Abstract
The increase in oxidative stress and inflammatory responses associated with neurodegenerative diseases has drawn considerable attention towards understanding the transcriptional signaling pathways involving NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and Nrf2 (Nuclear Factor Erythroid 2-like 2). Our recent studies with immortalized murine microglial cells (BV-2) demonstrated effects of botanical polyphenols to inhibit lipopolysaccharide (LPS)-induced nitric oxide (NO) and enhance Nrf2-mediated antioxidant responses (Sun et al., 2015). In this study, an immortalized rat astrocyte (DI TNC1) cell line expressing a luciferase reporter driven by the NF-κB or the Nrf2/Antioxidant Response Element (ARE) promoter was used to assess regulation of these two pathways by phytochemiscals such as quercetin, rutin, cyanidin, cyanidin-3-O-glucoside, as well as botanical extracts from Withania somnifera (Ashwagandha), Sutherlandia frutescens (Sutherlandia) and Euterpe oleracea (Açaí). Quercetin effectively inhibited LPS-induced NF-κB reporter activity and stimulated Nrf2/ARE reporter activity in DI TNC1 astrocytes. Cyanidin and the glycosides showed similar effects but only at much higher concentrations. All three botanical extracts effectively inhibited LPS-induced NF-κB reporter activity. These extracts were capable of enhancing ARE activity by themselves and further enhanced ARE activity in the presence of LPS. Quercetin and botanical extracts induced Nrf2 and HO-1 protein expression. Interestingly, Ashwagandha extract was more active in inducing Nrf2 and HO-1 expression in DI TNC1 astrocytes as compared to Sutherlandia and Açaí extracts. In summary, this study demonstrated NF-kB and Nrf2/ARE promotor activities in DI TNC1 astrocytes, and further showed differences in ability for specific botanical polyphenols and extracts to down-regulate LPS-induced NF-kB and up-regulate the NRF2/ARE activities in these cells.
Keywords: Ashwagandha, Açaí, Sutherlandia, quercetin, ARE reporter, NF-κB reporter
1. Introduction
Astrocytes comprise a major immune active glial cell type and provide nutrients that regulate the homeostatic balance of neurotransmitters and ions in the central nervous system. Under pathological conditions such as cerebral ischemia and traumatic brain injury, astrocytes surrounding the site of injury become reactive (Lin et al., 2004, Sofroniew and Vinters, 2010, Parpura et al., 2012). Reactive astrogliosis is associated with release of free radicals and inflammatory factors which are basis for secondary injury. In cultured astrocytes, pro-inflammatory cytokines, chemokines, and cytotoxic factors prime activation of metabolic pathways required for inflammatory responses, leading to oxidative and nitrosative stress (Glass et al., 2010). In our previous studies with an immortalized rat astrocyte DI TNC1 cell line, up-regulation of the NF-κB pathway by pro-inflammatory cytokines, such as TNFα and IL-1β, led to the release of sPLA2-IIA, an inflammatory enzyme known to play a role in infection and neuroinflammation. Botanical polyphenols such as resveratrol from grape and EGCG from green tea effectively inhibited cytokine-induced sPLA2-IIA mRNA expression (Jensen et al., 2009).
A number of compounds with electrophilic properties are capable of activating the antioxidant response pathway that involves the Kelch-like ECH-associated protein 1(Keap1)/Nrf2 complex, which is responsible for transcriptional activation of a large number of genes regulated by the ARE (Hybertson et al., 2011, Hybertson and Gao, 2014). In this pathway, Keap1 serves as an adaptor protein for an E3 ubiquitin ligase that targets Nrf2 for ubiquitin-dependent degradation by the proteasome (Zhang and Hannink, 2003, Zhang et al., 2004). Structurally diverse compounds, including a number of phytochemicals, can perturb Keap1-mediated repression of Nrf2, leading to the release of this transcription factor into the cytoplasm, and its translocation to the nucleus and subsequent binding to ARE sequences in promoters for genes of the phase II enzymes (Scapagnini et al., 2011, Cardozo et al., 2013, Reuland et al., 2013, Niture et al., 2014). These enzymes play an important role in detoxification and cytoprotection, as well as regulation of redox homeostasis. Recent emphasis has been directed to the induction of HO-1, an anti-oxidative enzyme that degrades heme and generates CO, biliverdin and free iron (Abraham and Kappas, 2008). The Nrf2 pathway appears to be ubiquitously expressed among neurons and glial cells, although the mechanism(s) for its regulation has not been clearly understood (Huang et al., 2015). In astrocytes, tert-butylhydroquinone (tBHQ), an anti-oxidant compound, was shown to exert effects through activation of the Nrf2 pathway and induction of HO-1 (Park and Kim, 2014). Lipoxin A4, an eicosanoid product, was also shown to attenuate oxygen-glucose deprivation (OGD) damage and oxidative stress in astrocytes through activation of the Nrf2 pathway and HO-1 production (Wu et al., 2015). In studies with microglial cells, there is evidence that botanical polyphenols (such as quercetin) inhibit LPS-induced inflammatory responses (e.g., production of iNOS/NO) in part due to upregulation of the Nrf2 pathway and synthesis of HO-1 (Chen et al., 2005, Kang et al., 2013, Lee et al., 2014a, Sun et al., 2015).
There is substantial interest to understand molecular cross-talk between NF-kB and Nrf2 pathways (Wardyn et al., 2015). Since astrocytes exhibit both similar and different properties as compared with microglial cells, understanding how phytochemicals regulate the oxidative (NF-κB) and anti-oxidative (Nrf2) pathways may offer important insights into therapeutic interventions for cerebral ischemia, traumatic injury and chronic neurodegenerative diseases (Calabrese et al., 2010, Lee et al., 2014b). In this study, the immortalized rat astrocyte (DI TNC1) cell line was stably transfected either with the NF-κB or the ARE promoter coupled to the luciferase reporter gene (Mossine et al., 2013), and was used to assess relative NF-κB and ARE activities in response to quercetin and cyanidin (flavonoids enriched in berries) and their glycosides, as well as extracts from Ashwagandha, Sutherlandia and Açaí, some have been used as dietary supplements in different parts of the world. In addition, the ability for botanicals to stimulate Nrf2 and HO-1 expression in DI TNC1 astrocytes was also assessed and compared with their effects on ARE reporter activity. Results from this study demonstrated similarities and differences in the NF-κB and Nrf2 responses between DI TNC1 astrocytes and the murine BV-2 microglial cells (Simonyi et al., 2015, Sun et al., 2015), and established protocols for screening regulators of oxidative and anti-oxidative pathways in response to astrogliosis.
