Abstract
Carotid bodies play a critical role in protecting against hypoxemia and their activation increases sympathetic activity, arterial pressure and ventilation, responses opposed by acute stimulation of the baroreflex. While chemoreceptor hypersensitivity is associated with sympathetically-mediated hypertension, the mechanisms involved and their significance in the pathogenesis of hypertension remains unclear. We investigated the chronic interactions of these reflexes in dogs with sympathetically-mediated, obesity-induced hypertension based on the hypothesis that hypoxemia and tonic activation of carotid chemoreceptors may be associated with obesity. After 5 weeks on a high-fat diet, the animals experienced a 35–40% weight gain, increases in arterial pressure from 106±3 to 123±3 mm Hg and respiratory rate from 8±1 to 12±1 breaths/min along with hypoxemia (PaO2= 81±3 mm Hg) but eucapnia. During 7 days of carotid baroreflex activation by electrical stimulation of the carotid sinus, tachypnea was attenuated and hypertension was abolished before these variables returned to pre-stimulation values during a recovery period. Following subsequent denervation of the carotid sinus region, respiratory rate decreased transiently in association with further sustained reductions in PaO2 (to 65±2 mm Hg) and substantial hypercapnia. Moreover, the severity of hypertension was attenuated from 125±2 to 116±3 mm Hg (45–50% reduction). These findings suggest that hypoxemia may account for sustained stimulation of peripheral chemoreceptors in obesity and that this activation leads to compensatory increases in ventilation and central sympathetic outflow that contributes to neurogenically-mediated hypertension. Furthermore, the excitatory effects of chemoreceptor hyperactivity are abolished by chronic activation of the carotid baroreflex.
Keywords: hypertension, blood pressure, baroreflex, sympathetic nervous system, obesity, carotid bodies, hypoxemia
Introduction
Stimulation of peripheral chemoreceptors by hypoxemia plays a key role in the reflex regulation of respiration.1 In addition to raising the arterial partial pressure of oxygen (PaO2) by increasing minute ventilation, activation of the carotid bodies also increases sympathetic outflow. Based on exaggerated increases in sympathetic activity, arterial pressure, and ventilation to chemoreceptor stimulation by hypoxia2–3 and to normalization of these variables by deactivation of chemoreceptors by hyperoxia,4–7 studies in the spontaneous hypertensive rat (SHR) and in patients with primary hypertension support the speculation that tonic increases in peripheral chemoreceptor activity may contribute to sympathetically-mediated hypertension. However, the mechanisms that account for the development of carotid body hyperactivity and the potential for this in contributing to the sustained sympathetic activation that is prevalent in primary and resistant hypertension remain unclear.
Recent experimental studies and clinical trials have led to a resurgence of interest in the hypothesis that carotid bodies may drive the neurogenic component of some forms of hypertension.1,8 Chronic studies in the SHR support the hypothesis that chemoreceptor hyperactivity may contribute to sustained increases in sympathetic activity, including renal sympathetic activity (RSNA), that lead to hypertension.7,9 In these studies, denervation of the carotid bodies (CBD) by bilateral carotid sinus nerve ligation produced sustained suppression of heightened RSNA and attenuated both the development and maintenance of hypertension in the SHR. These findings are of particular interest given the clinical need for non-pharmacological approaches for the treatment of resistant hypertension. This, in addition to the potential translational significance of these observations in the SHR has led to ongoing proof-of-concept clinical trials designed to evaluate the antihypertensive efficacy of unilateral carotid body resection in this patient population with a high risk for morbidity and mortality (clinicaltrials.gov identifier: NCT1745172, NCT02099851).
Activation of the arterial baroreflex inhibits sympathetic activity and lowers arterial pressure, responses opposite to those produced by stimulation of the carotid bodies. Furthermore, in work by Heistad et al.10–11 and Somers and associates12 acute activation of the baroreflex has been shown to diminish the cardiovascular and ventilatory responses seen with stimulation of peripheral chemoreceptors. However, it remains unknown whether the baroreflex can chronically oppose the effects of peripheral chemoreceptor activation. One technique for evaluating the potential chronic role of the baroreflex in cardiovascular homeostasis uses electrical stimulation of the carotid baroreflex, which provides a non-pharmacological method for chronically suppressing sympathetic activity and lowering arterial pressure. Using this technology, significant antihypertensive responses have been reported in both experimental animals and in patients with resistant hypertension.13–17
Obesity is common in resistant hypertension and likely contributes to the prevailing sympathetic activation and hypertension.13–15,18–20 However, the afferent mechanisms that account for sympathetic overactivity in obesity are incompletely defined. Since increased metabolic rate and impaired respiratory mechanics are prevalent in obesity,21–24 it is possible that chronic hypoxemia may provide a tonic drive for chemoreflex activation and subsequent sympathoexcitation in obesity. Thus, we hypothesized that hypoxemia and ensuing peripheral chemoreceptor hyperactivity are sustained responses in a canine model of obesity-induced hypertension that mimics many of the hemodynamic, neurohormonal, renal, and metabolic changes associated with obesity hypertension in humans.20,25–26 We further hypothesized that the excitatory effects of heightened chemoreceptor activation that lead to increased arterial pressure and respiratory rate would be attenuated by chronic electrical stimulation of the carotid baroreflex and finally that CBD would diminish chemoreceptor driven increases in respiratory rate and arterial pressure.
Methods
Animal Preparation
All procedures were performed in accordance with National Institutes of Health (NIH) Guidelines and approved by the Institutional Animal Care and Use Committee. Surgical procedures were conducted under isoflurane anesthesia (1.5–2.0%) after pre-medication with acepromazine (0.15 mg/kg, sq) and induction with thiopental (10mg/kg, sq). Carprofen (Rimadyl), 4mg/kg, was administered for 3 days postoperatively for analgesia. The specific surgical procedures for implantation of catheters and carotid sinus electrodes (Barostim neo system) and for denervation of carotid bodies are described in the online-only Data Supplement. It should be noted that the procedure we used for used for CBD by stripping the nerves in the carotid sinus area eliminates baroreceptor as well as chemoreceptor input into the central nervous system. General methods for experimentation during the development and maintenance of obesity-hypertension are also described in the online-only Data Supplement. Experiments were conducted in four male dogs weighing 23–26 kg.
