Abstract
Renal denervation (RDN) has been postulated to reduce sympathetic drive during heart failure (HF), but the central mechanisms are not completely understood. The purpose of the present study was to assess the contribution of neuronal nitric oxide synthase (nNOS) within the paraventricular nucleus (PVN) in modulating sympathetic outflow in rats with HF that underwent RDN. HF was induced in rats by ligation of the left coronary artery. Four weeks after surgery, bilateral RDN was performed. Rats with HF had an increase in FosB-positive cells in the PVN with a concomitant increase in urinary excretion of norepinephrine and both of these parameters were ameliorated after RDN. nNOS-positive cells immunostaining, diaphorase staining, and nNOS protein expression were significantly decreased in the PVN of HF rats, findings that were ameliorated by RDN. Microinjection of nNOS inhibitor L-NMMA into the PVN resulted in a blunted increase in lumbar sympathetic nerve activity (ΔLSNA: 11 ± 2 vs. 24 ± 2%) in HF than in sham group. This response was normalized after RDN. Stimulation of afferent renal nerve (ARN) produced a greater activation of PVN neurons in rats with HF. ARN stimulation elicited a greater increase in LSNA in rats with HF compared to sham rats (ΔLSNA: 45 ± 5 vs. 22 ± 2%). These results suggest that intact renal nerves contribute to the reduction of nNOS in the PVN, resulting in activation of the neurons in the PVN of rats with HF. RDN restores nNOS and thus attenuates the sympathoexcitation commonly observed in HF.
Keywords: renal afferent nerve, sympathetic nerve activity, central nervous system, heart failure, nitric oxide synthase
Introduction
Enhanced sympathetic nerve activity is a risk factor that influences the progression of heart failure (HF) and mortality in patients. Although most therapeutic pharmaceutical strategies target the peripheral symptoms of the disease, they may not influence the enhanced sympathetic nerve activity. Indeed, various studies have detailed the role of altered baroreceptor and chemoreceptor afferent mechanisms in the elevated sympathetic drive in HF, 1, 2 but these mechanisms do not fully account for the total sympathoexcitation observed in HF.
In the central nervous system, the paraventricular nucleus (PVN) of the hypothalamus mediates sympathetic nerve activity and influences the cardiovascular system. 3–5 Our previous studies have shown that the PVN is activated in rats with HF in conjunction with enhanced glutamatergic tone and blunted nitric oxide (NO) mechanism within the PVN. 6–9 Specifically, neurons within the PVN exhibit an increased hexokinase and FosB expression, which are markers of chronic neuronal activation. 5, 10–12 Furthermore, rostral ventrolateral medulla (RVLM) projecting PVN neurons are more active in rats with HF than in sham operated controls suggesting that preautonomic neurons within the PVN are activated and contribute importantly in initiating sympathoexcitation in HF. 13 The discharge frequency of putative vasopressinergic magnocellular neurosecretory neurons in the PVN is increased during stimulation of afferent renal nerves (ARN) and during the activation of specific renal receptors. 14 Recently we have shown that ARN stimulation activates RVLM projecting PVN neurons. 15 Stimulation of ARN also increases sympathetic activity and arterial pressure. 15, 16 In addition, neurons containing Fos-like immunoreactivity were observed to be increased after ARN stimulation in the PVN, indicated that the PVN neurons were activated by ARN stimulation. 17 These observations suggest that afferent information from the kidney is important in the coordination of neural and hormonal activity concerned with body fluid balance and the regulation of arterial blood pressure in normal and disease conditions. 17–21
Renal denervation (RDN) has been shown to reduce arterial pressure and sympathetic outflow in various animal models of hypertension as well as in patients with resistant hypertension. 22–24 Catheter-based RDN has been shown to reduce blood pressure in treatment-resistant hypertensive patients for the primary endpoint at 6 months. 25 In animal studies, RDN has been shown to decrease sympathetic activity and arterial pressure in a neurogenic form of hypertension produced by sectioning of aortic depressor nerves. Concurrently, noradrenergic activity is altered in the hypothalamus after RDN suggesting that the sympathoinhibitory effects of RDN are mediated centrally at the level of the hypothalamus. 26 Studies in experimental models of HF have previously induced RDN either before or immediately after the induction of HF 27, 28 but not in the chronic stage of HF, which is clinically more relevant.
The present study was conducted to examine the hypothesis that the activation of the PVN in HF is mediated and conveyed by intact renal nerves. Further, this renal nerve mediated information contributes to the down regulation of neuronal NO synthase (nNOS) within the PVN of rats with HF, leading to the enhanced activation of neurons in the PVN that translates ultimately to the activation of sympathetic nervous system in HF.
Methods
Animals
All procedures used for this study were approved by University of Nebraska Medical Center Institutional Animal Care and Use Committee and conducted according to the NIH guiding principles for the research involving animals. Male Sprague-Dawley rats weighing 220 to 250 g were purchased from Sasco Breeding laboratories (Omaha, NE). Animals were housed with a 12-hour light-dark cycle at ambient 22°C 30–40% relative humidity. Laboratory chow and tap water were available ad libitum. After acclimatization for 1 week, rats were assigned randomly to one of four groups: sham, HF, sham+RDN, HF+RDN (n = 15–18/group, total number of rats = 66).
Induction of heart failure
Rats were randomly assigned to either a sham-operated control group or a HF group. HF was produced by left coronary artery ligation, as previously described. 29 The rats were anesthetized with isoflurane gas starting in an anesthesia chamber from 3–4%. During the procedure, isoflurane (2 to 2.5%, gas vaporizer) was used. The degree of left ventricular dysfunction and HF was determined by using both hemodynamic and anatomic criteria. Rats with both left ventricular end-diastolic pressure (LVEDP) > 15 mmHg and infarct size > 30% of total left ventricular wall were considered to be in HF. Six rats in the HF group had infarct sizes < 30% and were excluded from data analysis.
Renal denervation
Four weeks after ligation surgery, rats underwent RDN under anesthesia. Complete RDN was achieved by cutting bilaterally all the visible renal nerves from the renal artery and vein, and painting the vessels with 70% ethanol. This method has been shown to ablate the afferent and efferent renal nerves. 30–32
One week after RDN (and 5 weeks after coronary ligation), rats were either euthanized to collect tissues for molecular or immunohistochemical studies or anesthetized for terminal electrophysiological studies to record PVN neuronal activity or lumbar sympathetic nerve responses to afferent renal nerves or lumbar sympathetic nerve responses to microinjections of L-NMMA into the PVN. Blood was collected for analysis of angiotensin II and aldosterone levels. Urine was collected for analysis of NE excretion. Additional methodologies and detailed description of procedures including NE, angiotensin II and aldosterone measurements, immunohistochemistry, NADPH-diaphorase activity, Western blot analysis, lumbar sympathetic nerve activity recording, microinjection into the PVN, extracellular single-unit recordings, electrical stimulation of afferent renal nerves are available in Methods section in the online-only Data Supplement.