2. Materials and Methods
2.1. Materials
Dulbecco's modified Eagle's medium (DMEM), penicillin, streptomycin, 0.05% (w/v) trypsin/EDTA and phosphate-buffered saline (PBS) were obtained from GIBCO-BRL (Gaithersburg, MD, USA). Fetal bovine serum was from Atlanta Biologicals (Lawrenceville, GA, USA). Cytokines (TNFα, IL-1β and IFNγ) were purchased from R & D Systems (Minneapolis, MN, USA). Lipopolysaccharide (LPS) (rough strains) from Escherichia coli F583 (Rd mutant) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Premixed WST-1 cell proliferation reagent was obtained from Clontech (Mountain View, CA). BCA protein assay kit was from Pierce Biotechnology (Rockford, IL). Reporter Lysis Buffer (E397A) and Firefly luciferase kits (PR-E4030 or PR-E2610) were from Promega (Madison, WI). Antibodies for Western blot analysis: goat anti-rabbit IgG-horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, rabbit polyclonal anti-Nrf2 (C20, sc-722) and rabbit polyclonal anti-HO-1 (H-105, sc-10789), were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-β-actin (sc-2004) was from Sigma-Aldrich (St. Louis, MO). Precision Plus Protein molecular weight standards were obtained from (Bio-Rad, Hercules, CA).
2.2. Botanical extracts
Sutherlandia frutescens
(L.) R.Br. (Sutherlandia) is one of botanicals used in the Center for Botanical Interaction Studies (CBIS) of the University of Missouri, Columbia, MO. Sutherlandia milled vegetative parts were purchased from Big Tree Nutraceutical (Fish Hoek, South Africa). Samples (50 g) were extracted with 500 mL of 95% (v/v) ethanol at room temperature on a rotating shaker and vacuum-filtered, and the solids returned to the flask and extracted twice more with ethanol. The combined filtrates were evaporated to dryness under vacuum, weighed and re-suspended in dimethyl sulfoxide (DMSO) prior to use in cell culture (Jiang et al., 2014). Samples were aliquoted and stored in -80°C freezer until use.
Withania somnifera (L.) Dunal (Ashwagandha) was obtained from NOW Foods (Bloomingdale, IL) as an encapsulated root extract (450 mg per capsule) with a minimum of 2.5% (w/v) total withanolides. Briefly, the contents of several capsules were weighed and suspended in 95% ethanol. The mixture was left to stand overnight at room temperature, after which it was centrifuged and filtered (0.2 μm, Fisher Scientific, Pittsburgh, PA). The extract was dried under vacuum (CentrVap Concentrater, LABCONCO, Kansas City, MO), and the dried extract was weighed and re-suspended in dimethyl sulfoxide (DMSO), and were aliquoted and stored in -80°C until use. This extract was applied to the culture medium such that the final DMSO concentration was always 0.1% (v/v).
Euterpe oleracea Martius (Açaí) berries were obtained in Belém (State of Pará, Rio de Janeiro, Brazil), which prepares açaí (in three forms) in strict hygienic conditions that are monitored by the Ministry of Agriculture. Briefly, the fruit was washed and disinfected with chlorinated water, then transferred to pulpers to remove the edible portion (pulp + peel). The product was packaged in plastic bags, stored at -18°C. Samples were lyophilized and sent to Dr. Robert Smith (FDA laboratories in Lenexa, KS. These samples do not have any kind of chemical additives (preservatives, coloring agents, acids or emulsifiers). Lyophilized samples were extracted with dry methanol at 100°C and 10 MPa (100 atm) pressure in a sealed container using an Accelerated Solvent Extractor (ASE, ThermoFisher, Sunnyvale, CA), as described previously (Luo R et al., 2012, Richards et al., 2014). The methanol was evaporated and a portion of the residue was weighed, suspended in DMSO and stored at -80° C prior to use in cell culture.
2.3. Cell cultures and transfection of NF-κB and ARE promoter constructs
Immortalized rat astrocytes (DI TNC1) were developed by transfecting astrocytes from rat brain diencephalon with the polyomavirus middle T-antigen, and this cell line was purchased from ATCC (CRL-2005, Rockville, MD). DI TNC1 cells were cultured in DMEM (high glucose), 10% (v/v) FBS, 100 units/mL penicillin and 100 μg/mL streptomycin, and maintained in 5% CO2 at 37°C. To harvest DI TNC1 astrocytes, cells were tre ated with 0.05% (w/v) trypsin/EDTA for 2 min at 37°C, and centrifuged at 125 × g for 10 min. The cell pellets were re-suspended in culture medium. Cell density was determined by counting cells with a hemocytometer. Cells were subcultured in 12-well (0.4 × 106 cells) or 6-well (1.0 × 106 cells) plates for experiments.
For this study, we used rat DI TNC1 astrocytes stably transfected with reporter vectors containing an “insulated” NF-κB response element or an Nrf2-ARE, immediately followed by a minimal CMV promoter and the firefly luciferase gene (Mossine et al., 2013). In addition, the vectors contained the EF1-promoted destabilized copepod GFP and puromycin resistance encoding sequences. Cells stably transfected with the NF-κB or Nrf2-ARE constructs were cultured and plated in the same manner as described for the untransfected DI TNC1 astrocytes.
2.4. Cell viability assay
The WST-1 protocol was used for assessment of cell viability (Chuang et al., 2015). Briefly, after reaching 80-90% confluency, untransfected cells were serum starved for 3 h, followed by treatment with or without botanical compounds or extracts for 1 h and then stimulated with or without LPS for 6 h. After treatment, aliquots of the culture medium were used for cell viability assay by adding 10 μl of the WST-1 reagent (Roche, Mannheim, Germany). After gentle shaking, cells were incubated for 2 h at 37 °C and absorbance was read at 420-480 nm (with reference at >600 nm) using a Synergy4 Plate Reader (BioTek Instruments, Inc., Fisher Scientific).
