample sizes might have contributed to the nonsignificance of some comparisons.
CONCLUSIONS
Our study findings show that implementation of the eras colorectal guideline in Alberta resulted not only in clinical outcome improvements, but also in a significant beneficial effect on scarce health system resources. Although colon cancer patients reaped clinical benefits, and the health system, cost-savings from eras implementation, non-cancer patients were likely to reap even higher benefits because of a significant age difference between the two cohorts of patients and also because of a significantly higher requirement for open surgery in cancer patients. Our results therefore show that eras guideline implementation is potentially beneficial to cancer and non-cancer colorectal surgical patients alike.
ACKNOWLEDGMENTS
The eras project was funded by a Partnership for Research and Innovation in the Health System research grant from Alberta Innovates: Health Solutions.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Walter CJ, Collin J, Dumville JC, Drew PJ, Monson JR. Enhanced recovery in colorectal resections: a systematic review and meta-analysis. Colorectal Dis. 2009;11:344–53. doi: 10.1111/j.1463-1318.2009.01789.x. [Erratum in: Colorectal Dis 2010;12:728] [DOI] [PubMed] [Google Scholar]
- 2.Wind J, Polle SW, Fung Kon Jin PH, et al. on behalf of the Laparoscopy and/or Fast Track Multimodal Management Versus Standard Care Study Group and the Enhanced Recovery after Surgery Group Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800–9. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 3.Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis. 2009;24:1119–31. doi: 10.1007/s00384-009-0703-5. [DOI] [PubMed] [Google Scholar]
- 4.Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149:830–40. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Varadhan KK, Neal KR, Dejong CHC, Fearon KCH, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (eras) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–40. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–41. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 7.Lv L, Shao YF, Zhou YB. The enhanced recovery after surgery (eras) pathway for patients undergoing colorectal surgery: an update of meta-analysis of randomized controlled trials. Int J Colorectal Dis. 2012;27:1549–54. doi: 10.1007/s00384-012-1577-5. [DOI] [PubMed] [Google Scholar]
- 8.Eskicioglu C, Forbes SS, Aarts MA, Okrainec A, McLeod RS. Enhanced recovery after surgery (eras) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg. 2009;13:2321–9. doi: 10.1007/s11605-009-0927-2. [DOI] [PubMed] [Google Scholar]
- 9.Roulin D, Donadini A, Gander S, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. 2013;100:1108–14. doi: 10.1002/bjs.9184. [DOI] [PubMed] [Google Scholar]
- 10.Lee L, Mata J, Ghitulescu GA, et al. Cost-effectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg. 2015;262:1026–33. doi: 10.1097/SLA.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 11.Nelson G, Kiyang L, Crumley E, et al. Implementation of Enhanced Recovery After Surgery (eras) across a provincial healthcare system: the eras Alberta colorectal surgery experience. World J Surg. 2016;40:1092–103. doi: 10.1007/s00268-016-3472-7. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (eras) Society recommendations. World J Surg. 2013;37:259–84. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 13.Nygren J, Thacker J, Carli F, et al. on behalf of the Enhanced Recovery After Surgery (eras) Society, for Perioperative Care, the European Society for Clinical Nutrition and Metabolism, and the International Association for Surgical Metabolism and Nutrition Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (eras) Society recommendations. World J Surg. 2013;37:285–305. doi: 10.1007/s00268-012-1787-6. [DOI] [PubMed] [Google Scholar]
- 14.Bell GV. Sample size. In: Bell GV, editor. Statistical Rules of Thumb. 2nd ed. Hoboken, NJ: John Wiley and Sons; 2008. pp. 27–51. [DOI] [Google Scholar]
- 15.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–18. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 16.Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011;55:111–15. doi: 10.4103/0019-5049.79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cakir H, van Stijn MF, Lopes Cardozo AM, et al. Adherence to Enhanced Recovery After Surgery and length of stay after colonic resection. Colorectal Dis. 2013;15:1019–25. doi: 10.1111/codi.12200. [DOI] [PubMed] [Google Scholar]
- 18.Hendry PO, Hausel J, Nygren J, et al. on behalf of the Enhanced Recovery After Surgery study group Determinants of outcome after colorectal resection within an enhanced recovery programme. Br J Surg. 2009;96:197–205. doi: 10.1002/bjs.6445. [DOI] [PubMed] [Google Scholar]
- 19.Delaney C, Zutshi M, Senagore A, Remzi F, Hammel J, Fazio V. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum. 2003;46:851–9. doi: 10.1007/s10350-004-6672-4. [DOI] [PubMed] [Google Scholar]
- 20.García-Botello S, Cánovas de Lucas R, Tornero C, et al. Implementation of a perioperative multimodal rehabilitation protocol in elective colorectal surgery. A prospective randomised controlled study [Spanish] Cir Esp. 2011;89:159–66. doi: 10.1016/j.ciresp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Vlug MS, Wind J, Hollmann MW, et al. on behalf of the lafa study group Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (lafa-Study) Ann Surg. 2011;254:868–75. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Jiang Z, Zhao K, et al. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16:1379–88. doi: 10.1007/s11605-012-1880-z. [DOI] [PubMed] [Google Scholar]
- 23.Braga M, Frasson M, Zuliani W, Vignali A, Pecorelli N, Di Carlo V. Randomized clinical trial of laparoscopic versus open left colonic resection. Br J Surg. 2010;97:1180–6. doi: 10.1002/bjs.7094. [DOI] [PubMed] [Google Scholar]
- 24.King PM, Blazeby JM, Ewings P, et al. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006;93:300–8. doi: 10.1002/bjs.5216. [DOI] [PubMed] [Google Scholar]