Abstract
A 4-month-old boy diagnosed with acute myocarditis was treated with extracorporeal membrane oxygenation (ECMO). Follow-up echocardiography eight hours after ECMO revealed intracardiac thrombosis involving all four heart chambers. Because of the high risk of systemic embolization due to a pedunculated thrombus of the aortic valve, we performed an emergency thrombectomy. After the operation, the patient had a minor neurologic sequela of left upper arm hypertonia, which had almost disappeared at the last outpatient clinic two months later. He was diagnosed with a major mutation in MTHFR (methylenetetrahydrofolate reductase), which is related to thrombosis.
Keywords: Myocarditis, Extracorporeal membrane oxygenation, Thrombosis, Methylenetetrahydrofolate reductase
CASE REPORT
A 4-month-old boy was referred to Severance Cardiovascular Hospital for suspicion of myocarditis. His body weight was 7 kg and his medical history was unremarkable except a fever that had begun 7 days previously. A chest X-ray showed car-diomegaly (cardiothoracic ratio 0.59). Electrocardiography revealed sinus tachycardia with a heart rate of 247/min. Echocardiography showed a left ventricular ejection fraction of 25% with inotropic support including dopamine and dobut-amine, and bi-ventricular enlargement was noted. Initial laboratory tests revealed elevated levels of cardiac enzymes (creatine kinase–myocardial band was 39.1 ng/mL and troponin-T was 0.054 ng/mL), but normal levels of inflammation markers (C-reactive protein was 1.7 mg/L). The N-terminal pro-brain natriuretic peptide level was elevated above 35,000 pg/mL. As we suspected myocarditis, we decided to apply extracorporeal membrane oxygenation (ECMO). Venoarterial ECMO was instituted to the right internal carotid artery and the right internal jugular vein after infusion of 350 units of heparin. After ECMO insertion, the flow rate suddenly dropped from 0.5 to 0.1 L/min for 15 minutes. During that time, the mean arterial pressure was maintained below 30 mmHg and the pulse pressure disappeared, so we started an epinephrine infusion. After the infusion of epinephrine, the mean arterial pressure increased gradually to above 60 mmHg and a pulse pressure appeared. The ECMO flow rate increased and was maintained at 0.5 L/min with an activated clotting time of more than 170 seconds. However, follow-up echocardiography after eight hours revealed intracardiac thrombosis involving all four chambers (Fig. 1A, B and Videos 1, 2). Therefore, we decided to perform an emergency thrombectomy. After median sternotomy, cardiopulmonary bypass via the ascending aorta and inferior vena cava was prepared, and then ECMO was stopped. Under routine cardiopulmonary bypass, an additional cannula through the superior vena cava was inserted. After aortic cross-clamping, a right atriotomy and atrial septectomy were performed for exposure of the left side. Thrombectomy of both the atrium and ventricle was done and an aortotomy was performed to inspect the lower portion of the aortic valve. A muscle biopsy of the right ventricular septal wall was also performed. The thrombus was fresh and red. An atrial septectomy of 8 mm was allowed to remain for left side decompression. After ensuring that there was no intracardiac thrombus via epicardial echocardiography, the ECMO circuit was changed and restarted with a flow of 0.5 L/min. The cardiopulmonary bypass time was 91 minutes, and the aortic cross-clamping time was 46 minutes. The postoperative course was smooth except for a hematoma evacuation procedure one day after the operation. Heart function recovery was rapid and the patient was weaned from ECMO two days after surgery. The duration of ECMO was 60 hours. On postoperative day 4, we investigated a focal seizure. A brain magnetic resonance imaging study showed multifocal diffusion restriction in the bilateral cerebral hemispheres and left cerebellum. Thus, we started aspirin and a direct thrombin inhibitor, argatroban, due to the possibility of heparin-induced thrombocytopenia. The duration of mechanical ventilation and intensive care unit (ICU) stay was 11 days and 17 days, respectively. During the stay in the ICU, arrhythmic medication including flecainide, amiodarone, and digoxin was prescribed for intermittent tachyarrhythmia, which usually presented as paroxysmal supraventricular tachycardia. After transfer to the general ward, the patient was treated for left arm hypertonia and delayed development with the help of the department of rehabilitation. During the hospital stay, the patient was diagnosed with a major heterozygous mutation in the MTHFR (methylenetetra-hydrofolate reductase gene); however, the patient had a normal homocysteine level for an infant (2.91 μmol/L). Immunoassays for anti-platelet factor 4/heparin antibodies were negative. At postoperative day 23, a newly developed 5-mm thrombus was observed in the chordae of the tricuspid valve and the left pulmonary drainage site. Thus, we switched to warfarin as anticoagulation treatment to maintain an international normalized ratio of 2.0. He was discharged home with improvement of left arm motion. A follow-up echocardiography 11 months later showed a normal ejection fraction of 65% without thrombus.
