Abstract
Purpose
Measuring antiepileptic drug (AED) concentrations is common practice in nursing homes. Phenytoin (PHT) concentrations fluctuate substantially in many nursing home residents under constant dose conditions; however, the stability of other AED concentrations has not been studied. We investigated the variability of carbamazepine (CBZ) and valproate (VPA) concentrations under constant dose conditions in US nursing home residents.
Methods
A database of elderly persons (≥65 years) in 119 nursing homes throughout the US was reviewed for residents with at least one measurement of total PHT, CBZ or VPA. Inclusion criteria for this study were three or more serum concentration measurements while on the same dose of CBZ or VPA, a two-month minimum stay, and no interfering co-medications (inducers or inhibitors). Enrollment occurred over a 2-year period. Data were collected on residents for a minimum of 6 months.
Key Findings
Of the 593 residents identified, 245 had CBZ or VPA concentrations measured and 44 (18%) met inclusion criteria (22 on CBZ and 22 VPA). Some subjects had little variability in AED concentrations; others had large fluctuations. Total CBZ concentrations within individuals varied as little as 0 mg/L to as much as 6.3 mg/L and total VPA concentrations as little as 10.0 mg/L to as much as 77.6 mg/L.
Significance
The variability of PHT, CBZ, and VPA concentrations in many but not all nursing home residents implies that a re-evaluation of the role of AED concentration measurements in the management of patients is needed. Strategies for use and interpretation of AED concentration measurements are discussed.
Keywords: Carbamazepine, valproate, elderly, concentrations, variability
INTRODUCTION
A major premise in the clinical practice of treating epilepsy with antiepileptic drugs (AEDs) is that drug concentrations will vary within a relatively narrow range during steady state dosing. This is true in younger adults, in whom levels of phenytoin (PHT), carbamazepine (CBZ) and valproate (VPA) vary less than 25% in compliant populations (Leppik et al. 1979; Graves et al. 1988). In a previous report we found that in elderly nursing home patients, PHT concentrations fluctuated substantially in many but not all patients who did not have a change in PHT dose and were not taking potentially interfering co-medications (Birnbaum et al. 2003). Unlike PHT, CBZ and VPA do not exhibit saturable pharmacokinetics and differ in other chemical properties. It may be presumed, then, that concentrations of these AEDs would be more stable under steady dosing conditions in the nursing home population.
In the US and Europe, 5% to 10% of NH residents are being treated with AEDs. (Cloyd et al. 1994; Garrard et al. 2000; Garrard et al. 2003; Galimberti et al. 2006; Huying et al. 2006). There are an estimated 1.5 million persons residing in US nursing homes, implying that there may be as many as 150,000 patients receiving an AED (Garrard et al. 2003). In one large study from the US, approximately 60% of the prescriptions for AEDs were for PHT, 15% for CBZ, and 9% for VPA (Garrard et al. 2007). Use patterns differ in other countries, with VPA and CBZ often used more than PHT. In one study from Germany, CBZ was 37.1%, VPA was 25.9% and PHT was 14.8% of the total AEDs prescribed (Huying et al. 2006).
The purpose of this study was to determine the extent of fluctuations CBZ and VPA concentrations of elderly persons residing in nursing homes, receiving stable doses of these AEDs, not receiving inducers or inhibitors, and having three or more measurements of total CBZ or VPA concentrations available for analysis.
METHODS
Study Population
Subjects receiving PHT, CBZ or VPA (n=593) and who were residents of 119 Beverly Enterprises, Inc. nursing homes across the US constituted the study population. Data for CBZ and VPA were used in this analysis. Results for PHT were included in an earlier report (Birnbaum et al., 2003). Residents were included if they met the following criteria: 1) resided in a nursing home for a minimum of 2 months, 2) aged ≥ 65 years, 3) not a resident of a sub-acute unit, 4) received CBZ or VPA medication for any indication, 5) had at least three total CBZ or VPA concentrations documented in the nursing home record while on the same dose for 4 weeks or more, and 6) had no interfering co-medications, such as metabolic inhibitors or inducers. The University of Minnesota’s Institutional Review Board, Human Research Protection Program’s committee, approved this study.
