Abstract
Background
Heart-lung transplantation (HLT) has provided hope to patients with end-stage lung disease and irreversible heart dysfunction. We reviewed the clinical outcomes of 10 patients who underwent heart-lung transplantation at Asan Medical Center.
Methods
Between July 2010 and August 2014, a total of 11 patients underwent HLT at Asan Medical Center. After excluding one patient who underwent concomitant liver transplantation, 10 patients were enrolled in our study. We reviewed the demographics of the donors and the recipients’ baseline information, survival rate, cause of death, and postoperative complications. All patients underwent follow-up, with a mean duration of 26.1±16.7 months.
Results
Early death occurred in two patients (20%) due to septic shock. Late death occurred in three patients (38%) due to bronchiolitis obliterans (n=2) and septic shock (n=1), although these patients survived for 22, 28, and 42 months, respectively. The actuarial survival rates at one year, two years, and three years after HLT were 80%, 67%, and 53%, respectively.
Conclusion
HLT is a procedure that is rarely performed in Korea, even in medical centers with large heart and lung transplant programs. In order to achieve acceptable clinical outcomes, it is critical to carefully choose the donor and the recipient and to be certain that all aspects of the transplant procedure are planned in advance with the greatest care.
Keywords: Heart-lung transplantation, Complication, Mortality
INTRODUCTION
Since the first case of heart-lung transplantation (HLT) was conducted at Stanford University in 1981 [1], 3,755 adult HLTs (including re-transplantation) have been performed worldwide as of June 2013 [2]. Initially, the majority of HLTs were performed for pulmonary vascular disease and cystic fibrosis, which had previously been treated with lung transplantation alone. Recently, the major indications for adult HLT have been expanded to include congenital heart disease and idiopathic pulmonary artery hypertension [2]. HLT recipients have survival rates of 63% at one year, 52% at three years, and 45% at five years [2].
In Korea, a total of 17 HLTs have been performed between July 2010 and August 2014, 11 of which were performed at Asan Medical Center, according to the official report of the Korean Network for Organ Sharing (http://www.konos.go.kr). However, the survival rate of the Korean HLT recipients has not yet been investigated due to the low number of cases of HLT. Therefore, the purpose of this study was to evaluate the clinical outcomes of HLT at a single medical center over five years.
METHODS
1) Patients and surgical indications
Our study population consisted of all patients who underwent HLT between July 2010 and August 2014 at Asan Medical Center in Seoul, Korea. Both pediatric and adult patients were included. The patients were sorted through our prospectively maintained HLT registry database containing clinical information regarding the recipients and donors. Clinical outcomes including deaths and complications following HLT were meticulously reviewed using the patients’ electronic medical records of Asan Medical Center. The indications for HLT have changed over time. Initially, pulmonary hypertension with congenital or acquired heart disease were the main indications for HLT. As time passed, the indications expanded to include end-stage lung disease with pulmonary hypertension (HTN) caused by right heart failure. For all patients, the decision to perform concomitant HLT was confirmed by a team of both cardiologists and pulmonologists, who have performed 260 cases of heart transplantation and 40 cases of lung transplantation over 15 years. This study was approved by the ethics committee of institutional review board of Asan Medical Center. And the requirement for informed consent was waived.
2) Donor selection, organ procurement, and transplant technique
We have paid special attention to manage the donor organs properly from the donor selection stage. Techniques used to assess organ quality included chest radiography, arterial blood gas determination, bronchoscopy, echocardiography, and visual inspection of the heart and lungs [3,4]. We tried to perform flexible bronchoscopy in every case to evaluate the airways and to remove retained secretion.