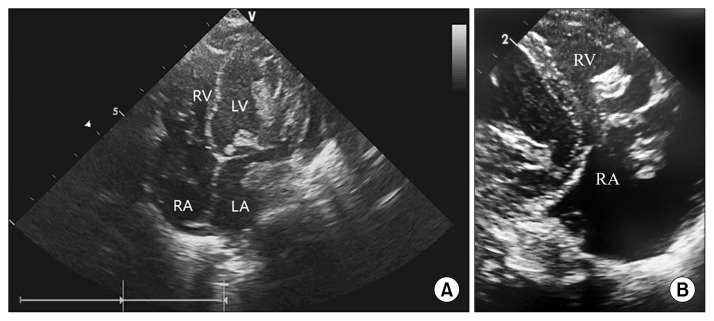
Fig. 1.
(A) Transthoracic echocardiography apical four chamber view demonstrating thrombi in all four chambers of the heart and the parasternal long axis view. (B) Transthoracic echocardiography demonstrating thrombus in the RA and RV. RV, right ventricle; LV, left ventricle; RA, right atrium; LA, left atrium.
DISCUSSION
During ECMO support, appropriate anticoagulation that balances hemorrhage and thrombosis is necessary. When thrombosis events occur, prompt recognition and treatment is important to prevent further sequelae including cerebrovascular thrombosis, pulmonary injury, and renal thrombosis. The most appropriate management of intracardiac thrombi in pediatric patients has not been well established.
Treatment options include either surgical thrombectomy or medical thrombolysis. The choice of treatment depends on the thrombus location (right chamber versus left chamber), size, response to previous therapy, and other risk factors. Right-chamber intracardiac thrombi are much more common and are usually treated with anticoagulation therapy. Medical treatment using tissue-type plasminogen activator for intra-cardiac and aortic thrombi has been reported [1,2].
Left-chamber cardiac thrombosis is rare and should be treated more aggressively. Surgery is often undertaken to prevent catastrophic systemic thromboembolic events, particularly when the thrombus is large. According to an analysis of 40 intracardiac thrombi from Texas, 18 of the 40 thrombi were found in the left chamber. Two patients with a total of four intracardiac thrombi were lost to follow-up after the initial echocardiograms, and unfortunately, their data were not shown in the published report [3]. Another paper from Korea reported that three of five intracardiac thrombi in children with dilated cardiomyopathy were found in the left ventricle. They were treated with heparin and warfarin, except one case, which was treated by thrombectomy [4].
To our knowledge, this is the first report describing the diagnosis and treatment of intracardiac thrombi appearing in all four chambers. The possible mechanisms are assumed to be related to MTHFR mutations, which impair the patient’s ability to metabolize homocysteine. This defective gene leads to elevated levels of homocysteine, resulting in an increased risk for coronary artery disease or venous thrombosis. Taking folic acid and vitamin B6 can lower elevated homocysteine levels [5].
Because of the high risk of systemic embolization due to a pedunculated aortic valve thrombus, we performed an emergency thrombectomy. After the operation, the patient had a minor neurologic sequela of left upper arm hypertonia, which had almost disappeared by the time of the last outpatient clinic two months later. If the operation had been delayed, the patient’s clinical status after the systemic embolization would have been fatal. The timely operation saved the patient’s life, which had been threatened by thrombi in four chambers after ECMO support.
In conclusion, we report an intracardiac thrombus involving four chambers after ECMO support and successful surgical treatment.
Supplementary Information
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via http://dx.do-i.org/10.5090/kjtcs.2016.49.3.207. Video 1. Transthoracic echo-cardiography apical four chamber view demonstrating thrombus in all four chambers of heart.
Supplementary materials can be found via http://dx.do-i.org/10.5090/kjtcs.2016.49.3.207. Video 2. Transthoracic echo-cardiography parasternal long axis view demonstrating thrombus in left atrium and left ventricle attached to a mitral and aortic valve.
REFERENCES
- 1.Garcia A, Gander JW, Gross ER, et al. The use of recombinant tissue-type plasminogen activator in a newborn with an intracardiac thrombus developed during extracorporeal membrane oxygenation. J Pediatr Surg. 2011;46:2021–4. doi: 10.1016/j.jpedsurg.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 2.Levitas A, Zucker N, Zalzstein E, Sofer S, Kapelushnik J, Marks KA. Successful treatment of infective endocarditis with recombinant tissue plasminogen activator. J Pediatr. 2003;143:649–52. doi: 10.1067/S0022-3476(03)00499-2. [DOI] [PubMed] [Google Scholar]
- 3.John JB, Cron SG, Kung GC, Mott AR. Intracardiac thrombi in pediatric patients: presentation profiles and clinical outcomes. Pediatr Cardiol. 2007;28:213–20. doi: 10.1007/s00246-005-1068-3. [DOI] [PubMed] [Google Scholar]
- 4.Choi SH, Jeong SI, Yang JH, et al. A single-center experience with intracardiac thrombosis in children with dilated cardiomyopathy. Pediatr Cardiol. 2010;31:264–9. doi: 10.1007/s00246-009-9602-3. [DOI] [PubMed] [Google Scholar]
- 5.Varga EA, Sturm AC, Misita CP, Moll S. Cardiology patient pages: homocysteine and MTHFR mutations: relation to thrombosis and coronary artery disease. Circulation. 2005;111:e289–93. doi: 10.1161/01.CIR.0000165142.37711.E7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.