Experimental Protocol
During the control period (the days immediately preceding fat feeding) and on the last 2 days of each protocol (see below), blood samples (~10 ml) were taken from one of the two arterial catheters. Arterial pressure was sampled continuously at 100 samples/s, 24-hours/day, using a Power Lab data-acquisition system (ADInstruments) and displayed and recorded on a computer for subsequent analysis.27 The daily values for mean arterial pressure (MAP) and heart rate were averaged between 11:30 AM and 7:30 AM.
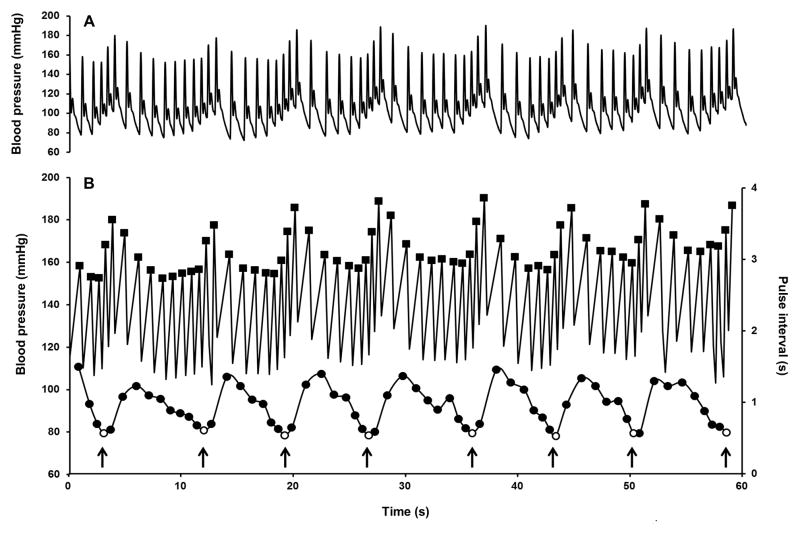
Estimation of respiratory rate was based on respiratory sinus arrhythmia (please see online-line Data Supplement for more detailed explanation). Since the heart period oscillates in synchrony with respiration, generating the phenomenon of respiratory sinus arrhythmia,28 respiration rate was estimated by counting the number of minima in the pulse interval time series every minute (corresponding to the periodic increase in heart rate during inspirations). Using a specially designed VBA® macro programing software and Excel® (Microsoft, WA), 18 h long segments originating from the blood pressure signal recorded continuously and sampled at 100 Hz were subjected to 3 consecutive moving window filters to extract successively local maxima (systolic blood pressure and dicrotic notch, first filter) and to generate time series of systolic blood pressure (second filter) and pulse interval (time difference between 2 successive peaks) and finally respiratory rate (third filter) (Figure 1). Respiratory oscillations that extended over two consecutive minute intervals were counted only once and finally a 3-point moving average smoothing function was applied to the daily time series.
Figure 1.
Representative 60 second recordings illustrating the method for estimation of respiratory rate by successive application of moving windows filters: A) Original recording of blood pressure in one dog sampled at 100 Hz; B) Upper panel: systolic blood pressure (■, first and second filter); Lower panel, pulse interval (●) with respirations (○ third filter). Arrows indicate detected breaths.
Summary of Protocols:
Control (days −2–0)
Days 1–35, high fat, (Developmental phase of obesity hypertension)
-
Days 36–70, reduced fat (Established phase of obesity hypertension)
Days 36–40, reduced fat
Days 41–47, baroreflex activation (7 days)
Days 48–56, recovery (9 days)
Day 57, bilateral CBD
Day 70, end of study (14 days after CBD)
During the first two days of baroreflex activation (days 41–42), the pulse generator was programed to deliver a continuous train of constant current impulses using the following parameters: 3–6 mA, 30 Hz, and 0.5 ms pulse duration. The intensity of activation was selected by adjusting the current to target a reduction in arterial pressure from hypertensive to control levels. To achieve this goal, small adjustments in current were needed during the first 48 hours, but no changes in the intensity of activation were made after the first 48 hours of stimulation.
Analytical Methods
Arterial blood gas samples were analyzed immediately on a blood-gas analyzer (ABL80 FLEX, Radiometer). All other blood samples were placed on ice, centrifuged, and the plasma stored at −80 C until analysis (with the exception of plasma protein concentration, which was measured before freezing). Plasma renin activity (PRA) and plasma levels of aldosterone, cortisol, and insulin were measured by radioimmunoassay in the Departmental Core facility.25–26,29 Plasma norepinephrine (NE) concentration was measured by high-performance liquid chromatography with electrochemical detection in the laboratory of Dr. David S.Goldstein.30 Plasma glucose concentration was measured with the glucose oxidation method.25–26 Standard techniques were used to measure hematocrit and the plasma concentrations of sodium, potassium, and protein.25–26,29
Breathing Rate Response to Baroreflex Activation in Nonobese Dogs
We also determined the effect of chronic baroreflex activation on respiratory rate in 6 nonobese normotensive dogs included in one of our recent studies.31 In this earlier study, there was a targeted and sustained reduction in MAP of ~15 mmHg during baroreflex activation.
Statistical Analyses
Results are expressed as means ± SE. One-way repeated-measures ANOVA, followed by the Holm-Sidak test for multiple comparisons (Prism 6.05, GraphPad Software), was used to compare the following experimental periods: 1) last 3 days of established obesity preceding baroreflex activation (days 38–40) vs. control (mean of days −2–0); 2) baroreflex activation (days 41–47) vs. baseline of established obesity (mean of days 39–40); 3) carotid body denervation (days 61–70) vs. recovery after baroreflex activation (mean of days 55–56). Statistical significance was considered to be P < 0.05.
Results
Developmental Phase of Obesity Hypertension
The hemodynamic, neurohormonal, and metabolic responses during the developmental phase of obesity were similar to those reported previously25–26 and are illustrated, in part, in Table 1. Further description of these changes is presented in the online-only Data Supplement. Respiratory rate increased from 8±1 to 12±1 breaths/ min with weight gain, a new finding not previously reported in this model of obesity hypertension.
Table 1.