Data Collection
Data were collected over a 2-year period on residents who were in the nursing home for a minimum of 6 months. Data were collected by pharmacists trained and certified in data abstraction as previously described (Birnbaum et al. 2003). Data included information on AED concentrations, formulation, dosing frequency, route of administration, date and time of drug administration, indication, and co-medications (prescription and non-prescription). Other information included: sex, birth date, total body weight, and height. For evaluation of variability of concentrations, records from residents receiving CBZ but who were also taking CBZ metabolic inducers (phenobarbital, PHT, primidone, Saint John’s Wort, rifampin, rifabutin) or CBZ metabolic inhibitors (clarithromycin, desipramine, diltiazem, erythromycin, fluconazole, fluoxetine, fluvoxamine, isoniazid, itraconazole, ketoconazole, lamotrigine, metronidazole, nefazodone, omeprazole, propoxyphene, sertraline, verapamil, and VPA) were excluded. Data from patients receiving VPA but who were also taking VPA metabolic inducers (CBZ, lamotrigine, phenobarbital, PHT, primidone, rifampin, rifabutin, Saint John’s Wort) or VPA metabolic inhibitors (fluoxetine, fluvoxamine, naproxen, sertraline) were excluded.
RESULTS
A total of 593 elderly nursing home residents were entered into our database; 245 were identified as receiving CBZ and/or VPA. Of these 245 residents, 44 subjects (18%) met inclusion criteria for the variability study of three or more measurements at steady dose conditions. Twenty-two residents (77.3% women; mean age 78.3 ± 8.1 years with range of 65.2 to 97.0) were receiving CBZ. Twenty-two (63.6% women; mean age 77.6 ± 8˙0 years with range of 65.9 to 93.3) were receiving VPA. These residents had routine orders for AED concentration measurements, but doses were not changed regardless of the levels. Overall there were 750 CBZ and VPA concentrations available for evaluation.
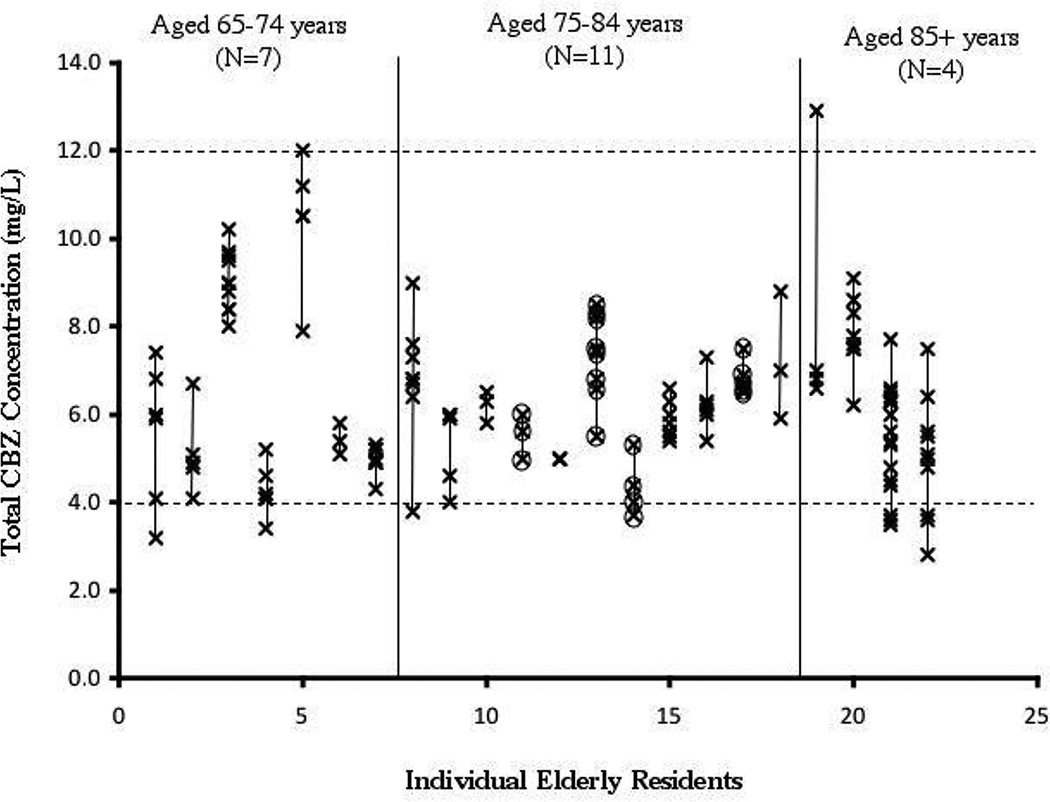
For CBZ, a majority of residents were taking this drug for seizures (Table 1). In the subset of CBZ residents who met the study criteria, there were 136 CBZ measurements, or an average of 6.2 measurements per subject with a range of 3 to 17 (Table 1). The individual total CBZ concentrations are presented in Figure 1. One resident had no difference in concentrations (all three measurements were 5.0 mg/L). The greatest difference in CBZ concentrations in the resident with the greatest variability was two-fold (6.6 mg/L to 12.9 mg/L). In this cohort, six residents had a total CBZ concentration below the suggested reference range (< 4 mg/L), and two residents had a total CBZ concentration higher (>12 mg/L). None of the residents had concentrations both below and above the reference range. There was no obvious trend of concentrations over time (Figure 3). None of the residents had a change in the formulation of CBZ during the study period. CBZ-10, 11-epoxide or unbound CBZ concentrations were not in the nursing home records and presumably had not been measured.
