Abstract
The association between visit-to-visit variability of blood pressure (BP) and cognitive decline over time remains incompletely understood in a general population of older adults. We assessed the hypothesis that higher visit-to-visit variability in BP, but not mean BP, would be associated with faster decline in cognitive function among community-dwelling older adults. This prospective cohort study comprised 976 adults who had 3 or 4 visits with BP measurements as part of the China Health and Nutrition Survey from 1991, up to their first cognitive tests, and completed cognitive screening tests at 2 or more visits in 1997, 2000 or 2004. Visit-to visit BP variability was expressed as the standard deviation (SD), coefficient of variation or as the variation independent of mean BP across visits conducted at a mean interval of 3.2y. Mean (SD) age at the first cognitive test was 64 (6)y. Using multivariable-adjusted linear mixed-effects models, we found higher visit-to-visit variability in systolic BP, but not mean systolic BP, was associated with a faster decline of cognitive function (adjusted mean difference [95% CI] for high vs. low tertile of SD variability: standardized composite scores −0.038 standardized units (SU)/y [−0.066, −0.009] and verbal memory −0.041 SU/y [−0.075 to −0.008]). Higher visit-to-visit variability in diastolic BP was associated with a faster decline of cognitive function, independent of mean diastolic BP, among adults 55–64y but not those ≥ 65y. Our results suggest that higher long-term BP visit-to-visit variability is associated with a faster rate of cognitive decline among older adults.
Keywords: Blood pressure, hypertension, cognition, aging, longitudinal studies
Introduction
Research relating blood pressure (BP) to cognitive disorders has focused on hypertension and mean BP levels. Although evidence demonstrates that midlife hypertension is a risk factor for cognitive decline, its association with late-life hypertension is less clear.1 In addition, randomized clinical trials have not shown a consistent effect of BP-lowering treatments for reducing the risk of cognitive decline2 or dementia.1
The clinical relevance of visit-to-visit BP variability has been dismissed until recently. Over the past 5 years evidence has accumulated that visit-to-visit BP variability may not be a random phenomenon or simply an unimportant measurement artifact, but may instead provide information on pathological process and be relevant for prognosis.3, 4 Recent studies have found higher visit-to-visit BP variability, over monthly or yearly visits, to be a strong risk factor for stroke,4 and of small and larger vessel cerebrovascular diseases,5, 6 which could lead to a cascade of changes related to cognitive decline and dysfunction.7
Limited research has examined the relationship between visit-to-visit BP variability and cognitive function, and the studies that have been conducted relied primarily on measurement of cognition at only one timepoint. Previous studies have found higher visit-to-visit BP variability to be associated with lower cognitive scores in populations taking a high number of BP-lowering drugs and other medications. Subjects were at a high risk of cardiovascular disease or a high prevalence of hypertension,8–10 or more than half of the samples were cognitively impaired.11 It remains uncertain whether visit-to-visit variability in BP is associated with a faster rate of cognitive decline in a general population of older adults. Using repeated measures of cognition, the current study examined the association between visit-to-visit variability in BP and cognitive decline among a sample of community-dwelling Chinese men and women.
Methods
Subjects
China Health and Nutrition Survey (CHNS) is an ongoing longitudinal open cohort study established in 1989. A multistage, random cluster process was used to draw the sample from nine provinces of China.12 In each wave of the CHNS, demographic, socioeconomic, lifestyle, and health information were collected. In 1997, 2000 and 2004, CHNS administered cognitive measures among participants 55 years or older, all of whom were community dwellers. Among 2,408 adults participated in a cognitive screening test (participation rate 73%), 1,677 had at least two measurement occasions of cognitive function. Among them, a total of 976 participants also had three or more waves of measurement of blood pressure (N=721 and 255 for three and four measures) at or before their first cognitive test. The temporal sequence of the assessment of blood pressure and cognitive function is shown in Supplemental Figure 1. Participants not included in the analytic sample were similar to those included regarding age, gender, antihypertensive treatment, and baseline systolic blood pressure (130.7 mm Hg and 128.9 mm Hg, respectively; P=0.07), but had slightly higher diastolic blood pressure (82.1 mm Hg and 80.5 mm Hg, respectively; P=0.01).
All participants in CHNS provided written informed consent. The institutional review committees of the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, approved this study.