The donor and recipient operations were performed using previously described techniques [5]. Following sternotomy, we opened mediastinal pleura of both side and carefully examined the condition of donor’s lung. If there was atelectasis, we requested to adjust the ventilation setting to expand the lungs fully. After dissection of the trachea, the superior vena cava (SVC), inferior vena cava (IVC), ascending aorta, and main pulmonary artery, cannulae were placed in order to provide preservative solutions to heart and lung. Before aorta cross-clamping, 25,000 units of heparin were administered intravenously and prostaglandin E1 (500 μg) was administered into the main pulmonary artery. When the systolic blood pressure dropped to under 90 mmHg following the administration of prostaglandin E1, aortic cross-clamping was carried out and cardioplegic infusion was started. The left atrial appendage was amputated in order to ensure complete emptying of the heart. The heart was preserved using 2 L of histidine-tryptophan-ketoglutarate solution (Custodiol HTK; Essential Pharmaceuticals, Newtown, PA, USA) infused through the aortic root cannula. The lungs were preserved using antegrade pulmoplegia with 5 L of low-potassium dextran solution (Perfadex; Vitrolife AB, Kungsbacka, Sweden) through the main pulmonary artery, with venting through the IVC and LA auricle. If the cold ischemic time was predicted to be more than 120 minutes, 1 L of Perfadex solution was infused again into the lung allograft at recipient’s operation room before anastomosis. At the time of tracheal stapling, the lungs were inflated enough to prevent atelectasis. Cold HTK solution was used for graft storage during returning to recipient’s operation room.
Standard median sternotomy was performed in eight recipients, while two patients underwent the clamshell approach due to severe adhesion. We used standard cardiopulmonary bypass (CPB) strategy including aortic and bicaval cannulations, and tried to cannulate the aorta, the SVC, and the IVC as distally as possible. After the initiation of CPB and cooling using ice slush, recipient cardiectomy and bilateral pneumonectomies were completed immediately after the arrival of the donor heart-lung bloc. The tracheal anastomosis was done first using a running polydioxanone 4-0 suture (PDS II; Johnson & Johnson K.K., Seoul, Korea) on the posterior side, and intermittent suturing was later performed on the anterior side using the same suture material. Next, the aortic anastomosis was performed. After the cross-clamp was removed using standard de-airing maneuvers, 500 mg of methylprednisolone was administered intravenously. Anastomosis of the SVC was performed, followed by anastomosis of the IVC. After weaned off from CPB, routinely 1 pericardial soft drain (Hemovac Evacuators; Zimmer, Warsaw, IN, USA), 1 mediastinal tube, and 2 pleural chest tubes in each side were placed. In every case, a pair of ventricular pacing wires was placed also.
3) Antimicrobial prophylaxis, immunosuppression, and postoperative management
As a prophylactic antimicrobiotics, cefepime was intravenously administered in patients who were reported as sputum-culture negative, or the appropriate antibiotics was chosen according to the result of the donor and recipient sputum cultures. For antiviral prophylaxis, Intravenous ganciclovir was given at a dose of 5 mg/kg every 24 hours from the first week to fourth week after the HLT, followed by 900 mg of oral valganciclovir once daily for six months. As antifungal prophylaxis, Voriconazole was intravenously administered 4 mg/kg every 12 hours with the target trough level of 1.5–5.5 mg/dL. After the recipient resumed normal diet, oral voriconazole was given. The total duration of voriconazole prophylaxis was six months. Cytomegalovirus and Aspergillus antigenemia assays were performed regularly for up to six months after HLT. Trimethoprim-sulfamethoxazole was then administered to prevent Pneumocystis jirovecii pneumonia throughout the patient’s life.
In all patients, induction of immune suppression was made using anti-interleukin-2 receptor monoclonal antibody (basiliximab) and mycophenolate mofetil (CellCept; Roche Laboratories, Nutley, NJ, USA). Basiliximab was intravenously administered one hour before the surgery and on the fourth post-transplant day at a dose of 20 mg in patients weighing over 35 kg. Mycophenolate mofetil was started pre-operatively and continued following surgery at a dose of 1.0 g every 12 hours, with a target trough level of 2 μg/mL (range, 1 to 3 μg/mL).
Postoperative immunosuppression consisted of a triple-maintenance therapy based on steroids, mycophenolate mofetil, and a calcineurin inhibitor. Among the calcineurin inhibitors, tacrolimus (FK506) was used first and cyclosporine (CsA) was used as a second choice when complications related to tacrolimus occurred. Tacrolimus was begun on the second postoperative day at a dose of 0.05–0.1 mg/kg for 24 hours, and the target continuing level was 10–15 ng/mL for six months and 8–12 ng/mL after three days. If CsA was used rather than tacrolimus, it was usually administered on the second postoperative day at an initial dose of 2–3 mg/kg or half the dose as a four-hour infusion twice a day, and was continued until the patient was able to take oral medication. A 500-mg dose of methylprednisolone was given intra-operatively after weaning of CPB. Two additional postoperative doses were given (1 mg/kg every 12 hours for 24 hours) until the day after the operation, and a half dose was then given for two weeks, based on the assumption that this would decrease the incidence of bronchial anastomotic complications. After two weeks, steroids were changed to oral prednisone with a daily dosage of 0.6 mg/kg, tapering to 0.2 mg/kg/day at six weeks and 0.1 mg/kg/day at six months.