2.5. Measurement of luciferase activity
Stably transfected DI TNC1-NF-κB or DI TNC1-ARE astrocytes were plated with 100 μL of DMEM at a density of 5,000–10,000 cells per well in a 96-well plate, and allowed to attach for at least 24 h in the standard cell culture conditions. Cells were serum-starved for 3 h, followed by dose-dependent treatment with phytochemicals or extracts for 1 h at 37°C. Cells were then incubated with or without LPS (100 ng/mL) for 6 h, after which the supernatant was removed, and cells were washed, followed by addition of 80 μL of Reporter Lysis Buffer (1×) for 30-40 min at room temperature. Fifty μL of the lysates were transferred to wells in a low-fluorescence black plate with a clear bottom, and fluorescence was measured at 485/528 nm. Afterwards, 30 μL of the lysates or blanks were transferred to a white 96-well plate, and 30 μL of luciferase substrate was added to each well, and followed by measuring luminescence within 5 min. Relative luciferase activity (fold induction) was calculated using the formula: RLA = (RLU/RFU)treated sample/(RLU/RFU)untreated control, where relative luminescence unit (RLU) and relative fluorescence unit (RFU) are the corresponding luminescence and fluorescence values less blanks.
2.6. Western blot analysis
Protocol for Western blot analysis was similar to that previously described (Sun et al., 2015). Briefly, cells were harvested in Laemmli buffer, centrifuged at 10,000 × g for 15 min at 4°C, and transferred to a clean tube to remove cell debris. The supernatant was collected in Eppendorf tubes and if necessary, the cell lysate could be frozen at -80°C until use. Protein concentration was measured and normalized with the BCA protein assay kit. Depending on the target of interest, 5-10 μg of total protein was loaded in SDS-PAGE for electrophoresis. Samples together with protein molecular weight (MW) standards were loaded in 10% SDS-PAGE gels and resolved at 100 V. After electrophoresis, proteins were transferred to 0.45 μm nitrocellulose membranes at 100 V for 1.5 h. The membrane was then cut into three strips using the MW ladder as guide: 25-37 kDA for HO-1 (MW 32 kDa), (37-50 kDa) for β-actin (MW 40 kDa), and 75-150 kDa for Nrf2 (MW 110 kDa). It is worth noting that the calculated molecular mass of Nrf2, based on amino acid sequence, is approximately 60 kD. However, the protein migrates through SDS-PAGE gels at approximately 100-110 kDa. This anomalous migration is a consequence of amino acids within the central region of the protein, as can be demonstrated by comparing a series of purified Nrf2 proteins that have sequential truncations from the C-terminus (note provided by M. Hannink). The membranes were blocked in Tris-buffered saline (TBS), pH 7.4, with 0.1% (v/v) Tween 20 (TBS-T) containing 5% (w/v) non-fat milk for 1.5 h at room temperature, and then incubated with anti-Nrf2 antibody (1:500) or anti-HO-1 antibody (1:500) overnight at 4°C. After repeated washing with 1× TBS-T, blots were incubated with goat anti-rabbit IgG-HRP antibody (1:6,000) for 1 h at room temperature, and then washed three times with 1× TBS-T. For loading control, blots were incubated with anti-β-actin antibody (1:50,000). Immuno-labeling was detected by chemiluminescence ECL/WestPico (Thermo Scientific, Rockford, IL). Films were scanned and the optical densities of protein bands were measured using the QuantityOne software program (BioRad, Hercules, CA).
2.7. Statistical analysis
Data are presented as means ± SEM of results from at least three independent experiments. Results were analyzed by either one-way or two-way ANOVA with Bonferroni post-tests (V4.00; GraphPad Prism Software Inc., San Diego, CA). Statistical significance was considered for p<0.05.
3. Results
3.1. Cytokines and LPS induce NF-κB luciferase activity in DI TNC1 astrocytes
Our previous studies with DI TNC1 astrocytes demonstrated the ability of pro-inflammatory cytokines and endotoxins to induce synthesis and release of sPLA2-IIA, an inflammatory enzyme known to be transcriptionally stimulated by the NF-κB pathway (Jensen et al., 2009, Sheng et al., 2011). In this study, we evaluated whether these pro-inflammatory cytokines (TNFα, IL-1β, and IFNγ, 10 ng/mL, each) as well as LPS (100 ng/mL) could stimulate activity of the luciferase reporter driven by the NF-κB promoter in stably transfected DI TNC1 astrocytes. Results indicate that TNFα, IL-1β and LPS, but not IFNγ, similarly stimulated NF-κB luciferase activity (Fig. 1A).
Fig. 1. Cytokines and LPS induce NF-κB-dependent luciferase activity in DI TNC1 astrocytes.
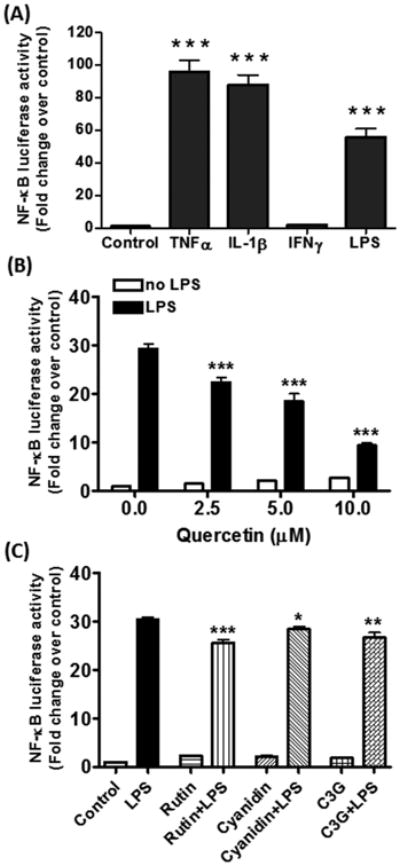
DI TNC1 astrocytes were stably transfected with a luciferase reporter gene driven by the NF-κB promoter. Cells were treated with (A) TNFα, IL-1β, and IFNγ (10 ng/mL each) or LPS (100 ng/mL) for 6 h,(B) quercetin (0-10 μM), or (C) rutin, cyanidin and cyanidin-3-O-glucoside (0 and 100 μM each) for 1 h followed by stimulation with LPS (100 ng/mL) for 6 h. Luciferase activity was determined, as described in Methods. Results are expressed as the mean ± SEM (n=3) and analyzed by one-way ANOVA (A) or two-way ANOVA (B, C) with Bonferroni post-tests. (A) ***p<0.001 vs. control. (B) *p<0.05, **p<0.01, ***p<0.001 vs. LPS alone.
