Abstract
The objective of the study was to investigate the pharmacokinetics (PK) of unbound and total plasma carbamazepine (CBZ) concentrations following simultaneous administration of intravenous and oral formulations. We tested the hypothesis that age-related alterations in physiology and patient characteristics influence CBZ disposition and protein binding. Patients (n = 113) on maintenance therapy received a 100 mg dose of a novel, intravenous, stable-labeled (SL) CBZ formulation as partial replacement of their morning CBZ dose. A two-compartment model described unbound and total SL-CBZ data. The stable-labeled intravenous dosing methodology enabled the estimation of the CBZ clearance (CL) and volumes of distribution. The CL of CBZ was dependent on race through the model equation unbound CL (L/hour) = 11.2 × (1.30)Race; where Race = 1 for Caucasian, 0 for African American. Total body weight explained 57% and 70% of the interindividual variability in the central and peripheral volumes of distribution, respectively. Age, sex, smoking, plasma albumin, and alpha 1-acid glycoprotein concentrations had no effect on CL, binding or volumes of distribution. The model was evaluated via bootstrap and predictive check. Results may support race specific dosing for CBZ where an average African-American individual would receive 70% of the standard dose prescribed for the Caucasian person.
Keywords: adults and elderly, carbamazepine, epilepsy, nonlinear mixed effects modeling, population pharmacokinetics, stable-labeled intravenous dosing
Epilepsy is a common neurologic disorder defined by a history of at least one seizure together with a diagnosed alteration in the brain that increases the likelihood of future seizures.1 It affects about 50 million people worldwide including approximately 3 million residents of North America.2 Carbamazepine (CBZ) is a standard treatment for partial seizures as well as trigeminal neuralgia and psychiatric disorders.3 In comparative trials with antiepilepsy medications, CBZ showed similar results to lamotrigine [time to 12-month remission (HR) 0.91 (95% C.I. 0.77–1.09)]4,5 but superior efficacy to phenobarbital and primidone for the treatment of partial seizures.6 However, 30–40% of patients with epilepsy respond poorly to CBZ or develop adverse effects which could be attributed to the complex pharmacokinetics (PK) of CBZ and the limited knowledge of individual factors influencing its disposition.7
CBZ is extensively metabolized by the liver, in part through the cytochrome P450 enzyme system (CYP3A4/5). Several factors may influence serum concentrations of CBZ and its metabolites. In the elderly (age ≥ 65–70), the population clearance (CL) of CBZ is reported to decrease by 25–40%8 and is could to be due to a decreased activity of CYP3A4.9 Co-prescribed drugs such as phenytoin and phenobarbital are reported to increase CBZ CL in both adults and children due to induction of the metabolizing enzymes.10,11 In addition, the unbound fraction of CBZ might be affected by changing levels of the major binding proteins, albumin, and alpha 1-acid glycoprotein (AAG).12
The variability in CBZ PK is presumably due to the complex PK and the narrow therapeutic window of the drug (unbound CBZ 0.87–2.80 mg/L).13 Elevated serum CBZ concentrations are associated with neurologic side effects, and hyponatraemia.14 Similarly, clinical effects of CBZ bear a relatively close relationship to serum drug concentration.15 CBZ PK following oral administration have been extensively studied,8,10,13–18 but there are a few reports involving the PK of IV CBZ in either healthy volunteers or epilepsy patients.19–22 The safety of an IV CBZ formulation in patients has previously been assessed.23 In the current study, we investigated the population PK of total and unbound CBZ from a novel stable-labeled (SL) IV formulation. This allowed us to examine CBZ disposition in community-dwelling elderly and younger adult patients with epilepsy without interrupting therapy.
Methods
Patient Selection and Recruitment
Patients were recruited from three epilepsy programs: the University of Minnesota and affiliated clinics, the University of Miami, and Emory University. Inclusion criteria included requirements that subjects be on CBZ monotherapy or taking non-interacting medications and were over the age of 18 years. In addition, patients had to be on a stable maintenance CBZ regimen with no dosage adjustments within 2 weeks prior to the first day of the study. Exclusion criteria included patients with serious medical problems that could compromise their tolerance to IV CBZ or influence the drug disposition. The University of Minnesota Institutional Review Boards (IRB), Miami Veteran Affairs Medical Center IRB, and Emory University IRB approved the study protocol and all patients provided informed, written consents prior to enrollment.
Study Design
Upon admission to the research center, detailed medication history was collected and a brief neurological examination was performed on every patient by the clinical site neurologists. Prior to drug administration, a normal saline infusion was started at 50 mL/hour and continued until 30 minutes after the infusion. Patients were maintained on steady-state oral CBZ therapy. On the day of the study, subjects received a total of 100 mg SL CBZ (10 mg/mL in 22.5% 2-hydroxypropyl-β-cyclodextrin) as an infusion over a period of 10–20 minutes. The SL isotope methodology allowed the IV CBZ to be analytically differentiated from the oral CBZ due to the different masses of CBZ in each formulation. Blood sampling was done at 0, 0.083, 0.25, 0.5, 1, 2, 4, 6, 10, 24, 48, 72, and 96 hours. At the end of the infusion, subjects were allowed to take their regular morning CBZ dose less 100 mg. Blood samples were centrifuged and the plasma was frozen at −80°C until assayed.