Assessments
Assessment of Cognitive Function
Cognitive screening items measured by CHNS in face-to-face interviews were derived from part of the Telephone Interview for Cognitive Status –modified (TICS-m).13 Similar to the Mini–Mental State Examination, TICS-m is able to detect cognitive decline over time,13, 14 which has been used by population based studies including the Chinese population.15, 16 In three waves (1997, 2000 and 2004), CHNS used identical cognitive screening items, including immediate and delayed recall of a 10-word list, counting backward from 20, serial 7 subtraction, and orientation. Both immediate and delayed recall scored from 0 to 10. Counting backward and serial 7’s scored from 0 to 7 and was used to assess attention and calculation. Orientation, with scores ranging from 0 to 3, was assessed by asking the current date (1 point each for year, month and date). Naming the tool usually used to cut paper was given 1 point. Higher scores on all items indicate better cognitive function.
Repeated measures of cognitive tests across waves were summarized as outcomes using three approaches: 1) a global composite cognitive score, which was the total of all cognitive screening items with scores ranging from 0 to 31 points; 2) a standardized composite score (in standardized units [SU]), created as an alternative global cognitive measure by averaging z-scores of verbal memory and the other items assessing attention, calculation and orientation; and 3) verbal memory scores (also in SU) which summarized scores for immediate and delayed recall of a 10-word list.
Blood Pressure Measurements
The same standard procedure and calibrated mercury sphygmomanometers were used for measuring BP in each wave since 1991. Detailed blood pressure measurement methods have been described elsewhere.17 Blood pressure was measured after a 5-minute seated rest by certified health workers or nurses who had passed a comprehensive reliability test. In each wave, three BP measurements were taken with a 30-second interval between cuff inflations and were averaged to derive the BP for that wave. To reduce potential concerns about reverse causation (e.g. blood pressure fluctuated due to stresses related to cognitive change), we used blood pressure measurements from 1991 through the first assessment of cognitive function (1997 or 2000).
For systolic and diastolic blood pressure, separately, we calculated the long-term mean, and BP variability across visits. Consistent with a number of other studies,4, 9, 11, 18–21 visit-to-visit BP variability was expressed as standard deviation (SD), coefficient of variation (CV), and variation independent of mean (VIM = SD/meanx, with x derived from curve fitting). Given the time interval between BP measurements, we also fitted a regression line for each participant to separate the two components of total BP variation: model variation and residual variation.
Statistical analysis
Two sets of analyses were performed: the analysis for visit-to-visit variability of systolic blood pressure (SBP) is described below with an identical approach followed for the analysis of visit-to-visit variability of diastolic blood pressure (DBP).
Characteristics of participants were calculated by tertile of SD of SBP. We used linear mixed-effects models to evaluate the association between SD of SBP and cognitive status over time with the lowest tertile of SD of SBP as the referent. The intercept and slope were fitted with random-effects components to account for inter-individual differences in baseline and rate of change of cognitive function. The first model adjusted for age, gender, education (highest level of education attained primary vs. less), time (year since baseline), and time interactions with the above covariates. The second model additionally adjusted for covariates considered as potential confounders or change in the effect estimate by ≥10%: urbanization index (a multicomponent continuous scale),22 ever smoking (yes/no), physical activity (tertile), antihypertensive treatment (self-report), mean SBP, and their time interactions. Linear trends were tested by modeling the median value of each tertile as a continuous variable.
Analyses were repeated modeling tertile of CV and VIM of SBP, as well as separate models exploring associations between cognitive decline and tertile of mean SBP (omitting mean SBP as a covariate) rather than BP variability. Since SBP increases with age, which can also lead to a large standard deviation of SBP, we evaluated if change in SBP over time (SBPlast visit-SBPfirst visit) was associated with cognitive function over time. The associations between SBP model variation or residual variation with cognitive decline were also evaluated.
We examined if the relationship between SD of SBP and cognitive decline was modified by one key factor, age, since previous evidence suggested a stronger association between BP and cognitive function in midlife vs. later-life.1 We also examined if the associations may be different between those with vs. without hypertension (defined as measured mean SBP/DBP ≥140/90 mm Hg or self-reported use of antihypertensive medications) by testing statistical interactions using product terms. A P-value<0.1 was defined as statistical significance for interaction, while P<0.05 was used for main effects.