3.2. Polyphenols and botanical extracts reduce LPS-induced NF-κB activity in DI TNC1 astrocytes
We examined the effects of a number of polyphenols from berries and fruits, including quercetin and cyanidin and their glycosides, rutin and cyanidin-3-O-glucoside, respectively, on LPS-induced NF-κB activity. Among these compounds, quercetin was the most effective in attenuating the LPS-induced NF-κB activity, with an inhibition of ∼70% at 10 μM (Fig. 1B). Quercetin added to cells alone caused an insignificant increase in NF-κB activity (Fig. 1B). Similar to results with BV-2 microglial cells (Simonyi et al., 2015), rutin, cyanidin and cyanidin-3-O-glucoside, even added at 100 μM, were not as effec tive as quercetin in the inhibition of LPS-induced NF-κB activity (Fig. 1C).
Similar to botanical polyphenols, the botanical extracts (Ashwagandha, Sutherlandia and Açaí) alone also did not promote NF-κB activity (data not shown), but all three extracts inhibited LPS-induced NF-κB activity in a dose-dependent manner, but to different degrees (Fig. 2A-C).
Fig. 2. Effects of Ashwagandha (A), Sutherlandia (B), and Açaí (C) extracts on LPS-induced NF-κB-dependent luciferase activity in DI TNC1 astrocytes.
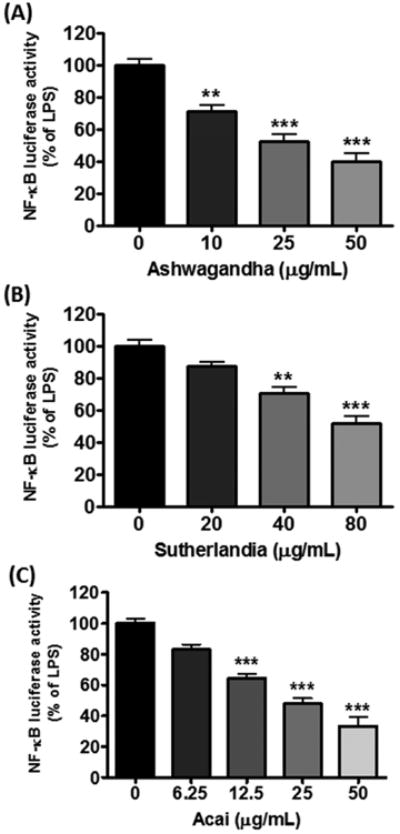
DI TNC1 astrocytes stably transfected with a luciferase reporter gene driven by the NF-κB promoter were treated with botanical extracts for 1 h followed by stimulation with LPS (100 ng/mL) for 6 h. Luciferase activity was determined, as described in Methods. Results are expressed as the mean ± SEM (n=3). Data were analyzed by one-way ANOVA followed by Bonferroni post-tests. **p<0.01, ***p<0.001 vs. LPS alone.
3.3. Polyphenols and botanical extracts enhance ARE–dependent luciferase activity in DI TNC1 astrocytes
Addition of quercetin to DI TNC1 astrocytes stably transfected with the Nrf2-ARE luciferase reporter vector led to an increase in luciferase activity in a dose-dependent manner, reaching a 20-fold increase at 10 μM quercetin (Fig. 3A). Cells treated with LPS (100 ng/mL) alone also caused a small (3-fold) increase in ARE activity (Fig. 3A). However, when quercetin was added prior to stimulation with LPS, ARE activity was consistently greater than that due to quercetin or LPS alone (Fig. 3A). We also tested the ability for rutin, cyanidin and cyanidin-3-O-glucoside to enhance ARE activity in DI TNC1 astrocytes. When these compounds were added to cells at 100 μM, only a 3-fold increase in ARE activity was observed, and upon stimulation with LPS, ARE activity increased up to 5-fold (Fig. 3B).
Fig. 3. Effects of quercetin (A), rutin, cyanidin and cyanidin-3-O-glucoside (B) on LPS-induced ARE-dependent luciferase activity in DI TNC1 astrocytes.
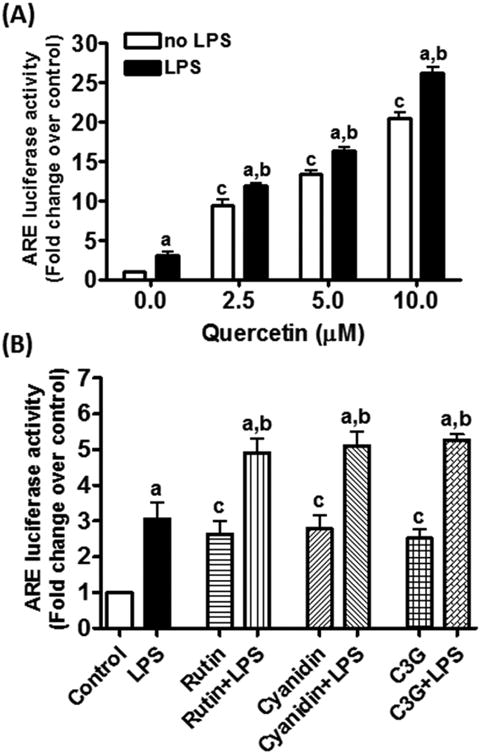
DI TNC1 astrocytes stably transfected with a luciferase reporter gene driven by the Nrf2/ARE were treated with quercetin (0-10 μM), rutin, cyanidin or cyanidin-3-O-glucoside (0 and 100 μM) for 1 h followed by LPS (100 ng/mL) or vehicle for 6 h. Luciferase activity was determined, as described in Methods. Results are expressed as the mean ± SEM (n =3) and analyzed by two-way ANOVA with Bonferroni post-tests where “a” denotes significant differences between LPS+polyphenol vs. polyphenol alone (p<0.05 for 0 and 2.5 μM quercetin and p<0.01 for all the other comparisons); “b” denotes significant differences between LPS+polyphenol vs. LPS alone (p<0.05 for rutin; p<0.01 for cyanidin; p<0.001 for quercetin and cyanidin-3-O-glucoside); “c” denotes significant differences as compared to 0 μM polyphenol (p<0.001 for quercetin and p<0.05 for each on Fig. 3B).
Extracts from Ashwagandha (10 - 50 μg/mL) and Sutherlandia (20 - 80 μg/mL) were capable of enhancing ARE activity in DI TNC1 cells in a dose-dependent manner with activity up to 50-60 fold above basal levels at high concentrations (Figs. 4A and B). When Ashwagandha and Sutherlandia extracts were pretreated in DI TNC1 cells for 1 h prior to stimulation with LPS, there was a small increase in ARE activities (Figs. 4A and B). On the other hand, addition of Açaí extract alone (6.25 - 50 μg/mL) only increased ARE activity by 2-3 fold, and pretreatment of Açaí followed by stimulation with LPS (100 ng/mL) increased ARE activity by 10-fold (Fig. 4C).