Table 1.
Patient demographics, dose, and concentration information for the patients included in the variability of AED analysis.
| CBZ | VPA | |
|---|---|---|
| Total numbers | 22 (17W, 5M) | 22 (13W, 9M, 1Unknown) |
| Number of Nursing Homes | 14 | 12 |
|
Mean Age (years) (±SD) (age at first blood draw) |
78.3 (8.1) | 77.6 (8.0) |
|
Mean Weight (kg) (±SD) (weight at first blood draw) |
68.6 (17.3) | 65.3 (17.8) |
| Mean Daily Dose (mg) (±SD) | 702.3 (381.9) | 1250.0 (642.0) |
|
Mean Daily Dose (mg/kg) (±SD) |
10.5 (5.4) | 18.7 (8.1) |
|
Plasma Concentration (mg/L) (±SD) |
6.3 (1.6) | 55.1 (16.6) |
|
Mean number of blood draws per person (±SD) |
6.2 (3.3) | 5.5 (2.3) |
| Indication | ||
| Seizure/epilepsy | 17 (77%) | 4 (18%) |
| Psychiatric | 2 (9%) | 17 (77%) |
| Unknown | 3 (14%) | 1 (5%) |
Figure 1. Individual Total Carbamazepine Concentrations Observed in Elderly Nursing Residents by Order of Ascending Age.
Each symbol (x) represents a single total CBZ measurement. A line connects all of the total CBZ measurements within an individual resident. Residents are segregated into age groups (65–74 years, 75–84 years, or 85+ years) according to their age at enrollment. The "therapeutic" range for CBZ is marked by a dashed line at 4 and 12 mg/L. Circled concentrations signify concentrations that were observed during administration of suspension. One subject represented by one x had no variability in 3 separate levels.
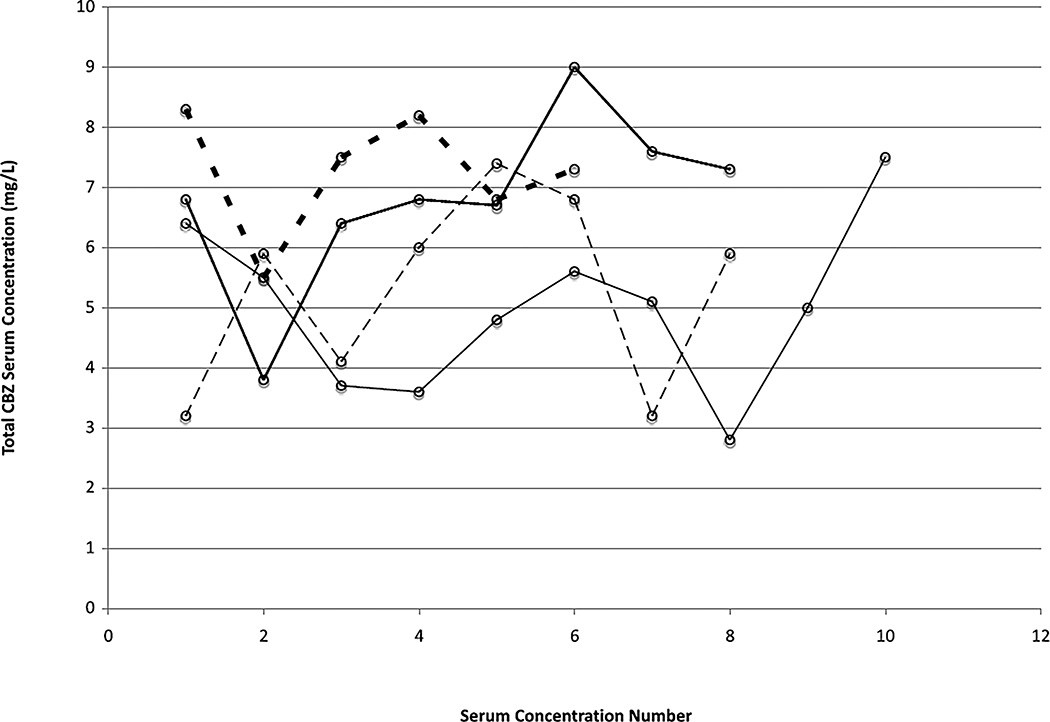
Figure 3.