Neurohormonal and Metabolic Responses to Baroreflex Activation and Carotid Body Denervation
| CONDITION | PRA (ng ANG I/mL/hr | PALDO (ng/dL) | PCORT (μg/dL) | PNE (pg/mL) | PINS (μU/mL) | PGLU (mg/dL) | BW (kg) |
|---|---|---|---|---|---|---|---|
| CONTROL | 1.0±0.1 | 5.5±0.8 | 2.0±0.4 | 121±36 | 8.2±0.8 | 108±5 | 24.7±0.3 |
| HIGH-FAT | 2.6±0.6 | 7.4±1.5 | 2.5±0.5 | 132±23 | 20.1±2.5* | 93±5 | 33.8±1.2 |
| REDUCED-FAT | 1.7±0.5 | 5.6±0.5 | 2.9±0.8 | 139±23 | 17.2±2.3* | 106±3 | 33.7±1.4 |
| BA | 1.0±0.3† | 4.3±0.6 | 2.5±0.4 | 83±8*† | 16.0±1.2* | 107±4 | 33.7±1.1 |
| RECOVERY | 1.2±0.2 | 4.6±0.4 | 2.0±0.6 | 112±15 | 17.4±2.7* | 110±3 | 33.6±1.2 |
| CBD | 1.2±0.2 | 5.0±0.6 | 2.3±0.3 | 110±4 | 19.7±2.8* | 106±2 | 33.7±1.1 |
Values are mean±SE: n=4. PRA indicates plasma renin activity; PALDO plasma aldosterone concentration; PCORT, plasma cortisol concentration; PNE, plasma norepinephrine concentration; PINS, plasma insulin concentration; PGLU, plasma glucose concentration. BA, baroreflex activation; CBD, carotid body denervation.
P<0.05 vs control,
P<0.05 vs reduced fat.
Established Phase of Obesity Hypertension
During the transition from high to reduced fat intake (days 36–40), there were no significant changes in sodium balance, arterial pressure, respiratory rate, or in any other measured variable including those listed in Table 1.
Responses to Baroreflex Activation
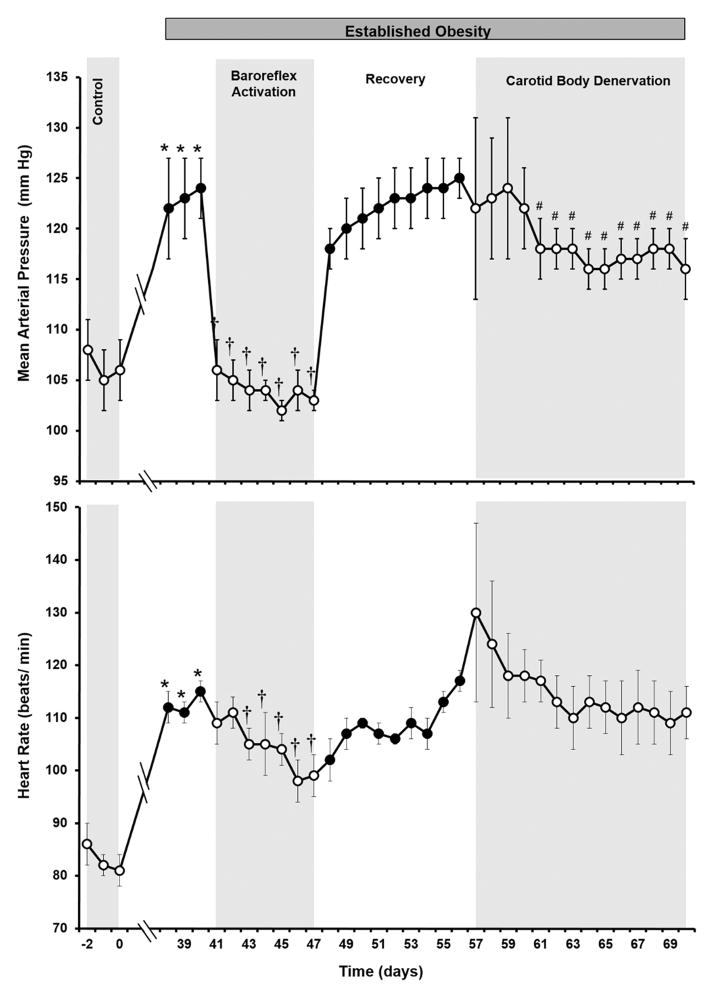
As illustrated in Figures 2 and 3, baroreflex activation completely abolished the hypertension and greatly diminished the tachycardia and tachypnea associated with weight gain. Further, the lowering of arterial pressure with baroreflex activation occurred concurrently with significant reductions in both plasma NE concentration and PRA (Table 1), consistent with previous observations in obese dogs.25–26 As reported previously,25–26 there were no significant changes in body weight or sodium balance during baroreflex activation when compared to pre-stimulation values (day 40). Other than the reductions in PRA and plasma NE concentration, there were no significant changes in the plasma levels of any measured hormones, glucose, electrolytes or in hematocrit. After terminating baroreflex activation, all of the above measures returned to values that were not significantly different from the pre-stimulation levels on day 40.
Figure 2.
Mean arterial pressure and heart rate responses to prolonged baroreflex activation and carotid body denervation in dogs with established obesity-induced hypertension. Values are mean±SE (n=4). *P<0.05 vs lean control; †P<0.05 vs days 38–40 of obesity; #P<0.05 vs recovery days 55–56 (immediate 4 postoperative days following CBD, days 57–60, excluded from analysis).
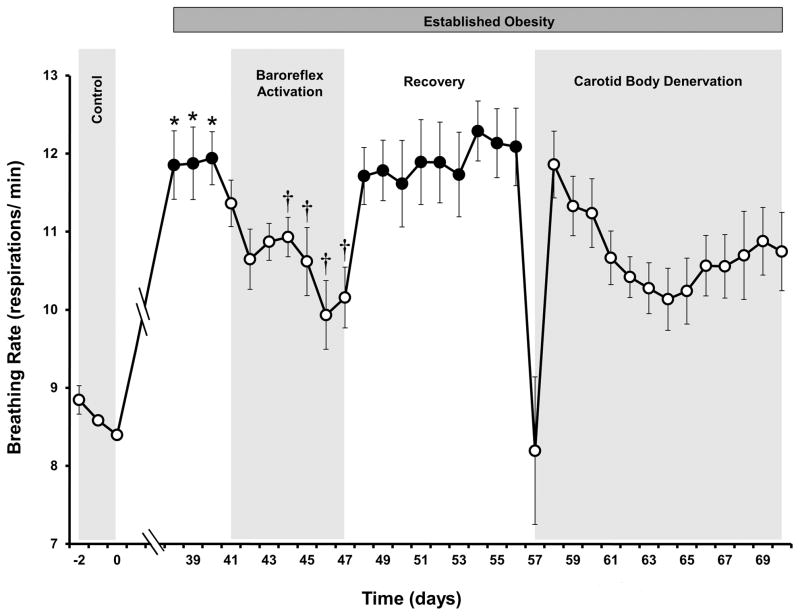
Figure 3.