Fig. 4. Effects of Ashwagandha (A), Sutherlandia (B), and Açaí (C) extracts on LPS-induced ARE-dependent luciferase activity in DI TNC1 astrocytes.
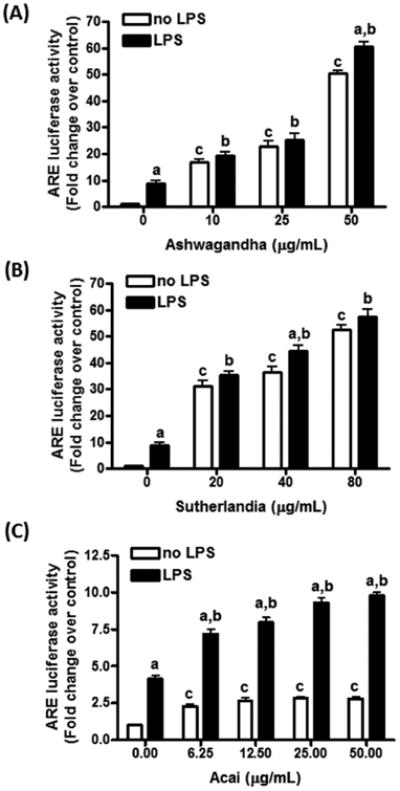
DI TNC1 astrocytes stably transfected with a luciferase reporter gene driven by the Nrf2/ARE were treated with the indicated concentration of botanical extracts for 1 h followed by LPS (100 ng/mL) or vehicle for 6 h. Luciferase activity was determined, as described in Methods. Results are expressed as the mean ± SEM (n=3) and analyzed by two-way ANOVA with Bonferroni post-tests, where “a” denotes significant differences between LPS+botanical extracts vs. extracts alone (p<0.05 for Sutherlandia; p<0.01 for Ashwagandha; p<0.001 for Açaí); “b” denotes significant differences between LPS+botanical extracts vs. LPS alone (p<0.001); “c” denotes significant differences as compared to 0 μg/mL extract (p<0.001 for each comparison except for Açaí at 6.25 μg/mL, where p<0.01).
3.4. Effects of quercetin and botanical extracts on Nrf2 and HO-1 protein expression in DI TNC1 astrocytes
Our earlier study with microglial cells provided evidence for polyphenol compounds such as quercetin to activate the Nrf2 signaling pathway leading to increased production of HO-1, one of the phase II anti-oxidative enzymes (Sun et al., 2015). In this study, Western blot analysis was used to test the ability of quercetin and botanical extracts to induce Nrf2 and HO-1 expression in untransfected DI TNC1 astrocytes. Similar to microglial cells, low levels of endogenous Nrf2 and HO-1 expression were found in control cells without addition of botanicals. Addition of quercetin to cells dose-dependently enhanced Nrf2 and HO-1 expression reaching a 3 and 5 folds increase, respectively, at 10 μM (Fig. 5A and B). Among the botanical extracts, Ashwagandha greatly induced Nrf2 and HO-1 expression, reaching 12- and 25-folds increase, respectively, at 50 μM (Fig. 5C and D). Sutherlandia (Fig. 5E and F) and Açaí (Fig. 5G and H) extracts were less effective, showing only 3-4 folds increase in Nrf2 and HO-1.
Fig. 5. Botanicals stimulate Nrf2 and HO-1 expression in untransfected DI TNC1 astrocytes.
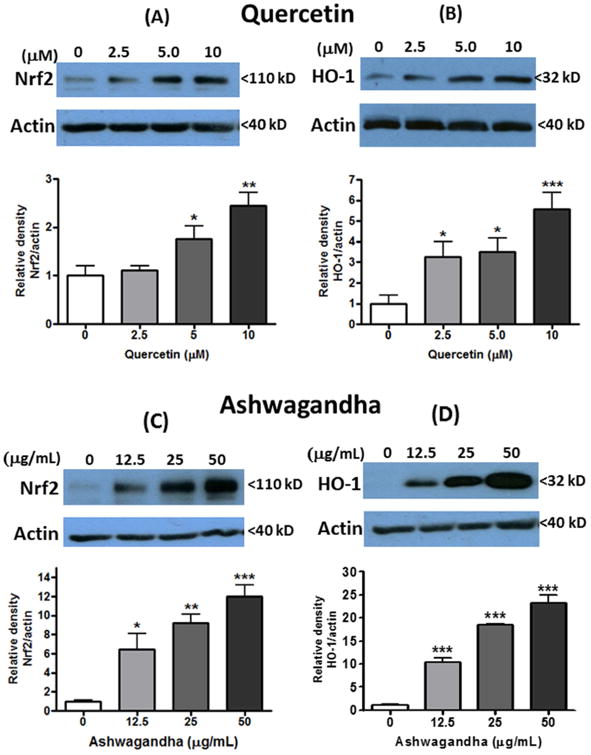
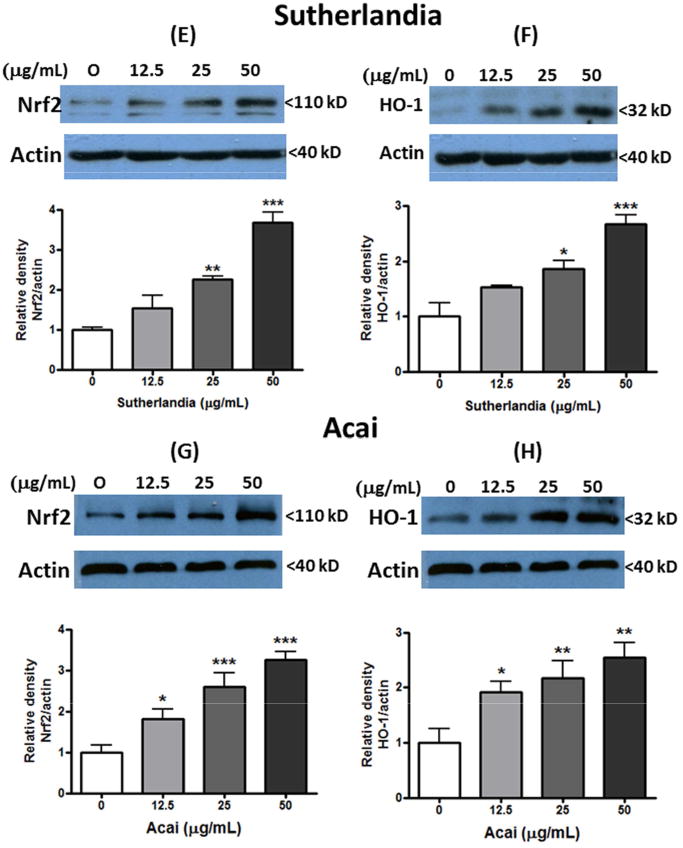
Untransfected DI TNC1 astrocytes were treated with quercetin (A, B), or Ashwagandha (C, D), or Sutherlandia (E, F) or Açaí (G, H) extracts at the indicated concentrations for 6 h. Nrf2 (A, C, E,F) and HO-1 (B, D, F, H) expression was determined by Western blot analysis, as described in Methods. Representative blots and quantitative analysis (bar graphs) are shown. Results are expressed as the mean ± SEM (n=3-6) and analyzed by one-way ANOVA with Bonferroni post-tests, where *p<0.05, **p<0.01, ***p<0.001 vs. control.