Intravenous Formulation
A general description of the synthesis and formulation of the SL-CBZ is reported in a previous publication.23
Carbamazepine Assay
Plasma concentrations of unbound and bound SL-CBZ were analyzed by a validated liquid-chromatography/mass spectroscopy. Carbamazepine-d10 (CBZ-d10, C/D/N Isotopes, Quebec, Canada) was used as the internal standard. 0.5 mL of patient plasma and 20 µL of internal standard were mixed with blank plasma and extracted with 3 volumes of ethyl acetate. Following each centrifugation, the organic layer was removed and evaporated under nitrogen to dryness. Each sample was then redissolved in 25 µL of ethyl acetate. Unbound drug was separated from the bound fraction by using Millipore-Amicon Centrifree filters. The mobile phase consisted of 50% 0.05 M ammonium acetate buffer (pH 4.7) and 50% methanol and was run at a flow rate of 0.4 mL/min on a Zobrax LC8 (3.0 mm × 150 mm, 3.5 µm) column. All samples from an individual patient were run at the same time along with a 7-concentration standard curve and low, medium, and high concentrations of quality control samples (range: 0.1–4 µg/mL). The lower limit of detection was 0.1 µg/mL for SL-CBZ. The assay was validated by being repeated five times on several days. The between-day and within day variability of the assay was 3.5% and 2.5%, respectively. Accuracy ranged between 83.7% and 102.6% for the analytical standards. Quality control samples were all within ≤10% with respect to variability.21
Population Pharmacokinetic Analysis
The population PK analyses were performed using the nonlinear mixed effects modeling approach as implemented in NONMEM (version VII, level 1.2). The first-order conditional estimation with interaction (FOCEI) was utilized throughout the model development process. Based on exploratory data analysis, the basic pharmacokinetic model was derived and fit to the data. A one-compartment (ADVAN1) and two-compartment model (ADVAN3) with first-order elimination were explored. The models were parameterized in terms of the unbound parameters including CL, volume of distribution (V1), unbound fraction (Fu), and for the two compartment model intercompartmental CL (Q), and peripheral volume (V2). In this analysis, the total concentration was assumed to be a function of the unbound concentration as follows:
where CTpred,ij and CUpred,ij are the j-th predicted total and unbound plasma concentration (mg/L), respectively, in the i-th individual, Fu,i is the unbound fraction for the i-th individual. This model assumes a constant Fu for an individual but can be modified to allow concentration or time dependencies. The effect of unbound drug concentration (Cu) on Fu was modeled through a linear slope function as follows:
where Fu,i,Base is the baseline Fu for the i-th individual and β is the estimated increase in Fu for a unit mg/L increase in the Cu. The inter-individual variability in the PK parameters was modeled through an exponential error model as given in Equation (1):
| (1) |
where βi is the individual PK parameter, TVβ is the population mean PK parameter, and ηi,β is the independent random error distributed normally with a mean zero and variance equal to , which specifies the between-subject variability around the TVβ.
Different error models were explored including an exponential, proportional, additive, and combined proportional and additive error model. Because unbound and total concentrations were simultaneously modeled, an indicator variable (TYPE) was used to build a separate residual error model for unbound and total concentrations as shown in Equations (2) and (3), respectively:
| (2) |
| (3) |
where CUobs,ij is the j-th observed unbound plasma concentration (mg/L) in the i-th individual, CTobs,ij is the j-th observed total plasma concentration (mg/L) in the i-th individual and -εij is the independent, normally distributed error, known as the residual error (between the predicted and observed concentration) with mean zero and variance . Model development was guided by examining diagnostic plots including plots of observed versus population and individual predicted concentrations and conditional weighted residuals versus predicted concentration and time plots.
Covariate Model
Scientific hypotheses regarding covariate effects on CBZ model parameters were tested. Covariates examined included age, body size measurements (including total body weight (TBW) in kg; body surface area [BSA]; ideal body weight [IBW]; and lean body weight [LBW]); demographic covariates (including race; sex; and clinical center), factors that may affect drug metabolism (smoking status; alcohol consumption; grapefruit juice consumption), and covariates that may influence drug binding (such as AAG, albumin, total protein concentration, and Cu).
Covariate modeling was done using the standard forward inclusion/backward elimination approach and was initially guided by observation of plots of posterior Bayesian estimates of PK parameters versus the different covariates. Continuous covariates, such as age, protein concentrations, and Cu were investigated through linear and non-linear regression on CL and free fraction. Age was additionally tested as a categorical covariate on both CL and protein binding (age <60 years, age ≥60 years). When evaluating TBW, allometric scaling was used.24 Categorical covariates were examined according to a multiplicative model in order to obtain the associated fractional change in pharmacokinetic parameters. The covariate model selection was done on the basis of the change in the NONMEM objective function value (OFV), clinical importance of the estimated effect, as well as visual inspection of diagnostic plots. A decrease in the NONMEM OFV of at least 7.88 (χ2, P ≤ .005, df = 1) and 10.83 (χ2, P ≤ .001, df = 1) was used as a cutoff value for forward inclusion and backward elimination, respectively.
