Abstract
Adipose tissue dysfunction related to obesity is overwhelmingly associated with increased risk of developing cardiovascular diseases. In the setting of obesity, (pro)renin receptor (PRR) is increased in adipose tissue of mice. We sought to determine the physiological consequences of adipocyte-PRR deficiency using Adiponectin-Cre mice. We report a unique model of adipocyte-PRR-deficient mice (PRRAdi/Y) with almost no detectable white adipose tissues. As a consequence, the livers of PRRAdi/Y mice were enlarged and demonstrated a marked accumulation of lipids. Adipocyte-specific deficiency of PRR increased systolic blood pressure (SBP) and the concentration of soluble PRR (sPRR) in plasma. To determine whether adipocyte PRR was involved in the development of obesity-induced hypertension, mice were fed a low-fat or a high-fat diet for 16 weeks. Adipocyte-PRR-deficient mice were resistant to diet-induced obesity. Both high- and low-fat-fed PRRAdi/Y mice had elevated insulin levels. Interestingly, adipocyte-PRR deficiency improved glucose tolerance in high-fat-fed PRRAdi/Y mice. In response to feeding either low-fat or high-fat diets, SBP was greater in PRRAdi/Y mice compared with control mice. High-fat feeding elevated sPRR concentration in control and PRRAdi/Y mice. In vitro knock down of PRR by siRNA significantly decreased mRNA abundance of PPARγ, suggesting an important role for PRR in adipogenesis. Our data indicate that adipocyte PRR is involved in lipid homeostasis and glucose and insulin homeostasis, and that soluble PRR may be a predictor of metabolic disturbances and play a role in SBP regulation.
Keywords: prorenin receptor, adipocytes, blood pressure, insulin, glucose, lipids
Hypertension is the major cause of cardiovascular diseases worldwide and, according to the NHANES III, the prevalence of hypertension continues to increase.1,2 Obesity is an important risk factor for hypertension.1 The renin angiotensin system (RAS) is recognized for playing a critical role in the regulation of blood pressure and sodium and water homeostasis. The deletion of components of the RAS, for instance angiotensinogen (AGT) in liver or adipose tissue, prevents obesity-related hypertension.3,4
Among the components of the RAS expressed in adipose tissue,5,6 (pro)renin receptor (PRR) is abundant and is up-regulated during the development of obesity.3,7–9 PRR is a 350 amino acid protein with a single transmembrane domain and has been first identified as the receptor for renin in its active form and for prorenin in its inactive form.10,11 Treatment of preadipocytes, extracted from adipose tissue, with AGT and renin results in a dose-dependent increase in angiotensin I (AngI) generation. Specific deletion of PRR in the brain attenuates angiotensin II–dependent hypertension, whereas human PRR transgenic rats exhibit elevated systolic blood pressure.12,13 Taken together, these results suggest that adipose PRR may potentially play a role in blood pressure control.7,11 In addition, PRR can be cleaved intracellularly by furin, resulting in the secretion of a soluble form of PRR (sPRR) in plasma14 and urine,15,16 which might bind renin and prorenin15 and participate in AngI formation.14–17 Previous studies have shown that increased plasma sPRR levels in early pregnancy are associated with the development of preeclampsia,18 a hypertension-related complication. Conversely, lower plasma sPRR levels were observed in patients treated with angiotensin II receptor blockers.19 The physiological consequences of changes in plasma sPRR levels during the development of obesity and hypertension remain unclear.
The function of PRR is not restricted to AngI generation and hypertension. The binding of renin or prorenin to PRR in mesangial cells11 and 3T3-L1 preadipocytes7,8 initiates an intracellular signaling cascade associated with the activation of the ERK1/2 pathway. In mesangial cells, the activation of the ERK1/2 pathway leads to the release of TGFβ1 and cytokines involved in inflammation. However, past attempts to generate complete PRR knock-out mice failed.20 Specific cardiomyocyte or podocyte deletion of PRR led to animal lethality 3 weeks after birth due to heart or kidney failure.21–24 Since PRR interacts with V-ATPase, the deletion of PRR may trigger destabilization of V-ATPase activity, leading to a decrease in vacuolar acidification and to the lethality of cells.21–24 Additionally, recent studies in xenopus have shown that PRR may link V-ATPase to the Wnt receptor protein, LRP6, and induce phosphorylation of LRP6.25,26 The objectives of our study were (1) to determine whether a specific adipocyte-PRR deficiency mouse model is viable, (2) to determine the physiological consequences of adipocyte-PRR deletion on blood pressure in normal physiology and during the development of obesity, and (3) to determine the relationship between adipocyte PRR, plasma sPRR concentrations, the RAS, and blood pressure.
Methods and Animals
All procedures involving animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky (University of Kentucky IACUC protocol number: 2013-1109). Female mice with loxP sites flanking exon 2 of the PRR gene (PRRfl/fl) were bred to transgenic male mice (PRRfl/Y) expressing Cre recombinase under the control of the adiponectin promoter (Strain Name: B6;FVB-Tg(Adipoq-cre)1Evdr/JB6;FVB-Tg(Adipoq-cre)1Evdr/J)24 (Figure 1A). Diets, plasma measurements, histology, radiotelemetry and statistical analyses are described in the online-only Data Supplement.