Breathing rate response to prolonged baroreflex activation and carotid body denervation in dogs with established obesity-induced hypertension. Values are mean±SE (n=4). *P<0.05 vs lean control; †P<0.05 vs days 38–40 of obesity; #P<0.05 vs recovery days 55–56 (immediate 4 postoperative days following CBD, days 57–60, excluded from analysis).
Responses to CBD
Respiratory rate decreased from 12±1 to 8±1 breaths/ min during the ~18 hour postoperative period following CBD but on subsequent days returned to levels not significantly different from the elevated pre-operative values associated with obesity (Figure 3). During the initial 4 postoperative days, there was fluid accumulation in the neck, resulting in occasional altered respirations and labored swallowing. These effects were largely subsided by day 5 postoperatively. To eliminate the potential influence of these untoward postoperative effects on the analysis for arterial pressure and heart rate, the initial 4 postoperative days were excluded from statistical evaluation of these variables. MAP on days 5–14 following CBD was significantly reduced when compared to the immediate preoperative hypertensive values (Figure 2). By day 14 of CBD, MAP was reduced from 125±2 to 116±3 mmHg. Based on control values of 105–106 mmHg, this represents ~ a 45–50% reduction in the severity of obesity-induced hypertension. Following CBD, there were no significant changes in heart rate or in any other measured variables with the exception of blood gases.
As indicated in Table 2, prior to CBD values for PaCO2 and pH in obesity were comparable to historical values in nonobese dogs.32–34 In marked contrast, PaO2 was substantially depressed. Furthermore, following CBD, there were further substantial reductions in PaO2 and a considerable increase in PaCO2, consistent with successful denervation of the carotid bodies.32
Table 2.
Arterial Blood Gas Determinations in Lean and Obese Dogs Before and after CBD.
| Condition | PaO2 (mmHg) | PaCO2 (mmHg) | pH |
|---|---|---|---|
| Control | 100±1 | 40±0.3 | 7.38±0.01 |
| Obesity | 81±3 | 39±0.3 | 7.39±0.01 |
| Obesity+CBD | 65±2* | 53±0.6* | 7.37±0.03 |
Values are mean±SE. Values in lean control dogs taken from Haskins et al.32, n=97. Values in obesity and obesity + CBD, n=4.
P<0.05, Obesity + CBD vs Obesity. PaO2, arterial partial pressure O2, PaCO2, arterial partial pressure CO2.
Breathing Rate Response to Baroreflex Activation in Nonobese Dogs
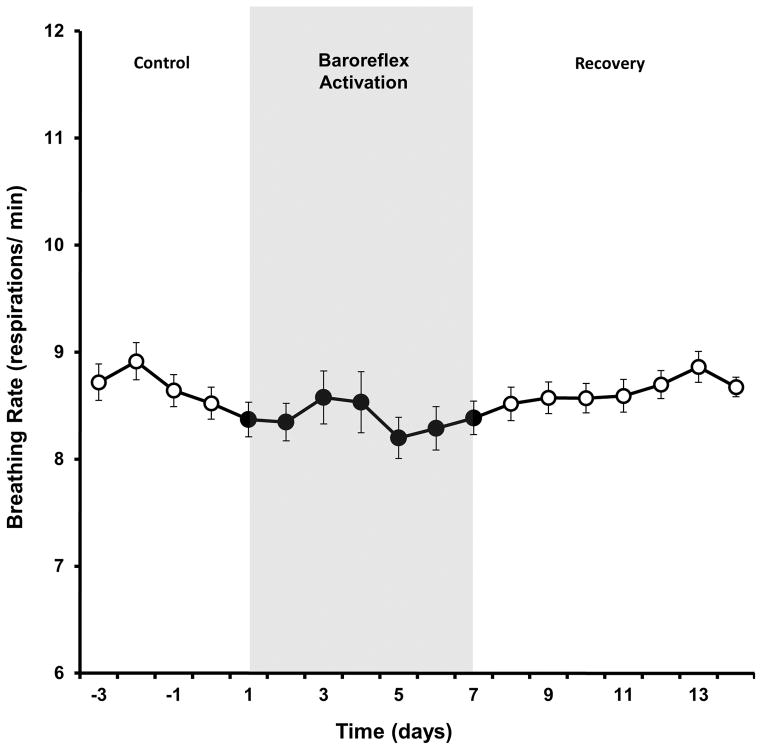
Despite chronically suppressing sympathetic activity, arterial pressure and heart rate,31 chronic baroreflex activation did not lower respiratory rate in normotensive nonobese dogs (Figure 4).
Figure 4.
Breathing rate response to prolonged baroreflex activation in lean dogs. Values are mean±SE (n=6). There were no significant changes in breathing rate.
Discussion
While significant progress has been made in understanding the mechanisms whereby increased efferent sympathetic activity leads to obesity hypertension, the determinants of this heightened sympathetic activity in obesity remain uncertain. In this regard, there are several novel findings in this study. The significant and sustained antihypertensive effects of CBD, an especially important new finding, supports the hypothesis that hyperactivity of carotid body chemoreceptors contributes to the increase in arterial pressure in this experimental model of obesity hypertension. Additionally, these findings implicate hypoxemia as a likely primary stimulus that increases chemoreceptor activity with weight gain. Finally, a most impressive new observation was that chronic activation of the baroreflex has a powerful effect to counteract the central effects of chemoreceptor stimulation in obesity that lead to sympathoexcitation, hypertension, and tachypnea.