Model Evaluation
The Nonparametric Bootstrap
The precision of the final parameter estimates were checked using 1000 separate NONMEM runs on resampled (with replacement) datasets. The bootstrap analysis was done through the model evaluation option in PDx-Pop 4.1 (ICON Development Solutions, Ellicott City, MD) interface for NONMEM. The results were imported to SAS (version 9.2) and the 2.5th and 97.5th percentiles of the rank ordered parameter values were computed as the lower and upper boundary of the bootstrap 95% confidence interval (CI). Finally, bootstrap parameter estimates as well as their 95% CI were compared to the NONMEM estimates from the original data set.
Model Qualification
The final model was qualified by visual predictive check (VPC) where the estimated parameters were used to simulate 1000 replicates of the original dataset. The 5th, 50th, and 95th quantiles of the simulated CBZ concentrations were plotted against observed concentrations. The simulations were done using the PDx-Pop 4.1 interface for NONMEM.
Results
Model Building Data Set
The final data set used comprised 833 observations of unbound concentrations and 1201 observations of total concentrations from 113 subjects: The University of Minnesota and affiliated clinics [median age 43, range (20–87)], the University of Miami [47 (22–82)], and Emory University [34 (19–61)]. Patients received a total infusion dose of SL-CBZ in the range of 87–130 mg at an average rate of 9 mg/min. Table 1 summarizes patient characteristics at baseline as well as potential covariates used in the analysis.
Table 1.
Baseline Characteristics of Patients Included in the Model Building Process
| Characteristic | Mean ± SD or Number |
|---|---|
| Weight (kg) | 80.6 ± 19.4 |
| Body surface area (m2) | 1.9 ± 0.2 |
| Lean body weight (kg) | 55.4 ± 11.3 |
| Ideal body weight (kg) | 63.7 ± 10.3 |
| Age (years) | 46.1 ± 14.9 |
| Young adults (Age <60) | |
| Number | 91 |
| Median age (range) | 42 (19–59) |
| Sex [women/men] | 46/45 |
| Race [Caucasian/African American] | 49/42 |
| Elderly (Age ≥60) | |
| Number | 22 |
| Median age (range) | 66 (60–87) |
| Sex [women/men] | 7/15 |
| Race [Caucasian/African American] | 21/1 |
| Women | |
| Number | 53 |
| Median Age (range) | 41 (20–72) |
| Age group [age < 60/age ≥60] | 46/7 |
| Race [Caucasian/African American] | 31/22 |
| Men | |
| Number | 60 |
| Median Age (range) | 48 (19–87) |
| Age group [age < 60/age ≥60] | 45/15 |
| Race [Caucasian/African American] | 39/21 |
| Total protein concentration (mg/dL) | 7.0 ± 0.5 |
| Albumin concentration (mg/dL) | 4.0 ± 0.4 |
| Alpha 1-acid glycoprotein concentration (mg/dL) | 79.7 ± 22 |
| Sex [women/men] | 53/60 |
| Race [Caucasian/African American] | 70/43 |
| Smoking history [smokers/nonsmokers/missing] | 22/47/44 |
| Alcohol consumption [consumers/non-consumers/missing] | 24/42/47 |
| Grape fruit consumption [consumer/non-consumers] | 71/42 |
Population Pharmacokinetic Analysis
The Structural Pharmacokinetic Model
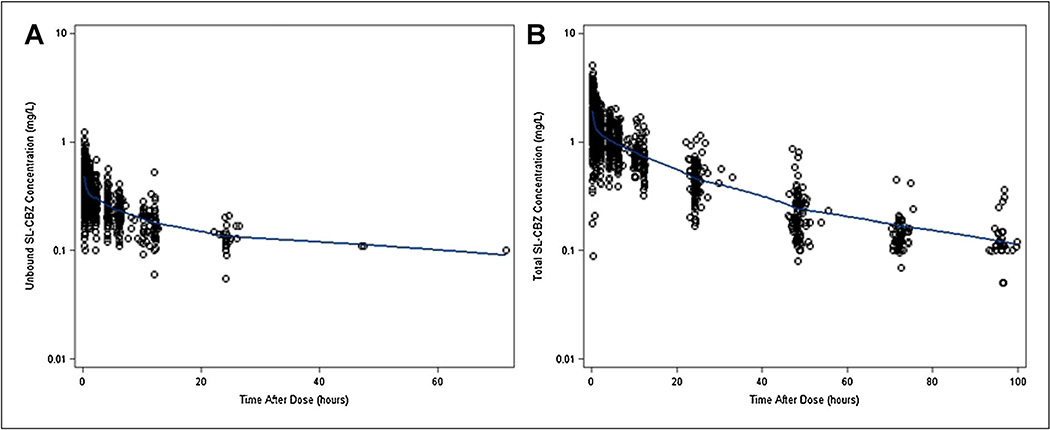
A two-compartment model with first order elimination (subroutine ADVAN3, TRANS4) adequately fit the data. Both alpha and beta phases of the two compartment disposition could be supported by the unbound and total SL-CBZ concentration-time profiles shown in Figure 1A and B. The one-compartment model resulted in a poorer fit to the data and a significantly higher OFV. The proportional error model and the combined proportional and additive error model best described the residual unexplained variability in the unbound and total concentrations, respectively.
