Abstract
Congenital cystic adenomatoid malformation is a rare, but well-known disease. It can be managed conservatively in patients without symptoms or require surgical removal when symptomatic. The surgical option of choice is en bloc resection of the affected lesion. We report an experience of life-threatening congenital cystic adenoid malformation in a low-birth-weight (1,590 g) premature neonate who was successfully treated with a lobectomy of the lung.
Keywords: Cystic adenomatoid malformation of lung, congenital; Infant, premature; Infant, low birth weight
CASE REPORT
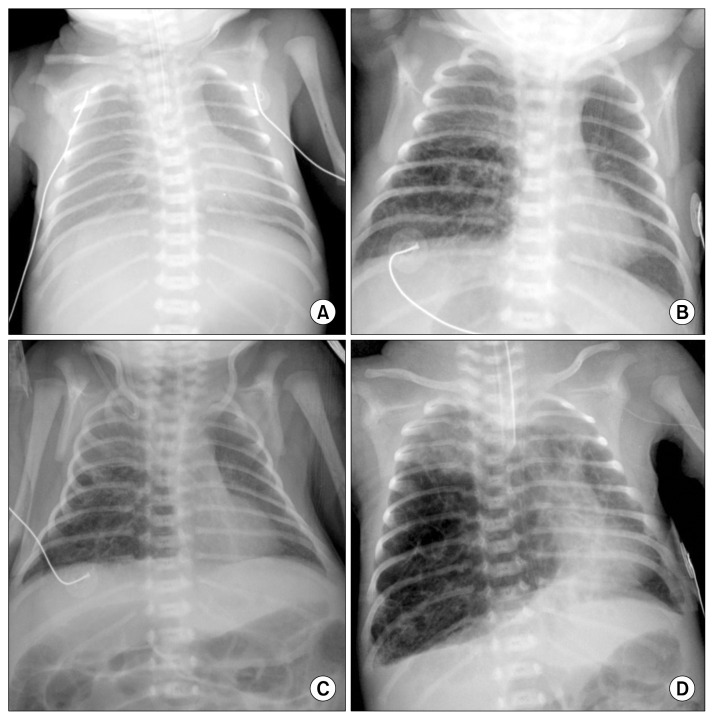
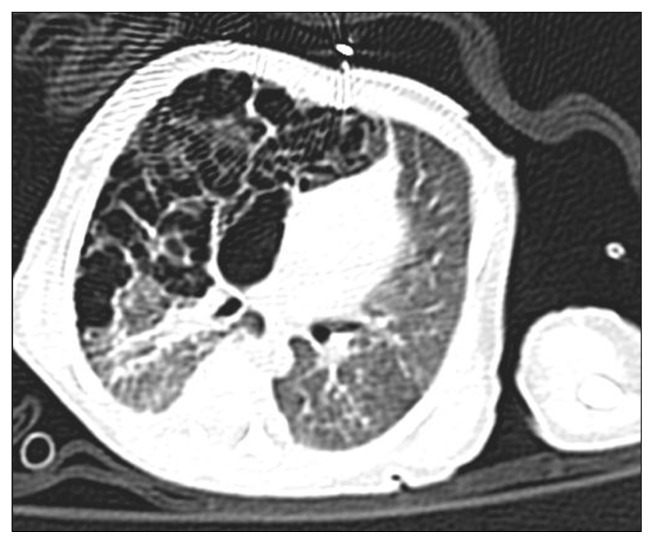
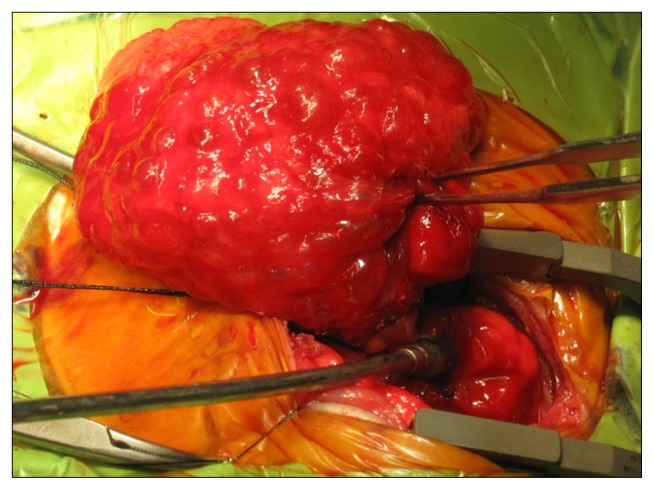
A female infant weighing 1,590 g was born at 31 weeks of gestation with acute chorioamnionitis and meconium aspiration by normal vaginal delivery. Apgar scores were 7 and 9 at 1 and 5 minutes. At birth, the vital signs of the patient included a blood pressure of 40/20 mmHg, heart rate of 156 beats per minute, respiratory rate of 63 per minute, and temperature of 37.2°C. The baby was admitted to the neonatal intensive care unit because of its prematurity and low birth weight. However, respiratory distress with subcostal retraction and cyanosis gradually became aggravated, and chest radiography showed diffusely increased opacities of both lungs (Fig. 1A). Laboratory data revealed marked leukocytosis of 39,400/mm3 and blood gas analysis showed severe respiratory acidosis (pH of 7.11, PaCO2 of 76 mmHg, PaO2 of 18 mmHg, and bicarbonate of 24.1 mmol/L). The baby was intubated, assisted by mechanical ventilation, and administered surfactant. Clinical symptoms and chest radiographic findings improved, and the baby was weaned from mechanical ventilation 4 days after birth. From day of 9 of life, the chest radiography showed a hyperlucent lesion on her right lower lung field (Fig. 1B). The hyperlucent lesion was extended to the left side, and computed tomography (CT) scan of the chest at day 14 of life revealed that the hyperlucent lung lesion was a congenital pulmonary airway malformation of the right middle lobe (Fig. 2). The hyperlucent lesion on chest posterior-anterior (PA) radiography enlarged gradually, but there were no symptoms or signs of respiratory distress (Fig. 1C). On day 24 of life, the baby’s hemodynamics were compromised (blood pressure of 20/10 mmHg, heart rate of 190/min, respiratory rate of 80/min, and desaturation), and mediastinal shifting was aggravated (Fig. 1D). A mechanical ventilator was applied due to respiratory