To assess the robustness of the findings, first we evaluated if the associations between SBP variability and cognitive decline were independent of the remote SBP (i.e. the first BP measurement), baseline SBP (i.e. the latest measure at or before cognitive assessment), or baseline hypertension status. Second, we repeated the main analyses between visit-to-visit BP variability and change in cognitive scores excluding participants who took antihypertensive medications (N=113), or who reported a history of MI, stroke or diabetes (N=51). Moreover, we repeated the main analyses among a total of 1,213 participants who had at least two waves of measurement of blood pressure (N=237, 721, 255 for two, three and four measures). All statistical analyses were performed using STATA (version 11.2; STATA Corp LP).
Results
Relationship between visit-to-visit variability in SBP and cognitive decline
Among all participants in the analytical sample, older age, higher urbanization index, lower education and physical activity, having a history of stroke and diabetes, and taking antihypertensive medication were associated with higher SD of SBP (Table 1). Also, mean SBP was progressively higher with increased SD of SBP (r=0.33).
Table 1.
Participant characteristics by tertile of standard deviation in SBP
| Tertile of standard deviation in SBP, range in mm Hg
|
||||
|---|---|---|---|---|
| Characteristics | T1 (N=327) <9.30 |
T2 (N=326) 9.30–15.00 |
T3 (N=323) ≥15.01 |
P-trend* |
| Age, y | 62.2 (6.3) | 63.1 (6.9) | 64.8 (6.9) | <0.001 |
| Women, % | 51.1 | 52.2 | 52.0 | 0.15 |
| North region, % | 25.7 | 30.1 | 22.6 | 0.59 |
| Mean urbanization index | 47.9 (16.5) | 49.6 (15.8) | 51.2 (16.5) | 0.05 |
| Graduated from primary or above, % | 40.7 | 45.4 | 34.1 | 0.005 |
| Ever smoking, % | 46.2 | 47.6 | 47.1 | 0.52 |
| Mean physical activity, % | ||||
| Low tertile | 27.2 | 32.5 | 36.8 | Ref |
| Middle | 33.6 | 29.8 | 32.5 | 0.19 |
| High | 39.1 | 37.7 | 30.7 | 0.04 |
| Mean body mass index, kg/m2 | 22.0 (2.8) | 22.4 (3.1) | 22.2 (3.4) | 0.14 |
| History of myocardial infarction, % † | 0.9 | 1.2 | 1.9 | 0.46 |
| History of stroke, % † | 0.9 | 1.2 | 3.7 | 0.01 |
| History of diabetes, % † | 1.2 | 2.2 | 3.4 | <0.001 |
| Mean SBP, mm Hg | 119.0 (14.7) | 122.4 (15.0) | 130.0 (18.0) | <0.001 |
| Mean DBP, mm Hg | 76.5 (8.9) | 78.7 (8.6) | 80.1 (9.4) | <0.001 |
| Standard deviation of DBP, mm Hg | 6.5 (4.1) | 8.2 (4.8) | 10.3 (5.6) | <0.001 |
| Antihypertensive medication, % † | 8.0 | 10.7 | 16.1 | <0.001 |
Numbers in table are mean (SD) or percentage. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Tests for P-trend were based on generalized linear regression with SD of SBP (continuous) as the independent variable.
History of myocardial infarction, stroke, diabetes and antihypertensive medication were self-reported information.
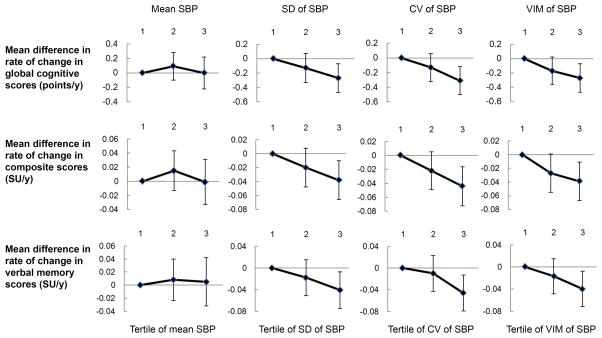
The mean follow-up time for estimating cognitive decline was 5.3 years. Mean SBP was not associated with the rate of cognitive change, while higher visit-to-visit variability of SBP was associated with a faster decline of cognitive function. Compared to the lowest tertile of SD of SBP, the unadjusted rate of global cognitive decline associated with the middle and the highest tertile was faster by −0.13 points per year (95% CI: −0.33, 0.07) and −0.26 points per year (95% CI: −0.45, −0.06) respectively (P-trend=0.01). Visit-to-visit variability of SBP remained associated with a faster decline of cognitive function after adjustment for age, gender, and education or further adjustment for urbanization index, ever smoking, physical activity, antihypertensive treatment, and mean SBP (Figure 1; Table 2). Results were consistent for CV and VIM (SD/mean1.4) of SBP.