4. Discussion
In this study, an immortalized rat astrocyte line (DI TNC1) was stably transfected either with the luciferase gene driven by NF-κB or Nrf2/ARE promoters and used to assess the ability of botanicals to regulate oxidative/inflammatory and anti-oxidative pathways. Based on our previous studies, indirect assays suggested that NF-κB activity in DI TNC1 astrocytes could be stimulated by TNFα, IL-1β or LPS, but not IFNγ (Jensen et al., 2009, Sheng et al., 2011). In this study, a similar profile was observed using cells transfected with the luciferase gene driven by the NF-κB promoter (Fig. 1A). Study with BV-2 microglial cells showed that polyphenols such as quercetin effectively inhibited LPS-induced iNOS/NO production, as an indirect indication of the NF-κB signaling pathway (Simonyi et al., 2015). In this study with DI TNC astrocytes, quercetin was shown to directly inhibit NF-κB promoter activity (Fig. 1B). Similar to microglial cells and cultured human keratinocytes, study here with DI TNC1 astrocytes also showed little effects for cyanidin, rutin and cyanidin-3-O-glucosides to mitigate LPS-induced NO production (Fig. 1C) (Ernst et al., 2012, Simonyi et al., 2015).
Aside from inhibition of LPS-induced responses, botanical polyphenols such as quercetin are active in stimulating the anti-oxidant pathway involving Nrf2 (Sun et al., 2015). Study here with DI TNC astrocytes showed that quercetin also was effective in stimulating the ARE promoter, whereas polyphenols such as cyanidin, rutin and C-3-G were less effective (Fig. 3A and B). However, unlike microglial cells, DI TNC1 astrocytes showed only a small increase in ARE activity when LPS was added after pretreatment with quercetin (Sun et al., 2015). The small increase in ARE activity in response to LPS in DI TNC1 astrocytes is probably due to the low levels of Toll-like receptors in these cells as compared to that in microglial cells. The present study demonstrates that different polyphenols and botanical extracts produce NF-κB- and ARE-dependent responses to different extents in astrocytes (Dajas et al., 2013).
In agreement with the mechanism for release of Nrf2 through the Keap1 pathway, expression of Nrf2 in DI TNC astrocytes are normally maintained at low levels in the absence of stimuli or electrophiles that target the Keap1 E3 ubiquitin ligase (Zhang et al., 2004). Increasing levels of quercetin and botanical extracts induced increasing expression of Nrf2 and HO-1. However, differences in ARE activity and HO-1 expression exist among different botanical extracts. Of particular interest is Ashwagandha extract, which showed higher levels of Nrf2 and HO-1 expression than the Sutherlandia and Açaí extract (Fig. 5). Ashwagandha belongs to the Solanaceae or nightshade family and has been known for decades as an Ayurvedic herb that is one of the most effective remedies for promoting health and quality of life (Singh et al., 2011). Recent clinical trials and cell and animal studies also indicate that Ashwagandha extracts have immune-modulative, anti-oxidative and anti-inflammatory properties, and ameliorate both acute and chronic disorders of the CNS (Mishra et al., 2000, Ven Murthy et al., 2010). Besides the roots, extracts of Ashwagandha leaves also have neuroprotective effects, including reversal of Alzheimer's and Parkinson's disease pathologies, protection from environmental neurotoxins and enhancement of memory (Wadhwa et al., 2015). The observation that Ashwagandha extract has exceptional ability to modulate the Nrf2 pathways in astrocytes may have important implications for its use as an adaptogen to enhance brain activity.
Sutherlandia is a medicinal plant indigenous to southern Africa and used in folk and contemporary remedies for stress, chronic diseases, cancer, and HIV/AIDS (van Wyk and Albrecht, 2008, Aboyade et al., 2014). In vitro and in vivo studies with extracts prepared from the leaf and whole plant have provided evidence for its anti-proliferative, anti-viral, anti-stress, anti-diabetic, anti-inflammatory, anti-mutagenic, anti-bacterial, anti-oxidant, and immunostimulatory properties (van Wyk and Albrecht, 2008, Aboyade et al., 2014, Tobwala et al., 2014, Lei et al., 2015b). Our previous studies with microglial cells showed that ethanol extracts of Sutherlandia suppressed LPS- and IFNγ-induced ROS and NO production by inhibition of the JAK/STAT and ERK1/2 signaling pathways (Jiang et al., 2014). Similar anti-inflammatory activity was reported in murine macrophages, but did not appear to be mediated by sutherlandiosides or sutherlandins, which are glycosylated derivatives (Lei et al., 2015a). Moreover, our recent in vivo studies demonstrated that dietary Sutherlandia mitigates cerebral ischemia-induced neuronal damage, in part by attenuating p47phox and phospho-ERK1/2 expression in microglial cells(Chuang et al., 2014). The present study showed that Sutherlandia extracts not only inhibit LPS-induced NF-κB activity (Fig. 2B) but also stimulate ARE activity (Fig. 4B) and HO-1 production (Fig. 5F) in DI TNC1 astrocytes.Euterpe oleracea Mart. is a fruit-bearing palm tree from the Amazon River basin. Açaí fruit, the palm berries, is an important food for indigenous people and different parts of the plant have been traditionally used in folk medicine (Schreckinger et al., 2010, Yamaguchi et al., 2015). Açaí contains a wide variety of phytochemicals, anthocyanins, proanthocyanidins and other flavonoids as well as lignans (Schauss et al., 2006b, Chin et al., 2008). Many studies have demonstrated high anti-oxidant capacity (Schauss et al., 2006a, Jensen et al., 2008), strong anti-inflammatory, anti-oxidant, anti-carcinogenic and neuroprotective activities for this fruit (Jensen et al., 2008, Xie et al., 2012, Poulose et al., 2014). In agreement with reports indicating the ability for açaí to inhibit iNOS expression and COX activity (Matheus et al., 2006, Poulose et al., 2012), our study additionally demonstrates its ability to inhibit LPS-induced NF-kB promoter activity in DI TNC astrocytes (Fig. 2C). Study here also demonstrated ability for açaí extract to stimulate ARE reporter activity and to induce both Nrf2 and HO-1 expression in DI TNC1 cells. Our results with cell model are in line with a recent in vivo study showing a significant overexpression of Nrf2 in the hippocampus and frontal cortex of rats fed with an açaí-enriched diet (Poulose et al., 2016).