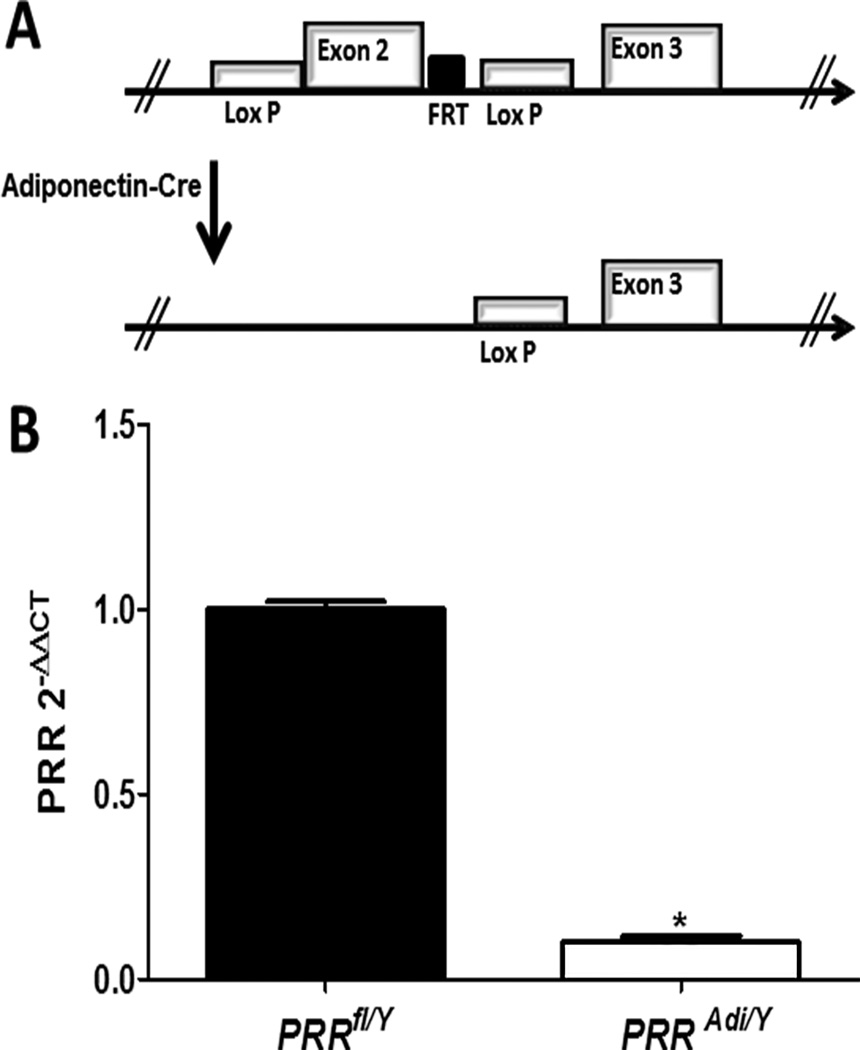
Figure 1.
(A) Schematic representation of the loxP-flanked PRR allele before (a) and after recombination with Adiponectin-driven Cre expression (b). (B) mRNA PRR abundance of differentiated adipocyte from subcutaneous adipose tissue of PRRfl/Y and PRRAdi/Y mice. Data are mean±SEM of 3 to 6 mice. * P<0.05 compared with PRRfl/Y mice.
Statistical analysis
Results are expressed as mean±SEM. All data were analyzed using Sigma Plot and Graph Prism. ANOVAs (and ANOVA repeated measures when appropriate) were used to compare diet and genotype effects, followed by post-hoc tests using Holm-Sidak or Bonferroni corrections for multiple comparisons. When the assumptions underlying the ANOVAs were not otherwise met, data were nonlinearly transformed; however, for ease of illustration, figures show untransformed data. GraphPad QuickCalcs (Grubbs’ test) was used to determine statistical outliers and a t-test was used to compare mean insulin levels between HF- and LF-fed mice. Statistical significance was defined as P<0.05.
Results
Generation of mice with adipocyte-PRR deficiency
To confirm efficiency of PRR deletion in adipocytes, pre-adipocytes from the stromal vascular fraction of subcutaneous adipose tissue of control mice (PRRfl/Y) and adipocyte-PRR-deficient mice (PRRAdi/Y) were differentiated into adipocytes (Figure 1A). PRR mRNA abundance was markedly reduced in adipocytes differentiated from PRRAdi/Y mice compared with control PRRfl/Y mice (Figure 1B; P<0.05) demonstrating the efficacy of the deletion.
Adipocyte deficiency of PRR drastically decreased adipose tissue mass in male mice fed a standard diet
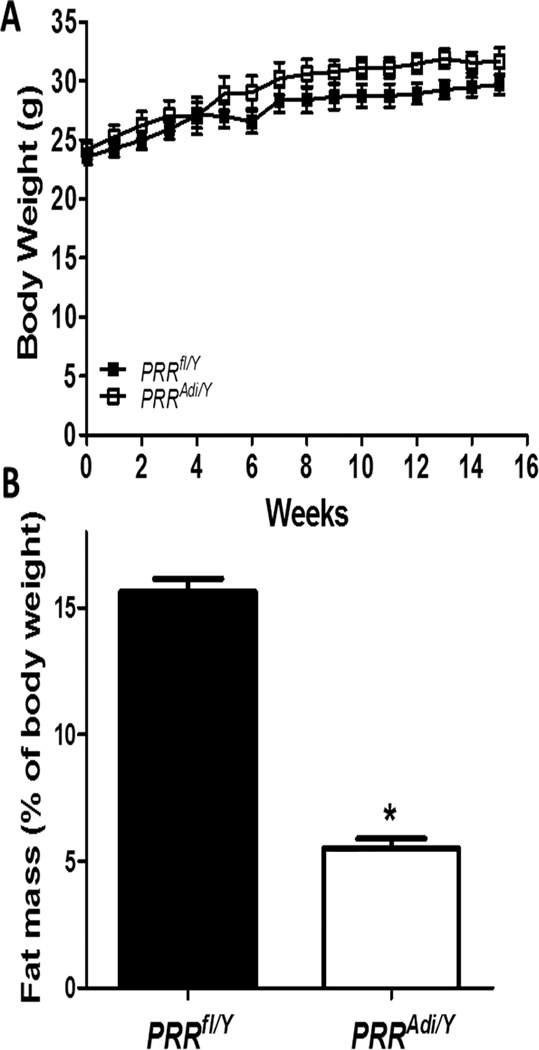
Body weight increased with age (Figure 2A) with no significant difference between genotypes. The fat mass was significantly reduced in PRRAdi/Y compared with control PRRfl/Y mice (Figure 2B) and did not increase with age in PRRAdi/Y mice suggesting PRRAdi/Y mice do not accumulate adipose tissue (Figure S1). The mass of all white adipose tissues was significantly reduced in PRRAdi/Y mice compared with PRRfl/Y mice (Table S1, P<0.05). The analyses of epididymal adipose tissue morphology from PRRfl/Y mice revealed the presence of differentiated adipocytes throughout the section, whereas histological analysis of residual adipose tissue from around the epididymis of PRRAdi/Y mice showed an unexpectedly very small number of differentiated adipocytes (Figure S2). To determine whether adipocyte PRR was involved in adipocyte differentiation, PRR was silenced in vitro in 3T3-L1-cells (Figure S3). PPARγ, an important gene involved in adipocyte differentiation, and fatty acid-binding protein 4 (Fabp4 or aP2), a marker for differentiated adipocytes and a carrier protein for fatty acids, were evaluated. The abundance of mRNA PPARγ and Fabp4 were significantly decreased in differentiated siPRR cells compared with control cells.