Figure 1.
Mean difference in rate of change in global cognitive scores, composite scores, and verbal memory scores by tertiles of mean, SD, CV or VIM of SBP. Values presented in Figure 1 are presented in Table 2 (model 2). CV, coefficient of variation; SBP, systolic blood pressure; SD, standard deviation; SU, standard unit; VIM, variation independent of mean.
Table 2.
Mean difference in rate of change in cognitive scores comparing tertiles of mean SBP, standard deviation, coefficient variation and variation independent of mean SBP (N=976)
| T1 | T2 | T3 | P-trend | |
|---|---|---|---|---|
| Global scores* mean change: −0.41 points/y | ||||
| Tertile of mean SBP | ||||
| <115.22 mm Hg | 115.22–128.76 mm Hg | ≥128.77 mm Hg | ||
| Model 1 | 0 (ref) | 0.09 (−0.10, 0.28) | 0.09 (−0.12, 0.29) | 0.43 |
| Model 2 | 0 (ref) | 0.09 (−0.10, 0.29) | 0.00 (−0.22, 0.22) | 0.99 |
| Tertile of Standard Deviation of SBP | ||||
| <9.30 mm Hg | 9.30–15.00 mm Hg | ≥15.01 mm Hg | ||
| Model 1 | 0 (ref) | −0.13 (−0.33, 0.06) | −0.24 (−0.43, −0.04) | 0.02 |
| Model 2 | 0 (ref) | −0.13 (−0.33, 0.06) | −0.27 (−0.47, −0.07) | 0.008 |
| Tertile of Coefficient of Variation of SBP | ||||
| <7.50% | 7.50–12.18% | ≥12.19% | ||
| Model 1 | 0 (ref) | −0.15 (−0.34, 0.05) | −0.31 (−0.51, −0.12) | 0.002 |
| Model 2 | 0 (ref) | −0.13 (−0.32, 0.06) | −0.31 (−0.50, −0.11) | 0.002 |
| Tertile of Variation Independent of Mean SBP | ||||
| <1.03% | 1.04–1.67% | ≥1.68% | ||
| Model 1 | 0 (ref) | −0.19 (−0.39, 0.00) | −0.28 (−0.47, −0.08) | 0.005 |
| Model 2 | 0 (ref) | −0.17 (−0.36, 0.02) | −0.27 (−0.47, −0.08) | 0.006 |
|
| ||||
| Composite scores* mean change: −0.062 SU/y | ||||
| Tertile of mean SBP | ||||
| Model 1 | 0 (ref) | 0.015 (−0.013, 0.043) | 0.010 (−0.020, 0.039) | 0.55 |
| Model 2 | 0 (ref) | 0.015 (−0.013, 0.043) | −0.001 (−0.033, 0.031) | 0.95 |
| Tertile of Standard Deviation of SBP | ||||
| Model 1 | 0 (ref) | −0.020 (−0.048, 0.008) | −0.034 (−0.062, −0.005) | 0.02 |
| Model 2 | 0 (ref) | −0.020 (−0.048, 0.008) | −0.038 (−0.066, −0.009) | 0.01 |
| Tertile of Coefficient of Variation of SBP | ||||
| Model 1 | 0 (ref) | −0.024 (−0.052, 0.004) | −0.045 (−0.073, −0.016) | 0.002 |
| Model 2 | 0 (ref) | −0.022 (−0.049, 0.006) | −0.044 (−0.072, −0.016) | 0.002 |
| Tertile of Variation Independent of Mean SBP | ||||
| Model 1 | 0 (ref) | −0.030 (−0.058, −0.002) | −0.040 (−0.068, −0.012) | 0.006 |
| Model 2 | 0 (ref) | −0.027 (−0.055, 0.001) | −0.039 (−0.067, −0.011) | 0.006 |
|
| ||||
| Verbal memory* mean change: −0.051 SU/y | ||||
| Tertile of mean SBP | ||||
| Model 1 | 0 (ref) | 0.008 (−0.025, 0.040) | 0.020 (−0.014, 0.054) | 0.25 |
| Model 2 | 0 (ref) | 0.008 (−0.024, 0.041) | 0.005 (−0.032, 0.042) | 0.79 |
| Tertile of Standard Deviation of SBP | ||||
| Model 1 | 0 (ref) | −0.017 (−0.050, 0.016) | −0.036 (−0.068, −0.003) | 0.03 |
| Model 2 | 0 (ref) | −0.018 (−0.051, 0.015) | −0.041 (−0.075, −0.008) | 0.02 |
| Tertile of Coefficient of Variation of SBP | ||||
| Model 1 | 0 (ref) | −0.013 (−0.045, 0.020) | −0.047 (−0.080, −0.014) | 0.004 |
| Model 2 | 0 (ref) | −0.010 (−0.043, 0.022) | −0.046 (−0.079, −0.013) | 0.006 |