Statistical analysis
Data were subjected to a two-way ANOVA followed by a Multiple Range (for multiple comparisons) or Student-Newman Keuls test. P < 0.05 were considered to indicate statistical significance.
Results
General characteristics
Table 1 presents morphological characteristics and left ventricular function parameters among the four experimental groups. The body weight, whole heart weight and heart weight/body weight ratio were significantly increased in HF group. RDN had no significant effects on these parameters in both sham and HF groups. Only rats with > 30% infarcts of the left ventricular wall were included in the study. Six rats in the HF group had infarct sizes < 30% and were excluded from data analysis. Sham rats had no visible myocardial damage. LVEDP was significantly increased in the HF rats compared to both sham groups and HF+RDN group. The magnitude of +dP/dt and −dP/dt were significantly decreased in the both HF and HF+RDN rats compared to both sham groups. LVEDP was partially reduced by RDN while +dP/dt and −dP/dt were not significantly affected by RDN. The data confirmed that rats in the HF groups were experiencing cardiac dysfunction and that RDN may contribute to partial improvement in cardiac function.
Table 1.
Baseline and left ventricular function parameters
| Measures | Sham (n = 10) | HF (n = 10) | Sham+RDN (n = 12) | HF+RDN (n = 12) |
|---|---|---|---|---|
| Body weight (g) | 408 ± 18 | 452 ± 26* | 386 ± 10 | 426 ± 21 |
| Heart weight (g) | 1.3 ± 0.2 | 2.2 ± 0.4* | 1.2 ± 0.2 | 2.0 ± 0.2* |
| Heart weight/Body weight ×1000 | 0.32 ± 0.05 | 0.49 ± 0.08* | 0.31 ± 0.05 | 0.47 ± 0.04* |
| Infarct size (% of epicardial LV) | 0 | 36 ± 6* | 0 | 35 ± 6* |
| LVEDP (mmHg) | 1 ± 1 | 25 ± 4* | 1 ± 1 | 12 ± 3*† |
| +dP/dt (mmHg/S) | 6899 ± 329 | 5016 ± 378* | 6856 ± 274 | 5366 ± 298* |
| −dP/dt (mmHg/S) | −5749 ± 328 | −3895 ± 277* | −6023 ± 314 | −4115 ± 169* |
| Plasma angiotensin II (pg/ml) | 68 ± 12 | 175 ± 31* | 53 ± 8 | 95 ± 12*† |
| Plasma aldosterone (pg/ml) | 1647 ± 117 | 2254 ± 74* | 1753 ± 91 | 2017 ± 29*† |
Data are means ± SE.
P < 0.05 compared to sham;
P < 0.05 compared to the group without denervation. LV: left ventricle; LVEDP, left ventricular end-diastolic pressure.
Urinary norepinephrine excretion measurements
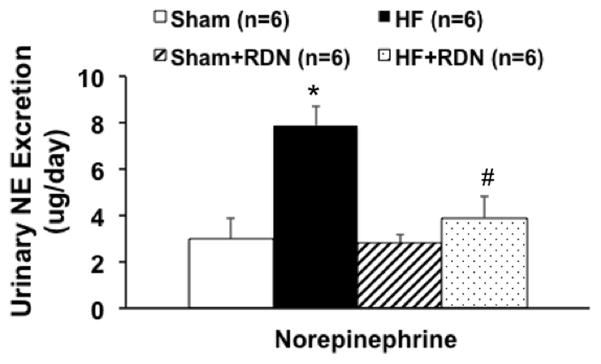
Urinary NE excretion was significantly greater in HF rats compared to sham operated controls and was used as an index of overall sympathetic activation. RDN reduced the urinary excretion of NE in rats with HF (4.2 ± 0.8 HF+RDN vs. 8.1 ± 0.3 μg/day HF, P < 0.05). There was no significant change in the sham rats with RDN suggesting that the RDN per se did not change the excretion of NE in the urine in control conditions (Figure 1).
Figure 1.
24 hours urinary NE excretion in sham and HF rats with/without RDN. * P < 0.05 vs. sham; # P < 0.05 vs. corresponding group without RDN.
Kidney NE content was significantly greater in HF rats compared to sham operated controls (325 ± 35 vs. 168 ± 36 ng/g, P < 0.05). RDN reduced the kidney content of NE to almost undetectable level in both sham and HF rats, which confirms the completeness of RDN.
Plasma angiotensin II and aldosterone measurements
Plasma angiotensin II and aldosterone was significantly greater in HF rats compared to sham operated controls. RDN significantly reduced the plasma angiotensin II and aldosterone in rats with HF (Table 1). RDN did not significantly change the levels of angiotensin II or aldosterone in the sham operated controls.
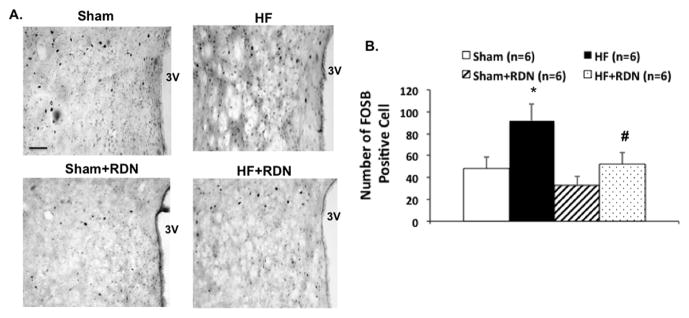
FosB immunohistochemistry in the PVN
The number of cells in the PVN (rostral-caudal level of the PVN) that stained positive for the neuronal activation marker, FosB, was increased in HF compared to the sham group (Figure 2). This enhanced activation was attenuated in the HF+RDN rats compared to the HF rats. These data indicate that the PVN is in an activated state during HF, perhaps contributing to the enhanced sympathetic activity. After RDN, the activation in the PVN is attenuated, paralleling the decrease in overall sympathetic activation as measured by urinary NE excretion (Figure 1).
Figure 2.
The effect of RDN on FosB immunohistochemistry staining in the PVN of rats with HF. Representative pictures of PVN with FosB staining (A) in four groups of rats, sham, HF, sham+RDN and HF+RDN. Bar = 100 μm. (B) Mean values of FosB staining in the PVN. *P < 0.05 vs. sham; #P < 0.05 vs. without RDN.