Figure 1.
(A) Scatter plot of observed unbound SL-carbamazepine concentration versus time after dose. (B) Scatter plot of observed total SL-carbamazepine concentration versus time after dose. The line through the data represents a Locally Weighted Scatterplot Smoother (LOESS) fit to the data.
Covariate Model
Final model parameter estimates as well as the precision associated with the estimation are shown in Table 2. During the forward selection process, race was found to be the only covariate that had a significant influence on CBZ unbound CL (ΔOFV = 13.4, P < .005). Caucasians were found to have an average of 30% greater unbound CL compared to African Americans. Body size measurements, age (tested as a continuous or categorical covariate), smoking category, alcohol use or grape fruit consumption, plasma albumin, and AAG concentration had insignificant effects on the unbound CL either through univariate inclusion or after accounting for the race effect (ΔOFV < 7.88, df = 1, ΔOFV < 10.8, df = 2; P > .005). No significant difference was found among the mean CL estimates in the three clinical centers. TBW (kg) was found to explain a significant proportion of the interindividual variability in the central (V1) and peripheral (V2) volumes of distribution. Approximately, 57% and 70% of the interindividual variability in V1 and V2, respectively, were explained by allometric scaling to a standard 70 kg individual. Neither age nor albumin, AAG or Cu had a significant effect on drug binding. Because Cu did not have an effect on binding, we further investigated the possibility that a fraction of individuals belong to a sub-population with concentration-dependent binding of CBZ via the implementation of mixture models in NONMEM. The mixture model indicated that 99.5% of the studied population had a constant binding (i.e. constant Fu) of CBZ over the studied range of Cu. The final model included a race effect on the unbound CL, and TBW as measured in kg on volumes of distribution. A backward elimination step was not performed since there was only one covariate effect per parameter included in the model.
Table 2.
Final Model Parameter Estimates and 95% CI From the Original Dataset and Bootstrap Analyses
| NONMEM Analysis | Bootstrap Analysis | |||
|---|---|---|---|---|
| Parameter | Estimate (% SE) | 95% CI | Median | 95% CI |
| CLun (L/h) | 11.2 (5.5) | 10–2.4 | 11.2 | 10.3–2.3 |
| V1 (L/70 kg) | 142 (4.8) | 128.6–55.4 | 143 | 127–179 |
| Q (L/h) | 444 (15.8) | 306.4–581.6 | 429 | 189–611 |
| V2 (L/70 kg) | 175 (4.2) | 160.5–189.5 | 175 | 131–191 |
| Fu | 0.25 (2.1) | 0.24–0.26 | 0.25 | 0.24–0.26 |
| θRACE | 1.30 (8.3) | 1.08–1.51 | 1.30 | 1.14–1.47 |
| Interindividual variance (% SE) | ||||
| IIV of CLun | 0.104 (28.5) CV% = 32.2 | 0.046, 0.162 | 0.101 | 0.065, 0.139 |
| IIV of V1 | 0.046 (29.8) CV% = 21.5 | 0.019, 0.073 | 0.052 | 0.018, 0.127 |
| IIV of Q | 1.78 (21.7) CV% = 133 | 1.02, 2.54 | 1.72 | 0.146, 2.52 |
| IIV of V2 | 0.055 (33.5) CV% = 23.5 | 0.019, 0.092 | 0.051 | 0.029, 0.149 |
| IIV of Fu | 0.036 (13.4) CV% = 18.9 | 0.026, 0.045 | 0.043 | 0.027, 0.045 |
| Residual variance (% SE) | ||||
| RUVun, Prop | 0.032 (16.4) CV% = 17.8 | 0.022, 0.042 | 0.031 | 0.022, 0.042 |
| RUVTot, Prop | 0.024 (14.4) CV% = 15.5 | 0.017, 0.031 | 0.021 | 0.018, 0.031 |
| RUVTot, additive SD (mg/L) = 0.05 | 0.002 (19.9) | 0.001, 0.003 | 0.002 | 0.001, 0.003 |
CI, confidence interval; CLun, unbound carbamazepine clearance estimated for an African American individual (considered to be the reference group); V1, central volume of distribution; Q, intercompartmental clearance; V2, peripheral volume of distribution; Fu, fraction unbound; θRACE, race effect, has a power of 1 if the patient is Caucasian, and a power of zero if the patient is African American; IIV, interindividual variability; %CV, percentage coefficient of variation; RUVun and RUVTot are residual unexplained variability in the unbound concentration and total concentration, respectively, expressed as a proportional error variance.
Equation (4) describes the final model for unbound CL of CBZ:
| (4) |
where Race [i] equals 1 if the patient is Caucasian or zero if the patient is African American.
Equations (5) and (6) describe the final model for the unbound volume of distribution in the central and peripheral compartment, respectively:
| (5) |
| (6) |
where WTi, is the i-th subject weight in kg.