Figure 2.
Adipocyte PRR deficiency reduced fat mass in mice fed a standard diet. (A) Body weight curves of PRRfl/Y and PRRAdi/Y mice. Data are mean±SEM of 4 to 6 mice. (B) Fat mass (% of body weight) for mice in each group. Data are mean±SEM of 4 to 6 mice. * P<0.05 compared with PRRfl/Y.
Tissue weights of liver, spleen and pancreas were significantly higher in PRRAdi/Y mice compared with PRRfl/Y mice (Table S1). Leptin plasma concentrations were lower in PRRAdi/Y mice than in PRRfl/Y mice (Table S1). Glucose tolerance did not differ between genotypes at week 5, 9 or 13 of the experiment (Figure S4).
Adipocyte-specific deficiency of PRR triggered lipid accumulation in livers of male mice fed a standard diet
Microscopic examination of liver sections revealed an increase in hepatic fat accumulation comprising small and large fat vacuoles in PRRAdi/Y mice compared with PRRfl/Y mice (Figure S5). Neutral lipids were significantly increased in liver of PRRAdi/Y mice compared with PRRfl/Y mice. Plasma triglycerides did not differ significantly between groups (Table S1).
Adipocyte-specific deficiency of PRR increased systolic blood pressure of male mice fed a standard diet
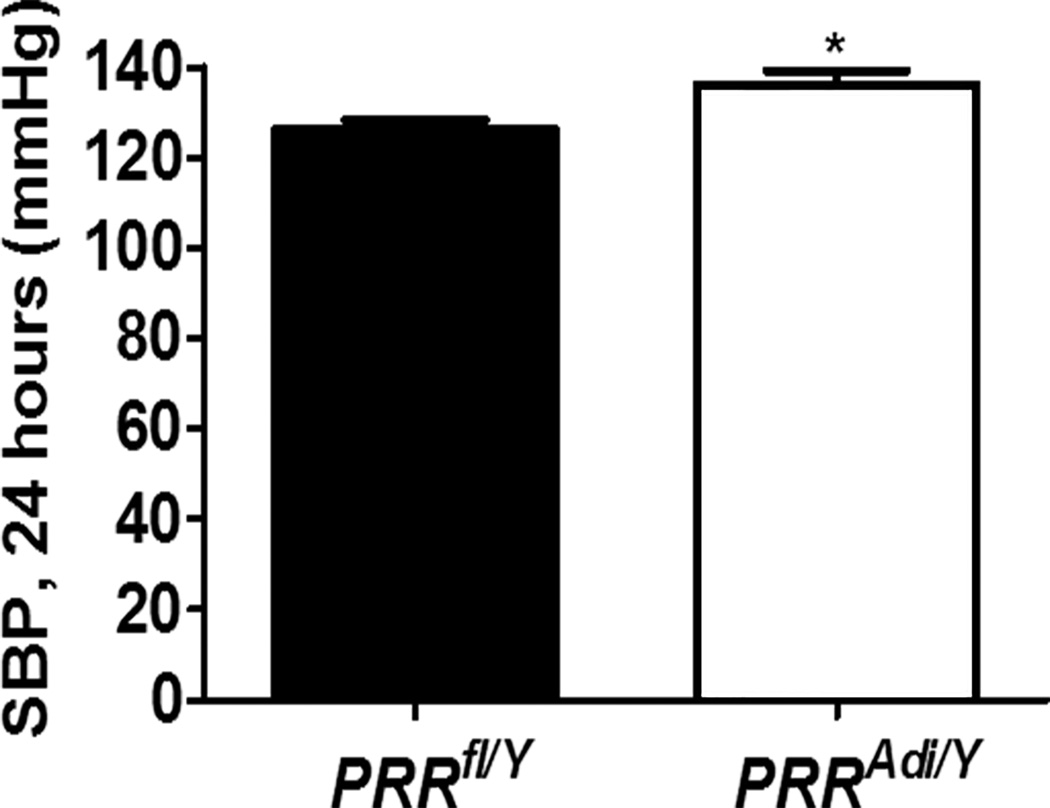
The SBP and the pulse pressure were significantly higher in PRRAdi/Y mice compared with PRRfl/Y mice (Figure 3). Mean arterial blood pressure, diastolic blood pressure, and heart rate did not differ significantly between groups (Table S2).
Figure 3.