| Tertile of Variation Independent of Mean SBP | ||||
| Model 1 | 0 (ref) | −0.020 (−0.052, 0.012) | −0.041 (−0.074, −0.008) | 0.01 |
| Model 2 | 0 (ref) | −0.017 (−0.049, 0.016) | −0.040 (−0.072, −0.007) | 0.02 |
Numbers in table are mean difference compared with tertile 1 (95% CI) unless otherwise specified. A negative β coefficient indicates faster rate of decline in cognitive performance. Model 1 adjusted for age, gender, education, time, and time interactions with each covariate. Model 2 additionally adjusted for urbanization index, ever smoking, physical activity, ever used antihypertensive treatment, mean SBP for variability analysis, and time interactions with each covariate.
Global score combined results of immediate recall of a 10-word list, delayed recall of a 10-word list, counting backward from 20, serial 7 subtraction, and orientation; Composite scores were computed by baseline z scores of memory scores and the rest of the cognitive scores, and were averaged into a single measure. Verbal memory score combined results of immediate and delayed recall of a 10-word list.
We did not find a significant association between change in SBP [mean change between the first and last visit 8.4 (SD 21.5) mm Hg] and global cognitive decline [Tertile (T)3 vs. T1: β=−0.13, 95% CI: −0.33, 0.06, P=0.09). After fitting a regression line from BP measurements over time, on average, model variation explained 51% of total variation. Greater model variation and residual variation of SBP were both associated with faster rate of cognitive decline (Supplemental Table 1).
The associations between visit-to-visit variability in SBP and cognitive decline were not modified by age. For example, compared to the lowest tertile of SD of SBP, the unadjusted rate of global cognitive decline associated with the highest tertile was different by −0.18 points per year (95% CI: −0.42, 0.07) among those 55–64 years and by −0.32 points per year (95% CI: −0.64, −0.01) among ≥65 years. The results were not modified by baseline hypertension status and sociodemographic characteristics such as gender and education. The associations remained unchanged after adjusting for remote SBP, baseline SBP, or baseline hypertension status. Limiting the analyses to participants who did not take antihypertensive medications, or who did not report a history of MI, stroke, or diabetes at baseline did not appreciably alter our results. Results materially remained the same in analyses including participants with two or more waves of measurement of blood pressure (N=1,213; Supplemental Table 2).
Relationship between visit-to-visit variability in DBP and cognitive decline
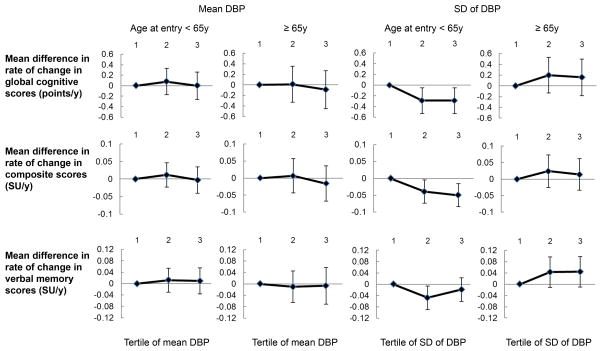
Since age significantly modified the association between DBP variability and cognitive decline (P=0.01), all subsequent analyses for DBP were stratified by age 55–64 (N=610), and ≥65 years (N=366). Increasing tertiles of SD of DBP were associated with higher mean DBP and antihypertensive medication in both age groups, and history of MI among participants aged ≥65 (Supplemental Table 3). The correlation between mean DBP and SD of DBP was r=0.14 among participants <65y and r=0.09 for those ≥65y.