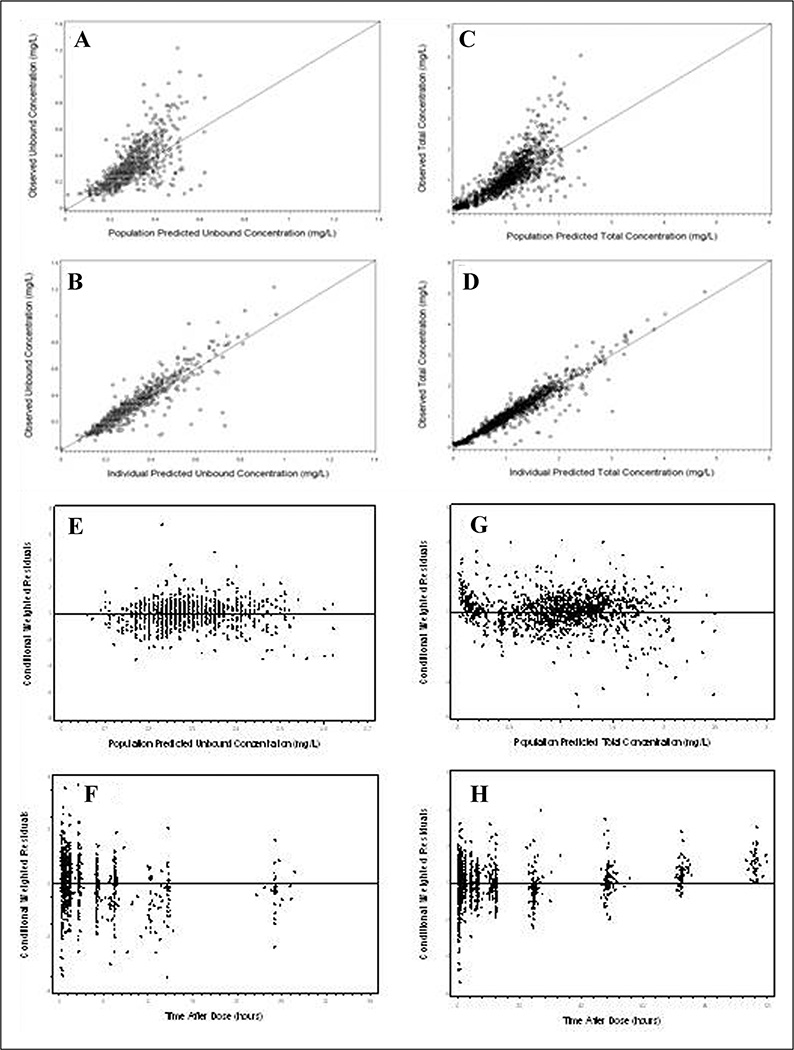
Goodness of fit plots from the final model are shown in Figure 2A–H. Both unbound and total SL-CBZ predicted concentrations showed a close agreement with the observed values (Figure 2A–D) and the conditional weighted residual plots showed lack of trend with respect to predicted concentrations and the time after dose (Figure 2E–H).
Figure 2.
Goodness of fit plots for the final population pharmacokinetic model. (A) Identity plot of observed versus population predicted unbound concentration. (B) Identity plot of observed versus individual predicted unbound concentration. (C) Identity plot of observed versus population predicted total concentration. (D) Identity plot of observed versus individual predicted total concentration. (E) Scatter plot of conditional weighted residuals (CWRES) versus population predicted unbound concentration. (F) Scatter plot for CWRES versus time after dose for unbound concentration fit. (G) Scatter plot for CWRES versus population predicted total concentration and (H) Scatter plot for CWRES versus time after dose for total concentration fit.
The Nonparametric Bootstrap
The results of the bootstrap validation step are presented in Table 2. From the 1000 bootstrap runs, 921 successful runs were pooled for the final analysis. The estimates of the fixed effects and random effects obtained from NONMEM were all within 10% of the bootstrapped means. Furthermore, the bootstrap 95% CI for all parameter estimates were comparable to NONMEM estimated CI.
Visual Predictive Check
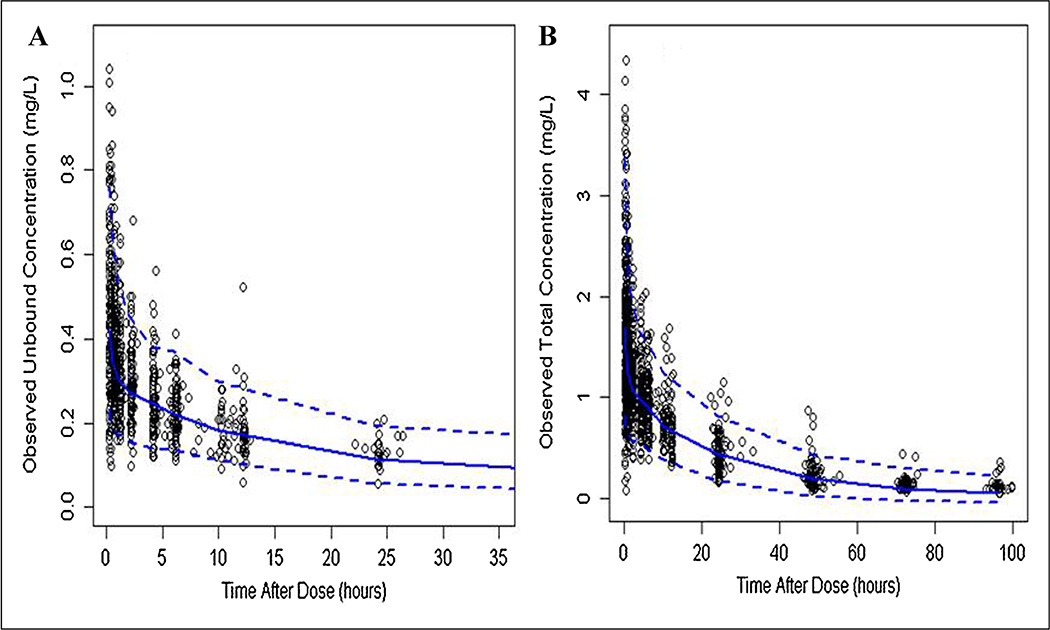
The predictive check plots of unbound and total SL-CBZ are shown in Figure 3A and B. Of the observed data, 91.3% fit within the 5th and 95th percentiles of the simulated datasets. This may indicate that the model adequately described the overall trend and variability in the observed data.