distress. Despite high frequency oscillatory ventilation with 100% of oxygen, arterial blood gas analysis of the patient showed severe respiratory acidosis. The patient was referred to department of thoracic and cardiovascular surgery and we performed emergency surgery. The expanded right middle lobe immediately began to bulge out after the thoracotomy (Fig. 3), and hemodynamics and tidal volume were recovered. We performed right middle lobe lobectomy and the pathologic result showed a type 2 congenital pulmonary airway malformation. The baby was extubated for 5 postoperative days and discharged home 3 weeks later, with follow-up for 5 years without complication.
Fig. 1.
(A) Chest PA radiography (PA) at birth. There are no specific findings in either lung. (B) Chest PA radiography at day 9 of life. Hyperlucent lesions appear in the right lung field. (C) Chest PA radiography at day 20 of life. (D) Chest PA radiography on the day of surgery. Mediastinal deviation and hyperlucent lesions appear in the right lower lung field. PA, posterior-anterior.
Fig. 2.
A chest computed tomography scan shows multiple thin-walled cysts in the right middle lobe.
Fig. 3.
Bulging out of the right middle lobe after thoracotomy with cystic change.
DISCUSSION
Congenital cystic adenomatoid malformation (CCAM) is a rare disease, with a reported incidence reported of 1 in 10,000–35,000 births. This congenital parenchymal lung disease is characterized as the over-proliferation and dilatation of terminal bronchioles with the absence of normal alveoli [1].
More than 99% of CCAMs are diagnosed by fetal sonography at gestational age of 18–20 weeks prenatally. In addition, it can be diagnosed by chest CT or magnetic resonance imaging (MRI) after birth. However, the sensitivity of simple chest radiography in the postnatal period is poor, at only 60%. However, the sensitivity of CT or MRI is 100% in detecting the disease in the postnatal period [2].
The good prognosis of patients with this condition when diagnosed during the fetal period is well known. Spontaneous regression in utero occurs in 43% to 86% of patients who have been diagnosed prenatally, resulting in an asymptomatic birth [3]. Others may experience compression of the normal lung, resulting in pulmonary hyperplasia, or may experience a mass effect including obstruction of the esophagus, poly-hydramnios, preterm labor, and compression of the heart leading to hydrops and tamponade. In several large series, it has been reported that 79% to 100% of fetuses with CCAM and hydrops die during the perinatal periods [4].