Expression of the nNOS (immunohistochemistry, diaphorase and protein) during HF and after RDN
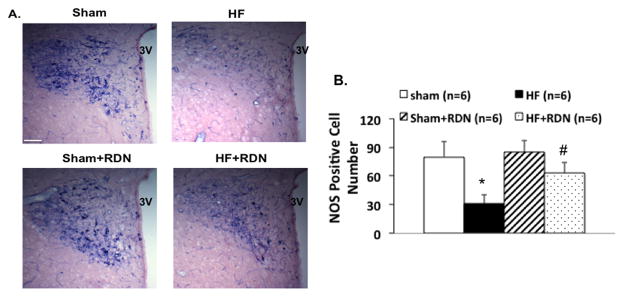
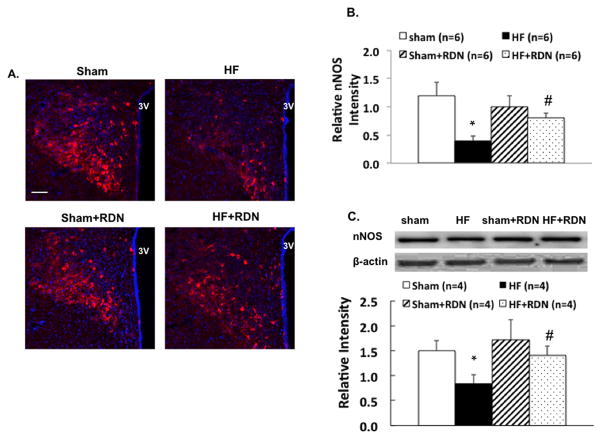
The number of cells in the PVN that stained positive for the NADPH-diaphorase activity was decreased in HF compared to the sham group. This reduction in NOS activity was attenuated in the HF rats after RDN (Figure 3). Immunostaining showed that the nNOS signaling in the PVN was decreased in HF compare to the sham group. This reduction in nNOS immunoreactivity was attenuated in the HF rats after RDN (Figure 4A, 4B). Furthermore, Western blot data showed similar pattern for reduction in the PVN; nNOS expression was decreased 48% in HF that was abrogated by RDN (Figure 4C). There was no difference in nNOS expression in the PVN after RDN between the sham and HF groups.
Figure 3.
The effect of RDN on NADPH-diaphorase in the PVN of rats with HF. Representative pictures of PVN with NOS positive staining (A) in four groups of rats, sham, HF, sham+RDN and HF+RDN. Bar = 100 μm. (B) Mean values of NOS positive cells in the PVN. *P < 0.05 vs. sham; #P < 0.05 vs. without RDN.
Figure 4.
A. Representative pictures of PVN with nNOS staining (A) in four groups of rats, sham, HF, sham+RDN and HF+RDN. Bar = 100 μm. B. Mean values of relative nNOS intensity in the PVN. C. nNOS protein expression in the sham and HF rats with/without RDN. *P < 0.05 vs. sham; #P < 0.05 vs. without RDN.
L-NMMA microinjection into the PVN
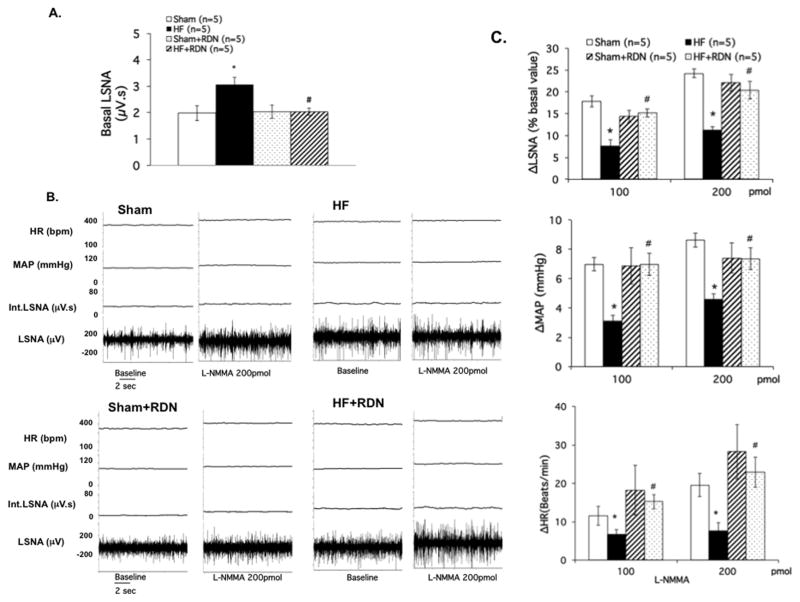
The basal lumbar sympathetic nerve activity (LSNA) was significantly increased in rats with HF compared to the sham rats (3.1 ± 0.3 vs. 2.0 ± 0.7 μV.s, P < 0.05). RDN significantly reduced the basal LSNA in HF group rats (2.0 ± 0.1 HF+RDN vs. 3.1 ± 0.3 μV.s HF, P < 0.05) (Figure 5A). To examine endogenous NO tone from the PVN, we blocked NOS within the PVN with microinjection of L-NMMA. L-NMMA microinjection into the PVN increased LSNA in both sham and HF groups as expected and shown previously while recording renal sympathetic nerve activity. 7 The percentage change in LSNA from basal values was smaller in HF compared with sham (11 ± 2% vs. 24 ± 2%, P < 0.05; Figures 5B, 5C). MAP and HR responses to L-NMMA were blunted in the HF condition. However, after RDN, these parameters were all normalized in HF rats.
Figure 5.
A. Basal lumbar sympathetic nerve activity (LSNA) in four groups of rats sham, sham+RDN, HF and HF+RDN. B. Raw tracings of changes in LSNA, BP and HR to administration of L-NMMA (200 pmol) in the PVN of four groups of rats. C. Summary data for the effect of L-NMMA in the PVN on changes in LSNA, mean arterial pressure (MAP) and heart rate (HR) in the four groups of rats. Data are presented as mean ± SE. *P < 0.05 vs. sham; #P < 0.05 vs. without RDN.