Individual total CBZ serum concentrations in four residents with six or more CBZ concentrations who exhibit fluctuation. The x-axis indicates the sequential number of the CBZ concentration and shows that the variability appears to be random over time.
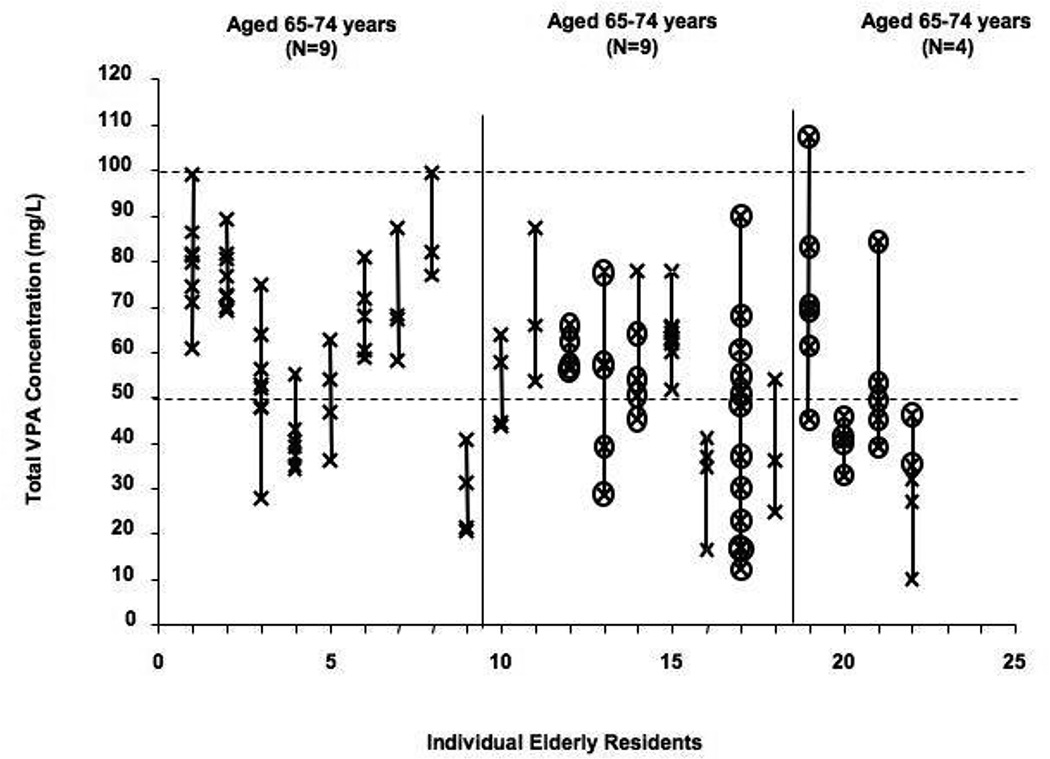
A majority of residents were taking VPA for psychiatric reasons (Table 1). In the subset of VPA residents who met the study criteria, there were 129 measurements, or an average of 5.5 measurements per subject (range 3 to 12). Each total VPA concentration from the variability analysis is presented in Figure 2. The resident with the least variability had a maximum difference in concentrations of only 10.0 mg/L. The person with the greatest difference in VPA concentrations had a six-fold difference (12.1 to 89.7 mg/L). During the study period, 14 residents had one total VPA concentration that was below the reference range (<50 mg/L); only one resident had a total VPA concentration >100 mg/L, and only one had concentrations both below and above the suggested therapeutic range. There was no obvious trend of concentrations over time (Figure 4). Two residents received more than one VPA formulation (syrup and oral solid) during the study period.
