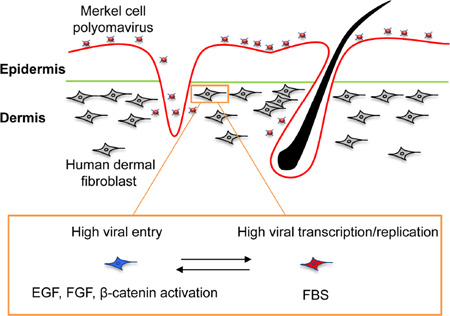
Summary
Infection with Merkel cell polyomavirus (MCPyV) can lead to Merkel cell carcinoma (MCC), a lethal form of skin cancer. However, the skin cell type productively infected by MCPyV remains a central question. We combined cell culture and ex vivo approaches to identify human dermal fibroblasts as natural host cells that support productive MCPyV infection. Based on this, we established a cell culture model for MCPyV infection, which will facilitate investigation of the oncogenic mechanisms for this DNA virus. Using this model, we discovered that induction of matrix metalloproteinase (MMP) genes by the WNT/β-catenin signaling pathway and other growth factors stimulates MCPyV infection. This suggests that MCC risk factors such as UV radiation, and aging, which are known to stimulate WNT signaling and MMP expression, may promote viral infection and thus drive MCC. Our study also introduces the FDA approved MEK antagonist trametinib as an effective inhibitor for controlling MCPyV infection.
eTOC Blurb
Merkel cell polyomavirus (MCPyV) infection can lead to Merkel cell carcinoma, a lethal skin cancer. Liu et al. identify dermal fibroblasts as the target of productive MCPyV infection in human skin. This study establishes a cell culture model and identifies a kinase inhibitor as a potential therapeutic agent against MCPyV.
Graphical abstract
Introduction
Merkel cell polyomavirus (MCPyV) is the first polyomavirus to be clearly associated with a human cancer, Merkel cell carcinoma (MCC)(Feng et al., 2008). MCC metastasizes rapidly. It is one of the most aggressive skin cancers, with a high mortality rate of 33%(Lemos and Nghiem, 2007) (which exceeds the mortality rate of melanoma) and a <45% five-year survival rate(Agelli and Clegg, 2003). Clonal integration of the MCPyV genomic DNA into the host cell genome has been observed in at least 80% of MCC cases(Feng et al., 2008). Continued expression of MCPyV viral oncogenes is required for MCC tumor cells to survive(Houben et al., 2010). These findings provide strong evidence that MCPyV plays an important causal role in the development of MCC skin cancer.
MCPyV is an abundant virus frequently detected on healthy human skin(Foulongne et al., 2012; Schowalter et al., 2010). Serological evidence confirms that exposure to the virus is essentially ubiquitous in the general population(Kean et al., 2009; Tolstov et al., 2009). Excessive exposure to sunlight and ultraviolet (UV) radiation, immune suppression, and advanced age are the most important risk factors for MCPyV-associated MCC(Chang and Moore, 2012). MCC is therefore more frequently observed among people with fair skin, the elderly, and organ transplant or AIDS patients(Engels et al., 2002; Locke et al., 2015). The incidence of MCC has tripled over the past 20 years as the aging population with prolonged sun exposure increases(Hodgson, 2005).
MCPyV has a circular, double-stranded DNA genome of ~5kb(Gjoerup and Chang, 2010). The viral genome contains the viral origin of replication and transcription regulatory elements, as well as the early and late coding regions(Gjoerup and Chang, 2010). The early region encodes large T (LT) antigen, small T (sT) antigen, the 57kT antigen, and a protein called alternative LT ORF (ALTO)(Carter et al., 2013; Gjoerup and Chang, 2010). The late region encodes the capsid proteins, VP1 and VP2(Schowalter et al., 2011). Although it is well established that clonal integration of MCPyV genomic DNA into the host genome precedes development of the majority of MCC cases(Chang and Moore, 2012), the mechanisms by which MCPyV infection contributes to MCC development and many aspects of the MCPyV infectious life cycle remain poorly understood. Mechanistic studies to fully investigate MCPyV molecular biology and oncogenic mechanisms have been hampered by the lack of knowledge of which host cell types are naturally infected by MCPyV.
Based on the expression of neuroendocrine markers, it has been suspected that MCC tumors arise from Merkel cells. Despite their neuroendocrine phenotype, Merkel cells are thought to be derived from the epidermal lineage(Morrison et al., 2009) and reside in the basal layer of the epidermis(Chang and Moore, 2012), whereas MCC tumors are usually isolated within the dermis or subcutis, without apparent connection to the epidermis(Calder and Smoller, 2010). This has led to speculation that MCC tumors may arise from pro-/pre-B cells whose gene expression patterns become deranged during the process of tumorigenesis(Zur Hausen et al., 2013). An additional puzzle is that Merkel cells are post-mitotic(Vaigot et al., 1987), and there are too few Merkel cells in the skin to account for the millions of copies of MCPyV DNA detected on healthy human skin(Schowalter et al., 2010). It is therefore believed that Merkel cells are unlikely to be the primary target of MCPyV infection or productive replication. Instead, the natural host cells of MCPyV could be one of the more abundant cell types in the human skin. An obvious hypothesis is that, analogous to skin-tropic papillomaviruses, new MCPyV virions are produced in terminally differentiated keratinocytes. This hypothesis has been difficult to test because MCPyV replicates poorly in a wide range of cell types, including keratinocytes and other cell types tested in our studies(Neumann et al., 2011; Schowalter et al., 2012; Tsang et al., 2014). We found that fewer than 0.1% of cells in cultures of various cell lines support high-level MCPyV transcription and replication(Tsang et al., 2014). Therefore, the host cells naturally infected by MCPyV are formally unknown. To tackle this fundamental question, we examined the MCPyV infectability of human skin cells using both MCPyV pseudovirus carrying a GFP reporter and MCPyV virions produced through co-transfection of VP1/VP2 expression plasmids and re-ligated recombinant MCPyV genome (Schowalter et al., 2011). Here we report our results identifying the natural host cells in the human dermis that are productively infected by MCPyV.
Results
MCPyV infects a sub-population of fibroblasts in the human dermis
To identify the natural host cells of MCPyV in the human skin, we produced recombinant MCPyV and MCPyV-GFP pseudovirus using our reported methods(Schowalter et al., 2011). MCPyV-GFP pseudovirus, which carries a GFP reporter construct instead of MCPyV genome within the viral capsid, is useful for visualizing the initial phase of viral infectious entry, whereas recombinant MCPyV, which carries the native viral genome, allows detection of viral transcription and productive replication in the infected cells.
Virions or reporter pseudovirions were applied to the total cell population isolated from human foreskin, including epidermal keratinocytes, dermal fibroblasts, Merkel cells, and other types of skin cells. We then examined MCPyV infectious tropism by performing immunofluorescent staining of cell type-specific markers to identify the GFP+ cells in the reporter pseudovirus-treated population. In addition, MCPyV virion-treated cells were double-stained with antibodies recognizing MCPyV LT and one of the cell type-specific markers to identify MCPyV-infected cells.
We first separately isolated epidermal and dermal cells from human foreskin. Because MCC tumors have sometimes been suspected of arising from the neural crest lineage(Tang and Toker, 1978), we initially speculated that the natural target of productive MCPyV infection in the skin could be derived from this lineage. Accordingly, we cultured the primary skin cells in medium containing growth factors such as Epidermal growth factor (EGF) and/or Fibroblast growth factor (FGF), but without fetal bovine serum (FBS), which could induce neural crest stem cell differentiation(Lee et al., 2007).