Carotid Body Hyperactivity in Hypertension
Based on exaggerated sympathetic3 and ventilatory2 responses to acute activation of chemoreceptors by hypoxia in hypertensive patients, studies conducted several decades ago led to the speculation that chemoreceptor hyperactivity may contribute to forms of hypertension that are sympathetically-mediated. This possibility is supported by additional acute observations showing that deactivation of carotid bodies with hyperoxia decreases efferent postganglionic muscle sympathetic nerve activity6 and arterial pressure5 in patients with essential hypertension, and RSNA and arterial pressure7 and ventilation4 in the SHR, responses that do not occur in the respective normotensive controls. However, until recently, the relevance of these acute observations to the pathogenesis of hypertension has not been critically tested.
The results of more recent studies strongly support the hypothesis that tonic activation of carotid chemoreceptors contribute to both the initiation and maintenance of hypertension in the SHR by increasing sympathetic activity. In a seminal study by Abdala et al. denervation of carotid chemoreceptors by sectioning the carotid sinus nerves bilaterally in pre-hypertensive SHRs prevented the full development of hypertension and appreciably attenuated established hypertension when the denervation was conducted in adult animals.9 In a follow-up investigation conducted in the adult SHR, McBryde et al. demonstrated that the chronic antihypertensive effects of bilateral carotid sinus ligation were associated with sustained suppression of RSNA,7 a response expected to lead to a chronic reduction in arterial pressure by increasing renal excretory function. Despite the significance of these studies, the mechanism for tonic chemoreceptor activation in hypertension was not identified.
Hypoxemia Drives Carotid Body Activation in Obesity Hypertension
Given the above observations in the SHR, we hypothesized that tonic stimulation of the carotid bodies may contribute to obesity hypertension. This hypothesis was based on the premise that hypoxemia, the primary physiological stimulus for peripheral chemoreceptor activation, drives carotid body hyperactivity in obesity. The rationale for this is based on the observation that obesity is associated with increases in metabolic rate and oxygen consumption along with impaired respiratory mechanics. Excessive deposition of adipose tissue around the thoracic cage and in the abdomen limit lung expansion during inspiration and reduce lung volumes, especially expiratory reserve volume and functional reserve capacity.21–24 These changes in lung volume cause gas trapping and small airway narrowing/closure during expiration, leading to ventilation-perfusion mismatch, and reduced PaO2.21–24 Hypoxemia may be an underappreciated outcome of these abnormalities in obese patients because arterial oxygen hemoglobin saturation rather than PaO2 is the more commonly measured variable. More specifically, arterial hemoglobin oxygen saturation may remain within the normal range despite modest but significant hypoxemia, as reflected by the plateau region of the oxyhemoglobin dissociation curve. However, despite impaired ventilation and greater CO2 production, most obese patients remain eucapnic due to compensatory increases in respiratory rate.21–24 The findings of hypoxemia (PaO2=81±3 mmHg) and eucapnia (PaCO2=39±0.3 mmHg) in the present study, in combination with increased respiratory rate, are consistent with these clinical observations. In a study conducted in 97 nonobese dogs, Haskins et al.33 reported that PaO2 and PaCO2 were 100±1 mmHg and 40±1 mmHg, respectively (see Table 2), values comparable to those reported by others in resting lean dogs.32,34 Although we did not measure blood gases during the control period prior to fat feeding, based on these historical measurements in nonobese dogs, we feel confident that the measured values for PaO2 and PaCO2 in this study during obesity reflect the occurrence of eucapnic hypoxemia. Therefore, these findings support our supposition that chronic hypoxemia is a likely stimulus for tonic activation of carotid bodies in obesity.
Baroreflex Activation Abolishes Obesity Hypertension and Attenuates Tachypnea
Studies in experimental animals and in human subjects have demonstrated that acute pressure-induced activation of the baroreflex attenuates chemoreceptor-mediated increases in sympathetic activity, peripheral resistance, and ventilation.10–12 The opposing effects of baroreceptor and chemoreceptor afferent input have been shown to include actions on the neurons within the nucleus tractus solitarius, the central site of termination of these afferent fibers.35 However, it is unclear whether the acute antagonism between the baroreflex and peripheral chemoreflex is relevant to long-term control of cardiovascular function. The ability to achieve chronically controlled increases in baroreceptor afferent activity by electrical stimulation of the carotid sinus provides a unique experimental approach to evaluate the long-term implications of these acute observations. The sustained suppression of sympathetic activity and concomitant abolition of obesity hypertension by baroreflex activation in the present study emphasizes the importance of increased sympathetic activity in mediating this form of hypertension. Furthermore, in the context of the present study identifying increased peripheral chemoreflex activation as a stimulus that contributes to obesity hypertension, these findings are consistent with the hypothesis that one component of sustained baroreflex-mediated sympathoinhibition includes reduced chemoreceptor-mediated sympathoexcitatory drive through the central interactions of these reflexes.35–36 It is also possible that baroreflex activation may diminish the tonic excitatory effects of peripheral chemoreceptor activity in obesity by reducing sympathetic tone to the arterioles perfusing the carotid bodies. This would increase blood flow and suppress chemoreceptor activation by raising PaO2 locally in the vicinity of the glomus cells or by reducing carotid body hyperactivity by other flow dependent mechanisms.1
Given that metabolic rate is increased in obesity, the finding of increased respiratory rate in this model of obesity hypertension is a new but rather predictable finding. A more significant novel observation was that the tachypnea of obesity was attenuated by baroreflex activation, while in nonobese dogs no effect on respiratory rate was seen despite appreciable baroreflex-induced blood pressure lowering and bradycardia (Figure 4). This suggests that the long-term antagonism between the baroreceptors and chemoreceptors on ventilation occurs only at heightened levels of chemoreceptor activation. In this regard it is relevant that activation of carotid chemoreceptor afferent fibers by electrical stimulation of the carotid sinus nerve may increase respiration and arterial pressure,36–38 changes opposite to those observed during carotid sinus stimulation in the obese dogs in the present study. However, despite carotid body afferents being in close proximity to carotid baroreceptors, there are no clinically relevant respiratory changes in hypertensive subjects with normal minute ventilation during electrical stimulation of the carotid sinus at intensities that reduce arterial pressure.39 Thus, electrical stimulation of the carotid sinus appears to selectively activate carotid baroreceptors without costimulation of neighboring chemoreceptors. The present study does not provide any insight into whether the reduction in respiratory rate in obesity during carotid sinus stimulation reflects baroreflex-mediated antagonism of the chemoreflex by actions in the brainstem, an effect of baroreflex activation to diminish the hypoxic stimulation of carotid chemoreceptors by increasing carotid body perfusion, or some other response not readily apparent from the data on hand.