Among adults aged less than 65 years, the unadjusted annual rate of cognitive decline was faster by −0.27 points (95% CI: −0.51, −0.03) and −0.27 points (95% CI: −0.51, −0.03) for the middle and the highest tertile of SD of DBP (P-trend=0.03). These associations persistent in age, gender-, education- adjusted and multivariate adjusted models (Figure 2; Supplemental Table 4). Results were consistent for CV and VIM of DBP, but no significant rate difference was found for verbal memory scores. On average, model variation explained 46% of total variation in DBP. No significant difference in global cognitive decline was associated with model variation (T3 vs. T1: β=−0.14, 95% CI: −0.38, 0.11; P-trend=0.45), but a difference was observed in association with the residual variation of DBP (T3 vs. T1: β=−0.27, 95% CI: −0.51, −0.03; P-trend=0.04) suggesting the relation between visit-to-visit DBP variability and cognitive decline is possibly due to the DBP fluctuation instead of the systematic pattern over time. SD, CV, or VIM of DBP was not associated with rates of cognitive decline for participants 65 years and older.
Figure 2.
Mean difference in rate of change in global cognitive scores, composite scores, and verbal memory scores by tertiles of mean DBP and standard deviation of DBP among different age groups. Values presented in Figure 2 are presented in Supplemental Table 4 (model 2). Age significantly modified the association between standard deviation of DBP and change in global cognitive scores (P=0.01). SD, standard deviation; SU, standard unit.
The associations between visit-to-visit variability in DBP remained unchanged for both age groups with additional adjustment for remote DBP, baseline DBP, or baseline hypertension status, or limited the analyses to participants who did not take antihypertensive medications or who did not report a history of MI, stroke, or diabetes. The associations between DBP variability and cognitive decline were not altered if analyses were modified to include participants with two or more waves of measurement of blood pressure, rather than three or more (Supplemental Table 5).
Discussion
In a cohort of community dwelling Chinese older adults, higher visit-to-visit variability in SBP was associated with a faster decline of global cognitive function and verbal memory over a mean follow-up of 5 years. Higher visit-to-visit DBP variability was associated with a faster decline of global cognitive function among adults 55–64 years of age. In contrast, we did not observe an association between mean SBP or DBP and cognitive change over time.
Few studies have evaluated the association between visit-to-visit BP variability and cognitive function. The results of our analyses are consistent with previous studies suggesting that long-term BP variability may be more informative than mean BP in predicting cognitive function in older adults.8–11 Our definition of visit-to-visit BP variability which used the SD, CV and VIM of measures at each time point, is consistent with previous studies.9–11, 18–20 In earlier studies, the interval between each BP visit ranged from one month to up to four years; the mean interval in our study was 3.2 years. In a US study, where more than half of the participants had cognitive impairment, visit-to-visit variability of SBP, but not DBP, was associated with worse episodic memory, executive and global cognitive function.11 In other studies where participants were at high risk of cardiovascular disease at entry, visit-to-visit SBP variability was associated with lower cognitive scores.8–10 One of these studies found both DBP and SBP variability were associated with cortical infarcts and lower hippocampal volume among a subgroup with magnetic resonance imaging (MRI) showed.9 Moreover, recent studies found higher visit-to-visit variability in both SBP and DBP among AD patients,19 and with higher incidence of dementia among participants 65+ years in which 60% were hypertensive at entry.20 Most of the previous studies were conducted among participants with high prevalence of BP-lowering treatments and other medication use. In contrast, our sample had an initial 22% prevalence of hypertension based on measured BP (mainly in 1991) and much lower level of diagnosis (7%) and treatment (4%), providing a less selective sample and a more natural study of the impact of visit-to-visit BP variability.
All of the above studies demonstrated that these associations were independent of mean BP. In fact, as in our data, very few of them found a significant relation between mean BP and cognitive outcomes. Consistent with these findings, clinical trials aimed at lowering BP to reduce the incidence of dementia1 or cognitive decline2 have not shown a consistent result. However, it is noteworthy that in the Syst-Eur trial, calcium-channel blockers were found to reduce the incidence of dementia,23 and separate studies have shown calcium-channel blockers to be most effective for reducing variability of BP among all antihypertensive drug classes.24
In the current study, while benefits of lower variability in SBP were observed for the full sample, we found that the association between DBP variability and cognitive decline was stronger at younger ages. We hypothesize that this may be due to fewer competing causes of cognitive disorders. Similar to our finding regarding the role of age, Rothwell and others found that BP variability, though increasing with age, was more predictive of stroke risk for younger than for older patients.4 Our findings of a null association between DBP variability and cognitive decline for adults aged 65 years and older were consistent with several other studies,8, 9 while our observations among adults below 65 years need to be confirmed by other studies.