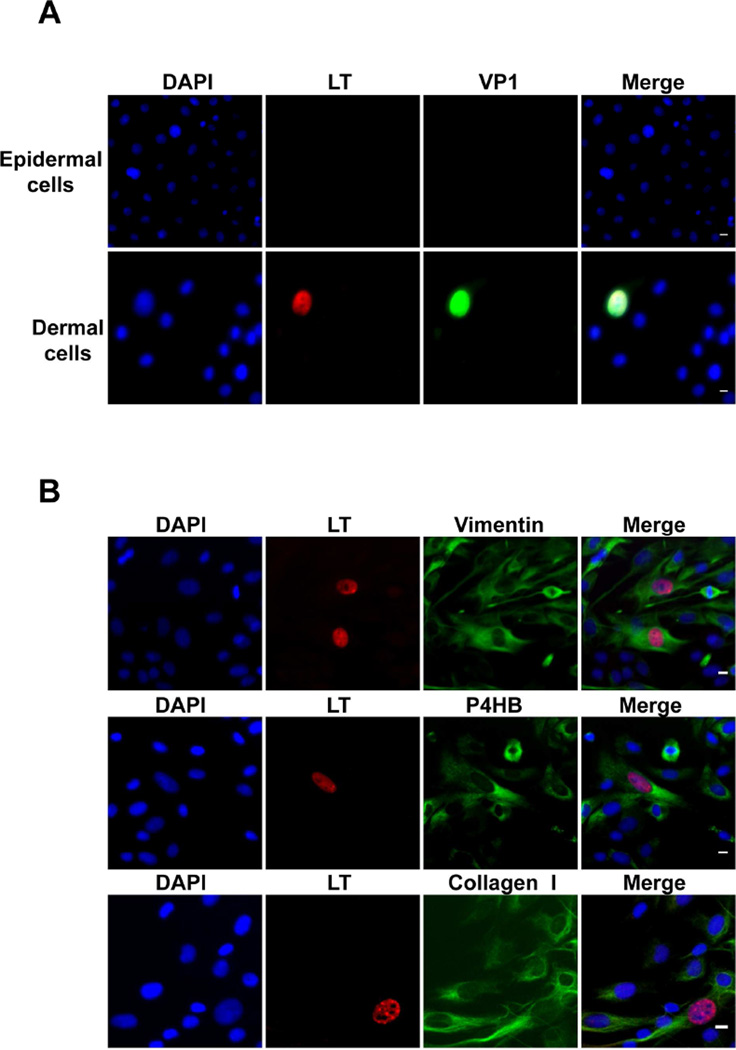
Consistent with past reports that MCPyV pseudovirions can deliver encapsidated reporter DNA to a wide variety of different cell types(Schowalter et al., 2012), GFP expression was observed in pseudovirus-exposed human foreskin keratinocytes (HFKs), marked by Cytokeratin 14 (K14), isolated from the epidermis, as well as in dermal cells (Table 1 and Figure S1). In contrast, after treatment with MCPyV virions, LT+ and VP1+ cells were only detected in the dermal cell population and none of the MCPyV virion-treated HFKs or other epidermal cells showed LT or VP1 expression (Figure 1A and Table 1). The results confirm that MCPyV infectious entry is relatively promiscuous but that viral gene expression is unexpectedly restricted to human dermal cells and not epidermal cells.
Table 1.
Skin cell markers tested in this study
| Cell Marker | Cell type | MCPyV-GFP | MCPyV |
|---|---|---|---|
| Cytokeratin 14 | Keratinocytes | Positive | Negative |
| Cytokeratin 20 | Merkel cells | Negative | Negative |
| S100 | Melanocytes /Schwann cells | Negative | Negative |
| Sox10 | Neural crest cells /Melanocytes | Negative | Negative |
| p75NTR | Neural crest cells | Negative | Negative |
| GFAP | Schwann cells/Glial cells | Negative | Negative |
| MAP2 | Neuron | Negative | Negative |
| Neurofilament-H | Neuron | Negative | N.A |
| β3-tublin | Neuron | Negative | Negative |
| FABP4 | Adipogenic cells | Negative | Negative |
| Osteocalcin | Osteogenic cells | Negative | N.A |
| CD73 | Mesenchymal stem Cells | Negative | N.A |
| CD105 | Mesenchymal stem Cells | Negative | N.A |
| STRO-1 | Mesenchymal stem Cells | Negative | N.A |
| Vimentin | Mesenchymal stem Cells/Fibroblasts |
Positive | Positive |
| Collagen I | Fibroblasts | Positive | Positive |
| PDPN | Fibroblasts | Positive | N.A |
| P4HB | Fibroblast/Keratinocytes | Positive | Positive |
| MyoD | Smooth muscle cells | Negative | N.A |
| α-SMA | Smooth muscle cells | Negative | N.A |
| CD3 | T cells | Negative | N.A |
| CD19 | B cells | Negative | Negative |
| CD11C | DC cells | Negative | N.A |
| CD56 | NK | Negative | N.A |
| CD146 | Endothelial Cell | Negative | N.A |
| CD31 | Endothelial Cell | Negative | N.A |
Figure 1. MCPyV preferentially infects dermal fibroblasts isolated from human foreskin.
Cells isolated from human foreskin epidermis and dermis (A) or from the dermis of human foreskin (B) were treated with recombinant MCPyV virions in DMEM/F12 with N2, B27, EGF and bFGF, but without FBS, for 2 days. After switching to the same fresh medium for 3 more days, cells were immunostained using the indicated antibodies and counterstained with DAPI. Bar, 10 µm. Shown are representative images from eight independent experiments. See also Figure S1.
Using immunofluorescent microscopy, we found that MCPyV-infected cells were not stained with antibodies recognizing a number of markers for various skin cell types including nerve cells, melanocytes, neural crest-like cells, smooth muscle cells, Schwann cells, mesenchymal stem cells, adipogenic cells, osteogenic cells, lymphocytes, and endothelial cells (Table 1). When stained with the antibody for Merkel cell marker Cytokeratin 20 (CK20), nearly no cells isolated from human foreskin were positive. The sample extracted from human fetal scalp containing CK20+ cells was therefore used to test MCPyV infection. When these cells were treated with MCPyV-GFP pseudovirus, nearly all of the CK20+ cells were negative for GFP signal (Figure S1B). However, one cell out of 122 CK20+ cells examined was transduced with MCPyV-GFP pseudovirus (Figure S1B). For the MCPyV-treated samples, all of the 42 CK20+ cells examined were negative for MCPyV LT signal (Figure S1C). This negative result could be due to the fact that the low number of CK20+ cells reduced the chance of detecting MCPyV LT+ cells. It could also be that CK20+ cells do not support robust MCPyV entry and viral transcription. In any case, the data suggested that MCPyV most likely productively infects other cells type(s) besides Merkel cells and may enter Merkel cells at a very low rate, which is consistent with the low incidence of MCPyV+ MCC tumors. In contrast, cells expressing GFP following infection with MCPyV-GFP pseudovirus were all positively stained for dermal fibroblast markers including vimentin, PDPN, collagen I, and P4HB (Figure S1D). More importantly, in the mixture of primary dermal cells treated with MCPyV virions, all of the LT+ cells were also positively stained for the dermal fibroblast markers (Figure 1B). These results suggest that dermal fibroblasts are likely the major cell type in the skin that is naturally infected by MCPyV.