Figure 2. Total Valproic Acid Concentrations Observed in Elderly Nursing Residents by Order of Ascending Age.
Each symbol (x) represents a single total VPA measurement. A line connects all of the total VPA measurements within an individual resident. Residents are segregated into age groups (65–74 years, 75–84 years, or 85+ years) according to their age at enrollment. The "therapeutic" range for VPA is marked by a dashed line at 50 and 100 mg/L. Circled concentrations signify concentrations that were observed during administration of syrup.
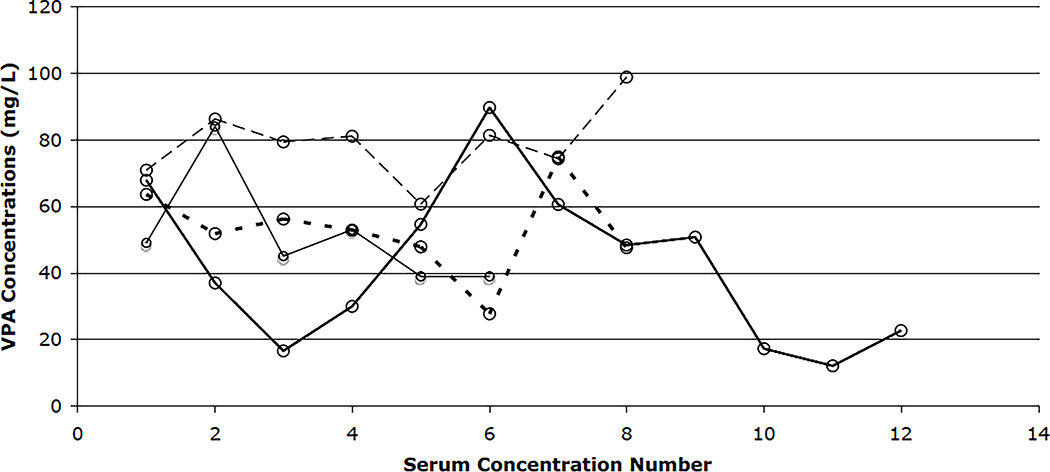
Figure 4.
Individual total VPA serum concentrations in four residents with six or more VPA concentrations who exhibit fluctuation. Each line represents a resident and connects individual total VPA concentrations. The x-axis indicates the sequential number of the total VPA concentration and shows that the variability appears to be random over time.
There were 495 AED concentrations in the database for the 201 persons receiving CBZ or VPA but not qualifying for the study, or an average of 2.3 tests per patient. Some of these had 3 or more AED measurements but did not meet the inclusion criteria because of changes in dose or the presence of interfering drugs.
Nursing administration records indicated that all doses had been given. Overall, 93.4% of the CBZ and 90.9% of the VPA samples were obtained six or more hours after the last dose was given and could be considered “trough” levels. The mean sampling time after dose for CBZ was 10.1 hours (SD±3.3) and VPA sampling was 9.4 hours (SD±3.1). Almost all samples were taken before the morning dose of these AEDs.