In this case, the patient was born at 31 weeks of gestation with a weight of 1,590 g due to intrauterine infection. Antenatal fetal sonography was performed by a local clinic, but CCAM was not found. Initial chest radiography showed consolidation of both lung fields and the patient was diagnosed with respiratory distress syndrome and meconium aspiration. The patient was treated with intravenous antibiotics and ventilator support and recovered fully.
Reported prognostic factors for CCAM are the size and type of the mass, the progression of lesions, cardiac axis deviation, the presence of hydrops, and associated anomalies [5]. In this case, the patient had macrocystic mass and rapid progression, but no hydrops was seen.
After birth, the presence of symptoms is the key element in deciding whether to perform a surgical intervention. Frequent pulmonary infections and the possibility of malignancy such as pulmonary rhabdomyosarcoma are reported among long-term outcomes [5].
In symptomatic patients, the treatment of choice is surgical resection. However, the treatment of patients without symptoms is still debated. Some authors advocate early intervention to prevent possible complications later in life, whereas others suggest perinatal observation to avoid possible surgical complications [6,7]. Most asymptomatic patients develop symptoms within 3 months to 2 years and have a good outcome with elective surgical resection. However, CCAM can produce respiratory distress and develop into a life-threatening condition due to mediastinal shifting, in which case emergency surgery is almost always needed [7].
On day 9 of life, radiolucent lesions appeared in chest PA radiography without symptoms. However, there was rapid progression to mediastinal deviation within 2 weeks. Because of hemodynamic compromise, we performed emergency right middle lobe lobectomy. Kim et al. [8] concluded that an early-stage operation before 1 month can be safely performed and does not exhibit increased morbidity. Stanton at el. [2] reviewed 41 reports between 1996 and 2008, describing 1,070 patients, of whom 79% were prenatally diagnosed, and concluded that elective surgery is safer than emergency surgery. They report that the average time of onset of symptoms is about 7 months for antenatally detected infants and about 10 months for all infants [2].
We report the successful experience of emergency lobectomy in a preterm low birth weight (1,590 g) infant with severe acute respiratory distress due to acute aggravation of CCAM. In the case of symptomatic CCAM, even in a low birth-weight premature infant, early surgical intervention can be considered for a favorable outcome.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Durell J, Lakhoo K. Congenital cystic lesions of the lung. Early Hum Dev. 2014;90:935–9. doi: 10.1016/j.earlhumdev.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Stanton M, Njere I, Ade-Ajayi N, Patel S, Davenport M. Systematic review and meta-analysis of the postnatal management of congenital cystic lung lesions. J Pediatr Surg. 2009;44:1027–33. doi: 10.1016/j.jpedsurg.2008.10.118. [DOI] [PubMed] [Google Scholar]
- 3.Kunisaki SM, Barnewolt CE, Estroff JA, et al. Large fetal congenital cystic adenomatoid malformations: growth trends and patient survival. J Pediatr Surg. 2007;42:404–10. doi: 10.1016/j.jpedsurg.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Miller JA, Corteville JE, Langer JC. Congenital cystic adenomatoid malformation in the fetus: natural history and predictors of outcome. J Pediatr Surg. 1996;31:805–8. doi: 10.1016/S0022-3468(96)90138-4. [DOI] [PubMed] [Google Scholar]
- 5.Duncombe GJ, Dickinson JE, Kikiros CS. Prenatal diagnosis and management of congenital cystic adenomatoid malformation of the lung. Am J Obstet Gynecol. 2002;187:950–4. doi: 10.1067/mob.2002.127460. [DOI] [PubMed] [Google Scholar]
- 6.Kim HK, Choi YS, Kim K, et al. Treatment of congenital cystic adenomatoid malformation: should lobectomy always be performed? Ann Thorac Surg. 2008;86:249–53. doi: 10.1016/j.athoracsur.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Muller CO, Berrebi D, Kheniche A, Bonnard A. Is radical lobectomy required in congenital cystic adenomatoid malformation? J Pediatr Surg. 2012;47:642–5. doi: 10.1016/j.jpedsurg.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim YT, Kim JS, Park JD, Kang CH, Sung SW, Kim JH. Treatment of congenital cystic adenomatoid malformation-does resection in the early postnatal period increase surgical risk? Eur J Cardiothorac Surg. 2005;27:658–61. doi: 10.1016/j.ejcts.2005.01.028. [DOI] [PubMed] [Google Scholar]