In previous studies, we showed that MCPyV LT colocalizes with BRD4 at MCPyV replication foci in cells co-transfected with MCPyV LT and origin constructs to reconstitute an in vitro replication system(Wang et al., 2012). In many of the MCPyV-infected human dermal fibroblasts, we also detected LT+ foci resembling the MCPyV replication foci described in our earlier study(Wang et al., 2012), with LT colocalizing with BRD4 in these foci (Figure S2A). This result indicates that MCPyV-infected dermal cells can support viral replication. In addition, human dermal fibroblasts transfected with re-ligated MCPyV genome could efficiently express LT and VP1 (Figure S2B). These studies suggest that dermal fibroblasts are not only infectable by MCPyV but also support viral transcription and replication.
Growth factors and WNT signaling activation stimulate MCPyV infection
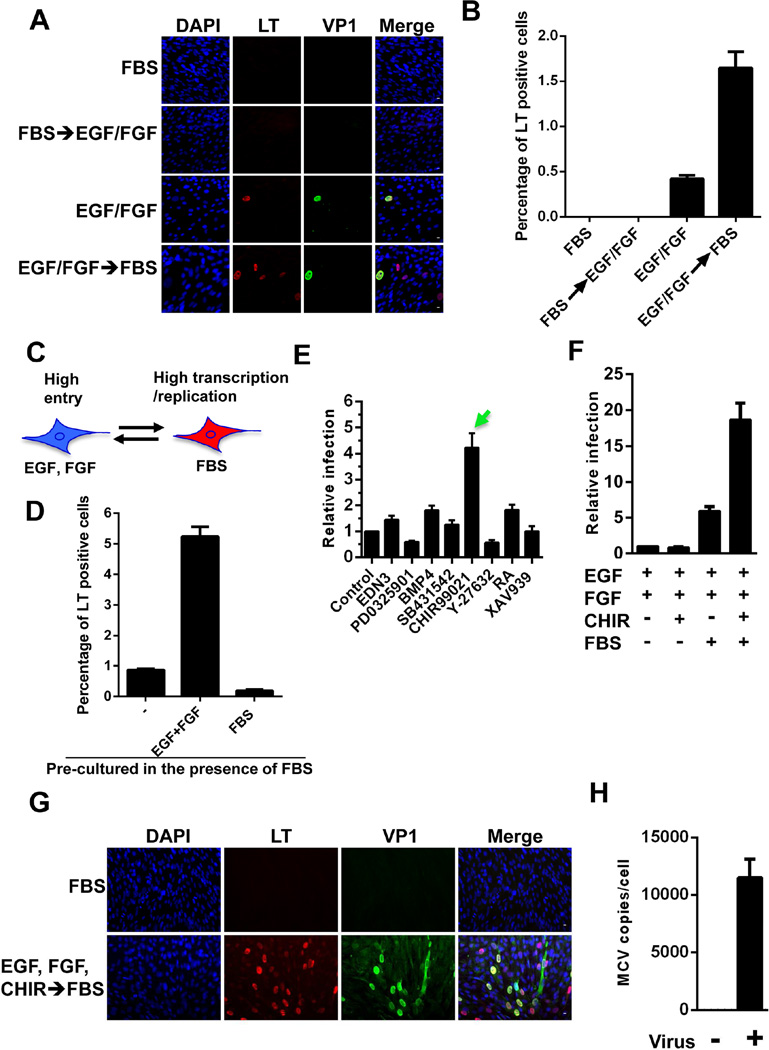
As described above, human dermal cells tested for MCPyV infection were initially cultured in the presence of EGF and FGF, without FBS, in order to mimic the culture conditions for neural crest stem cells. However, because dermal fibroblasts are normally cultured in FBS-supplemented medium, we tested whether FBS affects MCPyV infectious entry and gene expression. We discovered that treating dermal cell cultures with MCPyV for 2 days in the presence of EGF and FGF (without FBS) followed by a transfer to medium containing FBS caused an increase in the percentage of cells showing both LT signal and MCPyV replication foci relative to the no FBS condition (Figure 2A and 2B). Conversely, if the cells were first treated with MCPyV in FBS-supplemented medium and either grown continuously in medium with FBS, or switched to EGF/FGF-supplemented medium, we rarely detected either MCPyV LT/VP1 signals or replication foci (Figure 2A–2B). Both EGF and FGF could separately stimulate MCPyV infectivity and there was an additive effect when both were used during the viral entry phase (Figure S2C). To determine whether EGF and FGF enhance the infectious entry process or instead simply trigger an increase in MCPyV gene expression, we applied MCPyV-GFP pseudovirus to EGF/FGF-treated human dermal cells. Combination treatment increased the number of GFP-positive dermal cells by roughly 3.5-fold (Figure S2D). This suggests that the increased viral gene expression observed using MCPyV virions on EGF/FGF-treated dermal cells is due to an enhancement of infectious entry (Figure 2C). FBS, on the other hand, stimulated MCPyV gene expression only if it was added after the initial entry stage (Figure 2A–2B), and a dose-dependent effect was observed when up to 50% FBS was used (Figure S2E). Together, the MCPyV data suggested that there are two different phases of fibroblast proliferation; the EGF and FGF-induced phase supports MCPyV entry while the FBS culture condition promotes MCPyV gene expression and replication only after the infectious entry process is completed (Figure 2C). Under this condition, ~2% of the cells in the primary dermal culture were able to support viral entry and gene expression (Table S1).
Figure 2. The unique growth conditions that support effective MCPyV entry and transcription/replication events in human dermal fibroblasts.
(A) For the top two panels, dermal cells isolated from human foreskins were treated with MCPyV in medium containing FBS for 2 days and then either replaced with the same fresh medium (FBS) or switched to medium containing EGF and bFGF (FBS → EGF/FGF) for 3 more days. For the bottom two panels, dermal cells were treated with MCPyV in medium containing EGF and bFGF for 2 days and then either replaced with the same fresh medium (EGF/FGF) or switched to medium containing FBS (EGF/FGF → FBS) for 3 more days. All cells were immuno-stained using the indicated antibodies and counterstained with DAPI. (B) The percentage of LT+ cells in the samples described in (A). (C) MCPyV entry and transcription/replication occur in two different stages of dermal fibroblast proliferation. (D) Human dermal cells cultured in medium containing 10% FBS for multiple passages were treated with recombinant MCPyV in medium alone (−) or supplemented with either EGF/bFGF or FBS for 2 days. After culturing with fresh medium containing 10% FBS for 3 more days, the cells were stained with LT antibody. The percentage of LT+ cells was quantified and the value for the “medium alone” sample was set as 1. (E, F, and G) Human dermal cells were treated with MCPyV in medium containing EGF/bFGF and additional components, as indicated, for 2 days and then switched to medium containing 10% FBS for 3 more days. The cells were stained using LT and VP1 antibodies and counterstained with DAPI. The percentage of LT+ cells in the sample treated with only EGF and bFGF (control) was set as 1. EDN3: Endothelin 3; PD 0325901: a MEK1 and MEK2 inhibitor; BMP4: Bone morphogenetic protein 4; SB431542: an inhibitor of TGF-beta superfamily type I activin receptor-like kinase receptors; CHIR99021: the most selective inhibitor of GSK3; Y-27632: ROCK kinase inhibitor; RA: Retinoic acid; XAV939: tankyrase inhibitor. (H) Human dermal cells were treated with MCPyV in medium containing EGF, bFGF, and CHIR99021 before switching to medium containing 10% FBS. MCPyV genome copy numbers were determined by qPCR and normalized to GAPDH DNA levels. Error bars represent standard error of the mean (s.e.m.) of at least three independent experiments. See also Figure S2–S3.