Denervation of Carotid Bodies Attenuates Obesity Hypertension
To investigate the role of carotid bodies in the maintenance of obesity hypertension, we abolished their central input by stripping the area around the carotid sinus rather than ligating the carotid sinus nerves. Because both approaches abolish central input from carotid baroreceptors as well carotid chemoreceptors, elimination of carotid baroreflex-mediated inhibition of central sympathetic outflow may attenuate the reduction in sympathetic activity and the attendant fall in blood pressure following denervation of overactive carotid chemoreceptors. Despite the potential of this approach for underestimating the contribution of the carotid bodies to the hypertension, the hypertension in obese dogs was still reduced 45–50% following CBD. Therefore, this appreciable antihypertensive response to CBD indicates that tonic activation of carotid chemoreceptors contributes substantially to the neurogenically-mediated hypertension associated with weight gain. Additionally, the time course of this antihypertensive response to CBD, beginning 4–5 days postoperatively, was similar to that reported in the SHR where there were parallel reductions in arterial pressure and RSNA after bilateral carotid sinus denervation.7,9 Accordingly, because bilateral renal denervation abolishes obesity hypertension in dogs, but does not lower arterial pressure in lean normotensive canines,26,40 it is likely that suppression of renal sympathetic outflow played a key role in mediating the antihypertensive response to CBD in the present study. Finally, it is relevant that denervation of the carotid sinus or ligation of the carotid sinus nerves does not lead to chronic changes in arterial pressure in normotensive, nonobese rats or dogs.7,9,32,41–42 Thus, in contrast to the contribution of hyperactive carotid chemoreceptors to the hypertension of obesity, the central input from the carotid bodies in normotensive subjects, in the absence of hypoxemic activation, has no long-term influence on arterial pressure.
Although the direct effect of peripheral chemoreceptor activation includes a vagally-mediated bradycardia,1,43 heart rate did not increase after CBD, consistent with the findings in SHRs with established hypertension.7,9 Because decreased vagal tone mediates the tachycardia of obesity,16,44–45 any influence on heart rate from sustained activation of the carotid chemoreflex is likely overridden by this primary mechanism.
The failure to measure significant reductions in plasma NE concentration after denervation of carotid chemoreceptors (Table 1) does not discount the probability that the antihypertensive response to carotid sinus denervation is mediated by suppression of sympathetic activity. For a number of reasons discussed by others,19 it is well established that measurement of plasma NE concentration is not sufficiently sensitive to capture relatively small, but physiologically significant changes in overall sympathetic activity. This is evident by the absence of statistically significant increases in plasma levels of NE during the development of obesity hypertension in the present study (Table 1) or in obese subjects when compared to lean controls.46 Nonetheless, circulating levels of NE fell significantly during the pronounced suppression of central sympathetic outflow and arterial pressure during baroreflex activation. This response to baroreflex activation is consistent with our previous observations in obese dogs.25–26 In contrast, plasma levels of NE were unchanged following CBD. In this regard, any sustained increases in sympathetic activity attributed to either denervation of carotid baroreceptors or pressure-induced unloading of aortic baroreceptors would be expected to attenuate the sympathoinhibition associated with loss of chemoreceptor hyperactivity, making it even more difficult to discern suppression of sympathetic activity by measurement of plasma NE concentration.
In keeping with the major function of the carotid bodies to sense hypoxemia and restore PaO2 to normal by increasing ventilation, previous studies indicate that these peripheral chemoreceptors play an important role in the regulation of respiration even in nonobese dogs during eupnea.32,34,47 The present study expands on these observations by providing comparative data in obesity hypertension. Following elimination of the carotid body drive for ventilation by denervation of the carotid sinus, Rodman et al. reported sustained reductions in respiratory rate and alveolar ventilation along with a decrease in PaO2 from 95±4 to 86±7 mmHg and an increase in PaCO2 from 40±3 to 51±1 mmHg over a 3 week observational period in nonobese dogs.32 In comparison, in the obese dogs of the present study the corresponding changes in blood gases, particularly PaO2, were more pronounced as evident by a decrease in PaO2 from 81±3 to 65±2 mmHg and an increase in PaCO2 from 40±3 to 53±0.6 mmHg. These exaggerated changes in blood gases in obese dogs were even more remarkable in light of the progressive recovery in respiratory rate that followed the initial sharp reduction in the tachypnea after CBD. Using a similar technique for estimation of respiratory rate as in this study, Abdala et al. also reported only a transient reduction in respiratory rate after CBD in SHRs with established hypertension; however, as blood gases were not measured in their study further comparison is not possible.9 Presumably the recovery in respiratory rate in the present study was due to these more pronounced changes in PaO2 and PaCO2 providing more intense activation of peripheral aortic and central chemoreceptors. However, given the magnitude of the tidal flow/airway closure limitations and attendant ventilation/perfusion mismatch associated with obesity (as discussed above), the compensatory increase in respiratory rate attributed to stimulation of these other chemoreceptors was presumably insufficient to improve overall alveolar ventilation and restore CBD-induced changes in blood gases. However, without additional measurements of respiratory parameters, the current findings provide little insight into the mechanisms that lead to the quantitatively greater changes in PaO2 and PaCO2 following CBD in obese than in nonobese dogs. Nonetheless, the data indicate an especially important role of carotid body chemoreceptors in the regulation of ventilation with weight gain.
Limitation
We acknowledge that the number of dogs included in this study may have been too few to detect significant changes in all measured variables during baroreflex activation and following CBD, reflecting the possibility of a type II error. However, this is unlikely a major limitation in the present study for the following reasons: 1) responses to baroreflex activation and carotid sinus denervation were compared to control values in the same animal, minimizing the need for a larger data set, 2) experimental reproducibility through recovery from baroreflex activation (the first 56 days) was controlled by duplicating the protocol used in two of our previous studies in obese dogs,25–26 and the measured variables were comparable, and 3) the key novel findings in the last half of the current study--the marked fall in PaO2 and attenuation of obesity hypertension after CBD--were statistically significant. Parenthetically, the announcement by NIH that class B dogs could no longer be used for NIH supported research precluded acquiring additional class B dogs for this study.