All patients were required to undergo follow-up at our transplant outpatient clinic, which is associated with the department of pulmonology and critical care medicine. Peak expiratory flow was calculated daily in all patients using a portable peak flow meter to detect acute rejection or early lung injury. Routine spirometry was performed during the first six months after transplantation. Thereafter, bronchoscopies were only performed for special indications such as infection or rejection. Acute rejection was diagnosed by chest computed tomography and bronchoscopy if pulmonary function decreased. Chronic rejection, including restrictive allograft syndrome or bronchiolitis obliterans syndrome (BOS), was also made using concurrent forced vital capacity and forced expiratory volume in one second [6].
4) Surveillance and treatment of rejection
The patients did not undergo surveillance endomyocardial biopsies, but did have a routine transthoracic echocardiogram once a week until discharge and two, four, six, and 12 months after discharge.
The surveillance bronchoscopy was routinely performed at two, four, six, and 12 months and annually thereafter. Either endomyocardial or lung biopsies were obtained only when rejection was suggested clinically. Patients were followed up at the out-patient clinic of the department of pulmonology and critical care medicine. In cases of the patients showed signs of moderate or severe rejection, intravenous methylprednisolone (1 g/day for three days) was injected, followed by a two-week tapering course of prednisone. Persistent rejection was treated with an additional course of steroids followed by everolimus and low-dose cyclosporine.
5) Statistical analysis
Statistical analysis was performed using IBM SPSS software ver. 22.0 (IBM Co., Armonk, NY, USA). Data are shown as the mean plus or minus the standard error of the mean. The overall survival included all deaths from any cause during the follow-up period, whereas live patients were right-censored at the last available follow-up.
RESULTS
1) Patient demographics
Between July 2010 and August 2014, a total of 11 patients underwent HLT in our medical institution, one of whom was excluded due to undergoing simultaneous heart, lung, and liver transplantation. Table 1 shows the demographic and clinical characteristics of these patients. The median patient age was 44 years, ranging from five to 62 years. The reasons for HLT were interstitial lung disease with rapidly deteriorating pulmonary function (n=3), toxic acute respiratory distress syndrome (n=2), atrial septal defect with pulmonary HTN (n=1), dilated cardiomyopathy with pulmonary HTN (n=1) with a previous heart transplantation, ischemic cardiomyopathy with acute respiratory distress syndrome (n=1), angiosarcoma (n=1), and bronchiectasis (n=1). The mean patient height was 165 cm (range, 109 to 170 cm), and the mean weight was 62 kg (range, 18 to 74 kg). Eight patients (80%) were on pre-operative ventilatory support, the median duration of which was 20.5 days (range, 2 to 104 days). Six patients (60%) required extracorporeal membrane oxygenation (ECMO) therapy preoperatively, the median duration of which was 14.5 days, (range, 2 to 100 days). The median waiting time from registration to transplantation was 31.5 days (range, 3 to 180 days). The mean follow-up time was 26.1±16.7 months.
Table 1.
Demographic and clinical characteristics of the heart-lung transplant recipients
| Characteristic | Value |
|---|---|
| Age (yr) | 44 (5–62) |
| Gender (male) | 6 (60) |
| Disease leading to transplantation | |
| Interstitial lung disease | 3 (30) |
| Toxic ARDS | 2 (20) |
| Ischemic cardiomyopathy with ARDS | 1 (10) |
| Dilated cardiomyopathy with pulmonary HTN | 1 (10) |
| Atrial septal defect with pulmonary HTN | 1 (10) |
| Bronchiectasis with corpulmonale | 1 (10) |
| Angiosarcoma | 1 (10) |
| Preoperative extracorporeal membrane oxygenation support | |
| Patients | 6 (60) |
| Duration (day) | 14.5 (2–100) |
| Preoperative mechanical ventilation support | |
| Patients | 8 (80) |
| Duration (day) | 20.5 (2–104) |
| Wait for transplantation | |
| Duration (day) | 31.5 (3–180) |
Values are presented as median (interquartile range) or numbers of patients (%), unless otherwise indicated.