Human dermal cells cultured for multiple passages in medium containing 10% FBS could be reactivated for viral entry by culturing in the presence of EGF and FGF (without FBS) before adding virus (Figure 2D, column 2). After switching the EGF/FGF- and virus-treated cells back into medium with 10% FBS, we again detected robust viral transcription and replication (Figure 2D, column 2). If these cells were treated with the virus in the medium containing only FBS, they again showed very low infection efficiency (Figure 2D, column 3). In fact, pre-culturing human dermal cells in medium containing 10% FBS appears to select for the dermal fibroblasts that support MCPyV infection, as the percentage of cells showing LT and VP1 expression increased from ~1.6% in the primary dermal cells freshly isolated from human foreskin to ~5% in the cells pre-cultured in the presence of FBS (compare Figure 2B and 2D, Table S1). In contrast, HFKs treated with the same conditions did not show any detectable LT expression, suggesting that the stimulation effect of growth factors and FBS is specific for dermal fibroblasts. Immunofluorescent staining showed that nearly 100% of the human dermal cells pre-cultured in medium containing 10% FBS were positively stained for dermal fibroblast markers, vimentin, and collagen I, and negative for the HFK marker, K14 (Figure S2F), confirming that the culture process enriched for dermal fibroblasts that support MCPyV infection.
In light of the EGF/FGF stimulation effect on MCPyV infection of dermal fibroblasts, a screen was performed to identify additional chemical compounds and/or growth factors that might promote MCPyV infection. For this study, human dermal cells were first inoculated with MCPyV in the presence of EGF, FGF, and one of the factors shown in Figure 2E for 2 days and then switched to medium with 10% FBS. Among all of the reagents tested, CHIR99021, a highly selective inhibitor of GSK3, was the most efficient at stimulating MCPyV infection (Figure 2E).
Adding CHIR99021 to cells treated with EGF and FGF but without subsequent FBS stimulation did not enhance MCPyV infection (Figure 2F). In contrast, for cells treated with EGF/FGF before infection and FBS after infection, adding CHIR99021 inhibitor during MCPyV treatment dramatically increased the MCPyV infection efficiency. These results suggest that CHIR99021 functions to stimulate viral entry, but not the later events such as transcription and replication. When human dermal cells were treated with MCPyV in the presence of EGF, FGF and CHIR99021, the percentage of cells showing LT and VP1 expression was higher than 20% (Figure 2F–2G), with the viral genome number reaching an average of about 12,000 copies per cell (Figure 2H).
CHIR99021 is known to inhibit GSK3, thereby activating β-catenin in the WNT signaling pathway(Bennett et al., 2002). The efficacy of the drug on dermal fibroblasts was confirmed using a TOP-Flash reporter system (Figure S3A). The reporter assays showed that CHIR99021 specifically stimulated the β-catenin associated TCF/LEF transcription activation of the TOP-Flash reporter by ~8000-fold but had no significant effect on a negative control FOP-Flash reporter containing a mutated TCF binding site (Figure S3A). Another GSK3 inhibitor, BIO, which showed a similar effect in β-catenin activation assays (Figure S3A), also enhanced MCPyV infection (Figure S3B). Furthermore, GSK3 knockdown increased MCPyV infection (Figure S3C–S3D). These results suggest that activation of the WNT/β-catenin signaling pathway is important for MCPyV infection.
Induction of matrix metalloproteinase (MMP) genes is crucial for MCPyV infection
To understand how treating dermal fibroblasts with EGF, FGF and CHIR99021 stimulates MCPyV infection, we performed RT-qPCR to survey a large number of genes that might be involved in the process (Figure 3A and S4). Remarkably, several genes of the matrix metalloproteinase (MMP) family, including MMP1, 3, 7, 9, 10, 11 and 13, were robustly induced in the dermal cells treated with EGF, FGF and CHIR99021 (Figure 3A). The MMP induction was also confirmed using Western blotting, which revealed that the active (cleaved) forms of MMP1, MMP3, and MMP10 were dramatically increased in the cells treated with EGF, FGF and CHIR99021, compared to the untreated cells or cells treated with FBS (Figure 3B).
Figure 3. Activation of MMP genes supports MCPyV infection.
(A) Human dermal cells were cultured in DMEM/F12 alone or supplemented with either FBS or EGF, bFGF, and CHIR99021. After 48 h, the mRNA levels of the MMP genes were measured by RT-qPCR and normalized to GAPDH mRNA. The values for the sample grown in medium only were set as 1. (B) Cell lysates of human dermal cells cultured as in (A) were immunoblotted with the indicated antibodies. ACTIN was used as a loading control. The experiment was performed at least three times with similar results. (C) Human dermal cells cultured in DMEM/F12 were either untreated or treated with different concentrations of collagenase type IV before incubation with MCPyV for 2 days. After switching to fresh DMEM/F12 containing FBS for 3 more days, the cells were stained with LT antibody. The percentage of LT+ cells in the untreated sample was set as 1. (D) Human dermal cells were treated with MCPyV in medium containing EGF, bFGF, CHIR99021 and either DMSO (Control) or the inhibitors for 2 days. After switching to fresh medium containing FBS for 3 more days, the cells were stained with LT antibody. The percentage of LT+ cells was calculated. The value for the Control group was set as 1. (E) Human dermal cells cultured in DMEM/F12 containing EGF, bFGF, CHIR99021, and 1 mg/ml collagenase type IV were either untreated (Mock) or treated (Infected) with MCPyV for 2 days. After switching to fresh DMEM/F12 containing FBS for 3 more days, the cells were co-stained with LT and VP1 antibodies and counterstained with DAPI. (F) Human dermal cells treated with MCPyV as in (E) were co-stained with LT and vimentin antibodies and counterstained with DAPI. Bar, 10 µm. All error bars represent s.e.m. of three independent experiments. See also Figure S4.
Because these MMP enzymes are capable of degrading extracellular matrix proteins(Overall and Lopez-Otin, 2002), we reasoned that they might stimulate MCPyV infection by disrupting the extracellular matrix of the host cells. To test this hypothesis, we treated human dermal cells being infected with MCPyV using collagenase type IV, a member of the MMP family, to mimic the MMP induction. Indeed, collagenase treatment stimulated MCPyV infection in a dose-dependent manner (Figure 3C). In contrast, treatment with MMPs inhibitors, Marimastat and Batimastat, dramatically inhibited MCPyV infection (Figure 3D). Nearly 50% of the cells expressed LT and VP1 at the optimum collagenase dose (Figure 3E–3F). Many of the infected cells not only had highly expressed LT and VP1 but also showed robust MCPyV LT+ replication foci (Figure 3E–3F). This strong viral transcription and replication was not observed in any of the other cells we and others have examined(Neumann et al., 2011; Schowalter et al., 2012; Tsang et al., 2014). Since all of the LT+ cells were also positively stained for the dermal fibroblast marker, vimentin (Figure 3F), this result provides strong evidence to support the idea that human dermal fibroblasts are an important target of productive MCPyV infection.