Adipocyte PRR deficiency increased systolic blood pressure (SBP; 24 h) in mice fed a standard diet. (A) SBP (24 h) for male PRRfl/Y and PRRAdi/Y mice. Data are mean±SEM of 4 mice. *P<0.05 compared with PRRfl/Y mice.
Adipocyte-specific deficiency of PRR increased plasma sPRR concentrations
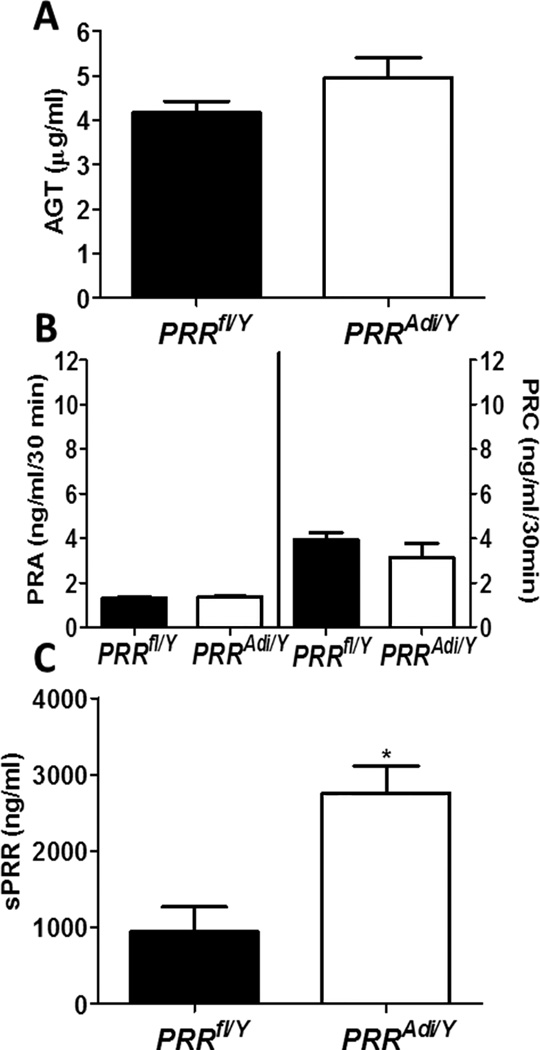
Adipocyte-specific deficiency of PRR did not change plasma AGT concentration (Figure 4A). Plasma renin activity (PRA) and plasma renin concentration (PRC) did not differ significantly between PRRAdi/Y mice and PRRfl/Y mice (Figure 4B), suggesting that adipocyte-specific deficiency of PRR did not influence AGT, PRA or PRC. Surprisingly, plasma sPRR levels increased by threefold in PRRAdi/Y mice compared with PRRfl/Y mice (Figure 4C).
Figure 4.
Adipocyte PRR deficiency increased plasma sPRR. (A) Plasma AGT concentrations in male PRRfl/Y and PRRAdi/Y mice. (B) Plasma renin activity (PRA; left y axis) and concentrations (PRC; right y axis) in male PRRfl/Y and PRRAdi/Y mice. (C) Plasma sPRR concentrations in male PRRfl/Y and PRRAdi/Y mice. Data are mean±SEM of 4 to 6 mice. * P<0.05 compared with PRRfl/Y mice.
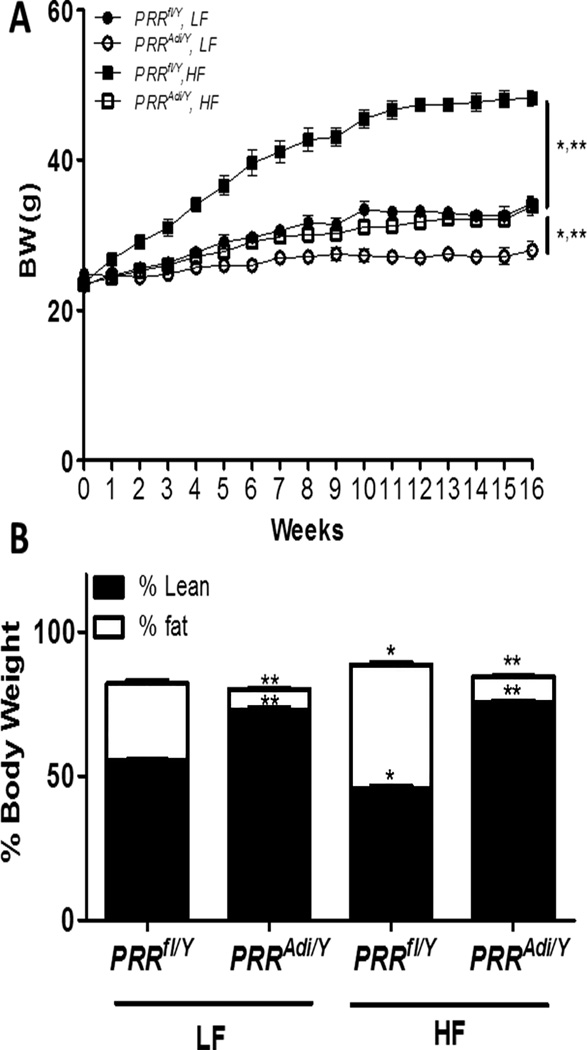
Adipocyte-specific deficiency of PRR prevented the development of obesity and the accumulation of fat mass in high-fat-fed mice
PRRAdi/Y mice were resistant to HF-diet-induced obesity (Figure 5A). The fat mass of PRRAdi/Y mice was lower by about −70% compared with LF-fed PRRfl/Y mice and was lower by almost −80% compared with HF-fed PRRfl/Y mice (Figure 5B). When challenged with a HF diet, tissue weights of liver, heart, and kidney were significantly higher in PRRAdi/Y mice compared with PRRfl/Y mice (Table S3). Adipocyte-PRR deficiency did not significantly affect kidney structure (Figure S6). HF- and LF-fed PRRAdi/Y mice had increased lipid accumulation in liver compared with PRRfl/Y mice (Figure S7). Adipocyte-PRR deficiency did not change PRR mRNA levels in kidney and liver (Figure S8A and S8B). The HF-diet induced a significant increase in plasma cholesterol, which did not differ between genotypes.