Discussion
In an earlier study from this database, we found that PHT concentrations fluctuated widely and unpredictably in many but not all elderly residents in NHs (Birnbaum et al., 2003). The widest variation in concentrations was an individual whose lowest PHT concentration was 9.7 mg/L and whose highest was 28.8 mg/L (Birnbaum et al. 2003). PHT is poorly water-soluble, is absorbed only in the small intestine where the pH becomes alkaline (due to PHT's high pKa), and exhibits non-linear kinetics due to saturable metabolism, with small changes in bioavailability leading to large changes in concentration. CBZ does not exhibit saturable metabolism but it does induce its own metabolism. It binds to alpha-1 acid glycoprotein (AAG) in addition to albumin. VPA is quite water-soluble, is absorbed throughout the gastrointestinal tract, and exhibits linear kinetics in regards to metabolism. Its protein binding is saturable so that total concentrations (bound and unbound) increase more slowly at lower doses than total concentrations at higher doses. Because CBZ and VPA have less complicated pharmacokinetics and different chemical properties than PHT, we hypothesized that CBZ and VPA concentrations would be more stable. However, we found that the extent of variability in total CBZ and VPA concentrations within a person was as much as 2-fold for CBZ and 6-fold for VPA.
The variability observed in this and the previous study could be due to several factors such as compliance, absorption, distribution, metabolism, and elimination differences within an individual over time, or storage conditions of the drugs. Non-compliance is a major factor for fluctuations of AED concentrations (Leppik et al. 1979; Graves et al. 1988). These patients had medications administered in a nursing home setting, and medication errors, although possible, would have had to be frequent to account for the extent of fluctuations. Daily nurse dispensing records were available and we included only persons for whom records indicated that no doses were missed seven days prior to the blood sample. Patients could have "cheeked" medications, but this is unlikely, as this behavior requires planning and effort. Non-compliance is thus not likely to be a significant factor in the variability observed. Storage conditions could be an issue; however, all medication storage conditions for an individual would be the same within a single nursing home and could not account for this degree of variability.
A population pharmacokinetic study in a larger group of residents from this nursing home population showed that VPA clearance changes are affected by the use of VPA syrup (Birnbaum et al. 2007). Thus use of a liquid formulation should be considered as a source of variability. Formulations dispensed (liquid or solid) were recorded in the nursing records for each daily dose (Figures 1 and 2). Only two of the residents with the most variable concentrations had formulation switches between oral solid and syrup. Of the residents taking VPA syrup at all time points, three exhibited the most and two the least variability. Therefore, the use of the VPA syrup formulation cannot be the sole cause of fluctuations in VPA concentrations in this population. The use of CBZ suspension showed no more variability in CBZ concentrations than the use of the oral solid. Thus, use of liquid formulations may account for some but cannot account for all of the variability. Only one resident was taking an extended release formulation of CBZ (#18, Figure 1).
Changes in metabolism within an individual over time are possible. CBZ induces its own metabolism; however, residents were already on CBZ maintenance therapy and were taking CBZ for at least four weeks. Induction of enzymes should have reached a maximum although it is possible that the induction of metabolism may have a different and less predictable time course in elderly nursing home patients than in younger adults. The few studies which have examined VPA pharmacokinetics in the elderly involved community-dwelling elderly, not nursing home residents (Bryson et al. 1983; Perucca et al. 1984; Bauer et al. 1985). In our previous study of NH residents we found that although VPA dose decreased with age, there was also a corresponding decrease in drug concentration (Birnbaum et al. 2004). Within the elderly age group, clearance values for elderly nursing home residents in our prior study were similar regardless of age (Birnbaum et al. 2004; Birnbaum et al. 2007). If metabolism decreases over time due to age, one would expect a gradual increase in concentrations over time within a person. Our results showed that over time the changes in concentrations within a person (Figures 3 and 4) are random and therefore not due to metabolism changes with aging.
Timing of sampling could have been a factor. The half-life of CBZ can be as short as 5 hours and as long as 26 hours during multiple dose administration in younger adults (Morselli 1995). In elderly nursing home residents, the half-life of VPA ranges from 6˙5 to 15˙8 hours as calculated from clearance data (Birnbaum et al. 2007). In this study the average times after dose for CBZ and VPA were 10˙1 (SD±3˙3) and 9˙4 (SD±3˙1) hours respectively. Thus the majority of the concentrations were measured post-absorption, and fluctuations due to sampling near peak concentrations were minimized.
Although CBZ is moderately bound to plasma albumin, it also binds to AAG. Non-glycated albumin, the major ligand for CBZ binding in serum, decreases and CBZ free fraction increases with age (Koyama et al. 1999). We observed no steady change with age. AAG concentrations do not change with age and CBZ binding would not be affected by this factor (Veering et al. 1990). VPA binds to plasma albumin at approximately 85% (Kober et al. 1980). Although binding could explain some of the variability, the marked degree and seeming randomness in fluctuating CBZ and VPA concentrations we observed is more than can be ascribed to changes in binding.