ARDS, acute respiratory distress syndrome; HTN, hypertension.
2) Organ donor and transplant operations
Five patients were female and five were male. The mean donor age was 29.1±13.7 years (range, 11 to 52 years) (Table 2). The causes of brain death were cerebrovascular accident (40%), head trauma (30%), hanging (20%), and traffic accident (10%). The median total ischemic time of the donor harvest was 192 minutes (range, 100 to 313 minutes). The median warm ischemic time was 89 minutes (range, 69 to 127 minutes), and the cold ischemic time was 104 minutes (range, 28 to 245 minutes). Five donors were in Seoul (mean ischemic time of less than three hours) and four donors were in Gyeonggi-do, Jeolla-do, and Daejeon (mean ischemic time of less than four hours). One donor was located in Ulsan, which led to an ischemic time of 331 minutes. During the HLT, the median surgery and CPB times were 587 minutes (range, 384 to 663 minutes) and 262 minutes (range, 181 to 439 minutes), respectively, and the median duration of aortic cross-clamping was 140 minutes (range, 128 to 173 minutes).
Table 2.
Donor demographics
| Variable | Value |
|---|---|
| Donor age (yr) | 29.1±13.7 (range, 11–52) |
| 10–19 | 2 (20) |
| 20–29 | 5 (50) |
| 30–39 | 1 (10) |
| 50–59 | 2 (20) |
| Gender (male/female) | 5/5 |
| Cause of death | |
| Cerebrovascular accident | 4 (40) |
| Head trauma | 3 (30) |
| Hanging | 2 (20) |
| Traffic accident | 1 (10) |
| Location within Korea | |
| Seoul (≤3 hr of transportation time) | 5 (50) |
| Gyeonggi-do, Jeolla-do, Daejeon (3–4 hr of transportation time) | 4 (40) |
| Ulsan (5–6 hr of transportation time) | 1 (10) |
Values are presented as mean±standard deviation or numbers of patients (%), unless otherwise indicated.
3) Morbidity and mortality
The morbidity and mortality data are presented in Table 3. Early mortality (less than 30 days following surgery or in-hospital mortality) occurred in 20% (n=2) of the patients and was caused by septic shock. One of the patients who was diagnosed with dilated cardiomyopathy underwent heart transplantation 15 days before HLT. On postoperative day 1, the patient required ECMO support for right heart failure, and progressive pulmonary HTN made it impossible to wean the patient from ECMO. After two episodes of bleeding control surgery, the patient expired nine days later due to septic shock. The other patient underwent ECMO–assisted emergency coronary artery bypass grafting surgery due to an acute myocardial infarction three months before HLT. After postoperative bleeding control, acute renal failure and acute respiratory distress syndrome also took place, followed by continuous renal replacement therapy and tracheostomy. The patient underwent HLT, although the total ischemic time of the donor heart-lung bloc was 313 minutes. Eventually, the patient suffered from low cardiac output syndrome and expired 31 days following the surgery due to septic shock.
Table 3.
Complications and causes of death
| Variable | No. (%) |
|---|---|
| In-hospital complications | |
| Early death | 2 (20) |
| Cases 1 and 2: septic shock | |
| Early morbidity among early survivors | |
| Prolonged mechanical ventilation (>72 hr) | 7 (70) |
| Acute renal insufficiency | 4 (40) |
| Pulmonary infection | |
| Bacterial | 2 (20) |
| Viral | 2 (20) |
| Biliary infection | 2 (20) |
| Hematogenous infection | 4 (40) |
| Postoperative diabetes mellitus | 2 (20) |
| Vocal nerve injury | 1 (10) |
| Brain hemorrhage | 1 (10) |
| Late complications | |
| Late deaths | 3 (38) |
| Cases 3 and 4: BOS | |
| Case 5: septic shock | |
| Late morbidity among early survivors | |
| BOS | 2 (25) |
| Chronic renal insufficiency | 2 (25) |
| Pulmonary infection | |
| Fungal | 1 (13) |
| Hematogenous infection | 2 (25) |
BOS, bronchiolitis obliterans syndrome.