Figure 5.
Adipocyte PRR deficiency prevented the development of obesity and decreased fat mass. (A) Body weight curve of PRRfl/Y and PRRAdi/Y mice fed a low-fat (LF) or high-fat (HF) diet. Data are mean ± SEM from n = 5 to 8 mice/group. (B) Fat and lean mass (% of body weight) of PRRfl/Y and PRRAdi/Y mice fed a LF or HF diet. Data are mean±SEM of 3 to 8 mice.
When challenged with HF diet, adipocyte-specific deficiency of PRR improved glucose homeostasis
Glucose tolerance did not differ between genotypes after 16 weeks of LF-diet (Figure S9A and S9B). However, HF-fed PRRAdi/Y mice exhibited improved glucose tolerance compared with HF-fed PRRfl/Y mice. Fasting glucose levels were significantly lower in HF-or LF-fed PRRAdi//Y mice compared with PRRfl/Y mice (Figure S9C). Adipocyte-PRR deficiency induced a significant increase in plasma insulin levels regardless of diet (Table S3).
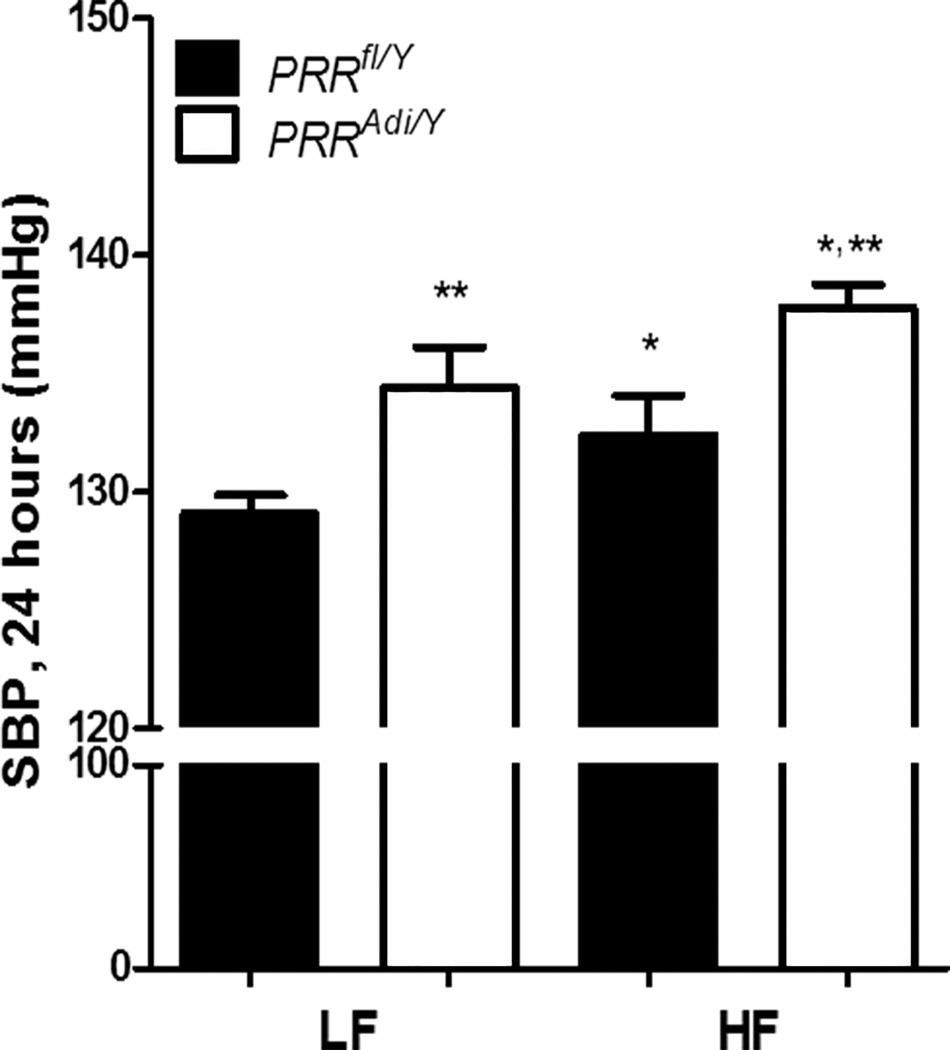
Despite the resistance to HF-diet-induced obesity, adipocyte-specific deficiency of PRR further increased SBP
Adipocyte-specific deficiency of PRR induced a significant increase in SBP in LF-fed mice (Figure 6). The increase in SBP, resulting from PRR deficiency, was further exacerbated when PRRAdi/Y mice were fed a HF diet. These data suggest that adipocyte-specific deficiency of PRR aggravated HF-diet-induced elevation of SBP. Mean arterial pressure and heart rate were higher in PRRAdi/Y mice regardless of diet (Table S4 and S5).
Figure 6.
Adipocyte PRR deficiency exaggerated diet-induced increase of blood pressure. SBP (24 h) of male PRRfl/Y and PRRAdi/Y mice fed a low-fat (LF) or high-fat (HF) diet. Data are mean±SEM from n = 5 to 8 mice. * P<0.05 compared with LF diet. ** P<0.05 compared with PRRfl/Y mice.