In summary, results from this cell-based study reveal similarities and differences between quercetin and several botanical extracts in the regulation of NF-κB- and Nrf2-mediated responses in DI TNC1 astrocytes. This study also provides protocols for screening the effects of phytochemicals and botanical extracts on oxidative and anti-oxidative responses in astrocytes. However, more studies are needed to test whether and how these botanicals may function as potential therapeutic targets to reduce neurodegenerative diseases.
Highlights.
DI TNC1 astrocytes stably express luciferase reporters for NF-κB or Nrf2/ARE promoter.
Phytochemicals down-regulate NF-κB and up-regulate Nrf2/ARE activity.
Ashwagandha can effectively stimulate Nrf2 and HO-1 in DI TNC1 astrocytes.
Acknowledgments
The açaí was provided by Armando U.O. Sabaa-Srur, from the Federal University of Rio de Janeiro, Brazil. This work is supported by NIH grants from the NCCAM, ODS and NCI [1P50 AT006273] and an Interdisciplinary Intercampus Research Program grant from the University of Missouri.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboyade OM, Styger G, Gibson D, Hughes G. Sutherlandia frutescens: the meeting of science and traditional knowledge. J Altern Complement Med. 2014;20:71–76. doi: 10.1089/acm.2012.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo LF, Pedruzzi LM, Stenvinkel P, Stockler-Pinto MB, Daleprane JB, Leite M, Jr, Mafra D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. 2013;95:1525–1533. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Chen JC, Ho FM, Pei-Dawn Lee C, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung W, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chin YW, Chai HB, Keller WJ, Kinghorn AD. Lignans and other constituents of the fruits of Euterpe oleracea (Acai) with antioxidant and cytoprotective activities. J Agric Food Chem. 2008;56:7759–7764. doi: 10.1021/jf801792n. [DOI] [PubMed] [Google Scholar]
- Chuang DY, Cui J, Simonyi A, Engel VA, Chen S, Fritsche KL, Thomas AL, Applequist WL, Folk WR, Lubahn DB, Sun AY, Sun GY, Gu Z. Dietary Sutherlandia and elderberry mitigate cerebral ischemia-induced neuronal damage and attenuate p47phox and phospho-ERK1/2 expression in microglial cells. ASN neuro. 2014;6 doi: 10.1177/1759091414554946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DY, Simonyi A, Kotzbauer PT, Gu Z, Sun GY. Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J Neuroinflammation. 2015;12:199. doi: 10.1186/s12974-015-0419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas F, Andres AC, Florencia A, Carolina E, Felicia RM. Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features. Cent Nerv Syst Agents Med Chem. 2013;13:30–35. doi: 10.2174/1871524911313010005. [DOI] [PubMed] [Google Scholar]
- Ernst IM, Wagner AE, Huebbe P, Rimbach G. Cyanidin does not affect sulforaphane-mediated Nrf2 induction in cultured human keratinocytes. Br J Nutr. 2012;107:360–363. doi: 10.1017/S0007114511002984. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li W, Su ZY, Kong AT. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015 doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybertson BM, Gao B. Role of the Nrf2 signaling system in health and disease. Clin Genet. 2014;86:447–452. doi: 10.1111/cge.12474. [DOI] [PubMed] [Google Scholar]
- Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG, Scherwitz L, Beaman R, Endres JR, Schauss AG. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem. 2008;56:8326–8333. doi: 10.1021/jf8016157. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Sheng W, Simonyi A, Johnson GS, Sun AY, Sun GY. Involvement of oxidative pathways in cytokine-induced secretory phospholipase A2-IIA in astrocytes. Neurochem Int. 2009;55:362–368. doi: 10.1016/j.neuint.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Chuang DY, Zong Y, Patel J, Brownstein K, Lei W, Lu CH, Simonyi A, Gu Z, Cui J, Rottinghaus GE, Fritsche KL, Lubahn DB, Folk WR, Sun GY. Sutherlandia frutescens ethanol extracts inhibit oxidative stress and inflammatory responses in neurons and microglial cells. PloS one. 2014;9:e89748. doi: 10.1371/journal.pone.0089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CH, Choi YH, Moon SK, Kim WJ, Kim GY. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-kappaB pathway and activating the Nrf2-dependent HO-1 pathway. Int Immunopharmacol. 2013;17:808–813. doi: 10.1016/j.intimp.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Lee DS, Kim KS, Ko W, Li B, Keo S, Jeong GS, Oh H, Kim YC. The neoflavonoid latifolin isolated from MeOH extract of Dalbergia odorifera attenuates inflammatory responses by inhibiting NFkappaB activation via Nrf2-mediated heme oxygenase-1 expression. Phytother Res. 2014a;28:1216–1223. doi: 10.1002/ptr.5119. [DOI] [PubMed] [Google Scholar]
- Lee J, Jo DG, Park D, Chung HY, Mattson MP. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol Rev. 2014b;66:815–868. doi: 10.1124/pr.113.007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Browning JD, Jr, Eichen PA, Brownstein KJ, Folk WR, Sun GY, Lubahn DB, Rottinghaus GE, Fritsche KL. Unveiling the anti-inflammatory activity of Sutherlandia frutescens using murine macrophages. Int Immunopharmacol. 2015a;29:254–262. doi: 10.1016/j.intimp.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Browning JD, Jr, Eichen PA, Lu CH, Mossine VV, Rottinghaus GE, Folk WR, Sun GY, Lubahn DB, Fritsche KL. Immuno-stimulatory activity of a polysaccharide-enriched fraction of Sutherlandia frutescens occurs by the toll-like receptor-4 signaling pathway. J Ethnopharmacol. 2015b;172:247–253. doi: 10.1016/j.jep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He YY, Xu J, Sun AY, Hsu CY, Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- Luo R, Tran K, Levine RA, Nickols SM, Monroe D, Sabaa-Srur AUO, RE S. Distinguishing components in Brazilian acai and in products obtained in the USA by using NMR. The Natural Products Journal. 2012;2:86–94. [Google Scholar]