Current treatment decisions for high BP are based on the mean value, which are typically assessed over several visits. 25 However, recent findings suggest that variability of monthly or yearly BP measures predict the risk of stroke,4 cardiovascular morbidity and mortality,26 and overall mortality independent of mean BP.18 Our study adds to the evidence that long-term BP variability is also relevant to cognitive functioning in older adults.
Potential mechanisms linking visit-to-visit BP variability and impaired cognitive function have been proposed. First, MRI findings support the hypothesis that the peripheral BP fluctuations could lead to cerebral hypoperfusion and silent vascular brain lesions,5, 6, 9 which in turn increase the risk of cognitive deterioration.27, 28 These studies have found that higher visit-to-visit BP variability, based on BP measurements during 3 study visits at 1 to 3-year intervals, was related to white matter hyperintensities or lesions,5, 6 and a recent study with visits at 3-months intervals found visit-to-visit BP was associated with decreased hippocampal volume, cerebral microbleeds and cortical infarcts.9 Second, it is also possible that higher BP variability may represent an augmented hemodynamic instability, which could lead to the damage of microvasculature with changes in brain structure and function.29 Consistent with this notion, higher BP variability was associated with endothelial injury and impairment.30 It is hypothesized that cerebral microcirculatory endothelial dysfunction may influence the integrity of the blood-brain barrier, cerebral auto-regulation and other physiologic processes, with subsequent impact on the genesis of brain infarcts and the development of cognitive impairment.31
A number of limitations of the current study should be considered. First, although a wide array of covariates were adjusted in the model, measures of potential confounders (e.g., depression, heart rate) were not available, and some residual confounding is possible. Second, BP for a given participant was not systematically assessed at the same time of day across the multiple follow-ups which could contribute to the random error of BP variability and attenuated the associations. Third, like some other studies,18, 20 BP variability in our study was calculated from 3 or 4 visits, which has limited the precision and reproducibility of estimating BP variability compared to more measurements. In addition, only 58% of participants were eligible for main analysis due to the open cohort design, but selection bias was not a strong concern given the similar findings when more participants were included in the sensitivity analysis. The relatively narrow scope of cognitive screening items adopted in CHNS is another limitation. The association between BP variability and cognitive decline may be strengthened if more sensitive tools for cognitive screening were available.
Perspectives
The current study supports the hypothesis that higher long-term BP variability independent of mean BP is associated with a faster rate of cognitive decline among older adults. Controlling BP instability could possibly be a strategy in preserving cognitive function among older adults, but intervention trials and more longitudinal studies are needed to confirm this finding.
Supplementary Material
Novelty and Significance.
What is New?
Utilizing repeated measures of cognitive function, this prospective study shows that higher long term visit-to-visit variability in systolic blood pressure, but not mean blood pressure, is associated with faster rate of cognitive decline in community dwelling older adults.
The association between visit-to-visit variability in diastolic blood pressure and cognitive decline is age-dependent: higher visit-to-visit variability in diastolic blood pressure was associated with a faster decline of cognitive function among adults < 65 years but not those ≥ 65 years.
What is Relevant?
The present study suggests that controlling blood pressure instability could be a potential strategy in preserving cognitive function among older adults.
Summary
In a cohort of community dwelling older adults, higher visit-to-visit variability in systolic blood pressure was associated with a faster decline of global cognitive function and verbal memory. Higher visit-to-visit diastolic blood pressure variability was associated with a faster decline of global cognitive function among adults 55–64 years. Mean SBP or DBP was not associated with cognitive change.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, Carolina Population Center [5 R24 HD050924], the University of North Carolina at Chapel Hill, the NIH [R01-HD30880, DK056350, R24 HD050924, and R01-HD38700] and the Fogarty NIH grant [5 D43 TW009077] for financial support for the China Health and Nutrition Survey (CHNS) data collection and analysis files. The first author received financial support from Sanofi/UNC Global Nutrition Fellowship and the Fogarty NIH grant [5 D43 TW009077].
Footnotes
Disclosures
None.