Figure 3.
Visual predictive check plots of observed unbound (A) and total (B) SL-CBZ concentrations (open circles), median (solid line), and 5th and 95th quantiles (dashed lines) of 1 000 simulated data sets.
Discussion
We characterized for the first time, the population PK of IV SL-CBZ under steady-state conditions in community-dwelling young adult and elderly patients. The use of a SL IV formulation coupled with a rich sampling schedule allowed the rigorous estimation of the absolute PK parameters under a clinically relevant setting without interruption of drug therapy. The major results from this study of community dwelling adults and elderly epilepsy patients are that the unbound CL of CBZ was dependent on race, but not on age, sex, smoking, plasma albumin or AAG concentrations. Total body weight explained 57% and 70% of the interindividual variability in the unbound central and peripheral volumes of distribution, respectively. Age, sex, smoking, plasma albumin, and AAG concentrations had no effect on protein binding or distribution volumes. The stable-labeled intravenous dosing methodology enabled the estimation of the CBZ CL and volumes of distribution.
Unbound and total SL-CBZ concentration time profiles were best described by a linear 2-compartment model. Previous studies suggest that CBZ PK follow a one-compartment disposition behavior8,10,13–17,23; but none of these studies used IV CBZ. The high variability and prolonged absorption of CBZ from oral tablets and capsules may mask CBZ distribution and elimination characteristics in a sparse sampling setting.
The actual body weight was found to be a significant covariate for the volume of distribution in the central and peripheral compartments, whereas elimination CL of CBZ was found to be independent of body weight. The lack of a weight effect on CL may be attributed to the relative homogeneity of body weight in the studied population (inter-quartile range 66–92 kg). Additionally, the effect of weight could be confounded by a variety of physical and hormonal factors.25 Previous population pharmacokinetic studies reported weight as a significant covariate on CBZ CL, but most of these studies included a wider range of body sizes than in our study.10,18
Covariate modeling results from our study suggested that CBZ PK were not influenced by age and sex. Regarding age, our results are in agreement with Hockings et al.,17 who reported an insignificant change in the PK of CBZ in five elderly compared with six young adults after a single oral dose of 400 mg. In a study of 879 ambulatory patients, Graves et al.,8 showed that only subjects 70 years or older had a 25% reduction in oral CBZ CL. In our study, the small number of elderly patients above the age of 60 (22/91) might have resulted in insufficient power to identify such an effect.
The effect of sex on the activity of CYP3A4 remains unclear; however, the enzyme is reported to have a greater activity in women than men for certain substrates.26–28 Using a noncompartmental approach, Marino et al.,21 reported a significantly greater CBZ CL in women than men aged less than 60 years (P < .007). We found a mean reduction in the unbound CBZ CL in young men compared to young women, however, the effect was reversed in the elderly, but the effects did not achieve a statistical significance.
Amongst all of the tested covariates, only race emerged as a predictor of CBZ CL, which was 30% higher in Caucasians compared to African Americans. Several studies have found differences in CL of specific drugs between Caucasians and African Americans.21,29,30 Racial effects on drug disposition are thought to be caused by differences in drug metabolizing capacity. Perhaps the high incidence of the variant allele CYP3A4*1B in African Americans compared to Caucasians (χ2 = 48.9, P < .001)31 partly explains the difference in CL between the two groups. CYP3A4*1B is hypothesized to be associated with a decreased enzymatic activity.32–34 which may confer a low CL of CBZ in African Americans compared to Caucasians.
CBZ is approximately 75% bound to serum proteins, mainly to serum albumin and to a lesser extent to AAG.35 Levels of the latter protein are elevated in inflammation, cancer and in elderly subjects.36 We found no significant effect of albumin or AAG levels or age on CBZ free fraction. The patients in our study were relatively healthy with plasma albumin and AAG levels homogenously distributed within the normal range. This may explain the relatively small inter-individual variability (%CV ~ 19%) encountered in the CBZ free fraction. Similarly, Koyama et al.,12 found no correlation between AAG level and CBZ free fraction. The binding of CBZ was shown to be constant (Fu ~ 0.25) over a range (0.1–1 mg/L) of unbound concentration. This finding does not exclude the possibility of a nonlinear binding for CBZ at higher concentrations than in our study.