The immediate postoperative course was complicated in one patient (10%) by excessive bleeding that required re-sternotomy within the first 24 hours. The bleeding focus of the patient was proven to be from the collateral vessels in the posterior mediastinum or in the chest wall. The most common postoperative complication was respiratory failure requiring prolonged mechanical ventilation (defined as assisted ventilation for >72 hours or reintubation), which occurred in seven early survivors (70%), all of whom required tracheostomy. The median ventilation support time was 12 days (range, 3 to 87 days). Acute renal failure requiring dialysis occurred in four patients (40%), three of whom already required continuous postoperative renal replacement therapy. The median length of the intensive care unit (ICU) stay among the survivors was 31 days (range, 5 to 124 days), and the median length of their hospital stay was 88 days (range, 42 to 379 days). Eight early survivors among the transplant recipients were discharged from the hospital. No prolonged air leak or acute graft failure was observed among the early survivors.
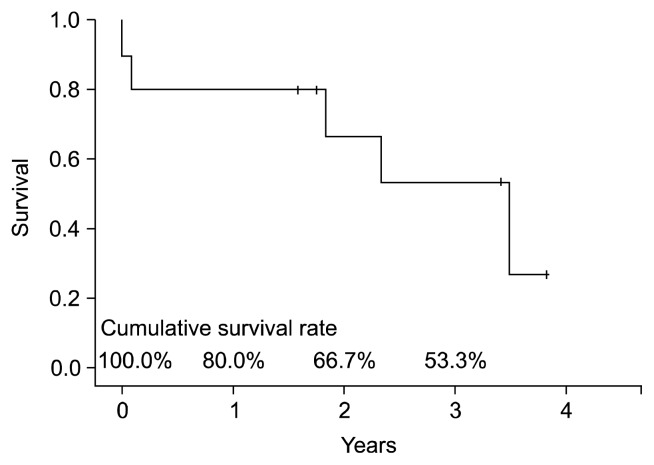
Late mortality (after postoperative day 30) occurred in 38% (3/8) of the remaining patients. The causes of late mortality included chronic allograft failure as a result of BOS (n=2) and septic shock (n=1). The survival duration of these patients was 22, 28, and 42 months, respectively. The actuarial survival rates at one, two, and three years after HLT were 80%, 67%, and 53%, respectively (Fig. 1). The common late complications were BOS in two patients (25%), chronic renal failure in two patients (25%), and hematogenous infection in two patients (25%). All survivors were in New York Heart Association class I or II after discharge.
Fig. 1.
Overall survival curves after heart-lung transplantation, using the Kaplan-Meier method.
DISCUSSION
In this study we presents the clinical outcomes of HLTs performed at the Asan Medical Center over a five-year period, with acceptable short- and mid-term survival rates. The survival rates at one year and three years in this study were 80% and 53%, whereas they were 63% and 52% in the (ISHLT) study [2]. According to the ISHLT study, the most common causes of death during the first 30 days after heart-lung transplantation were graft failure, technical complications, and non-cytomegalovirus infections. In late period, BOS/late graft failure (lung or heart) and non-cytomegalovirus infections were the common causes of death [2]. Due to the low number of cases, we were unable to analyze the cause of early and late deaths in order to determine statistically significant trends in the cause of death. However, the leading causes of early and late mortality were septic shock and BOS.
During the last 34 years, HLT has provided hope to a large number of patients and a great benefit to many. According to the International Society for Heart and Lung Transplantation study [2], the major indications for HLT are congenital heart disease (35.5%), idiopathic primary pulmonary hypertension (27.4%), cystic fibrosis (13.9%), and acquired heart disease (5.4%). The decision to list patients with idiopathic primary pulmonary hypertension for HLT rather than for bilateral lung transplantation was made depending on the right heart function and its predicted recovery after isolated lung transplantation [7]. However, until recently it has been difficult to assess the reversibility of right ventricular function in patients with right heart failure due to the lack of definite criteria. Consequently, the decision to perform HLT was made by experienced cardiologists who had specialized in performing heart transplantation for 20 years in consultation with pulmonologists based on the echocardiogram, cardiac computed tomography, and/or information derived from cardiac catheterization. According to the Asan Medical Center protocol, six patients with end-stage lung disease underwent HLT rather than isolated lung transplantation due to moderate-to-severe right heart failure with pulmonary HTN, although the ejection fraction was sometimes within the normal range. Three patients had heart failure (congenital or acquired) and their pulmonary function was irreversibly aggravated.