- Matheus ME, de Oliveira Fernandes SB, Silveira CS, Rodrigues VP, de Sousa Menezes F, Fernandes PD. Inhibitory effects of Euterpe oleracea Mart. on nitric oxide production and iNOS expression. J Ethnopharmacol. 2006;107:291–296. doi: 10.1016/j.jep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- Mossine VV, Waters JK, Hannink M, Mawhinney TP. piggyBac transposon plus insulators overcome epigenetic silencing to provide for stable signaling pathway reporter cell lines. PloS one. 2013;8:e85494. doi: 10.1371/journal.pone.0085494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kim HS. Regulation of hemeoxygenase-1 gene expression by Nrf2 and c-Jun in tertiary butylhydroquinone-stimulated rat primary astrocytes. Biochem Biophys Res Commun. 2014;447:672–677. doi: 10.1016/j.bbrc.2014.04.073. [DOI] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose SM, Bielinski DF, Carey A, Schauss AG, Shukitt-Hale B. Modulation of oxidative stress, inflammation, autophagy and expression of Nrf2 in hippocampus and frontal cortex of rats fed with acai-enriched diets. Nutritional neuroscience. 2016 doi: 10.1080/1028415X.2015.1125654. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Fisher DR, Bielinski DF, Gomes SM, Rimando AM, Schauss AG, Shukitt-Hale B. Restoration of stressor-induced calcium dysregulation and autophagy inhibition by polyphenol-rich acai (Euterpe spp.) fruit pulp extracts in rodent brain cells in vitro. Nutrition. 2014;30:853–862. doi: 10.1016/j.nut.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Fisher DR, Larson J, Bielinski DF, Rimando AM, Carey AN, Schauss AG, Shukitt-Hale B. Anthocyanin-rich acai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J Agric Food Chem. 2012;60:1084–1093. doi: 10.1021/jf203989k. [DOI] [PubMed] [Google Scholar]
- Reuland DJ, Khademi S, Castle CJ, Irwin DC, McCord JM, Miller BF, Hamilton KL. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med. 2013;56:102–111. doi: 10.1016/j.freeradbiomed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Richards KM, Tran K, Levin RA, Lou R, Maia JGS, Sabaa-Srur AAU, Maciel MIS, Melo EdA, Moraes MR, Godo HT, Chaves MA, Sacramento CKd, Thomas AL, Monroe D, Smith RE. Improved extraction of soluble solids form some Brazilian and North American fruits. The Natural Products Journal. 2014;4:201–210. [Google Scholar]
- Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Molecular neurobiology. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauss AG, Wu X, Prior RL, Ou B, Huang D, Owens J, Agarwal A, Jensen GS, Hart AN, Shanbrom E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae mart. (acai) J Agric Food Chem. 2006a;54:8604–8610. doi: 10.1021/jf0609779. [DOI] [PubMed] [Google Scholar]
- Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, Kababick JP. Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae mart. (acai) J Agric Food Chem. 2006b;54:8598–8603. doi: 10.1021/jf060976g. [DOI] [PubMed] [Google Scholar]
- Schreckinger ME, Lotton J, Lila MA, de Mejia EG. Berries from South America: a comprehensive review on chemistry, health potential, and commercialization. J Med Food. 2010;13:233–246. doi: 10.1089/jmf.2009.0233. [DOI] [PubMed] [Google Scholar]
- Sheng W, Zong Y, Mohammad A, Ajit D, Cui J, Han D, Hamilton JL, Simonyi A, Sun AY, Gu Z, Hong JS, Weisman GA, Sun GY. Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA(2)-IIA expression in astrocytes and microglia. J Neuroinflammation. 2011;8:121. doi: 10.1186/1742-2094-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Chen Z, Jiang J, Zong Y, Chuang DY, Gu Z, Lu CH, Fritsche KL, Greenlief CM, Rottinghaus GE, Thomas AL, Lubahn DB, Sun GY. Inhibition of microglial activation by elderberry extracts and its phenolic components. Life sciences. 2015;128:30–38. doi: 10.1016/j.lfs.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8:208–213. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Chen Z, Jasmer KJ, Chuang DY, Gu Z, Hannink M, Simonyi A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PloS one. 2015;10:e0141509. doi: 10.1371/journal.pone.0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobwala S, Fan W, Hines CJ, Folk WR, Ercal N. Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Complement Altern Med. 2014;14:271. doi: 10.1186/1472-6882-14-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk BE, Albrecht C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae) J Ethnopharmacol. 2008;119:620–629. doi: 10.1016/j.jep.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ven Murthy MR, Ranjekar PK, Ramassamy C, Deshpande M. Scientific basis for the use of Indian ayurvedic medicinal plants in the treatment of neurodegenerative disorders: ashwagandha. Cent Nerv Syst Agents Med Chem. 2010;10:238–246. doi: 10.2174/1871524911006030238. [DOI] [PubMed] [Google Scholar]
- Wadhwa R, Konar A, Kaul SC. Nootropic potential of Ashwagandha leaves: Beyond traditional root extracts. Neurochem Int. 2015 doi: 10.1016/j.neuint.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NFkappaB response pathways. Biochemical Society transactions. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Li HH, Wu Q, Miao S, Liu ZJ, Wu P, Ye DY. Lipoxin A4 Activates Nrf2 Pathway and Ameliorates Cell Damage in Cultured Cortical Astrocytes Exposed to Oxygen-Glucose Deprivation/Reperfusion Insults. J Mol Neurosci. 2015;56:848–857. doi: 10.1007/s12031-015-0525-6. [DOI] [PubMed] [Google Scholar]
- Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S, Wu T, Wu X. The acai flavonoid velutin is a potent anti-inflammatory agent: blockade of LPS-mediated TNF-alpha and IL-6 production through inhibiting NF-kappaB activation and MAPK pathway. J Nutr Biochem. 2012;23:1184–1191. doi: 10.1016/j.jnutbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Yamaguchi KK, Pereira LF, Lamarao CV, Lima ES, da Veiga-Junior VF. Amazon acai: chemistry and biological activities: a review. Food Chem. 2015;179:137–151. doi: 10.1016/j.foodchem.2015.01.055. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