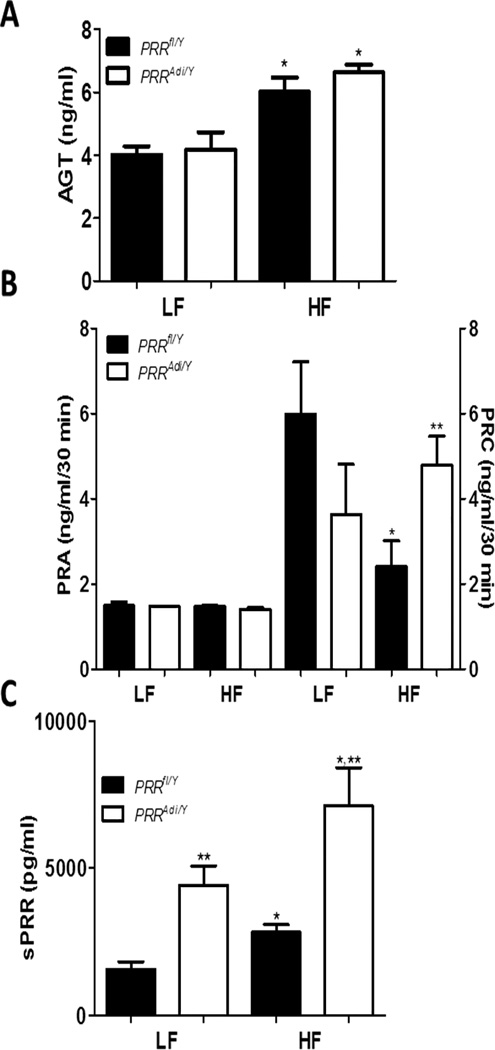
In obese mice, adipocyte-specific deficiency of PRR exaggerated the elevation of plasma sPRR levels
High-fat feeding induced a significant increase in plasma AGT concentrations in control PRRfl/Y mice (Figure 7A). However, plasma AGT concentrations did not differ between PRRAdi/Y and PRRfl/Y mice regardless of diet. PRA was not influenced by the diet or by adipocyte-specific PRR deficiency (Figure 7B). When challenged with HF diet, PRRfl/Y mice exhibited a lower PRC and a lower total prorenin/renin concentration than LF-fed PRRfl/Y mice (Figure 7B, Table S3). PRC and total prorenin/renin concentration in LF- and HF-fed PRRAdi/Y mice did not differ from those of LF-fed PRRfl/Y mice.
Figure 7.
(A) Plasma AGT concentrations in male PRRfl/Y and PRRAdi/Y mice fed a LF- or HF-diet. (B) Plasma renin activity (PRA; left y axis) and concentration (PRC; right y axis). (C) Plasma sPRR concentration. Data are mean±SEM of 5 to 8 mice. * P<0.05 compared with LF diet. ** P<0.05 compared with PRRfl/Y mice.
Plasma sPRR levels were significantly increased in LF-fed PRRAdi/Y mice compared with LF-fed PRRfl/Y mice. HF feeding induced a threefold increase in plasma sPRR levels in HF-fed PRRfl/Y mice compared with LF-fed PRRfl/Y mice (Figure 7C). Plasma sPRR levels were more than twofold higher in HF-fed PRRAdi/Y mice compared with HF-fed PRRfl/Y mice. Plasma sPRR concentration was positively correlated with SBP (P<0.05) in PRRfl/Y mice and PRRAdi/ mice combined (Figure S10A) and in PRRfl/Y mice alone; the correlation was weaker (P>0.05) in PRRAdi//Y mice alone. However, plasma insulin levels were not correlated with SBP (Figure S10B).
Discussion
This study examined the role of adipocyte-derived PRR in blood pressure control and the physiological consequences of the deletion of PRR in adipocytes of male mice during the development of obesity. The deletion of adipocyte PRR induced a marked reduction in all white adipose tissues with no abnormal distribution of adipose tissue pads. In vitro studies demonstrated that PRR regulated PPARγ and Fabp4. The lipodystrophy was accompanied by hepatic steatosis. When challenged with HF feeding, adipose PRR-deficient mice were resistant to the development of obesity and had improved glucose tolerance. Despite the absence of white adipose tissue and the resistance to diet-induced obesity, mice with adipocyte-PRR deficiency had elevated blood pressure. This blood pressure elevation in adipocyte-PRR-deficient mice appeared to be independent of systemic AGT and renin concentrations. Surprisingly, plasma sPRR concentrations were increased with HF diet and markedly elevated in adipocyte-PRR-deficient mice.
Deletion of adipocyte PRR led to a reduction of adipose tissue mass and an increase in lipid deposition in liver, suggesting lipodystrophy accompanied by liver steatosis. In vitro PRR silencing revealed a significantly decrease of PPARγ and Fabp4 suggesting that PRR is a master regulator of adipocytes differentiation. Additionally, since fatty acid binding proteins are important carriers for fatty acids uptake and fatty acids transport to sites of esterification into triglycerides,27our data suggest an important role of PRR in fatty acid trafficking and storage in adipocytes.
Our phenotype has been observed in other models of lipodystrophy such as the A-ZIP/F, aP2/DTA, SREBP-1c, or fatty liver dystrophy (fld) transgenic mouse models.28,29 In contrast, PRRAdi/Y mice fed a HF diet demonstrated much greater glucose sensitivity than HF-fed control mice. Our results differ from those of other mouse models of lipodystrophy, in which hyperglycemia and hypertriglyceridemia are commonly observed. In addition, the plasma insulin levels in PRRAdi/Y mice increased modestly, and PRRAdi/Y mice did not present severe hyperinsulinemia. Our data are nevertheless in agreement with the phenotype of the PPARγP465L/+ mouse model,30 which had improved ability to respond to acute glucose overload compared with controls when challenged with HF feeding. As suggested by Tsai et al.,30 the expansion of pancreatic islets likely could contribute to this increased responsiveness to glucose. Similar to our model, CGI-58β mouse31 model developed hepatic steatosis but were protected against obesity and glucose intolerance. A reduction of body weight may also have contributed to better glucose sensitivity.