Human dermal fibroblasts support the full MCPyV life cycle
Using the optimum MCPyV infection condition established above, including treatment with EGF, FGF, CHIR99021, and collagenase before adding virus and FBS after viral entry, LT protein was detected in the infected cells by Western blotting (Figure 4A). The replicated viral genome was measured by qPCR to be ~15,000 copies per cell (Figure 4B). Fluorescence in situ hybridization (FISH) analysis using in situ DNA-hybridization chain reaction (HCR) technique also revealed high concentrations of MCPyV DNA around the putative replication foci in the infected cells (Figure 4C). The replicated MCPyV genome and RNA were also detected in the infected cells using single molecule fluorescence in situ hybridization (smFISH) (Figure S5A). More importantly, immuno-DNA HCR FISH that simultaneously detects viral protein and DNA showed that the replicated MCPyV genomes were enriched at the MCPyV LT+ foci in the infected cells (Figure 4D). This finding confirmed that MCPyV sets up replication factories in the infected cells. Moreover, MCPyV LT and VP1 signals were still detected at 20–30 days after human dermal cells were treated with MCPyV (Figure 4E). These results suggest that MCPyV can be maintained in human dermal fibroblasts for an extended period.
Figure 4. Human dermal fibroblasts support the full MCPyV life cycle.
(A) Human dermal cells cultured as in Figure 3E were either untreated (−) or treated (+) with MCPyV. Cell lysates were immunoblotted with LT antibody. ACTIN was used as a loading control. The experiment was performed at least three times with similar results. (B) MCPyV copy numbers in the cells described in (A) were measured using qPCR and normalized to GAPDH DNA levels. (C) Human dermal cells treated with MCPyV as in Figure 3E were subjected to HCR FISH analysis. (D) Cells prepared as in (A) were subjected to immuno-FISH using LT antibody and MCPyV-specific DNA probes. HPV 16 probes were used as a negative control. The cells were also counterstained with DAPI. (E) Human dermal cells cultured in medium containing EGF, bFGF, and CHIR99021 were either untreated (Mock) or treated (Infected) with MCPyV for 2 days. The cells were then cultured in medium with FBS. On day 20, the cells were stained using the indicated antibodies and counterstained with DAPI. Shown are the representative images of the untreated and virus-infected dermal fibroblasts. (F) Human dermal cells were transfected with re-ligated MCPyV genome in DMEM containing 10% FBS for 1 day. After switching to fresh medium containing 10% FBS for 4 more days, the MCPyV virions were harvested and purified over an OptiPrep gradient. The viral genome copy number in each gradient fraction was measured using qPCR. (G) The virions purified in (F) were used to infect human dermal cells cultured as in Figure 3E. The cells were stained as in (E). All error bars represent s.e.m. of three independent experiments. Bar, 10 µm. See also Figures S5.
To test if human dermal fibroblasts allow MCPyV to complete its life cycle, we transfected these cells with re-ligated MCPyV genome in medium containing 10% FBS to stimulate viral transcription and replication. After lysing the cells, MCPyV virions were purified over an OptiPrep gradient (Figure 4F). The MCPyV virions were used to infect fresh human dermal fibroblasts treated with EGF, FGF, CHIR99021, and collagenase. After culturing the infected cells in medium with FBS, we again detected LT and VP1 signals as well as the LT+ replication foci in many of the infected cells (Figure 4G). This experiment further demonstrated that human dermal fibroblasts could support the completion of the MCPyV life cycle to produce infectious virions.
Besides the primary dermal fibroblasts isolated from human foreskin, dermal cells isolated from normal adult skin were also efficiently infected with MCPyV (Figure S5B). Interestingly, MCPyV also expresses LT and VP1 in fibroblast cell lines such as WI-38 and IMR-90 derived from lung tissues (Figure S5C).
MCPyV infects dermal fibroblasts in human skin cultured ex vivo
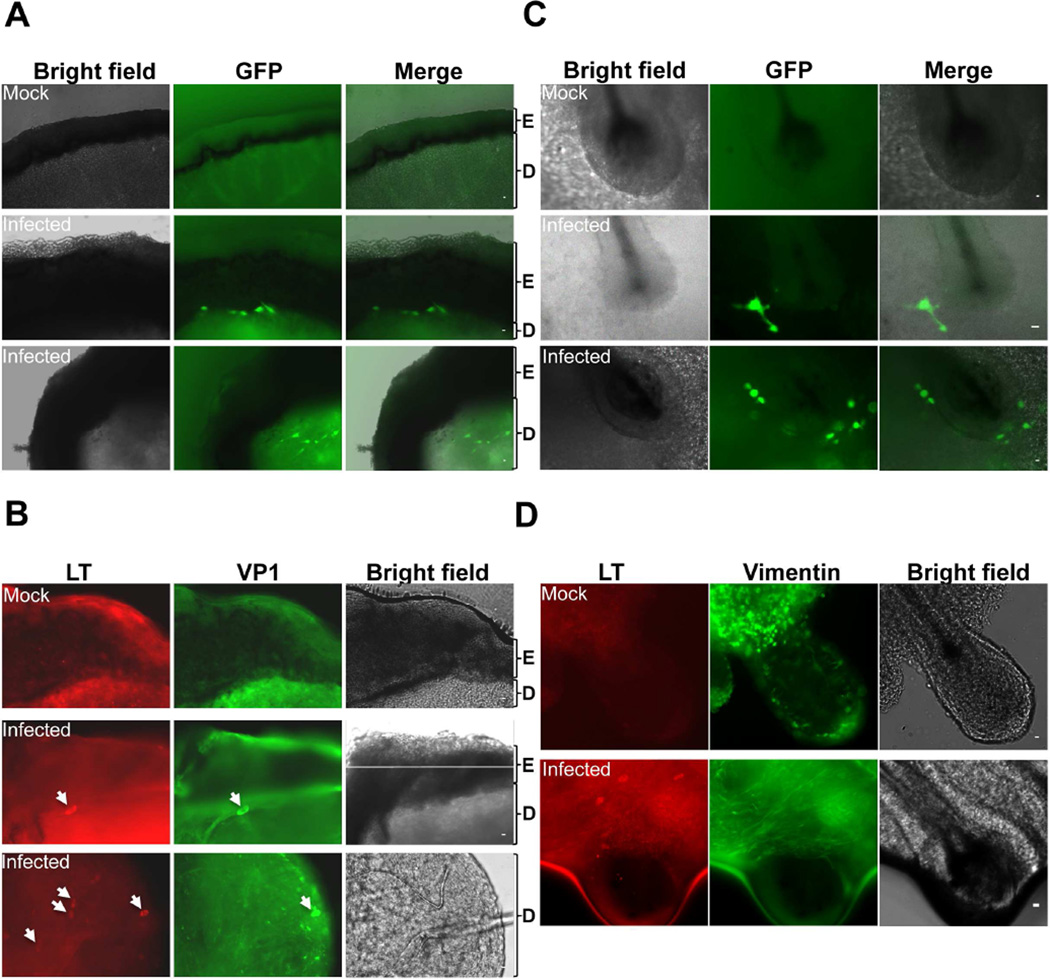
We also tested how MCPyV-GFP pseudovirions and MCPyV virions infect cells grown within the context of other cells in human skin ex vivo. In MCPyV-GFP pseudovirus-treated skin slices, GFP signal was detected in cells either at the boundary between epidermis and dermis or inside the dermis, whereas untreated skin slices showed negative GFP signal (Figure 5A). We also examined MCPyV infection in the human skin slices. Again, we detected the signals of both LT and VP1 either at the border between the epidermis and dermis or inside the dermis only in the infected skin slices (Figure 5B). Much of the GFP signal was lost during immunofluorescent staining of MCPyV-GFP pseudovirus-treated skin slices. However, we could clearly detect the remaining GFP+ cells present within the vimentin+ layer of the dermis (Figure S6A). Although HFKs could be easily transduced by MCPyV-GFP pseudoviruses when cultured in dish (Table 1 and Figure S1A), there were no detectable GFP+ epidermal cells in the human skin slices cultured with pseudovirions ex vivo (Figure 5A and S6A). These results confirmed that both MCPyV-GFP pseudovirus and MCPyV preferentially infect dermal fibroblasts in human skin cultured ex vivo.