Chronic allograft rejection after lung transplantation is manifested by ‘constrictive’ BOS and is physiologically characterized by progressive expiratory airflow obstruction seen on pulmonary function testing [7]. With regard to HLT, the lung grafts seem to protect the heart such that pulmonary infiltration usually precedes or prevents myocardial infiltration [8]. Meanwhile, as the heart does not provide immunoprotective effects for the lungs, HLT recipients exhibit BOS at a rate similar to lung transplantation recipients [9]. Therefore, BOS is a major factor limiting long-term survival after HLT as well as lung transplantation, affecting approximately one third of all patients within three years and half of all patients within five years [10]. In the past, BOS was considered an irreversible and incurable condition, whereas azithromycin can now improve airflow limitation in a significant proportion of patients with even long-standing BOS [11]. Tacrolimus conversion from CsA [12] or extracorporeal photopheresis [13] has also shown to be an effective adjunctive treatment. According to our transplant protocol, oral azithromycin is routinely administered at a dosage of 250 mg three times per week in order to prevent BOS. If BOS is suspected or diagnosed, CsA is switched to tacrolimus and high-dose steroids are used. In our study, BOS was diagnosed in two patients by transbronchial biopsy and both of them died, 22 months and 28 months following their surgery, respectively.
ECMO is a well-established bridging therapy in patients with cardiac or pulmonary failure and is used in order to maintain organ function. The use of ECMO as a bridge to lung transplantation is already an accepted therapy for patients with end-stage lung disease, although their survival rate is markedly worse when ECMO is necessary preoperatively [14]. Patients requiring mechanical ventilation (MV) at the time of lung transplantation are also known to have worse clinical outcomes [15]. In HLT patients, recipients bridged by MV or ECMO have been shown to have increased short- term. Long-term mortality, and longer hospital stays [16]. However, in our study, we could not confirm significant difference of survival outcomes of the patients who required ECMO support (n=6) and MV (n=8) before HLT when compared to unsupported group. This finding might be due to the small number of patients. Early mortality occurred pre-operatively in 17% (1/6) of the ECMO-supported patients and in 25% (1/4) of the unsupported patients. The survival rate of the ECMO-supported patients was 83% over the course of one to three years, whereas the survival rate of the unsupported patients was 75% at one year and 25% at three year, although this was not a statistically significant difference (p=0.495). The MV-supported patients had longer preoperative ICU stays than the unsupported patients (45 days vs. eight days), although this difference did not reach statistical significance (p=0.248). It is likely that these patients are susceptible to the same factors that cause worse outcomes in mechanically ventilated HLT patients, such as airway colonization and the risk of post-transplantation pneumonia [17]. Patients requiring MV are also often deconditioned [18,19], which can also delay their recovery from transplant surgery.
The main limitation of this study is that the number of the patients is small. However our study does provide valuable, recent, and detailed morbidity and mortality information regarding post-HLT patients in a Korean cohort. A cumulative analysis of HLT cases and a multicenter study will be required in order to further evaluate the actual survival outcomes and prognosis after HLT in Korean patients.
In conclusion, HLT is a procedure that is infrequently performed even in medical centers with large heart and lung transplant programs. It is critical that recipients be carefully chosen and that all aspects of the transplant procedure be meticulously planned in advance. These challenging procedures require experienced heart and lung surgeons with expertise in HLT in order to ensure the optimal utilization of these precious organs. Major advances in infection prophylaxis and treatment as well as continuously improved immunosuppressive regimens, together with our increasing clinical experience, allow us to expect a higher rate of mid- and long-term survivors in the future.