PRRAdi/Y mice exhibited elevated blood pressure similar to that reported in PPARγP465L/+ mice and humans expressing FPLD2 and FPLD3 mutations.28–30 In the latter instances, the cause of increased blood pressure is not well understood. Elevated leptin has been associated with elevated blood pressure32 but could protect against nonalcoholic fatty liver disease33. Thus, while it is unlikely that low levels of circulating leptin in PRRAdi/Y mice could have contributed to elevated blood pressure, low levels of circulating leptin may have contributed to the development of liver steatosis.
Insulin resistance can cause increased blood pressure, thus elevated insulin levels could have participate to the elevation of SBP in PRRAdi/Y mice.28 However, our results demonstrated that insulin levels were not correlated with SBP suggesting that elevated insulin might not be the origin of elevated blood pressure. In contrast, we have demonstrated that plasma sPRR levels increased with the development of obesity-induced hypertension. Surprisingly, the elevation of plasma sPRR concentrations was exacerbated by adipocyte-PRR deficiency. The elevation of plasma sPRR concentration during early pregnancy has been reported to predict both hypertension and preeclampsia risk in pregnant woman.18 Moreover, patients with heart failure have higher plasma sPRR levels than control subjects.34 However, our demonstration of a positive correlation between sPRR and SBP when control mice and PRRAdi/Y mice are combined or in control mice only suggests that sPRR could play a role in blood pressure control. Further investigation are needed regarding a direct effect of sPRR on SBP. The heart, brain, liver, kidney and smooth muscle express PRR gene and could potentially participate to the release of sPRR or be potential target tissues.13–18,34
Adipose tissue is one source of systemic angiotensin II, and both expansion or reduction of adipose tissue activates adipose RAS, thereby influencing blood pressure regulation.3,4,30,35,36 Since Tsai et al.30 demonstrated that expression of AGT and AT1R in adipose tissue is increased in PPARγP465L/+ mice, the increase in blood pressure in PRRAdi/Y mice could be attributed to a local activation of adipose RAS. In PRRAdi/Y mice, the reduction of adipose tissue weight in conjunction with elevated plasma AGT may have activated local adipose RAS. Unfortunately, due to the severe reduction in adipose tissue, we lacked sufficient adipose tissue weight to confirm this hypothesis. Hepatocyte AGT deficiency induced profound reductions in blood pressure, systemic AGT, and angiotensin II, which influences adipose RAS content and secretion despite the continued presence of obesity.4 Since the liver is an important source of renal angiotensin II,37 it may also be possible that other local RAS are activated to compensate for the absence of adipose tissue. In contrast to liver AGT deficiency, PRRAdi/Y mice demonstrated a radical shift in lipid distribution resulting in hepatic steatosis, which might also have influenced adipose and other local RAS systems.38 Indeed, despite lower body weights and adipose tissue weights in PRRAdi/Y mice, systemic AGT concentrations were not lower compared with those of obese control mice, suggesting substantial compensatory activation of systemic AGT from other tissues.
Perspectives
The remarkable phenotype of the adipocyte-PRR-deficient mouse model demonstrates the importance of adipocyte PRR in lipid and glucose and insulin homeostasis. Our results demonstrate the necessity of adipocyte PRR in the normal development of adipose tissue, beyond its potential role in local RAS activation. Further investigation is needed to determine the mechanism by which PRR regulates adipose cell formation and lipid homeostasis and blood pressure.
Supplementary Material
Novelty and Significance.
What is new?
Demonstration that adipocyte-derived PRR deficiency regulates fat mass growth
Demonstration that adipocyte-derived PRR influences lipid homeostasis and glucose and insulin homeostasis
Demonstration that adipocyte-derived PRR deficiency increases plasma sPRR and blood pressure
What is relevant?
This study demonstrates that adipocyte PRR is essential for the development of adipose tissue.
This study demonstrates that adipocyte PRR contributes to the control of blood pressure.
Summary
The effect of (pro)renin receptor to reduce fat mass is profound in mice with diet-induced obesity, demonstrating the important role of PRR in fat mass growth. Adipose PRR-deficient mice show elevated plasma insulin. In obese mice, adipocyte PRR deficiency improves glucose tolerance. Adipocyte PRR deficiency elevates SBP and increases plasma sPRR levels.
Acknowledgments
We thank Vickie English from the laboratory of Dr. Cassis for her assistance in the measurement of renin.
Sources of Funding
These studies were supported by grants from the American Heart Association (13SDG17230008), the National Institute of General Medical Sciences (P20-GM103527), and the University of Kentucky, Center for Clinical and Translational Sciences (UL1TR000117).
Footnotes
Disclosures
None.