Responses in RVLM projecting PVN neurons to stimulation of ARN
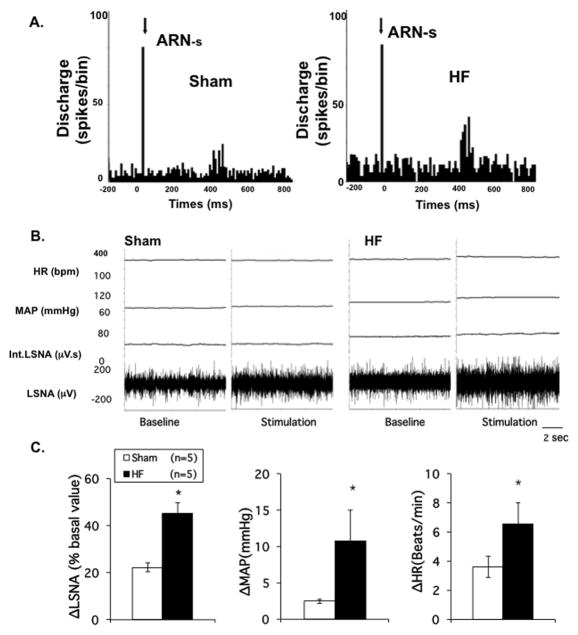
In 40 spontaneously active neurons recorded in the PVN, 10 units (5 from sham, 5 from HF rats) were antidromically activated from the RVLM in 6 rats. Stimulation of the ARN increased firing of RVLM projecting PVN neurons in both sham and HF groups. We have previously shown that RVLM projecting PVN neurons have a higher basal firing rate in the HF group. 13 Further, here we show that the percentage increase in firing of PVN/RVLM neurons from basal values was enhanced in HF compared with sham (22.7 ± 3.1% vs. 9.0 ± 1.3%, P < 0.05; Figure 6A). These data show that RVLM projecting PVN neurons in HF may have an exaggerated response to ARN stimulation.
Figure 6.
A. Discharge of RVLM projecting PVN neurons to ARN stimulation. Peristimulus histogram of spike occurrence triggered by electric stimulation of the afferent renal nerve with 100 sweeps, bin = 0.02 s. ARN-s: afferent renal nerve stimulation. Segments of original recordings of changes in discharge after ARN stimulation in sham and HF rats. B. Original recordings showing changes in LSNA, MAP and HR before and after ARN stimulation (40 Hz) in individual sham and HF rats. C. Mean percent change of LSNA, MAP and HR to ARN stimulation in sham and HF groups. * P < 0.05 vs. sham.
Responses in LSNA to stimulation of ARN
Stimulation of the ARN increased LSNA in both sham and HF groups. The percentage change in LSNA from basal values was enhanced in HF compared with sham (45 ± 5% vs. 22 ± 2%, P < 0.05) (Figure 6B, 6C). Similarly, MAP and HR responses to ARN were also elevated in the HF condition (11 ± 4 vs. 3 ± 1 mmHg and 7 ± 2 vs. 4 ± 1 bpm, P < 0.05, respectively) (Figure 6B, 6C). These data show that in HF there is an exaggerated response to ARN stimulation.
Discussion
The present study shows that removal of renal nerves by RDN abrogates the elevated neuronal activity in the PVN as well as the concomitant increase in global sympathetic outflow in HF, as indicated by the elevated excretion of NE in the urine. At the same time RDN restored the endogenous nNOS in the PVN that had been decreased in rats with HF. Consistent with these observations RDN normalized the blunted LSNA response to inhibition of endogenous NOS within the PVN observed in HF rats. Electrical stimulation of ARN resulted in an enhanced neuronal firing of RVLM projecting PVN neurons in rats with HF. As a corollary, ARN stimulation elicits a potentiated activation of LSNA in rats with HF. We propose that potentially tonic activation of ARN (due to the HF condition) contributes to the activation of preautonomic neurons within the PVN during HF. These results demonstrate a critical role for the PVN in relaying neural signals from the kidney that may be tonically active in the HF condition, and are abrogated by RDN. A possible mechanism for the therapeutic effects of RDN during HF may be through an NO-dependent mechanism within the PVN.
Renal denervation normalizes the global sympathoexcitation in HF
RDN in most experimental forms of hypertension as well as drug resistant hypertensive patient has been shown to reduce arterial pressure and sympathetic activity. 22, 23, 33 Since exaggerated sympathoexcitation is characteristic of both hypertension and HF, the efficacy of RDN to reduce sympathoexcitation has been explored in both ischemia-induced and pacing heart models of HF 27, 28, 34 and patients with HF. 35, 36 In previous studies that examined the effects of RDN in HF, RDN was performed either before or immediately after the induction of HF. 27, 28 Therefore, we investigated whether RDN reduces global sympathetic tone after the development of HF to further increase the clinical relevance. The results from the present study show that bilateral RDN was sufficient to reduce global sympathetic outflow, as evident by a reduction in urinary excretion of NE as well as basal level of LSNA.
Renal denervation abrogates activation of the PVN during HF
This study found that concomitant with the increase in global sympathetic outflow there was an activation of the PVN in HF. In HF rats, there was a two-fold increase in FosB-positive cells in the PVN compared to sham. In addition, HF rats exhibited greater protein expression of ΔFosB, which is a stable splice variant of FosB that is specifically upregulated during chronic neuronal activation. 37 Other studies have reported PVN activation using Fra-like immunoreactivity after induction of HF. 11, 38, 39 Extracellular recordings in the PVN have also shown that RVLM projecting PVN neurons are more active in rats with HF at 4 weeks. 13 Afferent neural information from renal sensory receptors has been shown to influence the activity of neurons at different levels of the neuraxis. 14, 17–19, 40–44 Furthermore, ARN stimulation has also been shown to activate the PVN. 14, 17, 20 In addition, a time course study examining inducible transcription factors in the PVN found that expression of neuronal activity marker cFOS was rapidly increased after ARN stimulation. 17 Therefore, since renal afferents are known to influence neuronal activity within the PVN, 17, 21, 45 it could be postulated that the enhanced FosB expression observed in the PVN of HF rats may be related to the potential tonic activation of afferent renal nerves during the HF condition. The level of this tonic activation as well as the exact modality of the stimulus from the kidney that activates the renal afferents in this disease condition remains to be elucidated. It should be noted that there are multiple triggers in the HF condition that have the potential for increased activation of the renal afferent nerve activity, including reduced perfusion pressure, increased venous pressure, increased inflammation, increased oxidative stress to name a few. 19
Additionally, HF rats exhibit increased sympathetic and hemodynamic responses to activation of the PVN. 6–9 In the current study, FosB results suggest that the PVN is activated during HF. Perhaps this activation of the PVN may be due to an exaggerated response to ARN activity during HF. This increased activity within the PVN translates to increased overall sympathetic outflow. However, RDN caused a decrease in the index of overall sympathetic activity, urinary excretion of NE in HF rats, while this response was minimal or absent in the sham group. One plausible interpretation of these data is that there may be a tonic level of ARN activity during the HF condition, which may contribute to the state of activation of the PVN resulting in an increased overall sympathoexcitation.