ACKNOWLEDGMENTS
The following members of Asan Medical Center Heart-Lung Transplantation Team contributed data and participated in the discussions: Hyeong Ruyl Kim1, Yong-Hee Kim1, Dong Kwan Kim1, Sung-Ho Jung1, Tae-Jin Yun1, Jae Won Lee1, Jae Suk Yoo2, Kyung-Wook Jo3, Tae Sun Shim3, Sang-Bum Hong3, Jae Joong Kim4, Sang-Oh Lee5
1Department of Thoracic and Cardiovascular Surgery, University of Ulsan College of Medicine, 2Department of Thoracic and Cardiovascular Surgery, Sejong General Hospital, Departments of 3Pulmonology and Critical Care Medicine, 4Cardiology, and 5Infectious Diseases, University of Ulsan College of Medicine
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund No. Fund No. KTCS04-042).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med. 1982;306:557–64. doi: 10.1056/NEJM198203113061001. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–24. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Potter CD, Wheeldon DR, Wallwork J. Functional assessment and management of heart donors: a rationale for characterization and a guide to therapy. J Heart Lung Transplant. 1995;14(1 Pt 1):59–65. [PubMed] [Google Scholar]
- 4.Tierney A, Foster R, Ogella D. A perfusionist’s role in lung transplant preservation. Perfusion. 2004;19:351–7. doi: 10.1191/0267659104pf767oa. [DOI] [PubMed] [Google Scholar]
- 5.Huddleston CB, Richey SR. Heart-lung transplantation. J Thorac Dis. 2014;6:1150–8. doi: 10.3978/j.issn.2072-1439.2014.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–33. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Deuse T, Sista R, Weill D, et al. Review of heart-lung transplantation at Stanford. Ann Thorac Surg. 2010;90:329–37. doi: 10.1016/j.athoracsur.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Novitzky D, Cooper DK, Rose AG, Reichart B. Acute isolated pulmonary rejection following transplantation of the heart and both lungs: experimental and clinical observations. Ann Thorac Surg. 1986;42:180–4. doi: 10.1016/S0003-4975(10)60514-0. [DOI] [PubMed] [Google Scholar]
- 9.Moffatt-Bruce SD, Karamichalis J, Robbins RC, Whyte RI, Theodore J, Reitz BA. Are heart-lung transplant recipients protected from developing bronchiolitis obliterans syndrome? Ann Thorac Surg. 2006;81:286–91. doi: 10.1016/j.athoracsur.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–18. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- 11.Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J. 2011;37:164–72. doi: 10.1183/09031936.00068310. [DOI] [PubMed] [Google Scholar]
- 12.Ross DJ, Lewis MI, Kramer M, Vo A, Kass RM. FK 506 ‘rescue’ immunosuppression for obliterative bronchiolitis after lung transplantation. Chest. 1997;112:1175–9. doi: 10.1378/chest.112.5.1175. [DOI] [PubMed] [Google Scholar]
- 13.Jaksch P, Scheed A, Keplinger M, et al. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2012;31:950–7. doi: 10.1016/j.healun.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Fischer S, Hoeper MM, Bein T, et al. Interventional lung assist: a new concept of protective ventilation in bridge to lung transplantation. ASAIO J. 2008;54:3–10. doi: 10.1097/MAT.0b013e318161d6ec. [DOI] [PubMed] [Google Scholar]
- 15.Mason DP, Thuita L, Nowicki ER, Murthy SC, Pettersson GB, Blackstone EH. Should lung transplantation be performed for patients on mechanical respiratory support?: the US experience. J Thorac Cardiovasc Surg. 2010;139:765–773.e1. doi: 10.1016/j.jtcvs.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Jayarajan SN, Taghavi S, Komaroff E, et al. Impact of extracorporeal membrane oxygenation or mechanical ventilation as bridge to combined heart-lung transplantation on short-term and long-term survival. Transplantation. 2014;97:111–5. doi: 10.1097/TP.0b013e3182a860b8. [DOI] [PubMed] [Google Scholar]
- 17.Baz MA, Palmer SM, Staples ED, Greer DG, Tapson VF, Davis DD. Lung transplantation after long-term mechanical ventilation: results and 1-year follow-up. Chest. 2001;119:224–7. doi: 10.1378/chest.119.1.224. [DOI] [PubMed] [Google Scholar]
- 18.Wust RC, Degens H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2:289–300. [PMC free article] [PubMed] [Google Scholar]
- 19.Enright S, Chatham K, Ionescu AA, Unnithan VB, Shale DJ. The influence of body composition on respiratory muscle, lung function and diaphragm thickness in adults with cystic fibrosis. J Cyst Fibros. 2007;6:384–90. doi: 10.1016/j.jcf.2007.02.006. [DOI] [PubMed] [Google Scholar]