References
- 1.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52 doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 3.Yiannikouris F, Gupte M, Putnam K, Thatcher S, Charnigo R, Rateri DL, Daugherty A, Cassis LA. Adipocyte deficiency of angiotensinogen prevents obesity-induced hypertension in male mice. Hypertension. 2012;60:1524–1530. doi: 10.1161/HYPERTENSIONAHA.112.192690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yiannikouris F, Wang Y, Shoemaker R, Larian N, Thompson J, English VL, Charnigo R, Su W, Gong M, Cassis LA. Deficiency of Angiotensinogen in Hepatocytes Markedly Decreases Blood Pressure in Lean and Obese Male Mice. Hypertension. 2015;66:836–842. doi: 10.1161/HYPERTENSIONAHA.115.06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10:93–98. doi: 10.1007/s11906-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111–117. doi: 10.1016/j.mce.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achard V, Tassistro V, Boullu-Ciocca S, Grino M. Expression and nutritional regulation of the (pro)renin receptor in rat visceral adipose tissue. J Endocrinol Invest. 2011;34:840–846. doi: 10.3275/7627. [DOI] [PubMed] [Google Scholar]
- 8.Achard V, Boullu-Ciocca S, Desbriere R, Nguyen G, Grino M. Renin receptor expression in human adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R274–R282. doi: 10.1152/ajpregu.00439.2005. [DOI] [PubMed] [Google Scholar]
- 9.Tan P, Shamansurova Z, Bisotto S, Michel C, Gauthier MS, Rabasa-Lhoret R, Nguyen TM, Schiller PW, Gutkowska J, Lavoie JL. Impact of the prorenin/renin receptor on the development of obesity and associated cardiometabolic risk factors. Obesity (Silver Spring) 2014;22:2201–2209. doi: 10.1002/oby.20844. [DOI] [PubMed] [Google Scholar]
- 10.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med. 2006;18:483–488. [PubMed] [Google Scholar]
- 11.Nguyen G, Blanchard A, Curis E, Bergerot D, Chambon Y, Hirose T, Caumont-Prim A, Tabard SB, Baron S, Frank M, Totsune K, Azizi M. Plasma soluble (pro)renin receptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertension. 2014;63:297–302. doi: 10.1161/HYPERTENSIONAHA.113.02217. [DOI] [PubMed] [Google Scholar]
- 12.Burckle A, Danser AH, Muller N, Garrelds I, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated Blood Pressure and Heart Rate in Human Renin Receptor Transgenic Rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Peng H, Cao T, Sato R, McDaniels SJ, Kobori H, Navar LG, Feng Y. Brain-targeted (pro)renin receptor knockdown attenuates angiotensin II-dependent hypertension. Hypertension. 2012;59:1188–1194. doi: 10.1161/HYPERTENSIONAHA.111.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53:1077–1082. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 15.Biswas KB, Nabi AN, Arai Y, Nakagawa T, Ebihara A, Ichihara A, Inagami T, Suzuki F. Qualitative and quantitative analyses of (pro)renin receptor in the medium of cultured human umbilical vein endothelial cells. Hypertens Res. 2001;34:735–739. doi: 10.1038/hr.2011.26. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57:859–864. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, Morimoto S, Jwa SC, Egawa M, Arai Y, Suzuki F, Sago H, Ichihara A. Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertension. 2012;60:1250–1256. doi: 10.1161/HYPERTENSIONAHA.112.197418. [DOI] [PubMed] [Google Scholar]
- 19.Hamada K, Taniguchi Y, Shimamura Y, et al. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol. 2013;17:848-586. doi: 10.1007/s10157-013-0803-y. [DOI] [PubMed] [Google Scholar]
- 20.Burckle C, Bader M. Prorenin and its ancient receptor. Hypertension. 2006;48:549–551. doi: 10.1161/01.HYP.0000241132.48495.df. [DOI] [PubMed] [Google Scholar]
- 21.Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res. 2010;107:30–34. doi: 10.1161/CIRCRESAHA.110.224667. [DOI] [PubMed] [Google Scholar]
- 22.Muller DN, Binger KJ, Riediger F. Prorenin receptor regulates more than the renin-angiotensin system. Ann Med. 2012;44(Suppl 1):S43–S48. doi: 10.3109/07853890.2012.660496. [DOI] [PubMed] [Google Scholar]
- 23.Reudelhuber TL. The interaction between prorenin, renin and the (pro)renin receptor: time to rethink the role in hypertension. Curr Opin Nephrol Hypertens. 2012;21:137–141. doi: 10.1097/MNH.0b013e3283500927. [DOI] [PubMed] [Google Scholar]
- 24.Riediger F, Quack I, Qadri F, Hartleben B, Park JK, Potthoff SA, Sohn D, Sihn G, Rousselle A, Fokuhl V, Maschke U, Purfurst B, Schneider W, Rump LC, Luft FC, Dechend R, Bader M, Huber TB, Nguyen G, Muller DN. Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol. 2011;22:2193–2202. doi: 10.1681/ASN.2011020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs Cl. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 26.Ray BSOf Wnt. Prorenin Receptor, and V-ATPase. Sci. Signal. 2010;3:329. [Google Scholar]
- 27.Zimmerman AW, Veerkamp JH. News insights into the structure and function of fatty acid-bunding proteins. Cell Mol Life Sci. 59:1096–1116. doi: 10.1007/s00018-002-8490-y. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reue K, Peterfy M. Mouse models of lipodystrophy. Curr Atheroscler Rep. 2000;2:390–396. doi: 10.1007/s11883-000-0077-1. [DOI] [PubMed] [Google Scholar]
- 29.Savage DB. Mouse models of inherited lipodystrophy. Dis Model Mech. 2009;2:554–562. doi: 10.1242/dmm.002907. [DOI] [PubMed] [Google Scholar]
- 30.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, Wilson MD, Liu X, Graham MJ, Lee R, Crooke R, Shulman GI, Xue B, Shi H, Yu L. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG, Licinio J, Brown RD, Enriori PJ, O'Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canbakan B, Tahan V, Balci H, Hatemi I, Erer B, Ozbay G, Sut N, Hacibekiroglu M, Imeryuz N, Senturk H. Leptin in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:249–254. [PubMed] [Google Scholar]
- 34.Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Abe T, Suga T, Takada S, Kadoguchi T, Okita K, Matsushima S, Tsutsui H. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int J Cardiol. 2013;168:4313–4314. doi: 10.1016/j.ijcard.2013.04.176. [DOI] [PubMed] [Google Scholar]
- 35.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 36.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 2001;15:2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 37.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll WX, Kalupahana NS, Booker SL, Siriwardhana N, Lemieux M, Saxton AM, Moustaid-Moussa N. Angiotensinogen gene silencing reduces markers of lipid accumulation and inflammation in cultured adipocytes. Front Endocrinol (Lausanne) 2013;4:10. doi: 10.3389/fendo.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.