RDN did improve overall sympathetic drive, as indicated by a decrease in excretion of urinary NE levels as well as reduction in baseline LSNA in HF rats. Reducing NE is very important, as HF patients with lower levels of plasma NE are given a better prognosis. 46 These results suggest that RDN may alter the activity of neurons in central cardiovascular regulatory sites, such as the PVN, thereby contributing to the improvement in sympathetic tone. Indeed, our results indicate that ARN-mediated sympathetic and hemodynamic responses are improved after RDN.
Renal denervation improves nNOS expression and functional NOS activity in the PVN during HF
Nitric oxide, which is well known to inhibit neurons, is decreased in terms of mRNA and protein expression and diaphorase staining in the PVN during HF. 47, 48 Sympathoexcitatory and hemodynamic responses to endogenous blockade of NO within the PVN were impaired in HF, 7 suggesting that the PVN contributes to the increased sympathoexcitation exhibited in HF via an altered NO mechanism. This study shows that RDN restores the levels of nNOS within the PVN of rats with HF suggesting that RDN restores the endogenous inhibitory nNOS mechanisms in rats with HF. The levels of diaphorase positive neurons are also restored in rats with HF suggesting that the NOS activity index is also restored after RDN. Consistent with these observations, the functional activity of NOS within the PVN of rats with HF is also restored. The improved LSNA responses to microinjection of L-NMMA into the PVN are concomitant with improved nNOS levels in the PVN of rats with HF after RDN.
Activation of the PVN by ARN stimulation
The kidneys have a dense afferent sensory and efferent sympathetic innervation and are thereby strategically positioned to be the origin as well as target of sympathetic nervous system activation. 19, 33, 49 A number of studies utilizing anterograde tract tracing of fluorescent dyes, horseradish peroxidase transport, or pseudorabies virus injected into the kidneys as well as electrophysiological evidence indicate that the renal afferent information is transmitted to sites within the spinal cord that relay information to the central nervous system associated with cardiovascular regulation, including nucleus tractus solitaries, rostral ventrolateral medulla, preoptic area, subfornical organ, lateral hypothalamus and the PVN in the hypothalamus. 19, 21, 50 Renal afferent nerve signals can elicit both inhibitory reno-renal reflexes as well as long looped supra-medullary reno-excitatory responses. 21, 51 Although the inhibitory reno-renal reflex is blocked by RDN or spinal cord transection at C2 it was unaffected by transection at pontinemedullary junction 51 suggesting that the inhibitory reflex operates at the spino-medullary level. Electrophysiological and morphological evidence suggest that majority (70%) of the fibers in the ARN are small unmylinated C-fiber or finely mylinated A-delta fibers. 18, 52, 53 It is postulated that most of the chemoreceptor input is carried by these fibers. Additionally, it is thought that the electrical stimulation (used in this study) mainly elicits the response due to activation of this group of small fibers causing an excitatory response. 14, 18, 21 Stimulation of the mylinated large fibers causes the inhibitory response. 19
Renal afferent nerve signals are centrally integrated and their activation results in an increase in sympathetic tone which is not only directed toward the kidneys, but also toward other organs that have a dense sympathetic innervation resulting in an increase in sympathetic outflow to cause a rise in blood pressure. 20, 21, 54–56 Renal afferent signals are also involved in spinal feedback loops, termed reno-renal reflexes, whereby afferent activity from one kidney can modulate ipsilateral and contralateral efferent renal nerve activity to regulate diuresis and natriuresis to balance overall renal function between the two kidneys. 19 In this regard, it is of interest to note that such inhibitory reno-renal reflexes are reported to be blunted in rats with HF. 57 Furthermore, it was proposed that this blunting was mediated by angiotensin II. This could be interpreted to imply a role for renal efferents in potentially reducing inhibitory input from the kidney. These data are also consistent with the concept that this reduced inhibitory input may be overwhelmed by excitatory input generated within the kidneys of rats during the HF condition, since RDN was able to abrogate the increase in global sympathetic activation. Therefore, the kidney is not only a target of sympathetic outflow, but also a source of signals that have the potential to directly modulate overall sympathetic outflow in disease conditions such as HF and hypertension. 19
Evidence for excitatory reflexes originating in diseased kidneys is derived from studies in rats with chronic renal failure and patients with renal failure. 19 There is also considerably strong evidence that the diseased kidneys exert an excitatory effect on sympathetic nerve activity in various pathological conditions involving renal injury, including hypertension, HF, chronic renal failure, diabetes, and obesity. 19, 49, 58–61 It has been proposed that renal inflammation is prevalent in many of these pathological conditions and may contribute to the increased sympathetic outflow via activation of afferent renal nerves. 19
The PVN is an important site that integrates and responds to a variety of neural and humoral signals regulating sympathetic drive and extracellular fluid volume. 9, 31, 62 Previous studies demonstrated that the discharge frequency of neurons in the PVN was increased during stimulation of ARN. 14 In this respect it is of interest to understand if pre-autonomic neurons in the PVN respond to ARN stimulation and how the PVN integrates signals from the ARN. In the present study electrical stimulation of the ARN activated RVLM projecting PVN neurons to a greater extent in rats with HF than sham rats. These data suggest that afferent signal from the kidneys of HF rats are more potent in eliciting activation of preautonomic neurons within the PVN. It is of interest to note that basal levels of RVLM projecting PVN neurons is also increased in HF rats, 13 suggesting that perhaps there is a potential for tonic ongoing activation by the ARN during HF condition, which may contribute to the state of activation of the PVN which would then translate into an increased overall sympathoexcitation under basal conditions. However, to conclusively demonstrated that renal afferents are activated tonically in the HF condition, either direct renal afferent recordings, or a selective interruption of renal afferents is required and remains to be explored.