Changes in weight could also affect volume of distribution that could contribute to variability. Weights were available throughout the study. A majority of the VPA residents had weight changes over all visits of five kilograms or less (68.2%), with 86.4% having changes of 10 kilograms or less. Residents who were taking CBZ had higher weight changes over the study period, resulting in 63.6% of residents experiencing a 10-kilogram or less change in weight. The CBZ residents who had the greatest changes in weight were not the residents who also had larger fluctuations in CBZ concentrations.
We have now demonstrated that three major AEDs with different chemical properties, routes of metabolism, and protein binding exhibit similar profiles of fluctuation in the nursing home population. An important finding of our studies is that while some individuals exhibit large fluctuations in concentrations over time, others do not. This clearly demonstrates that the NH population is not homogenous in factors that influence drug concentrations. Indeed, there probably are major differences in the clinical condition of those who maintain stable concentrations as compared to those whose levels fluctuate. Unfortunately, this database did not contain detailed information regarding the clinical condition of the patients on the day of blood sampling.
While our studies show that levels of commonly used AEDs fluctuate widely in many patients, it raises many questions. We were unable to identify the causes and consequences of these fluctuations. These questions are being presently studied in a prospective study that includes newer AEDs: levetiracetam, gabapentin and lamotrigine. The hypothesis being evaluated in the ongoing study is that persons who fluctuate may differ medically from those who do not and that clinical characteristics may help identify fluctuators from those whose levels are stable.
Use of AED concentrations to manage therapy is a widely accepted practice and can be useful to monitor compliance, manage drug interactions, and guide therapy. The use of AED measurements in the nursing home is problematic. To date the best advice based on very limited evidence is that obtaining a single blood sample for PHT, CBZ, or VPA should be interpreted with caution. It may be useful to obtain or examine (i.e., using historical samples) several concentrations in an individual to establish if they are prone to fluctuating blood concentrations. Interpretation of AED measurements in those who do not exhibit large fluctuations may be comparable to community dwelling elderly or younger adults; however, the interpretation of AED concentrations in residents who exhibit large fluctuations in AED concentrations may need to be reconsidered. This also illustrates the importance of obtaining AED concentration measurements in nursing home residents under conditions that will ensure the lowest variability possible in that individual (i.e., after steady-state is achieved, at the same time of day and time after dose, verification of possible interfering co-medication). Changes in dose should be monitored by serial levels to determine if a person is a fluctuator. However, until the causes of fluctuations can be determined, management of these patients must depend on clinical judgment. At this time there is no evidence to indicate that the newer AEDs are less prone to fluctuations.
Our findings have significant implications for drugs other than the AEDs. No systematic study of serial concentrations for drugs used to treat other neurological or medical conditions in the nursing home has been performed. It is unlikely that PHT, CBZ and VPA are the only ones that fluctuate. Our study suggests that drugs used for conditions other than epilepsy need to be studied and that major changes in practice and drug development may be required.