Perspectives
Both electrical stimulation of the carotid sinus and carotid body ablation are currently under investigation for the treatment of resistant hypertension. While there is virtually no information from prospective studies or current clinical trials relating to the antihypertensive effects of carotid body ablation in humans, recent clinical trials have clearly shown that baroreflex activation reduces arterial pressure in many but not all patients with resistant hypertension.13–17 The reasons for this variability are not well understood, but as both baroreflex activation and carotid body denervation lower arterial pressure by inhibiting sympathetic activity, the variable degree of sympathetic activation in this heterogeneous population may be a key determinant of the antihypertensive response.19 Obesity-induced hypertension is mediated by activation of the sympathetic nervous system and obesity is common in patients with resistant hypertension. Therefore, by showing that tonic activation of carotid bodies chemoreceptors contributes significantly to the hypertension of obesity and that this chemoreceptor activation is associated with hypoxemia, the present study may provide a better understanding of the stimuli that contribute to sympathetic activation in resistant hypertension. However, it is noteworthy that while hypoxemia has been reported in some subjects with obesity,21–24 its overall prevalence in obesity is unclear. Perhaps a critical issue is the fat distribution pattern in obesity as impaired ventilatory function and gas exchange are better correlated with fat mass present around the thorax and in the abdominal cavity than with body mass index.22,24,48–50 That is, hypoxemia may be more likely to occur when fat impairs expansion of the chest and descent of the diaphragm than when fat is devoid of mechanical effects on ventilatory function when present in the lower body cavity. If so, this may be one factor that accounts for the observation that visceral obesity elicits greater sympathetic activation than does subcutaneous obesity.51
Supplementary Material
Novelty and Significance.
What is new?
We found in a clinically relevant canine model of obesity that tonic activation of carotid body chemoreceptors contributes to the sympathetically-mediated hypertension. Hypoxemia appears to drive chemoreceptor hyperactivity.
The excitatory effects of heightened chemoreceptor activity that lead to hypertension and increased respiratory rate are abolished by chronic electrical stimulation of the carotid baroreflex.
What is relevant?
Because obesity is common in resistant hypertension, the present study suggests that chemoreceptor hyperactivity may contribute to the variable degree of sympathetic activation and the antihypertensive response to device-based therapies that lower arterial pressure by sympathoinhibition in this heterogeneous patient population.
Summary
Hypoxemia is associated with weight gain in dogs fed a high fat diet, suggesting that this may be a primary stimulus for increased chemoreceptor activation in obesity.
The severity of obesity hypertension was reduced 45–50% by carotid body denervation and abolished by baroreflex activation, indicating that baroreflex-mediated sympathoinhibition can offset the central sympathoexcitatory effects of chemoreflex activation that promote hypertension.
The hypoxemia of obesity is exacerbated by deafferentation of the carotid bodies.
Acknowledgments
We thank David S. Goldstein and Patti Sullivan (NIH/NINDS) for plasma NE determinations. We also thank Boshen Liu for his technical assistance and Dr. Robert L. Hester for many insightful discussions on the respiratory aspects of this study.
Sources of Funding
National Heart, Lung, and Blood Institute Grant HL-51971.
Footnotes
Disclosures
| Thomas E. Lohmeier | Consultant fees, Scientific Advisory Board, CVRx |
| Eric D. Irwin | Consultant fees, Scientific Advisory Board, CVRx |
References
- 1.Paton JFR, Ratcliffe L, Hering D, Wolf J, Sobotka PA, Narkiewicz Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep. 2013;15:273–280. doi: 10.1007/s11906-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trzebski A, Tafil M, Zoltowski M, Przybylski J. Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovas Res. 1982;16:163–172. doi: 10.1093/cvr/16.3.163. [DOI] [PubMed] [Google Scholar]
- 3.Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- 4.Przybylski J, Trzebski A, Czyzewski T, Jodkowski J. Responses to hypoxia, hypercapnia and almitrine in spontaneous hypertensive rats. Bull Europ Physiopath Respir. 1982;18:145–154. [Google Scholar]
- 5.Izdebska E, Cybulska I, Sawicki M, Izdebski J, Trzebski A. Postexercise decrease in arterial pressure, total peripheral resistance and in circulatory responses to brief hyperoxia in subjects with mild essential hypertension. J Hum Hypertens. 1998;12:855–860. doi: 10.1038/sj.jhh.1000716. [DOI] [PubMed] [Google Scholar]
- 6.Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A, Gaciong Z. Tonic activity of carotid body chemoreceptors contributes to increased sympathetic drive in essential hypertension. Hypertens Res. 2012;35:487–491. doi: 10.1038/hr.2011.209. [DOI] [PubMed] [Google Scholar]
- 7.McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJA, Sobotka PA, Paton JFR. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395–2405. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- 8.Paton JFR, Sobotka PA, Fudim M, Engleman ZJ, Hart ECJ, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 9.Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV, Paton JFR. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;17:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heistad DD, Abboud FM, Mark AL, Schmid PG. Interaction of baroreceptor and chemoreceptor reflexes: modulation of the chemoreceptor reflex by changes in baroreceptor activity. J Clin Invest. 1974;53:1226–1236. doi: 10.1172/JCI107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heistad DD, Abboud FM, Mark AL, Schmid PG. Effect of baroreceptor activity on ventilatory response to chemoreceptor stimulation. J Appl Physiol. 1975;39:411–416. doi: 10.1152/jappl.1975.39.3.411. [DOI] [PubMed] [Google Scholar]
- 12.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: mechanisms and potential for hypertension therapy. Hypertension. 2011;57:880–886. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmeier TE, Iliescu R. The sympathetic nervous system in obesity hypertension. Curr Hypertens Rep. 2013;15:409–416. doi: 10.1007/s11906-013-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gassler JP, Bisognano JD. Baroreflex activation therapy in hypertension. J Hum Hypertens. 2014;28:469–474. doi: 10.1038/jhh.2013.139. [DOI] [PubMed] [Google Scholar]