To our knowledge, there are no reports of integrated modeling of unbound/total IV CBZ PK. Therefore, the current model for CL was compared to other population CBZ PK studies involving oral CBZ taking into account an average estimate of bioavailability of 70%.19,21 Based on the estimates of absolute CL and bioavailability, the mean apparent CBZ CL would be 13.4 L/hour. In a study of Omani patients with epilepsy in which unbound CBZ CL was determined by routine therapeutic drug monitoring, Deleu et al.,13 found a population mean apparent CL of 13.2 L/hour. Our results are in close agreement with that report. Furthermore, given a population estimate of 0.25 for the fraction unbound in plasma (our study), the total CL of CBZ was estimated to be 3.35 L/hour which is consistent with estimates of the mean CBZ CL reported in other studies.10,18,37 The standard diagnostic plots suggested that the final model adequately described both unbound and total SL-CBZ data. The observed trend in the residuals at low prediction values or late sampling times (Figure 2G and H) might not be influential due to the possible low accuracy of the analytical assay at such low concentrations and potential errors in recording late sampling times.
In conclusion, we have characterized the steady-state compartmental PK of unbound and total CBZ using stable-labeled intravenous formulation given to patients with epilepsy on maintenance CBZ regimens. We found no effect of aging in relatively healthy young-old (60–74 years) patients. In contrast, the influence of race on CBZ CL was highly significant and warrants further investigation. Results from our study may support race specific dosing for CBZ where an African American patient would receive, on average, 70% of the standard dose prescribed for a Caucasian. The satisfactory performance of the proposed population model may justify its applicability in estimating the steady-state PK parameters for CBZ.
Acknowledgments
Funding
This study was supported by grants from the National Institute of Health [NINDS P50-NS16308, M01-RR00400, M01-RR00039, and M01 RR16587]; a graduate fellowship grant from the Government of Egypt for Ghada F. Ahmed [GM842]; and a K 01 grant for Dr. Marino [NS050309].
Footnotes
Declaration of Conflicting Interests
James C. Cloyd, Ilo E. Leppik & Angela K. Birnbaum have a royalty agreement with Lundbeck Inc. related to the development of intravenous carbamazepine.
References
- 1.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Theodore WH, Spencer SS, Wiebe S, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006;47:1700–1722. doi: 10.1111/j.1528-1167.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 3.Perucca E, Berlowitz D, Birnbaum A, et al. Pharmacological and clinical aspects of antiepileptic drug use in the elderly. Epilepsy Res. 2006;68:S49–S63. doi: 10.1016/j.eplepsyres.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Nieto - Barrera M, Brozmanova M, Capovilla G, et al. A comparison of monotherapy with lamotrigine or carbamazepine in patients with newly diagnosed partial epilepsy. Epilepsy Res. 2001;46:145–155. doi: 10.1016/s0920-1211(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 5.Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000– 1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic–clonic seizures. N Engl J Med. 1985;313:145–151. doi: 10.1056/NEJM198507183130303. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosio AF, Soares-Da-Silva P, Carvalho CM, Carvalho AP. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem Res. 2002;27:121–130. doi: 10.1023/a:1014814924965. [DOI] [PubMed] [Google Scholar]
- 8.Graves NM, Brundage RC, Wen Y, et al. Population pharmacokinetics of carbamazepine in adults with epilepsy. Pharmacotherapy. 1998;18:273–281. [PubMed] [Google Scholar]
- 9.Tanaka E. In vivo age-related changes in hepatic drug-oxidizing capacity in humans. J Clin Pharm Ther. 1998;23:247–255. doi: 10.1046/j.1365-2710.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan E, Lee HS, Hue SS. Population pharmacokinetics of carbamazepine in Singapore epileptic patients. Br J Clin Pharmacol. 2001;51:567–576. doi: 10.1046/j.0306-5251.2001.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlanut M, Montanari G, Bonin P, Casara GL. Carbamazepine and carbamazepine-10,11-epoxide serum concentrations in epileptic children. J Pediatr. 1985;106:491–495. doi: 10.1016/s0022-3476(85)80689-2. [DOI] [PubMed] [Google Scholar]
- 12.Koyama H, Sugioka N, Uno A, Mori S, Nakajima K. Age-related alteration of carbamazepine-serum protein binding in man. J Pharm Pharmacol. 1999;51:1009–1014. doi: 10.1211/0022357991773474. [DOI] [PubMed] [Google Scholar]
- 13.Deleu D, Aarons L, Ahmed IA. Population pharmacokinetics of free carbamazepine in adult Omani epileptic patients. Eur J Clin Pharmacol. 2001;57:243–248. doi: 10.1007/s002280100300. [DOI] [PubMed] [Google Scholar]
- 14.Martin ES, III, Crismon ML, Godley PJ. Postinduction carbamazepine clearance in an adult psychiatric population. Pharmacotherapy. 1991;11:296–302. [PubMed] [Google Scholar]