References
- 1.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 2.Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Joyce C, Levitan EB, Holt E, Shimbo D, Webber LS, Oparil S, Re R, Krousel-Wood M. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. J hypertens. 2011;29:2332–2338. doi: 10.1097/HJH.0b013e32834cf213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 5.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: The honolulu-asia aging study. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 7.Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: Evidence from clinicopathological studies in humans. Stroke. 2012;43:2526–2534. doi: 10.1161/STROKEAHA.112.655803. [DOI] [PubMed] [Google Scholar]
- 8.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: New independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J hypertens. 2012;30:1556–1563. doi: 10.1097/HJH.0b013e3283552735. [DOI] [PubMed] [Google Scholar]
- 9.Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, Sattar N, Jukema JW, van Osch MJ, van der Grond J, van Buchem MA, Westendorp RG, de Craen AJ, Mooijaart SP. Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ. 2013;347:f4600. doi: 10.1136/bmj.f4600. [DOI] [PubMed] [Google Scholar]
- 10.Böhm M, Schumacher H, Leong D, Mancia G, Unger T, Schmieder R, Custodis F, Diener H-C, Laufs U, Lonn E. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension. 2015;65:651–661. doi: 10.1161/HYPERTENSIONAHA.114.04568. [DOI] [PubMed] [Google Scholar]
- 11.Epstein NU, Lane KA, Farlow MR, Risacher SL, Saykin AJ, Gao S. Cognitive dysfunction and greater visit-to-visit systolic blood pressure variability. J Am Geriatr Soc. 2013;61:2168–2173. doi: 10.1111/jgs.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: The china health and nutrition survey—monitoring and understanding socio-economic and health change in china, 1989–2011. Int J Epidemiol. 2010;39:1435–1440. doi: 10.1093/ije/dyp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt J, Welsh KA, Breitner JC, Folstein MF, Helms M, Christian JC. Hereditary influences on cognitive functioning in older men: A study of 4000 twin pairs. Arch Neurol. 1993;50:599–603. doi: 10.1001/archneur.1993.00540060039014. [DOI] [PubMed] [Google Scholar]
- 14.Plassman BL, Newman TT, Welsh KA, Helms M, Breitner JCS. Application in epidemiological and longitudinal studies. Cogn Behav Neurol. 1994;7:235–241. [Google Scholar]
- 15.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: The health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162–171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss J, Lei X, Park A, Shen Y, Smith JP, Yang Z, Zhao Y. Health outcomes and socioeconomic status among the elderly in china: Evidence from the charls pilot. J Popul Ageing. 2010;3:111–142. doi: 10.1007/s12062-011-9033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du S, Neiman A, Batis C, Wang H, Zhang B, Zhang J, Popkin BM. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in china. Am J Clin Nutr. 2014;99:334–343. doi: 10.3945/ajcn.113.059121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from nhanes iii, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 19.Lattanzi S, Viticchi G, Falsetti L, Buratti L, Luzzi S, Provinciali L, Silvestrini M. Visit-to-visit blood pressure variability in alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:347–351. doi: 10.1097/WAD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 20.Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues J-F, Richard-Harston S, Tzourio C. Blood pressure variability and risk of dementia in an elderly cohort, the three-city study. Alzheimers Dement. 2014;10:S330–337. doi: 10.1016/j.jalz.2013.05.1777. [DOI] [PubMed] [Google Scholar]
- 21.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: The coronary artery risk development in young adults (cardia) study. Hypertension. 2014;64:983–988. doi: 10.1161/HYPERTENSIONAHA.114.03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones-Smith JC, Popkin BM. Understanding community context and adult health changes in china: Development of an urbanicity scale. Soc Sci Med. 2010;71:1436–1446. doi: 10.1016/j.socscimed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forette F, Seux M-L, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in europe (syst-eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 24.Webb AJS, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ Committee tNHBPEPC. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G, Messerli F, Bakris G, Zhou Q, Champion A, Pepine CJ. Blood pressure control and improved cardiovascular outcomes in the international verapamil sr-trandolapril study. Hypertension. 2007;50:299–305. doi: 10.1161/HYPERTENSIONAHA.107.090290. [DOI] [PubMed] [Google Scholar]
- 27.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 28.de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012 doi: 10.1155/2012/367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cecchi E, Giglioli C, Valente S, Lazzeri C, Gensini GF, Abbate R, Mannini L. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214:249–256. doi: 10.1016/j.atherosclerosis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Diaz KM, Veerabhadrappa P, Kashem MA, et al. Visit-to-visit and 24-h blood pressure variability: Association with endothelial and smooth muscle function in african americans. J Hum Hypertens. 2013;27:671–677. doi: 10.1038/jhh.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32:S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.