Figure 5. Both MCPyV-GFP pseudovirus and MCPyV infect dermal fibroblasts in human skin cultured ex vivo.
Human foreskin (A, B) and scalp (C, D) slices cultured ex vivo were treated with either MCPyV-GFP pseudoviruses (A, C) or recombinant MCPyV (B, D). The MCPyV-infected slices (B, D) were stained using the indicated antibodies. Bar, 10 µm. Shown are the representative images of both infected and uninfected (mock) skin slices from three independent experiments. Arrows mark the MCPyV-infected cells. E = Epidermis; D = Dermis. See also Figure S6.
As shown in Figure 2 and S3, activation of the WNT signaling pathway in cultured human dermal cells stimulates MCPyV infection. We next investigated how WNT signaling may contribute to MCPyV infection in the more physiological environment of the skin using ex vivo skin culture. It has been shown that WNT signals released from epidermal keratinocytes trigger the proliferation of dermal fibroblasts beneath the basal keratinocytes(Chen et al., 2012). This could explain why dermal fibroblasts underlying the basal layer of the epidermis were efficiently infected (Fig 5A and 5B). In addition, there is also a layer of basal keratinocytes comprising the outer root sheath of hair follicles. WNT signaling in the keratinocyte compartment of hair follicles is critical for hair follicle formation and regeneration(Chen et al., 2012). Therefore, we also tested if MCPyV preferentially infects the cells surrounding hair follicles compared to other dermal cells. Both MCPyV-GFP pseudovirions and MCPyV virions were used to treat slices of human scalp containing hair follicles. Remarkably, in MCPyV-GFP pseudovirus-treated samples, GFP signal was found in the dermal cells surrounding hair follicles (Figure 5C). Immunofluorescent staining of these skin slices using the antibody for the dermal fibroblast marker, vimentin, clearly highlighted the layers of dermal fibroblasts around hair follicles and revealed that the GFP signal was present in the dermal fibroblasts encircling hair follicles (Figure S6B). Similarly, in the samples infected with MCPyV virions, we detected LT expression in the cells near hair follicles (Figure 5D). These cells were also positively stained for vimentin (Figure 5D). Together, the data from both MCPyV-GFP pseudovirus and MCPyV virion demonstrated that the viruses preferentially infect the dermal fibroblasts around hair follicles.
Identification of a kinase inhibitor that efficiently blocks MCPyV infection
Uncontrolled MCPyV infection in immunocompromised individuals is thought to pose significant risk for the development of MCC(Chang and Moore, 2012). To explore possible ways to inhibit MCPyV infection, we set out to identify small molecules targeting the EGF, FGF, β-catenin, and FBS pathways that our work suggests are important for MCPyV infection. We screened a number of compounds, including several FDA approved kinase inhibitors, for inhibitory effects on MCPyV infection. Trametinib, a MEK1 and MEK2 inhibitor, dramatically inhibited MCPyV infection, whereas the Bruton's tyrosine kinase inhibitor Ibrutinib, the Phosphoinositide 3-kinase inhibitor Idelalisib, and many other compounds had no detectable inhibitory effect on MCPyV infection (Figure 6A and 6B). In addition, trametinib did not affect MCPyV-GFP pseudovirus transduction of human dermal fibroblasts (Figure S7), suggesting that this kinase inhibitor mainly blocks MCPyV transcription and/or replication in the infected cells. This specific inhibitory effect of the MEK1 and MEK2 inhibitor suggests that MCPyV infection may require activation of the MAP kinase pathway, which could be induced by EGF, FGF, and/or FBS(Katz et al., 2007; Whitmarsh et al., 1995).
Figure 6. Identification of a kinase inhibitor that efficiently blocks MCPyV infection.
(A) Human dermal cells cultured as in Figure 3E were treated with recombinant MCPyV for 2 days. After switching to medium containing FBS, the inhibitors were added to the culture for 3 days. The cells were stained with LT antibody and counterstained with DAPI. Bar, 10 µm. (B) The percentage of LT+ cells in the samples treated as in (A) was calculated. The value for the DMSO (Control) group was set as 1. Error bars represent s.e.m. of three independent experiments. (C) The working model for MCPyV infection of the human skin. See also Figure S7.
Discussion
As the first human polyomavirus clearly shown to cause human cancer, MCPyV represents an exciting opportunity to better understand polyomavirus-induced tumorigenesis. However, the lack of knowledge of the natural host cell tropism of MCPyV and the technical difficulties in propagating the virus in cell culture have hindered the discovery of its oncogenic mechanisms. In this study, we identified human dermal fibroblasts as a human skin cell type that supports robust MCPyV infectious entry, gene expression, and productive replication.
The discovery of human dermal fibroblasts as MCPyV natural host cells was made after establishing the cell culture conditions that could potentially mimic specialized cellular growth signals in the skin. For example, we found that culturing dermal cells with EGF and FGF is needed to stimulate MCPyV entry (Figure 2A–2D). After the viral infectious entry process is completed, FBS becomes critical for stimulating MCPyV transcription and replication. Furthermore, culturing human dermal cells in the presence of FBS for multiple passages enriches for the type of cells that could readily be primed for MCPyV infectious entry by EGF and FGF (Figure 2D). EGF and FGF are among the cocktail of growth factors induced in the proliferative fibroblasts at the wound site of damaged human skin(Martin, 1997). They are physiologically up-regulated in the dermis during wound healing and angiogenesis(Werner and Grose, 2003). These growth factors provide the signals for initiating wound healing and angiogenesis(Martin, 1997). Thus, cellular signaling associated with recovery from wounding may induce the skin environments that promote MCPyV infection. The subsequent burst of fibroblast proliferation may also enhance the viral transcription and replication activities.
In human skin, WNT signaling transduced through intercellular communication between epidermal keratinocytes and dermal fibroblasts or follicular keratinocytes and the surrounding dermal fibroblasts plays an important role in the proliferation of dermal cells and hair follicle regeneration, respectively (Chen et al., 2012). Using chemical screening, we discovered that chemical compounds, such as CHIR99021 and BIO, which activate the WNT/β-catenin signaling pathway, could further stimulate MCPyV transduction of human dermal fibroblasts in cell culture. Furthermore, in experiments using human skin slices cultured ex vivo to mimic the natural environment of the human skin, we showed that both MCPyV-GFP pseudovirus and recombinant MCPyV infect dermal fibroblasts located at the boundary between epidermis and dermis, and also in the regions surrounding hair follicles inside the dermis (Figure 5 and S6). Together, these results suggest that active WNT signaling in these skin locations may stimulate MCPyV infection in the physiological environment of the human skin.