- 15.Battino D, Croci D, Rossini A, Messina S, Mamoli D, Perucca E. Serum carbamazepine concentrations in elderly patients: a case-matched pharmacokinetic evaluation based on therapeutic drug monitoring data. Epilepsia. 2003;44:923–929. doi: 10.1046/j.1528-1157.2003.62202.x. [DOI] [PubMed] [Google Scholar]
- 16.Bondareva IB, Jelliffe RW, Gusev EI, Guekht AB, Melikyan EG, Belousov YB. Population pharmacokinetic modelling of carbamazepine in epileptic elderly patients: implications for dosage. J Clin Pharm Ther. 2006;31:211–221. doi: 10.1111/j.1365-2710.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 17.Hockings N, Pall A, Moody J, Davidson AV, Davidson DL. The effects of age on carbamazepine pharmacokinetics and adverse effects. Br J Clin Pharmacol. 1986;22:725–728. doi: 10.1111/j.1365-2125.1986.tb02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reith DM, Hooper WD, Parke J, Charles B. Population pharmacokinetic modeling of steady state carbamazepine clearance in children, adolescents, and adults. J Pharmacokinet Pharmacodyn. 2001;28:79–92. doi: 10.1023/a:1011569703060. [DOI] [PubMed] [Google Scholar]
- 19.Walzer M, Biton V, Bekersky I, Wesche D, Tolbert D, Cloyd JC. Bioequivalence of oral and intravenous carbamazepine in adult patients with epilepsy; Abstract presented at the Annual Meeting of American Epilepsy Society; 2010. [Abstract 1.259] [Google Scholar]
- 20.Walzer M, Johnson ALC, Collins SB, Cloyd WS, Tolbert JCD. Pharmacokinetics of oral and intravenous carbamazepine in adult patients with epilepsy; Abstract presented at the Annual Meeting of American Epilepsy Society; 2008. [Abstract 3.264] [Google Scholar]
- 21.Marino SE, Birnbaum AK, Leppik IE, et al. Steady-state carbamazepine pharmacokinetics following oral and stable-labeled intravenous administration in epilepsy patients: effect of race and sex. Clinical Pharmacology & Therapeutics. 2012;91:483–488. doi: 10.1038/clpt.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerardin A, Dubois JP, Moppert J, Geller L. Absolute bioavailability of carbamazepine after oral administration of a 2% syrup. Epilepsia. 1990;31:334–338. doi: 10.1111/j.1528-1157.1990.tb05384.x. [DOI] [PubMed] [Google Scholar]
- 23.Conway JM, White JR, Birnbaum AK, et al. Safety of an IV formulation of carbamazepine. Epilepsy Res. 2009;84:242–244. doi: 10.1016/j.eplepsyres.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmood I, Balian JD. Interspecies scaling: predicting clearance of drugs in humans. Three different approaches. Xenobiotica. 1996;26:887–895. doi: 10.3109/00498259609052491. [DOI] [PubMed] [Google Scholar]
- 25.Reith DM, Appleton DB, Hooper W, Eadie MJ. The effect of body size on the metabolic clearance of carbamazepine. Biopharm Drug Dispos. 2000;21:103–111. doi: 10.1002/1099-081x(200004)21:3<103::aid-bdd222>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Sakuma T, Kawasaki Y, Jarukamjorn K, Nemoto N. Sex differences of drug-metabolizing enzyme: female predominant expression of human and mouse cytochrome P450 3A isoforms. J Health Sci. 2009;55:325–337. [Google Scholar]
- 27.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 28.Cotreau M, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Johnson JA, Burlew BS. Racial differences in propranolol pharmacokinetics. Clin Pharmacol Ther. 1992;51:495–500. doi: 10.1038/clpt.1992.53. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JA. Influence of race or ethnicity on pharmacokinetics of drugs. J Pharm Sci. 1997;86:1328–1333. doi: 10.1021/js9702168. [DOI] [PubMed] [Google Scholar]
- 31.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 32.Sata F, Sapone A, Elizondo G, et al. CYP3A4 allelic variants with amino acid substitutions in exons 7 and 12: evidence for an allelic variant with altered catalytic activity. Clin Pharmacol Ther. 2000;67:48–56. doi: 10.1067/mcp.2000.104391. [DOI] [PubMed] [Google Scholar]
- 33.Lamba JK, Lin YS, Thummel K, et al. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics. 2002;12:121–132. doi: 10.1097/00008571-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Felix CA, Walker AH, Lange BJ, et al. Association of CYP3A4 genotype with treatment-related leukemia. Proc Natl Acad Sci USA. 1998;95:13176–13181. doi: 10.1073/pnas.95.22.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacKichan JJ, Zola EM. Determinants of carbamazepine and carbamazepine 10,11-epoxide binding to serum protein, albumin and alpha 1-acid glycoprotein. Br J Clin Pharmacol. 1984;18:487–493. doi: 10.1111/j.1365-2125.1984.tb02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grandison MK, Boudinot FD. Age-related changes in protein binding of drugs: implications for therapy. Clin Pharmacokinet. 2000;38:271–290. doi: 10.2165/00003088-200038030-00005. [DOI] [PubMed] [Google Scholar]
- 37.Bonneton J, Iliadis A, Genton P, Dravet C, Viallat D, Mesdjian E. Steady state pharmacokinetics of conventional versus controlled-release carbamazepine in patients with epilepsy. Epilepsy Res. 1993;14:257–263. doi: 10.1016/0920-1211(93)90050-h. [DOI] [PubMed] [Google Scholar]