Activation of the ARN produces exaggerated LSNA responses in rats with HF
Renal afferent information has been shown to influence baroreceptors and cardiac afferent reflexes to influence sympathetic tone. 15, 63 It should be noted that the cardiac afferent reflex is shown to have enhanced sympathoexcitatory responses in HF and hypertension. 3, 64, 65 Consistent with our findings of an over-active sympathoexcitatory state originating from the PVN during HF, the potentially tonic ARN stimulus may be a critical contributor during the HF condition. Previous studies have demonstrated the importance of the PVN in cardiovascular regulation during HF. 4–7, 9 More recently we have shown that there is a specific activation of RVLM projecting PVN by stimulation of ARN. 15 It is of interest to note that all the neurons that were responsive to ARN stimulation in that study were also sensitive to cardiac sympathetic afferents, a source of sympathoexcitatory input. It may well be that this convergence of input from the kidney and heart are synergistically driving these preautonomic PVN neurons in the HF condition. ARN stimulation induces sympathoexcitation via the PVN by a glutamatergic mechanism. 15 Therefore, the ARN-induced activation of the PVN may influence the neuronal activity in the RVLM among other sites and thereby contribute to the overall regulation of sympathetic activity. This does not also preclude the possibility that ARN may influence spinal cord-projecting PVN neurons to play an equal or greater role in the ARN-induced activation of the PVN and subsequent sympathoexcitation. 66 It may well be that presence of input from any of these afferents (renal, cardiac or chemoreceptors) may be sufficient to activate the PVN to drive the sympathetic overactivation since specific cardiac denervation or chemo denervation have also be shown to alleviate the overactive sympathoexcitation observed in HF. 67, 68
Direct electrical stimulation of the renal afferent nerves in animals has been shown to produce sympathoexcitation in various vascular beds and an increase in arterial pressure. 20, 69, 70 It is possible that a pathological signal, which remains to be identified, from the kidney may initiate an increase in ARN activity, whereby causing an increase in overall sympathetic tone which in turn exuberates the pathological signal (positive feedback loop). In the present study stimulation of the ARN produced an increase in LSNA and these responses were exaggerated in rats with HF, suggesting that the renal mediated activation of the sympathoexcitation is more potent in rats with HF, possibly contributing to the enhanced sympathoexcitation commonly observed in the HF condition.
Removal of efferent renal innervation
The potential effects of interrupting the renal efferent nerves should be considered as an alternative explanation for the changes in the nitroxidergic mechanisms within the PVN of rats with HF. It has been well documented that angiotensin II levels are elevated in HF and also in this model. 71, 72 One well-known function of the renal nerves is to stimulate renin release from the kidney and renal nerve activity has been reported to be elevated in HF. 33, 73 It is conceivable that this activation of efferent renal nerves may be causally related to increased levels of angiotensin II in HF. It has also been shown previously that angiotensin II causes an increased activation of the sympathetic activation via the PVN in HF. 74–76 Further, we have observed that angiotensin II may also cause a decrease in levels of nNOS in vitro as well as in the PVN of rats with HF. 77 So it is conceivable that the changes in the PVN are influenced by the removal of this angiotensin II component by removal of renal efferent nerves. The decline in circulating levels of angiotensin II and aldosterone after RDN in rats with HF are consistent with this overall hypothesis. However, it should be noted that the levels of angiotensin II are still significantly elevated after RDN in rats with HF suggesting that it might contribute to this response but may not be totally responsible for the changes observed after RDN. The causal role for angiotensin II in the effects of RDN on central regulation of sympathoexcitation in HF remains to be explored.
Activation of the PVN
Another intriguing alternative possibility for these observations is that since there was a slight improvement in cardiac function with RDN, this improvement (in cardiac function) may elicit a signal to improve central nNOS mechanism within the PVN, which in turn would alleviate the enhanced sympathoexcitation in rats with HF. Nevertheless, the improvement in centrally mediated sympathoinhibition by RDN appears to be mediated via the nitroxidergic mechanism within the PVN. As to the source of this signal or its modality remains to be examined but appears to be associated with intact renal nerves.
Perspectives
The current study offers insight into the mechanism of the positive effects of RDN during HF. To date, the mechanisms for the normalization of sympathetic outflow by RDN during HF have not been fully elucidated. Here, we found that RDN normalizes the activation of the PVN during HF, likely through an NO-dependent mechanism. RDN reversed FosB activation of the PVN during HF as well as ARN mediated sympathoexcitation. Additionally, there was increased response to L-NMMA microinjection after RDN in HF rats, suggesting restoration of nNOS inhibition in the PVN by RDN.
In conclusion, the increased activation of the PVN via possible activation of ARN during HF condition may contribute to the enhanced sympathetic drive exhibited in HF, and the PVN mediates the RDN effect of restoring sympathetic activity during HF. Additionally, we conclude that restoration of nNOS levels with RDN reverses enhanced PVN activation, as well as exaggerated systemic sympathetic activity commonly observed in the HF condition.
Supplementary Material
Novelty and Significance.
What is New?
The present study shows that removal of renal nerves by renal denervation (RDN) abrogates the elevated neuronal activity in the PVN as well as the concomitant increase in global sympathetic outflow in heart failure (HF) rats.
RDN restored the decreased endogenous nNOS in the paraventricular nucleus (PVN) of rats with HF.
Inhibition of endogenous NOS within the PVN produced an exaggerated increase in lumbar sympathetic nerve activity (LSNA) in HF rats after RDN.
What is Relevant?
A conceptually novel approach to reducing the enhanced sympathoexcitation in HF was to investigate the influence of RDN on PVN mediated sympathoexcitation in HF. Specifically, we hypothesized that increased renal afferent input in HF activates the pre-autonomic sympathetic neurons in the PVN leading to enhanced sympathoexcitation.
Studies utilizing RDN in hypertensive patients have provided exciting new insight into reduction of sympathetic tone. However the mechanism of action for these results remains unknown. This present study was specifically designed to elucidate the NO mechanism within a specific site, the PVN, to give further insight into potential driving mechanism and treatments for hyper-sympathetic states such as HF and hypertension.
Summary
Our studies reveal a possible mechanism for the therapeutic effects of RDN during HF and provide insight into how nNOS within the PVN, specifically may contribute to improving the elevated sympathetic tone during HF, likely through an NO-dependent mechanism.
Acknowledgments
Sources of Funding
This work was supported by NIH grants R56 HL124104 and P01 HL62222.
Footnotes
Disclosures
None.