Our gene expression profiling analysis showed that one of the combined effects of EGF, FGF and CHIR99021 is to robustly induce the expression of MMP genes (Figure 3A–3B). This result suggests that activation of these downstream MMP genes may contribute to MCPyV infection by disrupting the extracellular matrix of the host cells. Indeed, cells treated with collagenase to degrade their extracellular matrix efficiently stimulated MCPyV infection (Figure 3C and 3E). EGF, FGF, and β-catenin signaling have all been shown to stimulate MMP expression(Aho et al., 1997; Neth et al., 2007; Van Meter et al., 2004). This could partly explain why these components activate MCPyV infection. In the physiological skin environment, skin damage induced by UV/ionizing radiation (IR) and wounding processes can activate both WNT signaling and MMP expression(Cho et al., 2009; Fisher et al., 1996; Gill and Parks, 2008; Whyte et al., 2012). Aging skin also shows dramatically increased MMP expression and reduced collagen levels(Quan and Fisher, 2015; Varani et al., 2006). Therefore, it is possible that these changes in physiological conditions of the skin could stimulate viral infection by inducing the expression of MMP genes (Figure 6C). Moreover, MMPs play a critical role in tumor invasion and metastasis(Brown and Murray, 2015). The dysregulated MMP gene expression that supports MCPyV infection could also contribute to the virus-induced oncogenesis. Interestingly, a recent study from the Chang and Moore laboratory discovered that several MMP genes are induced in cells expressing T antigens derived from MCC tumors(Richards et al., 2015), which may further promote metastasis of MCPyV-induced MCC tumors.
Shed MCPyV virions can readily be detected at the surface of human skin(Schowalter et al., 2010). How MCPyV virions produced in the dermis could get to the surface of the skin remains an interesting unresolved question. Because we found that MCPyV infects dermal fibroblasts near hair follicles, it is possible that mature MCPyV virions could be released to the surface of human skin through hair follicles and/or associated sebaceous and sweat glands. This hypothesis is supported by the observation that MCPyV is frequently detected in eyebrow hair bulbs(Peretti et al., 2014). It is also possible that the infected dermal fibroblasts might die and the released virions could be carried to the skin surface by the flow of differentiating keratinocytes.
This study provides evidence to support the concept that human dermal fibroblasts are the primary host cell type naturally and productively infected by MCPyV. This finding is consistent with the fact that most MCC tumors are detected in the human dermis(Calder and Smoller, 2010). Because Merkel cells located in the basal layer of the epidermis are in the immediate vicinity of MCPyV-infected dermal fibroblasts (Figure 5 and S6), it is possible that MCPyV actively replicating in the dermal fibroblasts may accidently enter Merkel cells, as shown in Figure S1B, and cause MCC simply because Merkel cells represent a dead-end replication environment that favors viral integration and transformation over virally induced cell lysis. Alternatively, MCPyV infection of dermal fibroblasts could induce their transformation and activate gene expression profiles typical of MCC tumors under special physiological conditions that remain to be discovered.
The cell culture system for MCPyV infection established in this study provides a platform to elucidate the largely unknown MCPyV life cycle and MCPyV-induced oncogenic mechanism in the context of its natural host. The MCPyV infection model we report here also laid the groundwork for investigating how UV, IR, and other major MCC risk factors alter the natural MCPyV life cycle and normal skin physiology to cause a highly aggressive skin cancer.
Our identification of trametinib as an effective inhibitor of MCPyV infection indicates that MCPyV infection requires activation of the MAP kinase pathway. In fact, treating cells with EGF, FGF, or FBS all could lead to activation of the MAP kinase pathway(Katz et al., 2007; Whitmarsh et al., 1995). It is possible that trametinib inhibits MCPyV infection by antagonizing the effects of these growth factors. Although MCC is a highly aggressive carcinoma, currently there is no effective drug for curing this cancer. Identification of trametinib as an inhibitor for MCPyV infection could lead to the development of effective therapy for MCPyV infection. This finding could have an important impact on reducing the MCPyV viral load in immunocompromised patients with the goal of preventing MCC development.
Experimental Procedures
Cell culture
Human neonatal foreskins were obtained from Penn Skin Disease Research Center. Human fetal fibroblasts were isolated from 20-week-old fetal skin (Advanced Bioscience Resources; Alameda, CA). Adult human fibroblasts were obtained from discarded normal skin after surgery. All the protocols were approved by the University of Pennsylvania Institutional Review Board.
MCPyV virion and MCPyV-GFP pseudovirus preparation
Recombinant MCPyV virions and MCPyV-GFP pseudoviruses were prepared as previously described(Schowalter et al., 2011), with minor modifications specified in the Supplemental Experimental Procedures.
MCPyV infection of human dermal cells
Human dermal cells cultured in 10 cm dishes were washed once with 1X DPBS, and incubated with 0.05% Trypsin-EDTA at 37°C for 5 min. The cells were examined under the microscope to ensure that they were coming off the dish. After adding 10 ml DMEM/F12 medium to the dish, the cell solution was transferred to a 15 ml tube and centrifuged at 180 g for 2 min. The cells were resuspended in DMEM/F12 medium containing 20 ng/ml EGF, 20 ng/ml bFGF, and 3µM CHIR99021. As indicated in the figure legends, in some of the experiments, MMPs inhibitors, marimastat and batimastat, kinase inhibitors, trametinib, ibrutinib and idelalisib, or 1 mg/ml collagenase type IV was also used. This collagenase has very low tryptic activity, and therefore can limit the damage to membrane proteins and receptors. A total of 2×104 cells resuspended in 500µl medium were seeded into each well of a 24-well plate and 2×109 viral genome equivalents of MCPyV virions were added to the cells. After the cells were incubated at 37°C in 5% CO2 for 48–72 h, FBS was ad ded to each well to reach 10–50% final concentration. After 24 h, the medium was changed to DMEM/F12 medium with 10–50% FBS. The cells were incubated for 48–72 h before immunofluorescent staining.
MCPyV and MCPyV-GFP pseudovirus infection of human skin slices cultured ex vivo
A human foreskin-ring specimen was cut open. After trimming off fat and subcutaneous tissue, the skin was cut into 300-µm-thick slices using a McIlwain Tissue Chopper. The slices were transferred to DMEM/F12 medium containing 1% N2 supplement, 2% B27 supplement without vitamin A, 20 ng/ml EGF, 20 ng/ml bFGF, 3µM CHIR99021, and Antibiotic-Antimycotic. A total of 5×109 viral genome equivalents of MCPyV or MCPyV-GFP pseudovirus were added to the slice culture to initiate the infection. After 5–7 days, the medium was changed to DMEM/F12 medium with 10% FBS and incubated for 10–14 days before immunofluorescent staining.
For the human scalp experiments, the scalp samples were obtained from Advanced Bioscience Resources, Almeda, CA. The samples were cut into 300 µm slices, cultured, and infected with MCPyV or MCPyV-GFP pseudovirus as described above.
Immunofluorescent staining
Cells were fixed with 3% paraformaldehyde in PBS for 20 min. Immunofluorescent staining was performed as previously described (Liu et al., 2014).
In situ DNA-hybridization chain reaction (HCR) and immunofluorescent-HCR
Cells were fixed with 4% paraformaldehyde in PBS for 10 min. MCPyV FISH was performed using the HCR technique described previously (Choi et al., 2014).
A complete and more detailed description of the procedures performed in this study including reagent catalog numbers and the specifics of how the recombinant plasmid constructs were made can be found in the Supplemental Experimental Procedures. Primer sequences are shown in Supplemental Table S2.
Supplementary Material
Highlights.