- 16.Iliescu R, Tudorancea I, Lohmeier TE. Baroreflex activation: from mechanisms to therapy for cardiovascular disease. Curr Hypertens Rep. 2014;16:435–461. doi: 10.1007/s11906-014-0453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chobanyan-Jurgens K, Jordan J. Electrical carotid sinus stimulation: chances and challenges in the management of treatment resistant hypertension. Curr Hypertens Rep. 2015;17:71–77. doi: 10.1007/s11906-015-0587-4. [DOI] [PubMed] [Google Scholar]
- 18.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. doi: 10.1161/CIRCRESAHA.116.303604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension; interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood A. Altered resting and exercise respiratory physiology in obesity. Clin Chest Med. 2009;30:445–454. doi: 10.1016/j.ccm.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108:206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 23.Littleton SW. Impact of obesity on respiratory function. Respirology. 2012;17:43–49. doi: 10.1111/j.1440-1843.2011.02096.x. [DOI] [PubMed] [Google Scholar]
- 24.Verbraecken J, McNicholas WT. Respiratory mechanisms and ventilatory control on overlap syndrome and obesity hypoventilation. Resp Res. 2013;14:132–148. doi: 10.1186/1465-9921-14-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007;49:1307–1314. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 26.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension. 2012;59:331–338. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol. 2010;299:H402–H409. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayano J, Yasuma F. Hypothesis: respiratory sinus arrhythmia is an intrinsic resting function of cardiovascular system. Cardiovasc Res. 2003;58:1–9. doi: 10.1016/s0008-6363(02)00851-9. [DOI] [PubMed] [Google Scholar]
- 29.Lohmeier TE, Irwin ED, Rossing MA, Sedar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 30.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 31.Lohmeier TE, Liu B, Hildebrandt DA, Cates AW, Georgakopoulos D, Irwin ED. Global- and renal-specific sympathoinhibition in aldosterone hypertension. Hypertension. 2015;65:1223–1230. doi: 10.1161/HYPERTENSIONAHA.115.05155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- 33.Haskins S, Pascoe PJ, Ilkiw JE, Fudge J, Hopper K, Aldrich J. Reference cardiopulmonary values in normal dogs. Comp Med. 2005;55:156–161. [PubMed] [Google Scholar]
- 34.Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mifflin SW. Inhibition of chemoreceptor inputs to nucleus of tractus solitarius neurons during baroreceptor stimulation. Am J Physiol Regulatory Integrative Comp Physiol. 1993;265:R14–R20. doi: 10.1152/ajpregu.1993.265.1.R14. [DOI] [PubMed] [Google Scholar]
- 36.Katayama PL, Castania JA, Dias DPM, Patel KP, Frazan R, Jr, Salgado HC. Role of chemoreceptor activation in hemodynamic responses to electrical stimulation of the carotid sinus in conscious rats. Hypertension. 2015;66:598–603. doi: 10.1161/HYPERTENSIONAHA.115.05316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neil E, Redwood CR, Schweitzer A. Pressor response to electrical stimulation of the carotid sinus nerve in cats. J Physiol. 1949;109:259–271. doi: 10.1113/jphysiol.1949.sp004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy MN, Zieske H. Effect of carotid sinus nerve stimulation pattern on cardiorespiratory responses. Am J Physiol. 1976;230:H951–H958. doi: 10.1152/ajplegacy.1976.230.4.951. [DOI] [PubMed] [Google Scholar]
- 39.Alnima T, Goedhart EJBM, Seelen R, van der Grinten CPM, de Leeuw PW, Kroon AA. Baroreflex activation therapy lowers arterial pressure without apparent stimulation of carotid bodies. Hypertension. 2015;65:1217–1222. doi: 10.1161/HYPERTENSIONAHA.114.04354. [DOI] [PubMed] [Google Scholar]
- 40.Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA, Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension. 2007;49:373–379. doi: 10.1161/01.HYP.0000253507.56499.bb. [DOI] [PubMed] [Google Scholar]
- 41.Thrasher TN, Shifflett C. Effect of carotid or aortic baroreceptor denervation on arterial pressure during hemorrhage in conscious dogs. Am J Physiol Regulatory Integrative Comp Physiol. 2001;280:R1642–R1649. doi: 10.1152/ajpregu.2001.280.6.R1642. [DOI] [PubMed] [Google Scholar]
- 42.Van Vliet BN, Chafe LL, Montani J-P. Contribution of baroreceptors and chemoreceptors to ventricular hypertrophy produced by sino-aortic denervation. J Physiol. 1999;516(3):885–895. doi: 10.1111/j.1469-7793.1999.0885u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daly MD, Scott MJ. The cardiovascular responses to stimulation of the carotid body chemoreceptors in the dog. J Physiol. 1963:179–197. doi: 10.1113/jphysiol.1963.sp007051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Vliet BN, Hall JE, Mizelle HL, Montani J-P, Smith MJ., Jr Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol Heart Circ Physiol. 1995;269:H629–H637. doi: 10.1152/ajpheart.1995.269.2.H629. [DOI] [PubMed] [Google Scholar]
- 45.Iliescu R, Tudorancea I, Irwin ED, Lohmeier TE. Chronic baroreflex activation restores spontaneous baroreflex control of heart rate in obesity-induced hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1080–H1088. doi: 10.1152/ajpheart.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 47.Blain GM, Smith CA, Henderson KS, Dempsey JA. Contribution of the carotid body chemoreceptors to eupneic ventilation in the intact, unanaesthetized dog. J Appl Physiol. 2009;106:1564–1573. doi: 10.1152/japplphysiol.91590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function. Chest. 1997;111:891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- 49.Zavorsky GS, Murias JM, Kim DJ, Gow J, Sylvestre J-L, Christou NV. Waist-to-hip ratio is associated with pulmonary gas exchange in the morbity obese. Chest. 2007;131:362–367. doi: 10.1378/chest.06-1513. [DOI] [PubMed] [Google Scholar]
- 50.Sutherland TJT, Goulding A, Grant AM, Cowan JO, Williamson A, Williams SM, Skinner MA, Taylor DR. The effect of adiposity measured by dual-energy x-ray adsorptiometry on lung function. Eur Respir J. 2008;32:85–91. doi: 10.1183/09031936.00112407. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez G, Ballard T, Beske S, Davy K. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2003;287:H414–H418. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.