References
- 1.Brändle M, Wang W, Zucker IH. Hemodynamic correlates of baroreflex impairment of heart rate in experimental canine heart failure. Basic Res Cardiol. 1996;91:147–154. doi: 10.1007/BF00799687. [DOI] [PubMed] [Google Scholar]
- 2.Levett JM, Marinelli CC, Lund DD, Pardini BJ, Nader S, Scott BD, Augelli NV, Kerber RE, Schmid PG., Jr Effects of beta-blockade on neurohumoral responses and neurochemical markers in pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 1994;266:H468–H475. doi: 10.1152/ajpheart.1994.266.2.H468. [DOI] [PubMed] [Google Scholar]
- 3.Zucker IH, Wang W, Brändle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 4.Patel KP. Neural regulation in experimental heart failure. Bailliere’s Clin Neurol. 1997;6:283–296. [PubMed] [Google Scholar]
- 5.Patel KP, Zhang K. Neurohumoral activation in heart failure: Role of paraventricular nucleus. Clin Exp Pharmacol Physiol. 1996;23:722–726. doi: 10.1111/j.1440-1681.1996.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 6.Li YF, Cornish KG, Patel KP. Alteration of nmda nr1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within pvn of rats with heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H995–H1004. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension. 2011;58:966–973. doi: 10.1161/HYPERTENSIONAHA.111.176222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H527–537. doi: 10.1152/ajpheart.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng H, Sharma NM, Liu X, Patel KP. Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: Role of angiotensin ii. Am J Physiol Regul Integr Comp Physiol. 2012;303:R387–394. doi: 10.1152/ajpregu.00046.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vahid-Ansari F, Leenen FHH. Pattern of neuronal activation in rats with chf after myocardial infarction. Am J Physiol Heart Circ Physiol. 1998;275:H2140–H2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 12.Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R923–R928. doi: 10.1152/ajpregu.1993.265.4.R923. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Zheng H, Patel KP. Enhanced activation of rvlm projecting pvn neurons in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H1700–1711. doi: 10.1152/ajpheart.00722.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciriello J. Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1745–R1754. doi: 10.1152/ajpregu.1998.275.6.R1745. [DOI] [PubMed] [Google Scholar]
- 15.Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates rvlm-projecting pvn neurons. Am J Physiol Heart Circ Physiol. 2015;308:H1103–1111. doi: 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel KP, Knuepfer M. Effect of afferent renal nerve stimulation on blood pressure and heart rate and noradrenergic activity in conscious rats. J Auton Nerv Syst. 1986;17:121–130. doi: 10.1016/0165-1838(86)90087-1. [DOI] [PubMed] [Google Scholar]
- 17.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res. 1997;753:102–119. doi: 10.1016/s0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- 18.Day TA, Ciriello J. Effects of renal receptor activation on neurosecretory vasopressin cells. Am J Physiol. 1987;253:R234–241. doi: 10.1152/ajpregu.1987.253.2.R234. [DOI] [PubMed] [Google Scholar]
- 19.Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol. 1988;254:R531–543. doi: 10.1152/ajpregu.1988.254.3.R531. [DOI] [PubMed] [Google Scholar]
- 21.Ciriello J, Caverson MM. Central organization of afferent renal nerve pathways. Clin Exp Hypertens A. 1987;9(Suppl 1):33–46. doi: 10.3109/10641968709160162. [DOI] [PubMed] [Google Scholar]
- 22.Kline RL. Renal nerves and experimental hypertension: Evidence and controversy1. Can J Physiol Pharmacol. 1987;65:1540–1547. doi: 10.1139/y87-243. [DOI] [PubMed] [Google Scholar]
- 23.Krum H, Schlaich MP, Whitbourn R, Sobotka P, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler MD. Catheter-based renal sympathetic denervation for resistant hypertension. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 24.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 25.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 26.Patel KP, Kline RL. Influence of renal nerves on noradrenergic responses to changes in arterial pressure. Am J Physiol. 1984;247:R615–R620. doi: 10.1152/ajpregu.1984.247.4.R615. [DOI] [PubMed] [Google Scholar]
- 27.Nozawa T, Igawa A, Fujii N, Kato B, Yoshida N, Asanoi H, Inoue H. Effects of long-term renal sympathetic denervation on heart failure after myocardial infarction in rats. Heart Vessels. 2002;16:51–56. doi: 10.1007/s380-002-8317-8. [DOI] [PubMed] [Google Scholar]
- 28.Schiller AM, Haack KK, Pellegrino PR, Curry PL, Zucker IH. Unilateral renal denervation improves autonomic balance in conscious rabbits with chronic heart failure. Am J Physiol Regul Integr Comp Physiol. 2013;305:R886–892. doi: 10.1152/ajpregu.00269.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang K, Li YF, Patel KP. Reduced endogenous gaba-mediated inhibition in the pvn on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1006–1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Li Y-F, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. Am J Physiol Renal Physiol. 2006;291:F1148–1156. doi: 10.1152/ajprenal.00501.2005. [DOI] [PubMed] [Google Scholar]
- 31.Li YF, Mayhan WG, Patel KP. Role of the paraventricular nucleus in renal excretory responses to acute volume expansion: Role of nitric oxide. Am J Physiol Heart Circ Physiol. 2003;285:H1738–1746. doi: 10.1152/ajpheart.00727.2002. [DOI] [PubMed] [Google Scholar]
- 32.Patel KP, Zhang PL, Carmines PK. Neural influences on renal responses to acute volume expansion in rats with heart failure. Am J Physiol. 1996;271:H1441–H1448. doi: 10.1152/ajpheart.1996.271.4.H1441. [DOI] [PubMed] [Google Scholar]
- 33.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 34.Hu J, Ji M, Niu C, Aini A, Zhou Q, Zhang L, Jiang T, Yan Y, Hou Y. Effects of renal sympathetic denervation on post-myocardial infarction cardiac remodeling in rats. PLoS One. 2012;7:e45986. doi: 10.1371/journal.pone.0045986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohm M, Ewen S, Kindermann I, Linz D, Ukena C, Mahfoud F. Renal denervation and heart failure. Eur J Heart Fail. 2014;16:608–613. doi: 10.1002/ejhf.83. [DOI] [PubMed] [Google Scholar]
- 36.Schiller AM, Pellegrino PR, Zucker IH. The renal nerves in chronic heart failure: Efferent and afferent mechanisms. Front Physiol. 2015;6:224. doi: 10.3389/fphys.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nestler EJ, Kelz MB, Chen J. Deltafosb: A molecular mediator of long-term neural and behavioral plasticity. Brain Res. 1999;835:10–17. doi: 10.1016/s0006-8993(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 38.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H227–236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guggilam A, Cardinale JP, Mariappan N, Sriramula S, Haque M, Francis J. Central tnf inhibition results in attenuated neurohumoral excitation in heart failure: A role for superoxide and nitric oxide. Basic Res Cardiol. 2011;106:273–286. doi: 10.1007/s00395-010-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ammons WS. Renal and somatic input to spinal neurons antidromically activated from the ventrolateral medulla. J Neurophysiol. 1988;60:1967–1981. doi: 10.1152/jn.1988.60.6.1967. [DOI] [PubMed] [Google Scholar]