Merkel cell polyomavirus (MCPyV) preferentially infects dermal fibroblasts in human skin
Induction of MMPs by growth factors and WNT signaling stimulates MCPyV infection
Dermal fibroblasts represent a cell culture model supporting the full MCPyV life cycle
FDA approved kinase inhibitor Trametinib efficiently blocks MCPyV infection
Acknowledgments
The authors would like to thank Dr. Meenhard Herlyn (Wistar institute) for skin cell marker antibodies, Dr. M. Celeste Simon (U. Pennsylvania) for providing TOF-flash and FOP-flash plasmids, Dr. Ling Li for technical support, and Margo MacDonald for editing the manuscript. We also thank the members of our laboratories for helpful discussion. This work was supported by the National Institutes of Health (NIH) Grants R01CA148768, R01CA142723, R01CA187718, NIAMS AR064220, R01AR054593, P50CA174523, and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
W.L., R.Y., X.X., C.B.B., and J.Y. designed the experiments. W.L., R.Y., and M.E.S. performed the experiments and analyzed the data. A.S.P., R.M.S., C.B.B., and P.F.L. provided reagents and feedback. W.L., and J.Y. wrote the paper.
References
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–841. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- Aho S, Rouda S, Kennedy SH, Qin H, Tan EM. Regulation of human interstitial collagenase (matrix metalloproteinase-1) promoter activity by fibroblast growth factor. Eur J Biochem. 1997;247:503–510. doi: 10.1111/j.1432-1033.1997.00503.x. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- Brown GT, Murray GI. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2015;237:273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- Calder KB, Smoller BR. New insights into merkel cell carcinoma. Adv Anat Pathol. 2010;17:155–161. doi: 10.1097/PAP.0b013e3181d97836. [DOI] [PubMed] [Google Scholar]
- Carter JJ, Daugherty MD, Qi X, Bheda-Malge A, Wipf GC, Robinson K, Roman A, Malik HS, Galloway DA. Identification of an overprinting gene in Merkel cell polyomavirus provides evolutionary insight into the birth of viral genes. Proc Natl Acad Sci U S A. 2013;110:12744–12749. doi: 10.1073/pnas.1303526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Moore PS. Merkel cell carcinoma: a virus-induced human cancer. Annu Rev Pathol. 2012;7:123–144. doi: 10.1146/annurev-pathol-011110-130227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Shin MH, Kim YK, Seo JE, Lee YM, Park CH, Chung JH. Effects of infrared radiation and heat on human skin aging in vivo. J Investig Dermatol Symp Proc. 2009;14:15–19. doi: 10.1038/jidsymp.2009.7. [DOI] [PubMed] [Google Scholar]
- Choi HM, Beck VA, Pierce NA. Next-generation in situ hybridization chain reaction: higher gain, lower cost, greater durability. ACS Nano. 2014;8:4284–4294. doi: 10.1021/nn405717p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, Pariente K, Segondy M, Burguiere A, Manuguerra JC, et al. Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS One. 2012;7:e38499. doi: 10.1371/journal.pone.0038499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjoerup O, Chang Y. Chapter 1 - Update on Human Polyomaviruses and Cancer. In: George FVW, George K, editors. Advances in Cancer Research. Academic Press; 2010. pp. 1–51. [DOI] [PubMed] [Google Scholar]
- Hodgson NC. Merkel cell carcinoma: Changing incidence trends. Journal of Surgical Oncology. 2005;89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- Houben R, Shuda M, Weinkam R, Schrama D, Feng H, Chang Y, Moore PS, Becker JC. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Amit I, Yarden Y. Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta. 2007;1773:1161–1176. doi: 10.1016/j.bbamcr.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lemos B, Nghiem P. Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol. 2007;127:2100–2103. doi: 10.1038/sj.jid.5700925. [DOI] [PubMed] [Google Scholar]
- Liu W, Stein P, Cheng X, Yang W, Shao NY, Morrisey EE, Schultz RM, You J. BRD4 regulates Nanog expression in mouse embryonic stem cells and preimplantation embryos. Cell Death Differ. 2014;21:1950–1960. doi: 10.1038/cdd.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke FL, Rollison DE, Sondak VK. Merkel cell carcinoma and immunosuppression: what we still need to know. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju422. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol. 2009;336:76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neth P, Ries C, Karow M, Egea V, Ilmer M, Jochum M. The Wnt signal transduction pathway in stem cells and cancer cells: influence on cellular invasion. Stem Cell Rev. 2007;3:18–29. doi: 10.1007/s12015-007-0001-y. [DOI] [PubMed] [Google Scholar]
- Neumann F, Borchert S, Schmidt C, Reimer R, Hohenberg H, Fischer N, Grundhoff A. Replication, Gene Expression and Particle Production by a Consensus Merkel Cell Polyomavirus (MCPyV) Genome. PLoS One. 2011;6:e29112. doi: 10.1371/journal.pone.0029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- Peretti A, Borgogna C, Rossi D, De Paoli L, Bawadekar M, Zavattaro E, Boldorini R, De Andrea M, Gaidano G, Gariglio M. Analysis of human beta-papillomavirus and Merkel cell polyomavirus infection in skin lesions and eyebrow hair bulbs from a cohort of patients with chronic lymphocytic leukaemia. Br J Dermatol. 2014;171:1525–1528. doi: 10.1111/bjd.13215. [DOI] [PubMed] [Google Scholar]
- Quan T, Fisher GJ. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology. 2015;61:427–434. doi: 10.1159/000371708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KF, Guastafierro A, Shuda M, Toptan T, Moore PS, Chang Y. Merkel Cell Polyomavirus T Antigens Promote Cell Proliferation and Inflammatory Cytokine Gene Expression. J Gen Virol. 2015 doi: 10.1099/jgv.0.000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schowalter RM, Pastrana DV, Buck CB. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog. 2011;7:e1002161. doi: 10.1371/journal.ppat.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schowalter RM, Pastrana DV, Pumphrey KA, Moyer AL, Buck CB. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509–515. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schowalter RM, Reinhold WC, Buck CB. Entry Tropism of BK and Merkel Cell Polyomaviruses in Cell Culture. PLoS ONE. 2012;7:e42181. doi: 10.1371/journal.pone.0042181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer. 1978;42:2311–2321. doi: 10.1002/1097-0142(197811)42:5<2311::aid-cncr2820420531>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Tolstov YL, Pastrana DV, Feng H, Becker JC, Jenkins FJ, Moschos S, Chang Y, Buck CB, Moore PS. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250–1256. doi: 10.1002/ijc.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang SH, Wang X, Li J, Buck CB, You J. Host DNA Damage Response Factors Localize to Merkel Cell Polyomavirus DNA Replication Sites to Support Efficient Viral DNA Replication. J Virol. 2014;88:3285–3297. doi: 10.1128/JVI.03656-13. PMCID: PMC3957940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaigot P, Pisani A, Darmon YM, Ortonne JP. The majority of epidermal Merkel cells are non-proliferative: a quantitative immunofluorescence analysis. Acta Derm Venereol. 1987;67:517–520. [PubMed] [Google Scholar]
- Van Meter TE, Broaddus WC, Rooprai HK, Pilkington GJ, Fillmore HL. Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor-mediated signaling in gliomas. Neuro Oncol. 2004;6:188–199. doi: 10.1215/S1152851703000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li J, Schowalter RM, Jiao J, Buck CB, You J. Bromodomain protein Brd4 plays a key role in Merkel cell polyomavirus DNA replication. PLoS Pathog. 2012;8:e1003021. doi: 10.1371/journal.ppat.1003021. PMCID:PMC3493480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol. 2012;4:a008078. doi: 10.1101/cshperspect.a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur Hausen A, Rennspiess D, Winnepenninckx V, Speel EJ, Kurz AK. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry. Cancer Res. 2013;73:4982–4987. doi: 10.1158/0008-5472.CAN-13-0616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.