Acknowledgments
This project was supported by grants P50NS16308 from the National Institute of Neurological Disorders and Stroke (NINDS), 5R01AG026390 from the National Institute on Aging (NIA), and the University of Minnesota Undergraduate Research Opportunities Program (MAS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors has any conflicts of interest relevant to this research activity.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Bauer LA, Davis R, Wilensky A, Raisys V, Levy RH. Valproic acid clearance: unbound fraction and diurnal variation in young and elderly adults. Clin Pharmacol Ther. 1985;37:697–700. doi: 10.1038/clpt.1985.116. [DOI] [PubMed] [Google Scholar]
- Birnbaum A, Hardie N, Leppik I, Conway JM, Bowers SE, Lackner T, Graves NM. Variability of total phenytoin serum concentrations within elderly nursing home residents. Neurology. 2003;60:555–559. doi: 10.1212/01.wnl.0000052997.43492.e0. [DOI] [PubMed] [Google Scholar]
- Birnbaum AK, Ahn JE, Brundage RC, Hardie NA, Conway JM, Leppik IE. Population pharmacokinetics of valproic acid concentrations in elderly nursing home residents. Ther Drug Monit. 2007;29:571–575. doi: 10.1097/FTD.0b013e31811f3296. [DOI] [PubMed] [Google Scholar]
- Birnbaum AK, Hardie NA, Conway JM, Bowers SE, Lackner TE, Graves NM, Leppik IE. Valproic acid doses, concentrations, and clearances in elderly nursing home residents. Epilepsy Res. 2004;62:157–162. doi: 10.1016/j.eplepsyres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Bryson SM, Verma N, Scott PJ, Rubin PC. Pharmacokinetics of valproic acid in young and elderly subjects. Br J Clin Pharmacol. 1983;16:104–105. doi: 10.1111/j.1365-2125.1983.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd JC, Lackner TE, Leppik IE. Antiepileptics in the elderly. Pharmacoepidemiology and pharmacokinetics. Arch Fam Med. 1994;3:589–598. doi: 10.1001/archfami.3.7.589. [DOI] [PubMed] [Google Scholar]
- Galimberti CA, Mazzucchelli I, Arbasino C, Canevini MP, Fattore C, Perucca E. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569–1572. doi: 10.1111/j.1528-1167.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- Galimberti CA, Magri F, Magnani B, et al. Antiepileptic drug use and epileptic seizures in elderly nursing home residents: a survey in the province of Pavia, Northern Italy. Epilepsy Res. 2006;68:1–8. doi: 10.1016/j.eplepsyres.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Garrard J, Cloyd J, Gross C, Hardie N, Thomas L, Lackner T, Graves N, Leppik IE. Factors associated with antiepileptic drug use among elderly nursing home residents. J Gerontol A Biol Sci Med Sci. 2000;55:M384–M392. doi: 10.1093/gerona/55.7.m384. [DOI] [PubMed] [Google Scholar]
- Garrard J, Harms S, Hardie N, Eberly LE, Nitz N, Bland P, Gross CR, Leppik IE. Antiepileptic drug use in nursing home admissions. Ann Neurol. 2003;54:75–85. doi: 10.1002/ana.10593. [DOI] [PubMed] [Google Scholar]
- Garrard J, Harms SL, Eberly LE, Leppik IE. Use of antiepileptic medications in nursing homes. Int Rev Neurobiol. 2007;81:165–182. doi: 10.1016/S0074-7742(06)81010-X. [DOI] [PubMed] [Google Scholar]
- Graves NM, Holmes GB, Leppik IE. Compliant Populations: Variability in Serum Concentrations. In: Schmidt D, Leppik IE, editors. Compliance in Epilepsy. New York: Elsevier; 1988. pp. 91–101. [PubMed] [Google Scholar]
- Huying F, Klimpe SKW. Antiepileptic drug use in nursing home residents: A cross-sectional, regional study. Seizure. 2006;15:194–197. doi: 10.1016/j.seizure.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kober A, Olsson Y, Sjoholm I. Binding of drugs to human serum albumin. XIV. The theoretical basis for the interaction between phenytoin and valproate. Mol Pharmacol. 1980;18:237–242. [PubMed] [Google Scholar]
- Koyama H, Sugioka N, Uno A, Mori S, Nakajima K. Age-related alteration of carbamazepine-serum protein binding in man. J Pharm Pharmacol. 1999;51:1009–1014. doi: 10.1211/0022357991773474. [DOI] [PubMed] [Google Scholar]
- Leppik IE, Cloyd JC, Sawchuk RJ, Pepin SM. Compliance and variability of plasma phenytoin levels in epileptic patients. Ther Drug Monit. 1979;1:475–483. [Google Scholar]
- Morselli PL. Carbamazepine: absorption, distribution, and excretion. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic Drugs. New York: Raven Press, Ltd.; 1995. pp. 515–528. [Google Scholar]
- Perucca E, Grimaldi R, Gatti G, Pirracchio S, Crema F, Frigo GM. Pharmacokinetics of valproic acid in the elderly. Br J Clin Pharmacol. 1984;17:665–669. doi: 10.1111/j.1365-2125.1984.tb02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veering BT, Burm AG, Souverijn JH, Serree JM, Spierdijk J. The effect of age on serum concentrations of albumin and alpha 1-acid glycoprotein. Br J Clin Pharmacol. 1990;29:201–206. doi: 10.1111/j.1365-2125.1990.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]