- 41.Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: An anterograde transport study of horseradish peroxidase. J Auton Nerv Syst. 1983;8:273–285. doi: 10.1016/0165-1838(83)90110-8. [DOI] [PubMed] [Google Scholar]
- 42.Knuepfer MM, Schramm LP. The conduction velocities and spinal projections of single renal afferent fibers in the rat. Brain Res. 1987;435:167–173. doi: 10.1016/0006-8993(87)91598-8. [DOI] [PubMed] [Google Scholar]
- 43.Simon OR, Schramm LP. The spinal course and medullary termination of myelinated renal afferents in the rat. Brain Res. 1984;290:239–247. doi: 10.1016/0006-8993(84)90941-7. [DOI] [PubMed] [Google Scholar]
- 44.Vizzard MA, Standish A, Ammons WS. Effects of renal receptor stimulation on neurons within the ventrolateral medulla of the cat. Am J Physiol Regul Integr Comp Physiol. 1993;265:R290–R301. doi: 10.1152/ajpregu.1993.265.2.R290. [DOI] [PubMed] [Google Scholar]
- 45.Ciriello J, Calaresu FR. Hypothalamic projections of renal afferent nerves in the cat. Can J Physiol Pahrmacol. 1980;58:574–576. doi: 10.1139/y80-095. [DOI] [PubMed] [Google Scholar]
- 46.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. New Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 47.Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (nos) positive neurons in the hypothalamus of rats with heart failure. Brain Res. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2. [DOI] [PubMed] [Google Scholar]
- 48.Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Res. 1996;734:109–115. [PubMed] [Google Scholar]
- 49.Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 50.Kuo DC, Nadelhaft I, Hisamitsu T, de Groat WC. Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol. 1983;216:162–174. doi: 10.1002/cne.902160205. [DOI] [PubMed] [Google Scholar]
- 51.Saeki Y, Terui N, Kumada M. Participation of ventrolateral medullary neurons in the renal-sympathetic reflex in rabbits. Jpn J Physiol. 1988;38:267–281. doi: 10.2170/jjphysiol.38.267. [DOI] [PubMed] [Google Scholar]
- 52.Calaresu FR, Kim P, Nakamura H, Sato A. Electrophysiological characteristics of renorenal reflexes in the cat. J Physiol. 1978;283:141–154. doi: 10.1113/jphysiol.1978.sp012493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malliani A, Montano N. Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension. 2002;39:63–68. doi: 10.1161/hy0102.099200. [DOI] [PubMed] [Google Scholar]
- 54.Grisk O, Rettig R. Interactions between the sympathetic nervous system and the kidneys in arterial hypertension. Cardiovasc Res. 2004;61:238–246. doi: 10.1016/j.cardiores.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 55.Malpas SC, Ramchandra R, Guild SJ, McBryde F, Barrett CJ. Renal sympathetic nerve activity in the development of hypertension. Curr Hypertens Rep. 2006;8:242–248. doi: 10.1007/s11906-006-0057-0. [DOI] [PubMed] [Google Scholar]
- 56.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: Novel implications for an old concept. Hypertension. 2009;54:1195–1201. doi: 10.1161/HYPERTENSIONAHA.109.138610. [DOI] [PubMed] [Google Scholar]
- 57.Kopp UC, Cicha MZ, Smith LA. Impaired responsiveness of renal mechanosensory nerves in heart failure: Role of endogenous angiotensin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R116–124. doi: 10.1152/ajpregu.00336.2002. [DOI] [PubMed] [Google Scholar]
- 58.Giamouzis G, Butler J, Triposkiadis F. Renal function in advanced heart failure. Congest Heart Fail. 2011;17:180–188. doi: 10.1111/j.1751-7133.2011.00240.x. [DOI] [PubMed] [Google Scholar]
- 59.Henegar JR, Zhang Y, De Rama R, Hata C, Hall ME, Hall JE. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens. 2014;27:1285–1292. doi: 10.1093/ajh/hpu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linz D, Hohl M, Schutze J, Mahfoud F, Speer T, Linz B, Hubschle T, Juretschke HP, Dechend R, Geisel J, Rutten H, Bohm M. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: The role of renal sympathetic innervation. Am J Hypertens. 2015;28:256–265. doi: 10.1093/ajh/hpu123. [DOI] [PubMed] [Google Scholar]
- 61.Ott C, Mahfoud F, Schmid A, Ditting T, Veelken R, Ewen S, Ukena C, Uder M, Bohm M, Schmieder RE. Improvement of albuminuria after renal denervation. Int J Cardiol. 2014;173:311–315. doi: 10.1016/j.ijcard.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 62.Patel KP. Role of paraventrivular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 63.Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst. 1981;3:311–320. doi: 10.1016/0165-1838(81)90072-2. [DOI] [PubMed] [Google Scholar]
- 64.Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM, Zhu GQ. Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf) 2015;213:778–794. doi: 10.1111/apha.12447. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Ma R. Cardiac sympathetic afferent reflexes in heart failure. Heart Fail Rev. 2000;5:57–71. doi: 10.1023/A:1009898107964. [DOI] [PubMed] [Google Scholar]
- 66.Caverson MM, Ciriello J. Renal and cardiovascular afferent inputs to hypothalamic paraventriculo-spinal neurons. Neurosci Lett. 1988;95:167–172. doi: 10.1016/0304-3940(88)90651-9. [DOI] [PubMed] [Google Scholar]
- 67.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014;64:745–755. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chinushi M, Izumi D, Iijima K, Suzuki K, Furushima H, Saitoh O, Furuta Y, Aizawa Y, Iwafuchi M. Blood pressure and autonomic responses to electrical stimulation of the renal arterial nerves before and after ablation of the renal artery. Hypertension. 2013;61:450–456. doi: 10.1161/HYPERTENSIONAHA.111.00095. [DOI] [PubMed] [Google Scholar]
- 70.Spelman FA, Oberg PA. Continuous measurement of renal cortical blood flow and renal arterial blood flow during stimulation of the renal nerve. Med Biol Eng Compt. 1991;29:121–128. doi: 10.1007/BF02447096. [DOI] [PubMed] [Google Scholar]
- 71.Pedersen EB, Danielsen H, Jensen T, Madsen M, Sorensen SS, Thomsen OO. Angiotensin ii, aldosterone and arginine vasopressin in plasma in congestive heart failure. Eur J Clin Invest. 1986;16:56–60. doi: 10.1111/j.1365-2362.1986.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 72.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the pvn in heart failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1863–1872. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel KP, Zhang K, Carmines PK. Norepinephrine turnover in peripheral tissues of rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2000;278:R556–R562. doi: 10.1152/ajpregu.2000.278.3.R556. [DOI] [PubMed] [Google Scholar]
- 74.Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1364–1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H423–433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- 76.Huang BS, Zheng H, Tan J, Patel KP, Leenen FH. Regulation of hypothalamic renin-angiotensin system and oxidative stress by aldosterone. Exp Physiol. 2011;96:1028–1038. doi: 10.1113/expphysiol.2011.059840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma NM, Zheng H, Mehtam PP, Li YF, Patel KP. Decreased nnos in the pvn leads to increased sympathoexcitation in chronic heart failure: Role for capon and ang ii. Cardivasc Res. 2011;92:348–357. doi: 10.1093/cvr217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.